Abstract
Objectives
The canine model has been used extensively to improve the human pancreatic islet isolation technique. At the functional level, dog islets show high similarity to human islets and thus can be a helpful tool for islet research. We describe and compare 2 manual isolation methods, M1 (initial) and M2 (modified), and analyze the variables associated with the outcomes, including islet yield, purity, and glucose-stimulated insulin secretion (GSIS).
Methods
Male mongrel dogs were used in the study. M2 (n = 7) included higher collagenase concentration, shorter digestion time, faster shaking speed, colder purification temperature, and higher differential density gradient than M1 (n = 7).
Results
Islet yield was similar between methods (3111.0 ± 309.1 and 3155.8 ± 644.5 islets/g, M1 and M2, respectively; P = 0.951). Pancreas weight and purity together were directly associated with the yield (adjusted R2 = 0.61; P = 0.002). Purity was considerably improved with M2 (96.7% ± 1.2% vs 75.0% ± 6.3%; P = 0.006). M2 improved GSIS (P = 0.021). Independently, digestion time was inversely associated with GSIS.
Conclusions
We describe an isolation method (M2) to obtain a highly pure yield of dog islets with adequate β-cell glucose responsiveness. The isolation variables associated with the outcomes in our canine model confirm previous reports in other species, including humans.
Keywords: dog islets, insulin secretion, isolation, morphometry, purity, viability
Tislethe canine model has been often exploited to improve human isolations.1–6 Dog islet isolation procedures have technical difficulties similar to those occurring during isolation of human islets,7 probably due to the similarity in the pancreas anatomy and islet architecture between the species. For example, canine and human islets, in contrast to pig islets, are almost completely encapsulated, with very little direct exocrine-to-endocrine cell-cell contact.8 Histology studies show similar amounts of collagen in pancreata from humans and dogs, significantly higher than those from rats.9 Importantly, dog β cells occupy large areas of the islet core as well as the periphery,10 similar to human but not rodent islets.11 At the functional level, canine and human islets, in contrast to rodent islets, have similar ion-channel regulation of the electrical activity induced by glucose in β cells12 and thus can be a helpful tool for islet research.
Dog islet isolations have been performed using either manual1,13,14 or automated methods.13,15,16 The automated method, compared with manual procedures, has been shown to yield a higher number of islets in different species including dogs,13 pigs,17 and humans.18 Nevertheless, because of simplicity and lower cost,19 manual methods may be a good alternative to isolate islets from large nonhuman mammals for research purposes, because large-scale isolation might not be necessary. However, an optimal isolation method should guarantee a significant islet yield and, at the same time, minimal damage to islets. Enzymatic and mechanical disruption of pancreatic tissue during isolation can affect β-cell response to glucose because of an impairment of the islet architecture, as reported in dog20,21 and human studies.22 Moreover, islet purity, specifically presence of acinar tissue in the islet yield, has been shown to affect in vitro insulin secretion, at least in rats.23 Therefore, a method that minimizes those potential hazards to isolation outcomes is desirable. In the present study, we compared 2 manual isolation methods developed in our laboratory, an initial method and a modified method, the latter in an attempt to improve the isolation outcomes. Donor- and controlled isolation-related variables were recorded, enabling us to evaluate the contribution of specific variables to isolation outcomes, including islet yield, purity, and glucose-stimulated insulin secretion (GSIS).
MATERIALS AND METHODS
Animals
Male adult mongrel dogs (1–3 years old) were used in the study. Animals were housed in kennels at the vivarium of the Keck School of Medicine at the University of Southern California. This study was approved by the ethics committee of the University of Southern California and followed an approved Institutional Animal Care and Use Committee protocol.
Reagents
CMRL-1066 medium was supplemented with fetal bovine serum (10%, vol/vol), L-glutamine (2 mmol/L), sodium pyruvate (1 mmol/L), HEPES (10 mmol/L), and antibiotic (100 U/mL penicillin + 0.1 mg/mL streptomycin). Aforementioned reagents were purchased from Invitrogen (Carlsbad, Calif). Cold (4°C) Hanks balanced salt solution (HBSS) without any supplement was used to reconstitute the enzyme collagenase. For each isolation, HBSS was supplemented with 4% bovine serum albumin (wt/vol), the pH adjusted to 7.4, and the solution kept on ice for all the subsequent steps throughout the isolation. Polysucrose/sodium diatrizoate solution (PS-D, available as Histopaque, Sigma-Aldrich, St Louis, Mo) was used for islet purification, warmed up to room temperature before use. Krebs-Ringer bicarbonate buffer modified with HEPES (KRBH) contained 140 mmol/L NaCl, 3.6 mmol/L KCl, 0.5 mmol/L NaH2PO4·H2O, 0.5 mmol/L MgSO4·7H2O, 1.5 mmol/L CaCl2·2H2O, 2 mmol/L NaHCO3, 10 mmol/L HEPES, 0.1% bovine serum albumin, and 3 mmol/L glucose. Diphenylthiocarbazone (dithizone) was used for islet staining and was prepared as previously described24 with some modification. Briefly, dithizone (10 mg) was mixed with pure ethanol (25 μL) and dissolved in 1 N NaOH (50 μL) and 0.9% NaCl (10 mL), then filtered with a 0.8/0.2-μm syringe filter (Pall Corporation, Cornwall, UK) and used fresh. Fluorescein diacetate and propidium iodide were dissolved in dimethyl sulfoxide, aliquoted and stored at −20°C. Unless otherwise specified, all chemicals and reagents were purchased from Sigma-Aldrich (St Louis, Mo or Milwaukee, Wis).
Pancreas Resection
Surgery was performed under general anesthesia. Dogs were anesthetized with sodium thiopental (1.3–1.6 mg/kg, intravenously), and anesthesia was maintained with 3% isoflurane (Abbott Laboratories, North Chicago, Ill). After a median incision in the abdomen, the distal portion of the pancreas and its supplying vessels were carefully dissected. The main supplying pancreatic vessels (splenic and pancreatoduodenal) and the pancreas, in that order, were clamped, and then the pancreas was cut transversally (~10 cm distal from the tip) and immediately immersed in HBSS (4°C). After pancreas resection, dogs were killed under anesthesia with pentobarbital sodium plus phenytoin sodium (Virbac AH, Inc, Forth Worth, Tex). All the surgeries were performed by the same team (O.O.W. and E.L.K.).
