Polycystic Kidney Disease (PKD)
“Every one of us was once a cyst – a blastocyst”. Jared J. Grantham (1)
Polycystic kidney diseases are characterized by the presence of fluid-filled renal cysts as well as cysts in other epithelial organs (2–4). PKD patients exhibit progressive cyst formation and massive renal enlargement that often leads to end- stage renal disease (ESRD) (5). Normal adult human kidneys make up about one half of one percent of a person’s total body weight. In contrast, a PKD patient who underwent bilateral nephrectomy after reaching ESRD had a total kidney weight of 22kg, or about twenty percent of their body weight (6). While normal renal tubules are approximately 40µm in diameter, renal tubules in a PKD patient enlarge to form cysts that measure centimeters in diameter (7). Remarkably, cysts as large as 5 cms have been reported in PKD patients (8). PKD is inherited as an autosomal dominant (ADPKD) or an autosomal recessive (ARPKD) trait (2, 9).
Autosomal Recessive Polycystic Kidney Disease (ARPKD)
ARPKD has an incidence rate of 1 in 20,000 live births (10–11) and affects primarily neonates and children (2). ARPKD fetuses present with severe polycystis kidneys, biliary tract defects and oligohydramnios. Approximately half of the ARPKD neonates die shortly after birth due to respiratory insufficiency caused by pulmonary hypoplasia. In surviving patients, morbidity and mortality are mainly caused by systemic hypertension, renal dysfunction and portal hypertension that results from portal-tract hyperplasia and fibrosis (12). Cysts in these patients develop in utero and some transient proximal tubular cysts are seen during early feal development. However, the major manifestation is seen as fusiform dilatation of the collecting ducts (2). In ARPKD, cysts are attched and do not separate from the parental tubules (13).
Mutations in a single gene, PKHD1, which encodes a transmembrane protein known as fibrocystin or polyductin causes ARPKD. Fibrocystin/polyductin is present in cortical and medullary collecting ducts and biliary ducts, and the subcellular localization of the protein include basolateral plasma membrane, primary cilia, and the centrosome in renal epithelial cells (12–13). Fibrocystin/polyductin is thought to mediate terminal differentiation of the renal collecting ducts and intrahepatic biliary ducts (12).
Autosomal Dominant Polycystic Kidney Disease (ADPKD)
ADPKD is one of the most common hereditary disorders in humans. It has an incidence of 1 in 500 to 1 in 1000 individuals, and occurs both in children and adults (2). Although ADPKD occurs more frequently than other prominent genetic disorders - it is 10 times more common than sickle cell anemia, 15 times more common than cystic fibrosis, and 20 times more common than Huntington’s disease – it does not receive the same attention as these other diseases. ADPKD is a systemic disorder which can have an adult as well as in utero onset (14–16). Extra renal manifestations in ADPKD include cysts in other epithelial organs such as liver and pancreas. ADPKD patients also show connective tissue defects such as intracranial aneurysms, cardiac valve abnormalities, aortic dissection and abdominal wall hernias (17–18). Hypertension is a very common symptom in ADPKD patients which often precedes the biochemical and clinical manifestations of the disease (19). In addition to hypertension, other variables associated with a more rapid progression to ESRD include younger age at diagnosis, male gender, increased left ventricular mass, and three or more pregnancies (15, 20–21). Progressive loss of renal function is seen in ADPKD patients (9, 13). Renal enlargement in these patients leads to the displacement of other organs and with considerable renal enlargement, ADPKD patients look chronically pregnant. These patients account for approximately 10% of ESRD cases (9, 13). ADPKD is also the fourth leading cause of ESRD requiring dialysis and transplantation in the U.S. (16, 22). Medicare costs for treating ESRD in ADPKD patients were found to exceed $200 million per year according to a report in 1993 (14).
ADPKD genes
ADPKD is genetically heterozygous. Mutations in either of two genes, PKD1 or PKD2, cause ADPKD. Mutations in the PKD1 gene, which is located on chromosome 16p13.3, cause ADPKD in 85% of ADPKD patients. The remaining 15% patients with ADPKD have mutations in the PKD2 gene, which is located on chromosome 4q21–23 (2, 23). While it was previously postulated that there might be a third gene associated with ADPKD, since some patients did not show linkage to either the PKD1 or PKD2 genes, a re-evaluation of the families in question showed there was linkage to PKD1 or PKD2 (23–25). Identical renal and extrarenal manifestations are seen in patients with PKD1 or PKD2 mutations. However, PKD2 patients show a later onset, have longer renal survival, and present fewer complications, compared to PKD1 patients (2).
The PKD1 gene is a very large gene and has 46 exons within a 52 kb genomic DNA. A region in the PKD1 gene that spans from exons 1 to 33 is duplicated at six other sites on the same chromosome, making mutation analysis of the PKD1 gene difficult (2). The PKD1 gene has some unusual structural features such as high GC content and multiple simple repeats. In addition, there is a 2.5kb polypyrimidine tract in intron 21 that may interfere with its replication, transcription, and RNA processing (13). The PKD2 gene is comparatively smaller than the PKD1 gene and is 25% homologous to a region of the PKD1 gene (2). Screening of the ADPKD population has resulted in the identification of 864 PKD1 and 139 PKD2 germ-line mutations to date (pkdb.mayo.edu). Most of these mutations produce truncated protein products due to nonsense changes, splicing defects, frame-shift deletion or frame-shift insertions. However, some missense and in-frame mutations have also been described (24). The protein products of the PKD1 and PKD2 genes are collectively called polycystins.
ADPKD- a developmental disorder
In most ADPKD patients renal dysfunction does not become apparent until the fourth or the fifth decade of life. However, there is increasing evidence suggesting that cystogenesis occurs as early as in utero in these patients. Even with an ultrasound detection threshold of >7.0mm, cysts have been detected at birth in ADPKD patients (26). Cysts have also been detected in stillborn fetuses, live born babies immediately after birth, and in infants (27). Moreover, a study measuring the growth rates of individual cysts in adult ADPKD patients suggests that cysts detected in newborn patients must have grown at vigorous rates in utero and at slower rates thereafter (26, 28).
Mammalian kidney development
The nephron, the functional unit of the excretory system in vertebrates, has similar characteristic features in lower vertebrates as in higher vertebrates, such as mammals (29). As it develops, the mammalian kidney goes through three spatially and temporally different stages known as the pronephros, the mesonephros, and the metanephros (29–32). The mammalian kidney is derived from the intermediate mesoderm (IM), which lies between the axial and the lateral plate mesoderm (33–34). The different stages of mammalian kidney development in a mouse embryo are shown in Figure 1. The first step in mammalian kidney development, the primary nephric duct, also known as the Wolffian duct or the pronephric duct, arises from the IM and grows in the rostral to caudal direction (33, 35). The elongating pronephric duct induces the formation of two sets of tubules in the adjacent undifferentiated mesoderm. In the mouse embryo, these inductive events take place at embryonic day 8 (E8) and E10 to produce the pronephros and the mesonephros, respectively. Both of these structures are transient in mice and humans (35). In humans, the pronephros can be seen by E22 and the mesonephros by E24 (31). The pronephric tubules are rudimentary structures and do not have any functional role in the mouse embryo. However, the caudal mesonephric tubules are well developed with glomeruli and convoluted proximal tubule-like structures and serve as transient filtration units that empty into the nephric duct (33–35). The mesonephros degenerates as the metanephric kidneys form in mammals (33).