Islet Isolation
Comparison of initial (M1, n = 7) and modified isolation methods (M2, n = 7) was carried out in islets from dogs used in a diet-induced obesity model project. Dogs were fed a hypercaloric high-fat diet (~5000 kcal/d, 55% from fat) until the day before the isolations (18.3 ± 1.6 [SEM] and 17.6 ± 3.0 weeks, M1 and M2, respectively). Body weight at the time of the pancreas excision was recorded only in the M2 group (33.7 ± 1.2 kg, n = 7); therefore, body weight was not considered in the regression analysis.
After 2 to 5 minutes in HBSS, the resected pancreas was weighed and cannulated with a 24-gauge Teflon catheter (Terumo, Somerset, NJ), after which collagenase solution (~1 mL/g of tissue) was manually injected at a rate of ~10 mL/min. The distended portion was separated and immersed in a polyethylene vial containing collagenase solution (0.5 mL/g of distended tissue) and kept on ice. The nondistended portion was discarded. The concentration of collagenase solution depended on the method as indicated in Table 1. Digestion started ~10 minutes after distension, performed in a shaking water bath at 37°C, at variable rates and times (Table 1). Digestion was stopped with 20 mL of HBSS. Tissue was teased for 1 to 5 minutes, depending on the percentage of digestion, and washed 3 times with 50 mL of HBSS for 10 seconds at 100g at 4°C. The pellet was suspended in HBSS and passed 3 times through a 14-gauge needle. The tissue was then gently sieved through a 243-μm nylon mesh (Wildco, Buffalo, NY) into a 95-mm dish containing HBSS. The remnant tissue was immediately resuspended in HBSS and passed once through a 19-gauge needle, then sieved through a 500-μm nylon mesh (Wildco) to a different dish containing HBSS. The residual tissue was discarded.
TABLE 1.
Comparison of Isolation Variables Between Both Methods
| Initial Method (M1)
|
Modified Method (M2)
|
P | |
|---|---|---|---|
| n = 7 | n = 7 | ||
| Collagenase concentration, CDUs/mL | 2550 | 3800 | — |
| Collagenase injected, mL/g | 0.96 ± 0.02 | 1.0 ± 0.01 | 0.102 |
| Net collagenase activity, CDUs/g | 3100.2 ± 354.7 | 4729.2 ± 423.5 | 0.012 |
| Pancreas distension, % | 83.6 ± 6.4 | 93.6 ± 5.6 | 0.265 |
| Pancreas weight, g | 5.4 ± 0.4 | 4.5 ± 0.3 | 0.082 |
| Shaking speed, cycles/min | 88 | 300 | — |
| Time of digestion, min | 44.4 ± 0.8 | 29.4 ± 0.5 | <0.001 |
| Time of teasing, min | 4.0 ± 0.4 | 2.4 ± 0.3 | 0.003 |
| Gradient density, g/mL* | 1.119/1.083/1.008 and 1.119/1.077/1.008 | 1.119/1.077/1.008 | — |
| Purification temperature, °C | 22 | 4 | — |
See text for further details.
Thence, the sieved tissue obtained with each mesh was processed in separated 50-mL tubes (2 preparations for each dog isolation). The tissues were washed for 10 seconds at 100g (4°C) twice and for 10 seconds at 200g (4°C) once. The pellets were resuspended and gently homogenized with 15 mL of PS-D δ = 1.119 g/mL and slowly layered on top with 10 mL of PS-D of different densities, either 1.083 or 1.077 g/mL. For M1, we used δ = 1.083 for the first preparation and δ = 1.077 for the second preparation. For M2, we used δ = 1.077 for both preparations. Finally, 10 mL of HBSS (δ = 1.008 g/mL) was layered on top to build the 3-layer density gradient in each tube. The islet purification was performed at 750g in a centrifuge CR422 (Jouan, Winchester, Va) for 10 minutes without braking. Islets were handpicked from the top interface with glass Pasteur pipettes (Corning Inc, Corning, NY) and transferred to a tube containing HBSS. At this step, five 50-μL aliquots (dilution = 1:500) from each preparation were collected to assess the islet yield. The purified islet suspensions were washed for 10 seconds at 200g (4°C), then resuspended in HBSS. Both islet suspensions were additionally washed twice by manual pipetting onto 60-mm Petri dishes containing 10 mL of HBSS, and representative digital images were taken to assess the purity. Finally, islets were cultured in supplemented CMRL-1160 medium (pH 7.4) at 37°C, 5% CO2.
All the isolations with M1 were performed within a period of 10 weeks, using 2 vials of collagenase V (lot no. 047K7680, 563 collagenase digestion units [CDUs]/mg, for 6 isolations; and lot no. 026K8639, 517 CDUs/mg, for 1 isolation). All the isolations with M2 were performed within a period of 8 weeks, using 2 vials of collagenase of the same lot (077K8628, 593 CDUs/mg). Each dog islet isolation with M2 required less than 3 hours from the time of the abdominal incision until islet culture. All the isolations were performed by the same investigator (O.O.W.).
Islet Morphometry
Islet yields estimated from both preparations were summed to estimate the total number of islets per gram of tissue. Islet count (performed by the same examiner) was performed with an inverted microscope CKX41 (Olympus, San Diego, Calif) coupled to a camera Qicam Fast 1394 (Qimaging; Surrey, British Columbia, Canada), using a 4× lens objective (UplanFL N 0.13 Php; Olympus), with the help of a calibrated scale bar displayed on a PC screen using the software Image-Pro Express 5.1.0.12 (Media Cybernetics, Inc, Bethesda, Md). Islets smaller than 50 μm (maximum diameter) were excluded for manual count. We also captured images from the same aliquots to determine more accurately the mean diameter (mean geometric diameter, √ab) of each islet obtained from the major (a) and minor (b) diameters of the ellipse of equivalent area to the islet area calculated with the software ImageJ 1.4 g (National Institutes of Health). The mean geometric diameter was used for all calculations. The number of islet equivalents (IEQ, standard normalization of islet size to a 150-μm-diameter sphere) was estimated according to the conversion factors for each 50-μm mean diameter ranges, as previously described.25,26 Islets smaller than 2000 μm2 were excluded from automatic count. From these data, we also analyzed the frequency of islet size distribution and the islet volume, excluding islets with a mean geometric diameter less than 50 μm. For the estimation of the volume, islets were assumed to have a prolate spheroid shape.
Yield purity (%) from each preparation was estimated from the ratio obtained of the total number of islets (from 1 representative image) to the sum of (1) single exocrine fragments (>30 μm, arbitrarily chosen), (2) ductal tissue fragments, (3) lymph nodes, and (4) islets. The purities of the 2 preparations in each dog were averaged to obtain the total purity for each isolation.
Islet Viability
Islet Viability Stain
Fluorescein diacetate (0.67 μmol/L) and propidium iodide (75 μmol/L) were used to determine cell membrane integrity of pancreatic cells as previously described.27 Green- and red-stained cells (viable and nonviable, respectively) were detected with a fluorescence inverted microscope (IX70; Olympus).
Islet Static Incubation
After 24-hour culture, islets were washed by transferring them sequentially to 2 different dishes containing preincubated (37°C) basal KRBH (3 mmol/L glucose, pH 7.4). Then, batches of 10 to 15 islets (~100–200 μm) were handpicked and transferred to 4 independent wells (results were the mean of the 4 replicates) of a 24-well nontreated culture plate (Corning Incorporated Life Sciences, Lowell, Mass) containing 1 mL of preincubated basal KRBH. For M1, we used islets from the preparation purified with PS-D δ = 1.077 (Table 1); for M2, before islet handpicking, both preparations purified with PS-D δ = 1.077 were first combined. The plate was incubated at 37°C, 5% CO2, for 1 hour, then 200 μL of buffer was collected from each well and stored at −80°C. Glucose 50% (B/Braun, Irvine, Calif) was added to each well so the final glucose concentration was 15 mmol/L, and islets were incubated for 1 additional hour. After the stimulation period, 200 μL of buffer was collected and stored at −80°C. GSIS was expressed as the stimulation index: ratio of the insulin secreted ([pmol/L]/islet/h) during 15 mmol/L glucose stimulation to the insulin secreted with 3 mmol/L glucose.
Islet Perifusion
After 24- to 48-hour culture, 30 to 50 islets were hand-picked and plated onto 15-mm glass coverslips coated with poly-L-lysine (Sigma-Aldrich). Islets were incubated for 30 minutes at 37°C and 5% CO2, and then supplemented CMRL was added to culture them for 24 hours. Coverslips were transferred to a perifusion chamber RC-20H (Warner Instruments, Hamden, Conn), and islets were perifused at 200 μL/min with basal KRBH using a peristaltic pump (Ismatec SA, Glattbrugg, Switzerland). The whole system was kept at 37°C with a dual-channel temperature controller TC344B (Warner Instruments). After a 60-minute equilibrium period, islets were stimulated with 15 mmol/L glucose for 45 minutes, then switched to basal KRBH for 30 minutes. Samples were taken every minute starting 5 minutes before stimulation until 10 minutes after stimulation, followed by 2-minute sampling until minute 40, and then 5-minute sampling until the end of the experiment. B-cell function was expressed as [pmol/L]/islet and as percentage increases of insulin release (during stimulatory conditions) from baseline (at 3 mmol/L glucose).
Insulin Assay
Insulin was determined by enzyme-linked immunosorbent assay (human insulin kit; Millipore, St. Charles, Mo), using dog insulin (Novo Nordisk, Bagsvaerd, Denmark) to make the standards and dog plasma as quality controls. All samples were assayed in duplicate.
Statistical Analyses
All data were expressed as means ± SEM. Two-way t test for independent samples or one-way analysis of variance (ANOVA) (when appropriate) was used to compare differences between M1 and M2 groups. Multiple regression analysis was used to identify the variables associated with the isolation outcomes, including islet yield (islets/g of pancreas), yield purity, basal insulin secretion, and GSIS. The variables included for analysis of the isolation outcomes were the isolation method (categorical variable that included collagenase concentration [CDUs/mL], lot of collagenase, shaking speed, purification temperature, and type of gradient), duration of high-fat diet, amount of collagenase solution injected (mL/g of pancreas), distension of the pancreas (%), net collagenase activity (CDUs/g of pancreas), time of digestion, time of teasing, weight of the pancreas, and yield purity. We excluded the type of gradient for the analysis of the basal insulin and GSIS outcomes because assessment of insulin secretion was done only in islets purified with PS-D δ = 1.077. All analyses were performed using Statistica (StatSoft Inc, Tulsa, Okla). P < 0.05 was considered statistically significant.
RESULTS
Islet Morphometry
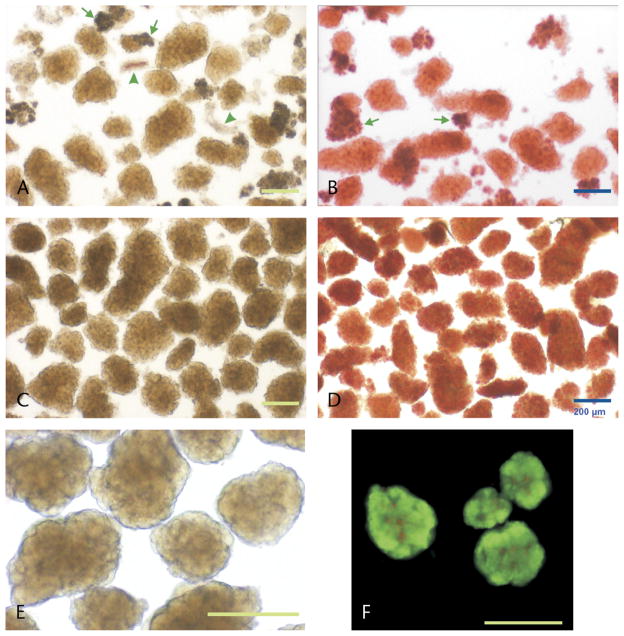
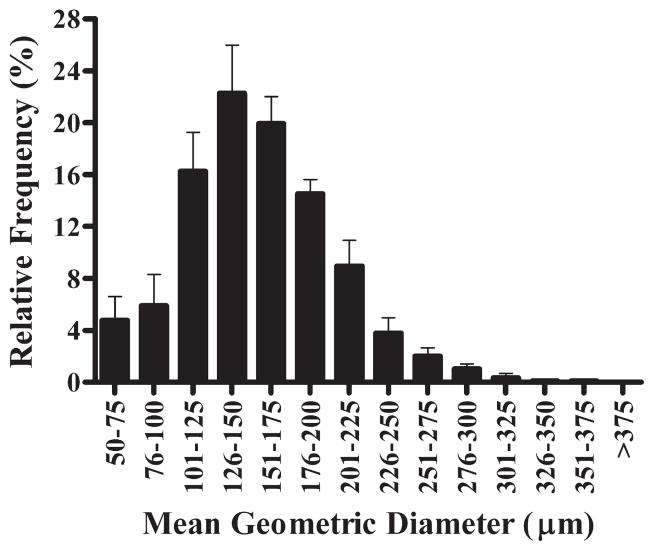
Most of the fresh islets had the appearance of brown spheroids, confirmed with dithizone (40–100 μL/mL). Islets initially did not have regular edges, but a more compact shape was apparent after 24-hour culture, indicating recovery after isolation (Figs. 1A–E). In a subset of isolations performed with M2 from fat-diet–fed dogs (n = 5, body weight: 34.0 ± 1.7 kg), we assessed the islet size using a computerized algorithm in ImageJ. Islets had a mean geometric diameter of 157.9 ± 1.9 μm, and the largest islet had a mean diameter of 356.8 μm (from a total of 644 islets analyzed). Islet size between 125 and 175 μm represented 42.2% ± 2.7% of the population (Fig. 2). The mean islet volume was estimated in 2.26 × 10−3 ± 0.09 μL with a wide range (0.07–21.75 × 10−3 μL).