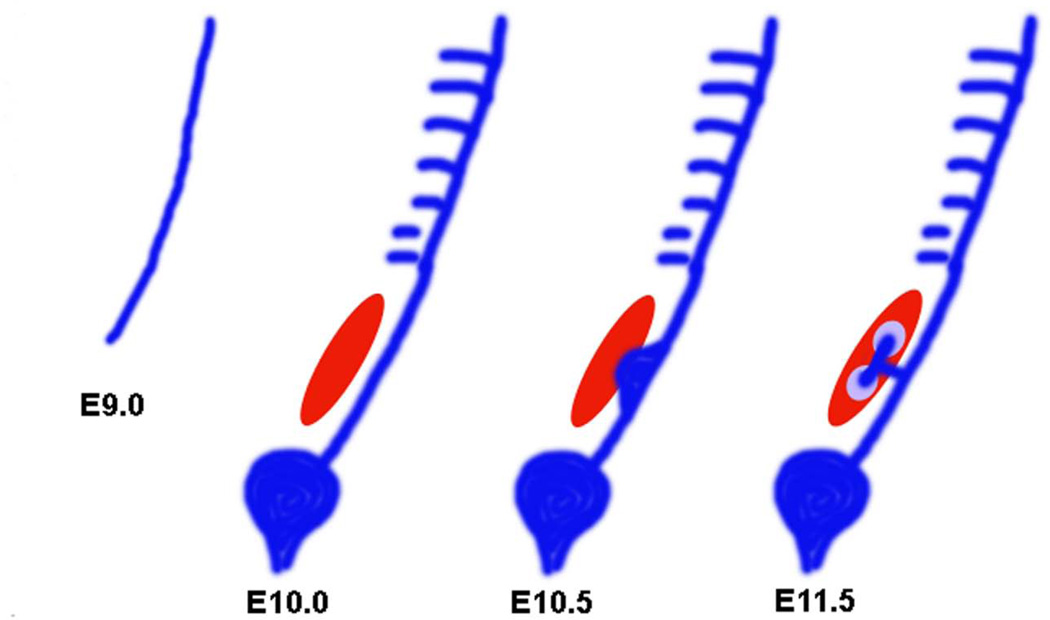
Figure 1. Stages of kidney development in the mouse embryo.
The pronephric duct arises from the intermediate mesoderm in the mouse embryo at E9.0. The nephric duct grows caudally until it reaches the cloaca. Mesonephric tubules can be seen at E10.0. The mesonephric tubules which are found more caudally, are more developed compared to the proximal ones. Posterior cells of the intermediate mesoderm specialize to form an aggregate called metanephric mesenchyme (red). The metanephric mesenchyme gives rise to all the segments of the nephron. The ureteric bud, an outgrowth from the nephric duct invades the MM by E10.5. The UB gives rise to the collecting system of the kidneys. The UB bifurcates and the induced mesenchyme, known as the cap mesenchyme (purple), surrounds the tips of the UB.
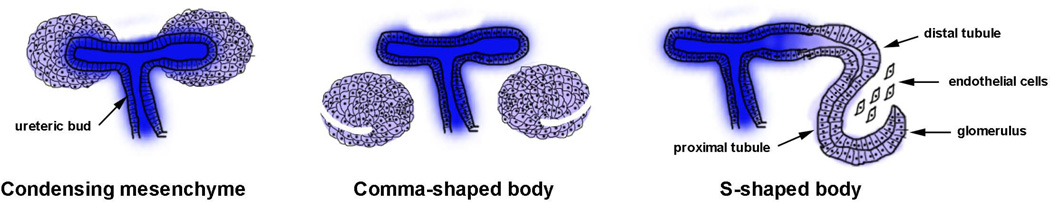
The functional kidney in adult mammals, the metanephros, forms at the posterior end of the IM (34). An outgrowth of the primary nephric duct called ureteric bud (UB) extends into a specialized surrounding region of the IM known as the metanephric mesenchyme (MM). The MM contains the progenitor cells of nephrons, the functional units of the adult kidney. In the mouse embryo, at E10.5, the MM also provides inductive signals for the primary nephric duct to evaginate and to form the UB at its caudal end. The epithelial cells of the UB invade the MM and undergo branching morphogenesis to generate the collecting system of the kidneys. At the same time, mesenchymal cells from the MM, aggregate around the tips of the newly formed UB branches and begin a mesenchyme-to-epithelial conversion (MET). This process generates the nephron epithelia and is therefore called nephrogenesis (33, 36). During nephrogenesis, the mesenchymal aggregates that form around the UB, called cap mesenchyme, go through three different stages known as the polarized renal vesicle, the comma-shaped body, and the S-shaped body. One end of the renal vesicle remains in contact with the UB epithelium and two clefts are formed in the renal vesicle, one after the other, to form the comma and the S-shaped bodies, respectively. The distal end of the S-shaped body that remained in contact with the UB fuses with it to form a single, continuous epithelial tubule. Subsequently, endothelial cells invade the more proximal cleft in the S-shaped body and form the glomerular tuft. The ureteric bud branches continuously, inducing new nephrons along the radial axis of the kidney. By virtue of this process, the oldest nephrons are found close to the medullary region while the youngest nephrons are located more peripherally in the nephrogenic zone in a developing kidney (33). In the mouse embryo, metanephric induction starts at E10.5 and nephrogenesis continues until a week after birth (34, 37). In humans, the metanephros develops at around E35 to E37 (31). The stages of nephrogenesis are shown in figure 2.
Figure 2. Stages of nephrogenesis.
The adult metanephric kidney in mammals develops by a reciprocal interaction between the epithelial ureteric bud (blue) and the metanephric mesenchyme (purple). During the condensation stage, the loose mesenchymal cells of the MM condense around the tips of the epithelial UB. After the condensation stage, the induced mesenchyme undergoes a mesenchyme-to-epithelial conversion forms the comma and S-shaped structures. Later, the tubules elongate and the podocytes in the glomeruli fold to give rise to a mature nephron.
The molecular signals underpinning the development of the kidney have been the subject of intense investigation. For the purposes of this review, we will focus on the signals involved in the development of the metanephros, the permanent kidney in mammals. As described above, the metanephros begins to form when an outgrowth of the nephric duct, called the ureteric bud, makes contact with an undifferentiated patch of mesenchymal cells, called the metanephric mesenchyme. The induction of ureteric bud growth is induced by the interaction of glial cell derived neurotrophic factor (gdnf), secreted by the metanephric mesenchyme, with the tyrosine kinase receptor c-ret, and its co-receptor Gfrα1 (38). c-ret and Gfrα1 are expressed along the length of the nephric duct prior to induction by gdnf. As development proceeds, this expression becomes restricted to the caudal portion of the duct. The relationship between gdnf and c-ret was further established experimentally. When a local source of gdnf is placed near the nephric duct, ectopic ureteric buds are induced to form and grow towards the source (39). The ability of gdnf to induce ectopic buds is restricted by signaling between the receptor Robo2 and its ligand Slit2, and by the transcription factor Foxc1 in metanephric mesenchyme (40–41). The expression of Slit2 is inverse to the expression of gdnf, suggesting that reduced Robo2 signaling permits gdnf expression (40). Within the nephric duct, an inhibitor of receptor tyrosine kinases, called Sprouty, inhibits ret-gdnf signaling, preventing ectopic UB formation (42–43). BMP4 is expressed in the mesenchymal cells surrounding the nephric duct where it functions to inhibit gdnf, further preventing the induction of multiple ureteric buds (44). The BMP4 antagonist Gremlin1 is expressed in the mesenchyme, permitting a narrow avenue of gdnf between the metanephric mesenchyme and the nephric duct regulating the point of ureteric bud outgrowth (44). Together, these factors precisely regulate the interaction of the ureteric bud with the metanephric mesenchyme ensuring that only a single ureter develops.
While the ureteric bud develops into the collecting duct system, the nephron proper arises from the cap mesenchyme. A number of transcription factors have been identified that function in the establishment and survival of the metanephric mesenchyme. These include Wt1, Pax2, Eya1, Sall1, and the three Hox11 paralogues (Hoxa11, Hoxc11, and Hoxd11) (45–47). Following the induction of mesenchymal condensation, a population of self-renewing cells is formed that are the stem cells of the nephron. These cells give rise to the epithelial cells that will form the glomerulus, proximal and distal tubules, and the loop of Henle. A host of signaling molecules and transcription factors have been identified that regulate the progression through an orderly sequence of developmental stages that can be recognized morphologically. These stages are the renal vesicle (stage I), comma and S-shaped bodies (stage II), developing capillary loop (stage III), and maturing glomerulus (stage IV). Several excellent reviews describing the molecular mechanisms regulating this process are available (38, 48–49).