FIGURE 1.
Pancreatic islets isolated from high-fat–fed dogs. A, Fresh isolated islets with the initial method (M1) after purification with polysucrose/sodium diatrizoate (PS-D). B, Islets from the same preparation as in A, stained with the zinc chelator dithizone. C–F, Representative images of islets isolated with the modified method (M2), after purification with PS-D. C, Fresh islets shown after purification. D, Islet stained with dithizone. E, Islets after 24-hour culture in CMRL medium at 37°C. Note the appearance of a more compact islet shape after culture (visual magnification of 100×). F, Viability stain with fluorescein diacetate and propidium iodide in islets cultured for 48 hours. Green stain indicates cell membrane integrity, whereas red stain represents nonviable cells. Arrows and arrowheads indicate exocrine and ductal tissue, respectively. Scale bar: 200 μm, for all insets.
FIGURE 2.
Frequency distribution of the size of islets isolated from high-fat–fed dogs using the modified isolation method (M2). Islets between 100 and 200 μm represent ~75% of the total population. Columns represent mean values (n = 5) ± SEM.
Islet Yield and Purity
After purification, cleaved islets were located virtually only in the upper interface. M1 and M2 yielded similar total number of islets (3111.0 ± 309.1 vs 3155.8 ± 644.5 islets/g, respectively; P = 0.951) (Fig. 3A). The number of IEQ/g yielded with M2 (n = 5) was 3653.3 ± 1073.0 (Fig. 3B). Weight of the pancreas was the only variable independently associated with the total islet yield (r = 0.68; P = 0.008). Multiple regression analysis showed that the weight of the pancreas (partial correlation = 0.82; β = 1.1; P < 0.001) and the purity of the yield (partial correlation = 0.63; β = 0.63; P = 0.022) together were directly associated with the islet yield (adjusted R2 = 0.61; P = 0.002).
FIGURE 3.
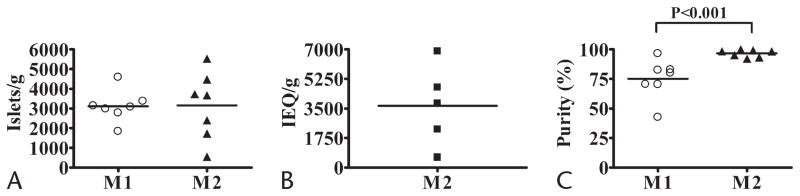
Yield and purity after gradient purification of pancreatic islets from high-fat–fed dogs. A, Total number of islets yielded with the initial isolation method (M1, n = 7) and the modified isolation method (M2, n = 7). B, Yield of islets expressed as IEQ (size normalized to 150-μm-diameter sphere) isolated with M2 (n = 5). C, Purity of the yield with M1 (n = 7) and M2 (n = 7). In each panel, horizontal bars indicate the mean values; plots (circles, triangles, and squares) represent individual values of each dog.
The purity of the yield was significantly higher with M2 compared with M1 (96.7% ± 1.2% [92.3%–100%] vs 75.0% ± 6.3%; P < 0.001; Fig. 3C). We found that PS-D δ = 1.077, when used with M2, was superior (96.7% ± 1.2%) to δ = 1.083 used with M1 (71.1% ± 6.7%; P = 0.012, ANOVA), but not significantly different to δ = 1.077 used with M1 (79.0% ± 6.8%; P = 0.584, ANOVA). Purity was independently associated with net collagenase activity (CDUs/g, r = 0.64; P = 0.013), time of digestion (r = −0.68; P = 0.008), and weight of the pancreas (r = −0.68; P = 0.007). High collagenase concentration, fast shaking speed, cold purification, and PS-D δ = 1.077 together (isolation method) accounted for the improvement in the purity of the yield (adjusted R2 = 0.44; β = 0.70; P = 0.006). Inclusion of weight of the pancreas in the model resulted in an improvement of the association (adjusted R2 = 0.58; P = 0.004), although this variable did not reach statistical significance independently (partial correlations: 0.58 [P = 0.04] and −0.55 [P = 0.051], isolation method and weight of the pancreas, respectively). Nevertheless, the last model indicates that there is a trend to a lower purity when the weight of the pancreas is higher.
Islet Viability
Islet Viability Stain
Fluorescent stain with fluorescein diacetate and propidium iodide, the current international standard to assess islet viability,28 showed integrity of the plasma membrane of pancreatic cells from islets isolated with M2. Only a small fraction of cells were not viable, particularly in the core (Fig. 1F).