Polycystins
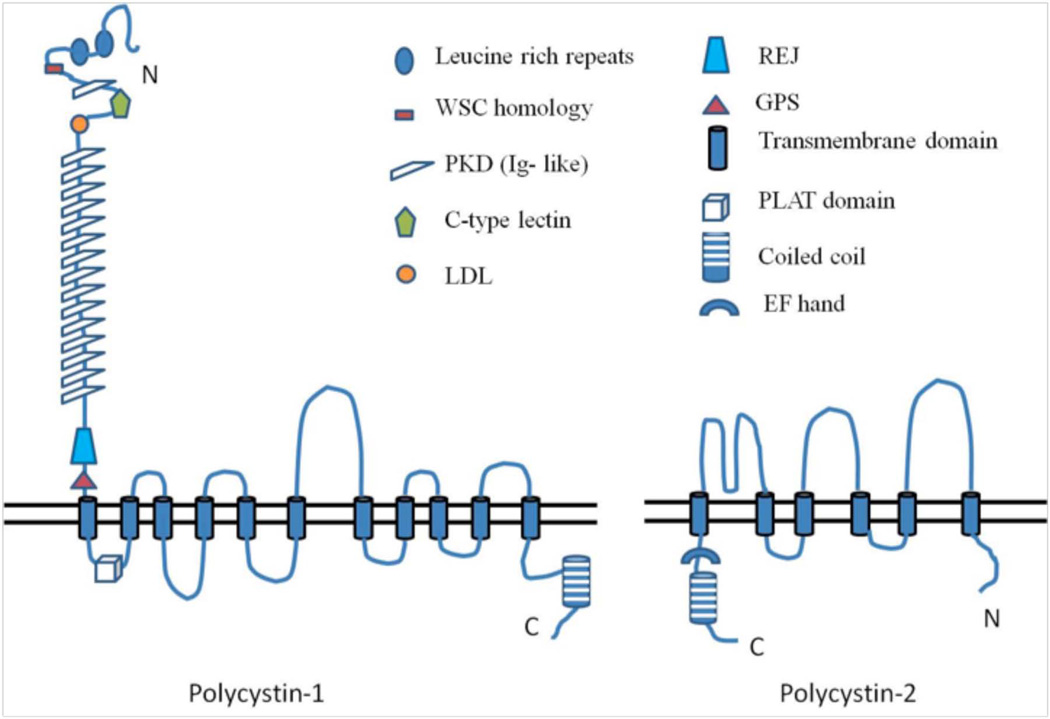
Polycystin-1 (PC1), the protein product of the PKD1 gene, is an integral membrane protein with a large extracellular N-terminal domain (~3000 amino acids), eleven transmembrane domains (~1000 amino acids), and a C-terminal cytosolic domain of about 200–225 amino acids (2, 50). The extracellular domain of PC1 contains a region that is homologous to a sea urchin protein, receptor for egg jelly, or REJ. Regulation of ion transport in sea urchin sperm involves the REJ module of the REJ protein. Likewise, the REJ domain in PC1 is thought to be involved in ion transport (51). The PC1 extracellular domain also contains several other domains such as leucine-rich repeats (LRRs), a C-type lectin domain, an LDL-A region, and multiple Ig-like domains or PKD domains, all of which are implicated in cell-cell or cell-matrix interactions (2, 13, 16, 52).
The first cytoplasmic loop of PC1 contains a lipoxygenase domain (PLAT domain named after polycystin-1, lipoxygenase and alpha toxin), which is essentially a β-sandwich domain. PLAT domains are usually involved in protein-protein or protein-lipid interactions and in PC1, the presence of a PLAT domain may indicate its interaction with other proteins (53).
The cytoplasmic tail of PC1 is found to bind heterotrimeric G proteins in vitro, activate the AP-1 transcription factor, and take part in the regulation of Wnt signaling (54). The cytoplasmic tail contains a coiled-coil domain with which it interacts with polycystin-2 (PC2) (13). A sequence motif that is rich in proline, glutamic acid, serine and threonine (PEST), which facilitates ubiquitin-mediated degradation, is also seen in the PC1 C-tail (55). In addition, the PC1 C-tail contains two stretches of basic amino acid residues that make up a putative nuclear localization sequence (NLS) (56).
PC1 is present on the plasma membrane at focal adhesions, desmosomes, tight junctions and adherens junctions, and also in the shaft and basal body of primary cilia (2, 23, 57). PC1 is also found in urinary exosomes, which are small vesicles (50–100nm) secreted by the renal epithelial cells into urine. Normal urine shows the presence of thousands of proteins, most of which are packaged into exosomes and thereafter shed into the urine. These exosomes are products of the multivesicular body sorting pathway (MVB). The MVB-sorting pathway consists of the endocytosis of the integral membrane proteins to form endosomes, fusion of these endosomes with the MVB, and finally fusion of the MVB with the apical plasma membrane releasing exosomes. Hogan et al reported the presence of PC1 and Polycystin 2 (PC2) in urinary exosomes that can interact with renal primary cilia (58–59) http://dir.nhlbi.nih.gov/papers/lkem/exosome/).
PC2, the protein encoded by the PKD2 gene, is also a membrane-associated protein with six transmembrane domains and cytoplasmic N- and C- terminal domains. PC2, also known as TRPP2, is a member of the family of transient receptor potential (TRP) ion channels. It is a non-selective cation channel permeable to Ca2+, Na+ and K+ ions. The level of intracellular calcium modulates the channel activity of PC2. PC2 is insensitive to two other ligand-gated calcium channels, IP3 receptors and ryanodine receptors, and is proposed to be a third class of calcium release channels in addition to the other two. PC2 has been shown to form heteromeric channels with two other TRP family members, TRPC1 and TRPV4 (57, 60).
PC2 is found on the apical and basolateral plasma membrane, endoplasmic reticulum, Golgi, shaft and basal bodies of the primary cilia, and in urinary exosomes (13, 23, 57). The trafficking of PC2 among the ER, Golgi, and plasma membrane is modulated by the phosphorylation of an acidic cluster present on its C-terminal tail by casein kinase II (57). Most of the cellular pool of PC2 is found in the intracellular compartments where it modulates the release of calcium from intracellular stores. There are at least two cytoplasmic domains in PC2, one in the N-terminus and the other in the C-terminus that contribute to the oligomerization of PC2. The C-terminus of PC2 contains a coiled coil domain with which it interacts with PC1, an EF hand characteristic of calcium binding proteins, and an ER retention signal that localizes PC2 to the ER and Golgi compartments (55).
Expression of polycystins during kidney development and in ADPKD
The expression pattern of the polycystins in the developing human fetal kidneys between the gestational ages of 13–40 weeks has been described. PC1 and PC2 are weakly expressed in early nephrogenic precursors such as comma and S-shaped bodies. Weak, but detectable expression of the polycystins was also seen in the proximal and distal branches of the ureteric bud (35, 55). Maturing proximal and distal tubules and collecting ducts showed a marked level of expression of the polycystins. While proximal tubules sustain polycystin expression until 28 weeks of gestation, both cortical and medullary collecting ducts maintain expression until 40 weeks of gestation. Analysis of the adult human kidneys also showed a weak expression pattern of polycystins in the proximal tubules. Continued expression and colocalization of PC1and PC2 were seen in the medullary collecting ducts (61).
Similar to the expression profile of PC1 in humans, murine PC1 was also widely expressed in the kidneys and other organs such as brain, liver, pancreas, small intestine, lung and heart (62). Developing kidneys showed the weakest expression of PC1 in the ureteric bud tips, suggesting that PC1 may not be required for nephrogenic induction. The UB derivatives, including the collecting ducts, papillary ducts and renal pelvis showed a strong expression of PC1 during later developmental stages. This tubular expression of PC1 continued after birth and was maintained until the third week postnatally, after which PC1 expression became undetectable (62). The absence of PC1 expression in the earliest stages of the developing collecting duct is consistent with the late onset of cystic disease in the Pkd1 knockout mouse models (discussed later in this review).
The murine embryonic ectoderm and endoderm showed the presence of PC2 as early as embryonic day 6. PC2 was widely expressed in the embryonic stages while the expression became more restricted after birth. By E12.5, the metanephric UB showed low-intensity staining for PC2. The developing proximal tubules showed a weaker staining while the distal tubules strongly expressed PC2 by E15.5. Medullary collecting ducts showed the expression of PC2 by postnatal day 14 (63).
Ward CJ et al described the expression pattern of PC1 in adult human tissues, in ADPKD kidneys and also in a polycystic liver (52). Quantification of PC1 expression in adult human tissues showed the highest PC1 expression was in the brain, the lowest was in the thymus, and an intermediate level of expression was found in the kidneys. This extensive expression of PC1 in a variety of tissues may explain the systemic nature of the disease. When analyzed, end-stage ADPKD kidneys and ADPKD polycystic livers, showed an approximately 2-fold increase in PC1 mRNA compared to normal kidneys and livers (52).
How do cysts form in ADPKD?
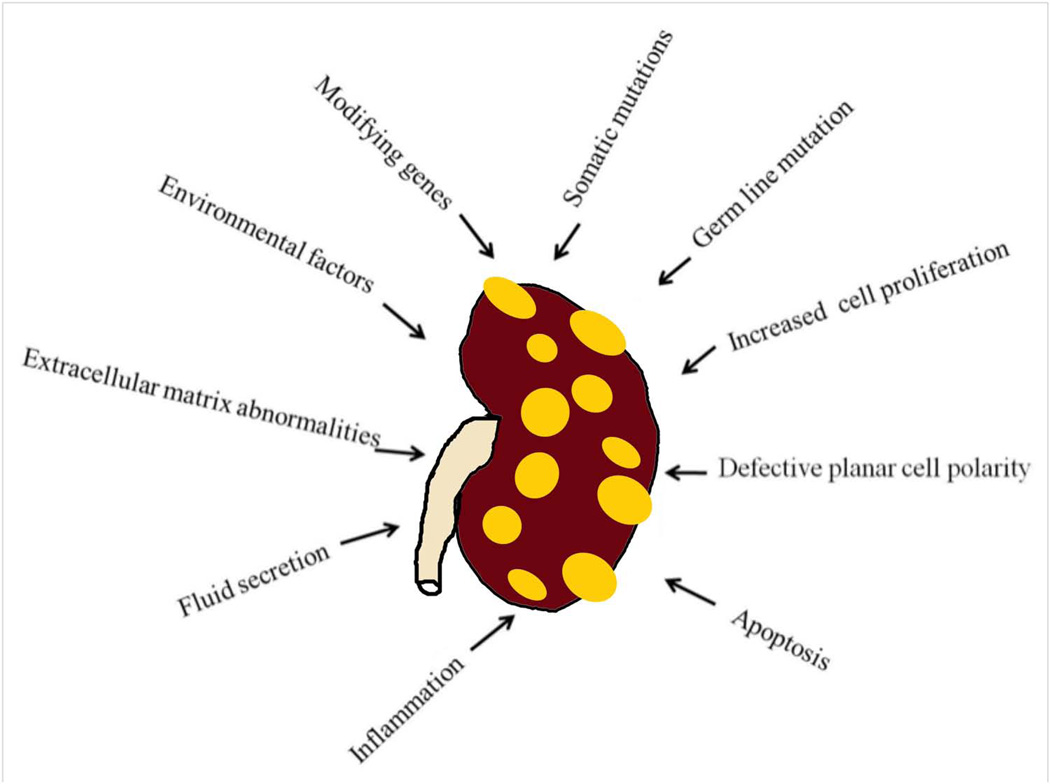
Cysts form as small dilations in renal tubules, which then expand to form fluid-filled cavities of different sizes. Factors that are thought to lead to cystogenesis include a germ-line mutation in one of the polycystin gene alleles, a somatic second hit which leads to the loss of the normal allele, and a third hit, which can be anything that triggers cell proliferation, leading to the dilation of the tubules. Continued dilation of the tubules through increased cell proliferation, fluid secretion, and separation from the parental tubule will lead to the formation of cysts. Other factors that are involved in cystogenesis and/or cyst progression include defective planar cell polarity, extracellular matrix abnormalities, inflammation, increased apoptosis, modifying genes, and environmental factors (2, 57).
There is controversy in the PKD field about some factors such as increased cell proliferation and defective planar polarity. The debate is whether they initiate cyst formation, or just assist in growing existing cysts. Recently, defective planar cell polarity was ruled out as an initiating mechanism for cystogenesis (discussed in detail below). Germ-line mutations that cause ADPKD are discussed above. Other mechanisms that cause and/or promote cystogenesis are discussed below.
The two-hit or second-hit model for cystogenesis
Microdissection studies on ADPKD kidneys revealed a focal nature for cyst formation in patients. Even though each human kidney is composed of about a million nephrons, cysts in ADPKD patients were derived from only a comparatively few nephrons (~1000). All of the cells, including all renal tubular cells, in ADPKD patients carry a germ-line mutation in one of the alleles of the polycystin genes. If the germ-line mutation by itself can cause cystogenesis, all these cells should develop cysts. However, cysts develop only in a fraction of these cells. A second-hit theory was proposed to explain this phenomenon (64–66). This theory states that in addition to the germ-line mutation in one of the PKD1 or PKD2 alleles, a somatic mutation must occur in the second normal allele leading to the complete loss of function of the polycystins causing cyst formation. Cultured epithelial cells from the cysts of ADPKD patients showed a loss of heterozygosity in two closely linked polymorphic markers located within the PKD1 gene in a subset of cysts (64). Further genetic analysis also confirmed the loss of the normal haplotype in this subset of cysts, thus favoring the second hit hypothesis (64).
Insights from mouse models
A number of animal models have been described for ADPKD. A Pkd1 knockout mouse model (Pkd1del34) was developed by homologous recombination strategy leading to a frameshift mutation in exon 33 of the Pkd1 gene. Kidneys from the Pkd1del34 homozygous mice developed normally until E14.5. Cystic dilations were noted in the proximal tubules at E15.5, which was followed by cysts crowding the entire medulla and most of the cortex in the mice, which survived to term. These results are consistent with the expression pattern of murine PC1, which is barely detectable in the ureteric bud tips and the early stages of nephrogenesis, but peaks at E15.5 in the more mature ureteric bud derivatives, including the collecting ducts, papillary ducts, and renal pelvis. This shows the probable role of polycystins in tubular elongation and maintenance of tubular architecture, rather than in nephron formation. Pancreatic duct dilations and hypoplastic lungs were some extrarenal manifestation seen in the Pkd1del34 mice (67). A Pkd2 knockout mouse model was also developed by gene targeting the Pkd2 locus. Similar to the Pkd1 knockout mice, the Pkd2−/− mice also showed normal kidney development until E14.5. Cysts began to form in the maturing kidneys by E15.5, and most of these mice died embryonically beginning at E16.5. Similar to PC1, these results suggest that PC2 is not required for nephrogenesis but required for the proper maintenance and subsequent elongation of the nephron segments. Extrarenal manifestations, such as cardiac defects and pancreatic duct dilations, were also seen in these mice (68). The knockout mouse models show that the loss of both alleles of either the Pkd1 or Pkd2 genes is sufficient to cause PKD, validating the two-hit hypothesis in animal studies. However, analysis of transgenic mice persistently expressing Pkd1, which also developed PKD, raises questions of whether the loss of both Pkd alleles is necessary (69–70). Another mouse model, expressing a hypomorphic Pkd1 gene also developed PKD (71). Recently, a hypomorphic mutation was identified in consanguineous families showing a relatively mild cystic disease in individuals who were homozygous for the mutation, a mis-sense R3277C variant in PKD1 (72). These individuals did not progress to end stage renal disease until the sixth or seventh decade. Moreover, adult relatives who were heterozygous for the mutation only had a few cysts. A mouse model carrying the same mutation has been generated that exhibits a slowly progressing disease as a homozygote. When combined with a null Pkd1 allele, the disease becomes rapidly progressive (73). These results suggest that dysregulation of polycystins leads to cystogenesis, rather than their loss or overexpression per se. These results suggest that the dysregulation of polycystins leads to cystogenesis, rather than their loss or overexpression per se (72–74).