Islet Static Incubation
Within-assay coefficient of variation of the insulin samples analyzed was 4.02% ± 0.6%. We found no differences in basal insulin secretion between M1 and M2 (3.5 ± 1.2 and 2.6 ± 1.2 [pmol/L]/islet/h, respectively; P = 0.566). There was no correlation between basal insulin secretion and other variables. Interestingly, islets isolated with M2 showed a 3-fold improvement in GSIS compared with those isolated with M1 (stimulation index: 11.5 ± 2.9 vs 3.4 ± 1.0; P = 0.021). Glucose-stimulated insulin secretion was inversely associated with the time of digestion (r = −0.61; P = 0.021). High collagenase concentration, fast shaking speed, cold purification, and PS-D δ = 1.077 together accounted for the higher GSIS (adjusted R2 = 0.32; β = 0.61; P = 0.021).
Islet Perifusion
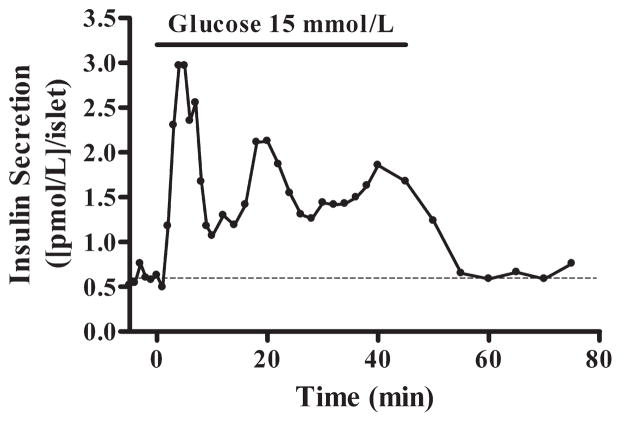
A separate group of dogs (n = 3; body weight, 28.2 ± 1.7 kg) was used to further evaluate viability of islets isolated with M2 by exploring the phases of insulin response to glucose. Islets stimulated with 15 mmol/L glucose secreted insulin in a biphasic manner (Fig. 4). The first peak of insulin secretion during high glucose stimulation showed an increase by 397.0% ± 140.0% of the baseline, whereas the area under the curve of insulin release throughout the stimulation period showed an increase by 222.3% ± 53.8%.
FIGURE 4.
Perifusion of dog pancreatic islets isolated with M2. Trace shows the typical experimental biphasic insulin secretion in response to 15 mmol/L glucose of islets cultured for 72 hours. Stimulated insulin secretion returns to baseline (dashed) after switching to 3 mmol/L glucose.
DISCUSSION
Islet Morphometry
As previously reported,29 fresh isolated dog islets had the appearance of spheroids, of brown color, with irregular edges. The highest frequency of islets had a diameter between 100 and 150 μm (Fig. 2). Similar islet size frequency distribution has been reported in lean mongrel dogs.30 In contrast, other studies found a predominant islet size between 50 and 100 μm in beagle31 and mongrel dogs.32 Because a higher peak of distribution frequency of less than 100 μm has been associated with islet fragmentation during isolations in dogs33 and rats,34 it is likely that the modified isolation method in the present study caused minimal islet fragmentation.
Islet Yield and Purity
After purification, we obtained a yield ~3600 IEQ/g (~3200 islets/g) with M2. Our results are similar to those in previous dog islet studies using manual isolation methods,1,13 but not superior to yields obtained with automated methods.35,36 However, the fact that islets were isolated from the distal region of the pancreas from high-fat–fed animals might have contributed in part to the high yield, because the distal region has been shown to have a higher islet density in dogs31 and data from human studies have shown that body weight is directly correlated with islet yield.37,38 Nevertheless, we found no correlation between islet yield and the duration of the high-fat diet.
Weight of the pancreas and purity of the yield together were directly associated with the yield. Because purity was also directly associated with the yield in our study, it suggests that improvements in the purity of the yield might contribute to obtain a higher islet yield. Similar association between prepurification islet yield and purity has been found in nonhuman primate isolations.39 Interestingly, we were able to reach a yield purity of ~97% (92.3%–100%) using M2, superior to those reported with previous dog islet isolation methods (46%–90%).3,13,15,40 However, in our study, we picked up islets only from the upper interface, which has been reported to contain more purified islets compared with lower interfaces.41 Nevertheless, there were few islets in lower interfaces and few very small islets trapped in the pellet (personal observation).
The isolation method (high collagenase concentration, lot of collagenase, fast shaking speed, cold purification and gradient of δ = 1.077) and the weight of the pancreas together accounted for the variability of the purity. It is possible that a bigger differential among solution densities used for the purification might have contributed to the better separation of islets from exocrine tissue in M2 compared with M1, because these factors have been shown to influence the differences in the density of both tissues in human islet isolations.42 However, given that there was no difference between δ = 1.077 and δ = 1.083 densities using the same method (M1), together these results suggest that other isolation variables might have also contributed to these differences. It should be noted that although the lot of collagenase was different between both methods, it is uncertain if that contributed to the differences in the isolation outcomes, particularly in the yield, as previously reported.43 However, we observed a marked intralot variability in M2 in terms of islet yield. Variations in enzyme content between and within lots of collagenases, even in purified preparations, have been reported by several groups.43–46 We speculate that higher concentrations of collagenase in M2 compared with M1 might have led to a more pronounced effect of other existing enzymes in the collagenase preparations such as clostripain, trypsin, and neutral proteases (specified by the manufacturer) on islet isolation, contributing to better yields in some isolations or resulting in lower yields in others, due to overdigestion, as previously reported.47,48 For human islet isolations, low amounts of endogenous pancreatic enzymes can remain active throughout the isolation process49; if this occurs in canine islet isolations, it might have also influenced in part our results. Alternatively, the presence of intraislet collagenase remaining after stopping digestion as demonstrated in human islets after ductal injection of the enzyme50 could also explain the higher variability of islet yield with M2, because injected collagenase concentration was higher in M2. Finally, intradonor features are important in islet yield outcomes51; however, in the present study, there were no significant differences in donor-related variables, nor did the regression analysis show any correlation with islet yield; therefore, we consider it unlikely that those variables accounted for islet yield variability with M2.