A critical window for cystogenesis
A study by Piontek et al, using an inducible Pkd1 mouse model in which Pkd1 was inactivated at different time points during and after renal development, has shed some light into the mechanism of cystogenesis (75). When Pkd1 was inactivated in mice at postnatal day 2 (P2), a time when cortical nephrogenesis is still ongoing, the mice developed severely cystic kidneys within 2 weeks. It was noted that the renal cortex in these cystic mice did not have any dysplastic or immature structures, further suggesting that Pkd1 is not required for the initial stages of nephrogenesis. However, when the Pkd1 gene was inactivated in mice at 3 and 6 weeks of age, these mice did not develop PKD until approximately 5 to 6 months of age. In order to dissect out this time dependent phenotypic difference in cystogenesis, the Pkd1 gene was inactivated at several time points between P2 and P21. Mice that had the Pkd1 gene inactivated at P12 or before, developed severe PKD within 3 weeks. In contrast, inactivation of Pkd1 in mice between P14 and P21 induced a late cystic phenotype at ~ 6 months of age. A microarray analysis comparing differences in gene expression in normal kidneys between the ages of P11 and P15 revealed significant differences between P11–P12 and P14–P15. Most of the genes that were differentially expressed were clustered into transporter and catalytic functions. In contrast, cell proliferation rates were relatively high at both P12–P14 in normal control kidneys, while proliferation was reduced by P16–P19. These results suggested that the rate of cyst development in these animals depended more upon the state of differentiation of the kidneys at the time point when Pkd1 is inactivated than the rate of cell proliferation. Moreover, the dramatic differences in cyst progression suggest that different pathways may be altered at these different time points leading to rapid cystogenesis at earlier time points of Pkd1 inactivation and slower cystogenesis at later time points of Pkd1 inactivation (75).
A recent report from Grantham et al followed the diameter, volume, and growth rates of cysts in eight ADPKD patients for 3 years. All of these patients had normal renal function as measured by their glomerular filtration rates (GFR). The rate of individual cyst growth in these patients ranged between 2.2 and 71.1 percent per year. An adult collecting duct tubule varies in diameter from 40µm to 100µm as demonstrated by microdissection studies, with fetal collecting ducts likely being smaller than this. Most of the cysts in ADPKD begin in utero in collecting ducts and cysts as big as 10mm in diameter or larger have been detected in ADPKD children who are as young as 1 year old. The authors questioned how fast the cysts needed to grow in order to reach a size detectable by the imaging techniques (2–10mm). For a tubule as big as 100µm in diameter, the growth rate should be 20%/year to grow into a cyst of 2mm in diameter. Even though these numbers could be justified in an adult patient, it would not account for those 2mm cysts that can be detected in ADPKD children, and suggests that renal cysts that are formed in fetal life must grow at a much faster rate in order to reach the detection threshold of the imaging techniques (26). The mouse model in which Pkd1 was inactivated between P2 and P12, which developed a rapid cystic disease, show that cystogenesis and growth were sensitive to the developmental factors that dissipate shortly after birth. Inactivation of the Pkd1 gene after this critical time window resulted in PKD that progressed at a much slower pace. In mice, nephrogenesis and tubular elongation continues until about two weeks of age, corresponding to the window of time where they develop rapid cystic disease. In contrast, in humans, nephrogenesis ceases at approximately 34 weeks of gestation. Thus, renal cysts that are detectable by imaging techniques at birth or in young children with ADPKD, likely progress at a high rate during fetal development, and thereafter, cyst expansion may proceed at a slower rate (26, 75).
Third hit
A more recent hypothesis that has emerged in the ADPKD field is the third hit for cystogenesis. This hypothesis is built upon the second hit hypothesis. The study by Piontek et al, described above, showed that late inactivation of the Pkd1 gene in adult kidneys resulted in a slow onset of cystogenesis in mice (75). Lantinga-van Leeuwen et al and Takakura et al also generated inducible Pkd1 mouse models in which they inactivated the Pkd1 gene in mature mouse kidneys (76, 77). Takakura et al argued that the slow onset of PKD when the Pkd1 gene was inactivated in adult animals could not be justified by the second hit hypothesis and proposed a third hit hypothesis. In a follow-up study the same group showed that renal injury resulting from unilateral ischemia reperfusion injury accelerated PKD in the adult inducible Pkd1 knockout mouse model (78). The third hit theory states that a cell may not be cystogenic just because it received the first hit (germ-line mutation) and the second hit (somatic inactivation of the normal allele), but that it also requires an additional event that leads to cell proliferation and cyst growth. Recent studies showing that nephrotoxic injury and ischemia induce rapid cyst growth when cystic mutations are induced in adult animals support this idea (79–81). A similar pattern of phenotype as seen in the inducible Pkd1 knockout models was observed when genes required for primary cilia formation were inactivated in developing or adult kidneys. Patel et al described a study using an inducible mouse model to knockout the Kif3a gene, which is required for cilia formation. While Kif3a inactivation and hence the loss of cilia at P2 led to PKD, inactivation of Kif3a at P10, P14 or P21 did not yield cystic disease when these mice were examined 2 months after gene inactivation (83). The inducible knockout of the Ift88 gene in adult kidneys also led to a slow onset cystic disease. More recently, another third hit, unilateral nephrectomy, was added to the list (79). Unilateral nephrectomy leads to the hypertrophy of the existing kidney and an increase in glomerular filtration rate as it compensates for the loss of the other kidney. As described above, mice that lost renal cilia (as a result of Ift88 inactivation) in the adult kidney did not develop cystic kidneys until after 6 months. However, inactivation Ift88 in 8 week-old mice, followed by unilateral nephrectomy 1 week later, resulted in severe cystic kidney disease in the contralateral kidney by 3 months of age. Activation of the mTOR (mammalian target of rapamycin) pathway was also observed in these mice, which has been previously documented in association with unilateral nephrectomy-induced hypertrophy. Activation of the mTOR pathway in these mice also led to increased cell proliferation could explain the rapid onset of cystic disease compared to that of non-nephrectomized control mice (79). However, increased cell proliferation alone was unable to induce rapid cystogenesis in the absence of nephrectomy (84).
ADPKD is a Ciliopathy
ADPKD, together with many other diseases that present with renal cysts, was inducted into a family of diseases known as ciliopathies (85). This came after the discovery that many cystoproteins that cause renal cysts localize to the once disregarded organelle, the non-motile primary cilia. Primary cilia are sensory organelles that protrude from the center of epithelial cells (86). They grow out from the basal bodies or centrosomes and are microtubule-based structures. Primary cilia are highly conserved in evolution and are used to detect a wide variety of physical and chemical stimuli that are of mechanical, photonic, olfactory, or hormonal nature (87).
The association between cilia and PKD was first discovered when mutations in Tg737, responsible for renal cystic disease in the orpk mouse model of PKD, showed stunted primary cilia in their renal epithelial cells. The orthologue of Tg737 in Chlamydomonas, IFT88, is required for intraflagellar transport and assembly of motile cilia. Subsequently it was shown that polycystins and fibrocystins also localize to the primary cilia. Other diseases such as nephronophthisis (NPHP), Bardet-Biedl syndrome (BBS), and Orofaciodigital syndrome, also present with renal cysts and the protein products of these genes all localize to the cilia, basal bodies, or centrosomes. In fact, the protein products of all the genes that cause renal cystic diseases in humans, mice, and zebrafish localize to the primary cilia, basal bodies, or centrosomes, placing primary cilia at the center of cystic kidney diseases. However, since the cystoproteins also localize to many other sub-cellular locations, a ciliary defect alone cannot be attributed as a primary defect in these diseases. Nevertheless, the systemic nature of different types of PKDs, BBS, and NPHP favors the ciliary hypothesis because of the presence of cilia in many of the organs affected (85, 87).