As mentioned before, weight of the pancreas together with the purity of the yield was directly associated with the islet yield. M2 effectively separated most of the islets from the exocrine tissue. Whether improvement in the yield purity in human islet isolations could lead to greater islet yields needs to be explored, because our method of islet purification differs from the semiautomated process used in human islet isolation.52,53
Islet Viability
Fluorescent staining showed a low proportion of nonviable cells in islets isolated with M2, even after 48-hour culture (Fig. 1F). It should be noted that the utility of fluorescein diacetate and propidium iodide has been questioned by some researchers,54 arguing that fluorescein produces intense staining that obscures the propidium iodide stain. Nevertheless, we also assessed islet quality by testing β-cell function in response to high glucose concentrations, which is considered under the current guidelines for human islet assessment pretransplantation.18,28 Basal insulin secretion rates during static incubation were similar between M1 and M2 (~2.6 and ~3.5 [pmol/L]/islet/h, respectively) despite differences in time of digestion. These results do not appear to reconcile with a previous rodent study showing a direct correlation between both variables.55 However, although the time of digestion was more prolonged in the case of M1, compared with M2, the net collagenase activity per gram of tissue was less in M1.
Islets isolated with M2 showed a higher GSIS during static incubation compared with islets isolated with M1 (stimulation index, 11.5 vs 3.4), even under the same high-fat-diet conditions. Because the present study is the first report of β-cell function in vitro in high-fat–fed dogs, we cannot compare our data with other studies. Regression analysis showed that the variables controlled in the isolation method were associated with GSIS. In addition, we found that the time of digestion was inversely associated with GSIS. This last finding is consistent with a previous human islet study showing a higher GSIS with shorter exposure to enzyme digestion.38 Together, these results highlight the importance of reducing the time of digestion during isolation. To further assess the viability of islets isolated with M2, we measured changes in insulin secretion in perifused islets during a continuous stimulation with 15 mmol/L glucose. Islets secreted insulin in a biphasic manner (Fig. 4), as typically reported in human islets in vitro.56,57 Despite the use of several tests to assess islet quality (for details, refer to Papas et al28), it is debatable whether the existing tests can predict islet transplant outcomes.51,58,59 More recently, a new method has been reported to assess islet viability based on quantification of insulin mRNA to predict islet transplant outcomes in streptozotocin-induced diabetic mice.60 Both insulin released over 16-hour culture period and insulin mRNA predicted blood glucose levels on day 21 after transplantation.
In summary, this study provides 2 main findings. First, we report a simplified manual dog islet isolation method (M2) that results in a highly pure yield of islets with good β-cell glucose responsiveness. The approach is more consistent than previous dog islet isolation protocols, and the islets show a good β-cell response to stimulatory glucose concentrations in vitro. Second, analyses of the donor- and isolation-related variables in our canine model confirm previous findings from human studies. The purity of the yield and the weight of the pancreas together were directly associated with the islet yield. High collagenase concentration, fast shaking speed, cold purification, and high differential gradient together accounted for the improvement of the yield purity. β-cell response to glucose was also associated with changes in the isolation method. Time of digestion was inversely associated with GSIS.
Despite anatomical and structural similarities between human and dog pancreata, extrapolation of our results to human isolations, such as by the current standard method developed by Ricordi et al,61 should be done with caution. Our study was conducted with a small sample size. However, the results were obtained under conditions more ideal than those normally attainable in studies of human isolations. In particular, the canine population we used was of relatively homogenous age, and the islet isolations were performed immediately after pancreas procurement in heart-beating animals. Donor age, pancreas procurement, and preservation are known to have major effects on the outcomes of human islet isolations.62,63 Pancreas preservation, particularly time of ischemia, correlates with islet functionality and islet yield.63–65
In conclusion, our manual isolation method M2 may be used to obtain dog islets of consistent, highly pure yield and that retain β-cell glucose responsiveness, as would be appropriate for islet research. The isolation variables associated with the outcomes in our canine model confirm previous reports in other species, including humans.
Acknowledgments
These studies were supported by the National Institutes of Health (grants DK29867 and DK27619 to R.N.B. and grants DK60623 and GM85791 to R.H.C.).
The authors thank Edward Zuñiga and Edgardo Paredes for their technical assistance with the animals. They also thank Rita Thomas for her help with the insulin assays.
Footnotes
The authors declare no conflict of interest.
References
- 1.Noel J, Rabinovitch A, Olson L, et al. A method for large-scale, high-yield isolation of canine pancreatic islets of Langerhans. Metabolism. 1982;31:184–187. doi: 10.1016/0026-0495(82)90133-0. [DOI] [PubMed] [Google Scholar]
- 2.Mehigan DG, Zuidema GD, Cameron JL. Pancreatic islet transplantation in dogs. Critical factors in technique. Am J Surg. 1981;141:208–212. doi: 10.1016/0002-9610(81)90158-6. [DOI] [PubMed] [Google Scholar]
- 3.Alejandro R, Cutfield RG, Shienvold FL, et al. Natural history of intrahepatic canine islet cell autografts. J Clin Invest. 1986;78:1339–1348. doi: 10.1172/JCI112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warnock GL, Rajotte RV. Critical mass of purified islets that induce normoglycemia after implantation into dogs. Diabetes. 1988;37:467–470. doi: 10.2337/diab.37.4.467. [DOI] [PubMed] [Google Scholar]
- 5.van der Vliet JA, Kaufman DB, Meloche RM, et al. A simple method of canine pancreatic islet isolation and intrahepatic transplantation. J Surg Res. 1989;46:129–134. doi: 10.1016/0022-4804(89)90215-1. [DOI] [PubMed] [Google Scholar]