Polycystins as mechanosensors
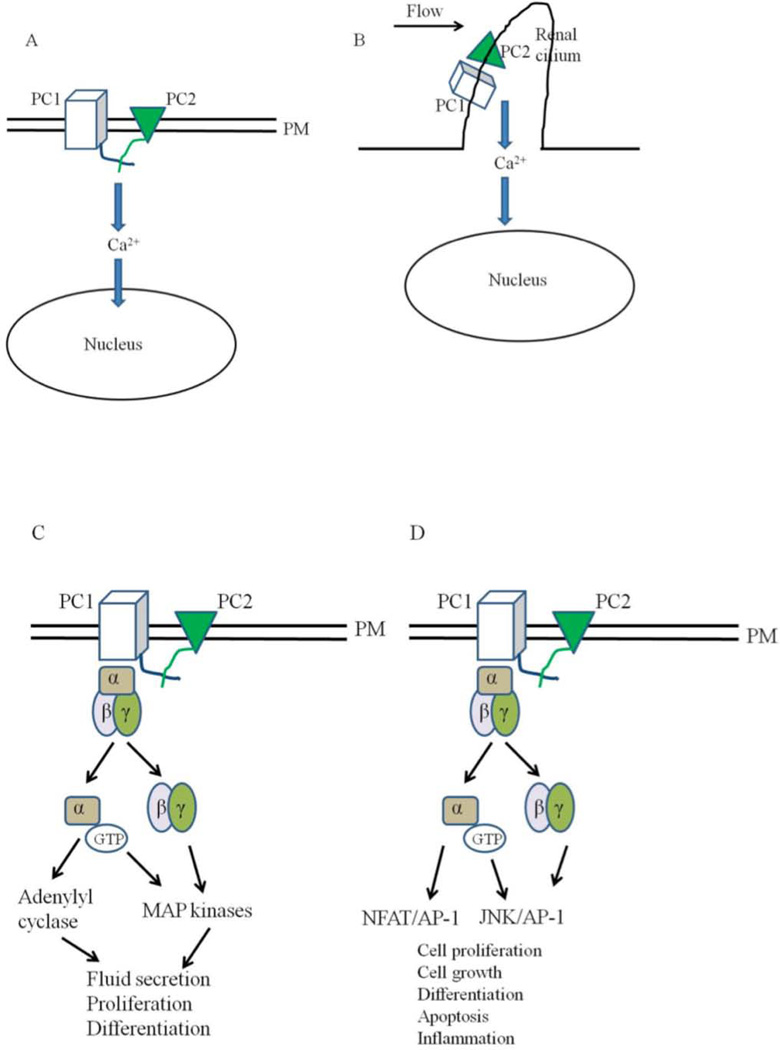
Renal tubular epithelial cells, including all the segments of the nephron and the collecting ducts, with the exception of intercalated cells, show the presence of a single primary apical cilium (15). Fluid flow in renal tubular cells bends the primary cilia on their surface. PC1, which is present on these cilia, is thought to sense the flow with its large extracellular domains, activating the associated PC2 calcium channels. This allows a calcium influx which results in a calcium induced calcium release from other intracellular Ca2+ stores, leading to various genetic changes in cells (Figure 4 B) (23, 88).
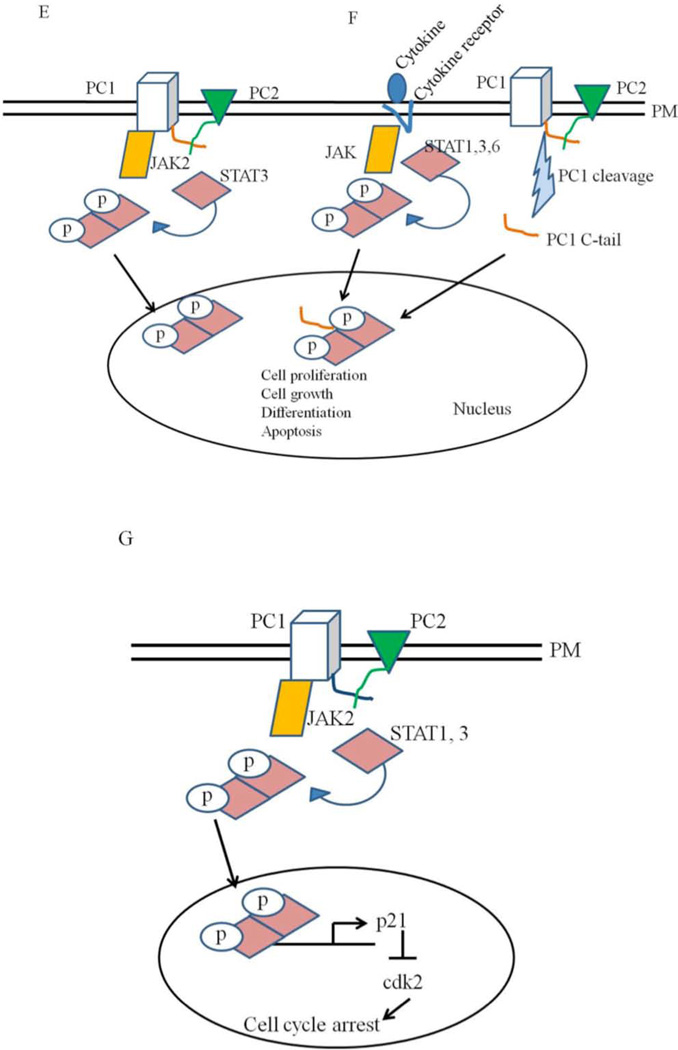
Figure 4. Models for the functions of polycystins.
A: PC1 and PC2 are found on the plasma membrane (PM) in this model. Activation of PC1 leads to the activation of PC2 which leads to calcium influx and changes in gene transcription. B: Polycystins signaling through the primary cilia. Renal cilia bend in response to fluid flow. Bending of the cilia leads to the influx of calcium through polycystins. C and D: Polycystins regulating G-protein signaling. Binding of PC1 to heterotrimeric G proteins activates the Gα subunit and the release of the βγ subunits. This leads to the activation of adenylyl cyclases and MAP kinases which can affect several cellular processes such cell proliferation, fluid secretion and differentiation. G protein signaling through polycystins can also affect the JNK/AP-1 and NFAT/AP-1 pathways. E: Polycystins signaling through the JAK-STAT signaling pathway. E: Membrane anchored PC1binds to JAK2 and activates it which then phosphorylates STAT3. F: PC1 C tail can translocate to the nucleus and coactivate STAT1, 3 and 6 which were already activated independently through cytokine signaling. Once in the nucleus, STAT transcription factors affect the expression of genes required for cell proliferation, cell growth, differentiation and apoptosis. G: Polycystins regulating the JAK-STAT signaling pathway. PC1, in a reaction requiring PC2, activates JAK2 which leads to the phosphorylation of STAT1. Phosphorylated STAT1 translocates to the nucleus and activates the cyclin-dependent kinase inhibitor, p21. Activation of p21 results in the subsequent inhibition of cdk2 and cell cycle arrest.
A flow-Ca2+ imaging system was used to test the functionality of PC1 on primary cilia. Mouse embryonic kidney cells responded to fluid flow at a rate comparable to the physiological urine flow rate. However, Pkd1 null cells failed to respond to fluid flow rates higher or lower than those detected by wild type cells, confirming that PC1 is required to detect fluid flow. Moreover, atomic force microscopy studies revealed that the PKD domains on PC1 exhibit remarkable mechanical strength further supporting the mechanosensory function of PC1 (57).
Channel activity of polycystins
The last six transmembrane domains of PC1 share significant sequence homology with the domains of Na+ and Ca2+ channels and TRP channels (60). However, by itself PC1 cannot form ion channels since PC1 overexpression alone does not yield any measurable channel activity (57).