- 6.van der Burg MP, Guicherit OR, Frolich M, et al. Assessment of islet isolation efficacy in dogs. Cell Transplant. 1994;3:91–101. doi: 10.1177/096368979400300113. [DOI] [PubMed] [Google Scholar]
- 7.Warnock GL, Kneteman NM, Evans MG, et al. Isolation of purified large mammal and human islets of Langerhans. Horm Metab Res Suppl. 1990;25:37–44. [PubMed] [Google Scholar]
- 8.Wang RN, Paraskevas S, Rosenberg L. Characterization of integrin expression in islets isolated from hamster, canine, porcine, and human pancreas. J Histochem Cytochem. 1999;47:499–506. doi: 10.1177/002215549904700408. [DOI] [PubMed] [Google Scholar]
- 9.van Suylichem PT, van Deijnen JE, Wolters GH, et al. Amount and distribution of collagen in pancreatic tissue of different species in the perspective of islet isolation procedures. Cell Transplant. 1995;4:609–614. doi: 10.1177/096368979500400610. [DOI] [PubMed] [Google Scholar]
- 10.Redecker P, Seipelt A, Jorns A, et al. The microanatomy of canine islets of Langerhans: implications for intra-islet regulation. Anat Embryol (Berl) 1992;185:131–141. doi: 10.1007/BF00185914. [DOI] [PubMed] [Google Scholar]
- 11.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson DA, Philipson LH. Action potentials and insulin secretion: new insights into the role of Kv channels. Diabetes Obes Metab. 2007;9(suppl 2):89–98. doi: 10.1111/j.1463-1326.2007.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warnock GL, Kneteman NM, Evans MG, et al. Comparison of automated and manual methods for islet isolation. Can J Surg. 1990;33:368–371. [PubMed] [Google Scholar]
- 14.van der Burg MP, Guicherit OR, Frolich M, et al. Impact of donor-related variables on islet isolation outcome in dogs. Diabetologia. 1994;37:111–114. doi: 10.1007/BF00428786. [DOI] [PubMed] [Google Scholar]
- 15.Lakey JR, Cavanagh TJ, Zieger MA, et al. Evaluation of a purified enzyme blend for the recovery and function of canine pancreatic islets. Cell Transplant. 1998;7:365–372. doi: 10.1177/096368979800700404. [DOI] [PubMed] [Google Scholar]
- 16.Tanioka Y, Hering BJ, Sutherland DE, et al. Effect of pancreatic warm ischemia on islet yield and viability in dogs. Transplantation. 1997;64:1637–1641. doi: 10.1097/00007890-199712270-00001. [DOI] [PubMed] [Google Scholar]
- 17.Toomey P, Chadwick DR, Contractor H, et al. Porcine islet isolation: prospective comparison of automated and manual methods of pancreatic collagenase digestion. Br J Surg. 1993;80:240–243. doi: 10.1002/bjs.1800800242. [DOI] [PubMed] [Google Scholar]
- 18.Ichii H, Ricordi C. Current status of islet cell transplantation. J Hepatobiliary Pancreat Surg. 2009;16:101–112. doi: 10.1007/s00534-008-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paget M, Murray H, Bailey CJ, et al. Human islet isolation: semi-automated and manual methods. Diab Vasc Dis Res. 2007;4:7–12. doi: 10.3132/dvdr.2007.010. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg L, Wang R, Paraskevas S, et al. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery. 1999;126:393–398. [PubMed] [Google Scholar]
- 21.Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163:181–190. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 22.Nano R, Clissi B, Melzi R, et al. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48:906–912. doi: 10.1007/s00125-005-1725-3. [DOI] [PubMed] [Google Scholar]
- 23.Kessler L, Jesser C, Belcourt A, et al. Influence of acinar tissue contamination on encapsulated pancreatic islets: morphological and functional studies. Transplantation. 1997;63:1537–1540. doi: 10.1097/00007890-199705270-00032. [DOI] [PubMed] [Google Scholar]
- 24.Fiedor PS, Oluwole SF, Hardy MA. Localization of endocrine pancreatic islets. World J Surg. 1996;20:1016–1022. doi: 10.1007/s002689900155. [DOI] [PubMed] [Google Scholar]
- 25.Ricordi C. Quantitative and qualitative standards for islet isolation assessment in humans and large mammals. Pancreas. 1991;6:242–244. doi: 10.1097/00006676-199103000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27:185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 27.Ichii H, Pileggi A, Molano RD, et al. Rescue purification maximizes the use of human islet preparations for transplantation. Am J Transplant. 2005;5:21–30. doi: 10.1111/j.1600-6143.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 28.Papas KK, Suszynski TM, Colton CK. Islet assessment for transplantation. Curr Opin Organ Transplant. 2009;14:674–682. doi: 10.1097/MOT.0b013e328332a489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alderson D, Kneteman NM, Olack BJ, et al. Isolation and quantification of canine islet tissue for transplantation. Transplantation. 1987;43:579–581. doi: 10.1097/00007890-198704000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Lakey JR, Cavanagh TJ, Zieger MA. A prospective comparison of discontinuous EuroFicoll and EuroDextran gradients for islet purification. Cell Transplant. 1998;7:479–487. doi: 10.1177/096368979800700507. [DOI] [PubMed] [Google Scholar]
- 31.Nagaya M, Kubota S, Isogai A, et al. Ductular cell proliferation in islet cell neogenesis induced by incomplete ligation of the pancreatic duct in dogs. Surg Today. 2004;34:586–592. doi: 10.1007/s00595-004-2789-2. [DOI] [PubMed] [Google Scholar]
- 32.Lakey JR, Woods EJ, Zieger MA, et al. Improved islet survival and in vitro function using solubilized small intestinal submucosa. Cell Tissue Bank. 2001;2:217–224. doi: 10.1023/A:1021171200127. [DOI] [PubMed] [Google Scholar]
- 33.Alejandro R, Strasser S, Zucker PF, et al. Isolation of pancreatic islets from dogs. Semiautomated purification on albumin gradients. Transplantation. 1990;50:207–210. doi: 10.1097/00007890-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Morini S, Braun M, Onori P, et al. Morphological changes of isolated rat pancreatic islets: a structural, ultrastructural and morphometric study. J Anat. 2006;209:381–392. doi: 10.1111/j.1469-7580.2006.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arita S, Une S, Ohtsuka S, et al. Increased islet viability by addition of beraprost sodium to collagenase solution. Pancreas. 2001;23:62–67. doi: 10.1097/00006676-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Tanioka Y, Sutherland DE, Kuroda Y, et al. Preservation of dog pancreas before islet isolation with the two-layer method. Transplant Proc. 1998;30:3419–3420. doi: 10.1016/s0041-1345(98)01085-9. [DOI] [PubMed] [Google Scholar]