In contrast, in response to local increases in intracellular calcium, PC2 releases calcium from intracellular stores. The loop between the fifth and the sixth transmembrane domains of PC2 are involved in the calcium conducting activity. The third transmembrane of PC2 is also involved, at least partially, in this process. In addition to its direct involvement in calcium induced calcium release, PC2 also indirectly regulates calcium by interacting with two intracellular calcium channels. PC2 inhibits ryanodine receptor mediated calcium release by binding to the channel in its open state, thereby decreasing its conductance. Direct binding between the C terminus of PC2 and IP3R (inositol 1,4, 5-triphosphate receptor) modifies IP-3 induced calcium flux (55).
An interaction between PC1 and PC2 generates a functional ion channel. It is not certain whether this is through the activation of PC2’s intrinsic channel properties, or from the formation of a functional complex between these two proteins (55). Because of the calcium channel activity of polycystins, genetic mutations in any of the polycystins disrupt intracellular calcium regulation leading to abnormal proliferation. The steady-state intracellular calcium levels have been measured in normal and ADPKD kidney cell cultures where it was found that calcium levels in ADPKD cells were 20nM lower compared to the normal renal cells (89).
Other functions of polycystins
PC1 found on the plasma membrane may interact with PC2 present on the adjacent endoplasmic reticulum (23). PC1 may also act as a receptor for a ligand yet to be identified. Upon receiving a stimulus, PC1 may signal to the interior of the cell by its interaction with PC2 on the plasma membrane. This signaling event can result in the activation of calcium channels, an increase in cytoplasmic calcium which leads to exocytosis, and changes in gene expression (Figure 1–4 A) (2).
Cellular signaling pathways modulated by the polycystins can be broadly classified into three categories: G- protein activation, growth regulation and Wnt pathway modulation (55).
G-protein activation
PC1 functions as a G-protein coupled receptor (GPCR) which can regulate cell proliferation, fluid secretion, cell polarity and differentiation (Figure 1–3 C and D) (2). PC1 contains sequences that are found in GPCRs. These include a proteolytic site domain and a polybasic domain found in its C-terminal tail that has been shown to activate Gi/G0 proteins in vitro. However, PC1 is an atypical GPCR since it has 11 transmembrane domains compared to the 7 transmembrane domains typically found in GPCRs. Regulator of G-protein signaling 7 (RGS7), a member of a family of proteins capable of accelerating the hydrolysis of GTP bound to the Gα subunit of G-proteins, was shown as a candidate modifier gene in a mouse model of PKD. Moreover, PC1 has also been shown to bind and stabilize RGS7 thereby regulating G-protein signaling (50).
Figure 3. Structure of polycystins.
Polycystin-1 has a large extracellular domain, 11 transmembrane domain and a short cytoplasmic tail. The coiled coil domain in the C-terminal end of PC-1 interacts with the C-terminal tail of Polycystin-2. Polycystin-2 has cytoplasmic N and C-terminus. Together, PC1 and PC2 mediate calcium entry into cells. (Modified from 2)
Downstream signaling through GPCRs can lead to the activation of c-Jun N-terminal kinase (JNK) and activator protein-1 (AP-1) pathways. Arnould et al demonstrated the activation of the JNK/AP-1 pathway via the PC1 C-terminal tail. Several components such as protein kinase C α (PKC α) and small G proteins such as Cdc42 and Rac1 were involved in mediating this signal (90). Parnell et al showed that the activation of JNK/AP-1 pathway is mediated by signaling through Gα and Gβγ subunits. The JNK/AP-1 pathway controls various cellular processes such as cell cycle regulation, cell growth, differentiation, apoptosis and inflammation. In Drosophila, JNK signaling is involved in the developmental regulation of planar polarity, epidermal adhesion and integrity (50). Many of these pathways controlled by JNK/AP-1 are also involved in the pathogenesis of PKD. Moreover, the activity of the AP-1 components ATF-2, c-jun, and c-fos, were increased in ADPKD patients as well as in a hypomorphic Pkd1 mouse model, further suggesting the involvement of JNK/AP-1 pathway in PKD pathogenesis (91).
Additional support for PC1 signaling through G-proteins comes from PC1 activation of phospholipase C (PLC) mediated by the Gαq and the subsequent activation of the calcineurin/NFAT (nuclear factor of activated T-cells) pathway. Signaling through this pathway also connects the function of polycystins as regulators of intracellular calcium levels. Exogenous expression of the PC1 C tail domain results in an increase in calcium level in a reaction requiring PLC β. This intracellular increase in calcium leads to the activation of calcineurin, a serine-threonine phosphatase that dephosphorylates NFAT. Activated NFAT translocates to the nucleus and regulates target genes at composite NFAT/AP-1 elements. In addition to the evidence for PC1 mediating NFAT activation, NFAT is co-expressed with PC1 in renal tubular epithelial cells of developing and adult mice, suggesting that NFAT and PC1 may work together in a pathway (92).
Growth regulation
In accordance with the cell proliferation defects seen in PKD, PC1 and PC2 are directly involved in regulating the cell cycle (93). The mechanisms utilized by PC1/PC2 include signaling through the JAK-STAT pathway (94). Signaling through this pathway leads to the activation of STAT1 and STAT3, subsequent upregulation of the cyclin-dependent kinase inhibitor (CKI) p21, and the inhibition of cyclin dependent kinase 2 (CDK2) which ultimately leads to cell cycle arrest at the G0/G1 transition (Figure 1–4 G) (2, 95–96).
Additional evidence for a direct role of polycystins in cell cycle regulation was shown by the direct interaction of PC2 with the helix-loop-helix (HLH) protein Id2, which regulates cell proliferation and differentiation. Phosphorylation of PC2 by PC1 leads to its interaction with Id2. Id2 is found in a complex with another HLH protein, E47, which transcriptionally activates p21. Id2 lacks a nuclear localization signal and can be transported to the nucleus only through its interaction with other proteins, such as E47. The interaction of the Id2-E47 complex with PC2 sequesters this complex outside the nucleus. When PC2 is unable to bind to this complex, Id2 is translocated to the nucleus and exerts its dominant negative effects on E47 and other HLH proteins. In this manner, the normal expression of PC1 and PC2 leads to cell cycle arrest by an increase in p21 while a mutation in one of the polycystins leads to the dysregulation of this pathway resulting in increased cell proliferation (97).
There are three proteolytic cleavage sites seen in PC1. PC1 undergoes partial cleavage at the extracellular GPS domain, although the N- and C-termini of the protein remain non-covalently linked. A missense mutation at the GPS domain, which makes it non-cleavable, causes ADPKD in patients. Moreover, a mutant PC1 that cannot undergo GPS cleavage was unable to rescue PC1-null cultured cells or transgenic mice. This suggests the requirement for PC1 cleavage at the N-terminal GPS domain to be fully functional. However, not all PC1 molecules in a cell are cleaved. Rather, they exist in a heterogeneous population of full length and GPS cleaved isoforms (13, 55).
In addition to the GPS cleavage site, cleavage of PC1 at the C-terminal tail (CTT) releasing a soluble ~ 35kD fragment has been described (56). This C-terminal cleavage suggests that PC1 may be involved in successive cleavage events, similar to that of the regulated intramembrane proteolysis (RIP) pathway. In the RIP model, described for various cell surface receptors such as Notch, APP, E-cadherin, ErbB4 and CD44, the cytoplasmic segment of a transmembrane protein enters the nucleus after its activation and modulates gene expression, bypassing adaptor proteins and kinase/phosphatase cascades (56). Consistent with this idea it was shown that the nuclear translocation of the PC1 CTT leads to the activation of the AP-1 pathway (56). PC2 seems to keep the PC1 CTT from entering the nucleus since co-transfection experiments using full length PC2 and PC1 CTT constructs led to the retention of PC1 CTT outside the nucleus and a reduction in AP-1 activity. PC2 must also interact with PC1 CTT for it to exert its inhibitory effect on PC1 since co-transfection experiments with a mutant PC2, which could not interact with PC1 and the PC1 CTT, only minimally reduced AP-1 activity. Unilateral ureteral ligation (leading to a reduction in fluid flow) and inactivation of Kif3a (resulting in the loss of the mechanosensor cilia where polycystins are localized) in mice led to an increase in the nuclear translocation of PC1 CTT. These results showed that PC1 CTT cleavage and nuclear translocation are associated with the mechanosensory effects of cilia. A reduction in fluid flow or loss of fluid flow sensitivity in renal epithelial cells can lead to an accumulation of PC1 CTT in the nucleus and transcription of its target genes (56). With its long extracellular domain, PC1 is thought to detect fluid flow in renal epithelial cells and PC2 has an inhibitory effect on the translocation of PC1 CTT. Hence, mutations in any of the polycystins can render renal epithelial cells insensitive to fluid flow and lead to the increased transcription of target genes.