- 37.Lakey JR, Warnock GL, Rajotte RV, et al. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation. 1996;61:1047–1053. doi: 10.1097/00007890-199604150-00010. [DOI] [PubMed] [Google Scholar]
- 38.Goto M, Eich TM, Felldin M, et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78:1367–1375. doi: 10.1097/01.tp.0000140882.53773.dc. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto S, Iwanaga Y, Okitsu T, et al. Analysis of large-scale nonhuman primate islet isolations. Transplant Proc. 2005;37:1317–1321. doi: 10.1016/j.transproceed.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 40.Hering BJ, Muench KP, Schelz J, et al. The evaluation of neutral density separation utilizing Ficoll-sodium diatrizoate and Nycodenz and centrifugal elutriation in the purification of bovine and canine islet preparations. Horm Metab Res Suppl. 1990;25:57–63. [PubMed] [Google Scholar]
- 41.Chadwick DR, Robertson GS, Contractor H, et al. Human islet purification: a prospective comparison of Euro-Ficoll and bovine serum albumin density gradients. Acta Diabetol. 1993;30:57–59. doi: 10.1007/BF00572876. [DOI] [PubMed] [Google Scholar]
- 42.Eckhard M, Brandhorst D, Brandhorst H, et al. Optimization in osmolality and range of density of a continuous ficoll-sodium-diatrizoate gradient for isopycnic purification of isolated human islets. Transplant Proc. 2004;36:2849–2854. doi: 10.1016/j.transproceed.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 43.Kin T, Zhai X, Murdoch TB, et al. Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. Am J Transplant. 2007;7:1233–1241. doi: 10.1111/j.1600-6143.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- 44.Bucher P, Mathe Z, Morel P, et al. Assessment of a novel two-component enzyme preparation for human islet isolation and transplantation. Transplantation. 2005;79:91–97. doi: 10.1097/01.tp.0000147344.73915.c8. [DOI] [PubMed] [Google Scholar]
- 45.Barnett MJ, Zhai X, LeGatt DF, et al. Quantitative assessment of collagenase blends for human islet isolation. Transplantation. 2005;80:723–728. doi: 10.1097/01.tp.0000174133.96802.de. [DOI] [PubMed] [Google Scholar]
- 46.Antonioli B, Fermo I, Cainarca S, et al. Characterization of collagenase blend enzymes for human islet transplantation. Transplantation. 2007;84:1568–1575. doi: 10.1097/01.tp.0000295719.88525.60. [DOI] [PubMed] [Google Scholar]
- 47.Bucher P, Bosco D, Mathe Z, et al. Optimization of neutral protease to collagenase activity ratio for islet of Langerhans isolation. Transplant Proc. 2004;36:1145–1146. doi: 10.1016/j.transproceed.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 48.Heiser A, Ulrichs K, Muller-Ruchholtz W. Isolation of porcine pancreatic islets: low trypsin activity during the isolation procedure guarantees reproducible high islet yields. J Clin Lab Anal. 1994;8:407–411. doi: 10.1002/jcla.1860080611. [DOI] [PubMed] [Google Scholar]
- 49.Rose NL, Palcic MM, Lakey JR. An evaluation of endogenous pancreatic enzyme levels after human islet isolation. Pancreas. 2003;27:167–173. doi: 10.1097/00006676-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Cross SE, Hughes SJ, Partridge CJ, et al. Collagenase penetrates human pancreatic islets following standard intraductal administration. Transplantation. 2008;86:907–911. doi: 10.1097/TP.0b013e318186df87. [DOI] [PubMed] [Google Scholar]
- 51.Kin T. Islet isolation for clinical transplantation. Adv Exp Med Biol. 2010;654:683–710. doi: 10.1007/978-90-481-3271-3_30. [DOI] [PubMed] [Google Scholar]
- 52.Mineo D, Ciancio G, Burke GW, et al. Islet and pancreas transplantation. In: Efrat S, editor. Stem Cell Therapy for Diabetes. New York, NY: Humana Press; 2010. pp. 41–83. [Google Scholar]
- 53.Lakey JR, Mirbolooki M, Shapiro AM. Current status of clinical islet cell transplantation. Methods Mol Biol. 2006;333:47–104. doi: 10.1385/1-59745-049-9:47. [DOI] [PubMed] [Google Scholar]
- 54.Barnett MJ, McGhee-Wilson D, Shapiro AM, et al. Variation in human islet viability based on different membrane integrity stains. Cell Transplant. 2004;13:481–488. doi: 10.3727/000000004783983701. [DOI] [PubMed] [Google Scholar]
- 55.Pai GM, Slavin BG, Tung P, et al. Morphologic basis for loss of regulated insulin secretion by isolated rat pancreatic islets. Anat Rec. 1993;237:498–505. doi: 10.1002/ar.1092370409. [DOI] [PubMed] [Google Scholar]
- 56.Henquin J-C, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55:3470–3477. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- 57.Ritzel RA, Veldhuis JD, Butler PC. Glucose stimulates pulsatile insulin secretion from human pancreatic islets by increasing secretory burst mass: dose-response relationships. J Clin Endocrinol Metab. 2003;88:742–747. doi: 10.1210/jc.2002-021250. [DOI] [PubMed] [Google Scholar]
- 58.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010;59:1285–1291. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertuzzi F, Ricordi C. Prediction of clinical outcome in islet allotransplantation. Diabetes Care. 2007;30:410–417. doi: 10.2337/dc06-1233. [DOI] [PubMed] [Google Scholar]
- 60.Omori K, Mitsuhashi M, Todorov I, et al. Microassay for glucose-induced preproinsulin mRNA expression to assess islet functional potency for islet transplantation. Transplantation. 2010;89:146–154. doi: 10.1097/TP.0b013e3181c4218d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ricordi C, Lacy PE, Finke EH, et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 62.Balamurugan AN, Chang Y, Bertera S, et al. Suitability of human juvenile pancreatic islets for clinical use. Diabetologia. 2006;49:1845–1854. doi: 10.1007/s00125-006-0318-0. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto T, Horiguchi A, Ito M, et al. Quality control for clinical islet transplantation: organ procurement and preservation, the islet processing facility, isolation, and potency tests. J Hepatobiliary Pancreat Surg. 2009;16:131–136. doi: 10.1007/s00534-009-0064-z. [DOI] [PubMed] [Google Scholar]
- 64.Lakey JR, Kneteman NM, Rajotte RV, et al. Effect of core pancreas temperature during cadaveric procurement on human islet isolation and functional viability. Transplantation. 2002;73:1106–1110. doi: 10.1097/00007890-200204150-00016. [DOI] [PubMed] [Google Scholar]
- 65.Lee TC, Barshes NR, Brunicardi FC, et al. Procurement of the human pancreas for pancreatic islet transplantation. Transplantation. 2004;78:481–483. doi: 10.1097/01.tp.0000128910.41921.4b. [DOI] [PubMed] [Google Scholar]