A more distal cleavage in the C-terminal domain of PC1 which generates a ~14–17kDa CTT fragment has been described by Low et al (98). This PC1 CTT fragment, which we will call PC1 CTT14, interacts with P100, a coactivator protein. The P100 protein has also been localized to the shaft and the basal bodies of renal cilia where PC1 is also found. P100 is overexpressed in the cyst-ling cells of ADPKD patients. The PC1 CTT14 fragment, together with P100, binds to the transcription factor STAT6 and leads to an enhancement of STAT6 dependent transcription. STAT6 is localized to the nucleus in MDCK and renal epithelial cells. However, subjecting MDCK cells to a constant flow of fluid (similar to the fluid flow in renal tubules) results in a translocation of STAT6 from the nucleus to the primary cilia. Moreover, high levels of nuclear STAT6 are seen in the cyst-lining cells of ADPKD patients, indicating that STAT6 dependent gene expression is upregulated in ADPKD (94, 99). Under normal fluid flow conditions PC1 keeps STAT6 sequestered to the cilia together with P100 preventing the transcription of P100/STAT6 genes. “No flow” conditions lead to the cleavage of the PC1 tail and the PC1 CTT14 fragment translocates to the nucleus with P100 and STAT6 to mediate gene transcription. No flow conditions have been observed in renal injury as well as in the cysts of PKD. Dilated tubules in PKD also show decreased of fluid flow (98).
A recent observation by Talbot et al shows the mechanism by which PC1 regulates STAT activity. Membrane anchored PC1 tail activates STAT3, however, activation of STAT1 and STAT6 requires the soluble PC1 tail as well as a previous activation of these STATs by cytokine signaling. The activation of STATs by PC1 after cytokine signaling is referred to as co-activation. STAT3, in addition to being activated by membrane-anchored PC1, can also be co-activated by the soluble PC1 tail. The co-activation process can lead to an exaggerated cytokine response. STAT3 activity is highly upregulated in ADPKD and PKD mouse models while it is downregulated in normal renal epithelial cells after differentiation (94, 100).
The mTOR pathway, which regulates protein translation, cell proliferation, and cell growth, has been shown as a target for PC1. Shillingford et al showed that PC1 C-terminal tail interacts with tuberin, the protein product of the TSC2 gene (101). Mutations in TSC2 lead to tuberous sclerosis, which is characterized by the presence of hamartomas in multiple organs and renal cysts. Tuberin regulates the kinase activity of mTOR through a small GTPase, Rheb. Activated mTOR phosphorylates and activates its downstream effectors - S6K1, S6K2 (ribosomal kinases), and 4E-BP1 and 4E-BP2 (eukaryotic initiation factor 4E-binding proteins) that lead to the stimulation of protein synthesis and proliferation. Shillingford et al also provided evidence for the increased activity of the mTOR pathway in the cyst-lining epithelial cells of ADPKD patients by showing an increase in activated phosphorylated mTOR and S6 kinase. Moreover, rapamycin, an immunosuppressant drug that is also a specific inhibitor of the mTOR pathway, was able to alleviate the cystic phenotype in two different PKD mouse models. These mouse models, and a rat PKD model in which mTOR inhibition also slowed the progression of PKD, did not have a primary mutation in polycystin genes. This suggests that activation of the mTOR pathway is common to all renal cystic diseases, despite their primary germ-line mutation (101–104).
PC1 also regulates mTOR activity by altering the subcellular localization of tuberin (102). Suppression of mTOR by tuberin takes place only when tuberin is tethered to the membrane. Phosphorylation of tuberin by PI3K/AKT leads to its binding to the 14-3-3 adaptor protein, which in turn leads to the partitioning of tuberin to the cytosol, making it unavailable to bind with Rheb and its activating partner, TSC1. In the presence of a functional membrane bound PC1 C-terminal tail, phosphorylation of tuberin by PI3K/AKT is inhibited and TSC2 becomes tethered to the membrane, repressing mTOR signaling. In the absence of a functional PC1, as in most cases of ADPKD, the mTOR pathway is activated (101–102).
Wnt pathway modulation
The Wnt signaling pathway, which is involved in embryonic induction, the generation of cell polarity, the regulation of cell proliferation, and the specification of cell fate, is also regulated by PC1. Wnts are secreted glycoproteins and canonical Wnt signaling regulates the cellular levels of a multifunctional polypeptide, β-catenin. The presence of Wnt ligands leads to the stabilization, accumulation, and nuclear translocation of soluble β-catenin. Secreted Wnts accomplish this by binding to frizzled receptors on the cell surface, activating disheveled proteins and inhibiting glycogen synthase kinase (GSK-3β). Once inhibited, GSK-3 can no longer target β-catenin for proteasomal degradation, and this leads to the accumulation and nuclear translocation of β-catenin. Inside the nucleus, β-catenin interacts with the TCF/LEF family of transcription factors to regulate gene expression (55, 105–106).
The expression of a stabilized β-catenin in renal tubular cells leads to PKD in animals (107). Moreover, microarray analysis has shown evidence for the activation of Wnt signaling in the cyst-lining cells of ADPKD patients (106). Experiments performed to demonstrate Wnt modulation by the PC1 C tail have produced contradictory results. In contrast to experimental results showing that the PC1 C tail activates canonical Wnt signaling (105), a more recent report suggests that the PC1 tail physically associates with β-catenin and acts as an inhibitor of intracellular Wnt signaling (106). PC1 exerts this inhibitory effect on Wnt signaling pathway by reducing the apparent affinity of the interaction between β-catenin and TCF. The inhibitory effect of the PC1 CTT on canonical Wnt signaling is consistent with the observation that the overexpression of c-Myc, a β-catenin/TCF regulated oncogene, induces a renal cystic phenotype in transgenic mice, and c-Myc is overexpressed in ADPKD and several other renal cystic diseases (106). The disparity between these results has been attributed to the use of a membrane anchored PC1 CTT in the early report (105) compared to the use of a soluble PC1 CTT in the latter study (106).
Finally, the deletion of PC2 results in an increase in β-catenin protein levels. However, it is not certain whether this effect is mediated directly by the inactivation of PC2 or through an indirect effect that PC2 might have on PC1 (55). Although these results suggest that the dysregulation of canonical Wnt signaling contribute to the pathogenesis of PKD, a recent study calls into question the importance of canonical Wnt signaling in the pathogenesis of PKD. Miller et al, using TCF-beta-catenin/lacZ reporter mice crossed with Pkd1 and Pkd2 mutant mice, reported a complete absence of Wnt signaling in the cysts of these mouse models (108).
One of the non-canonical Wnt signaling pathways, the planar cell polarity pathway, has also been implicated in PKD. Renal tubular cells generally divide parallel to the tubule axis. This oriented cell division (OCD) leads to the lengthening of the tubules rather than increasing its diameter. Loss of OCD leads to the division of cells at an angle leading to an expansion of tubule diameter. Defects in OCD have been reported in several mouse models of PKD (83, 109). Inversin, the gene mutated in nephronophthisis type II (a disease characterized by renal cysts) was shown to act as a switch between canonical and non-canonical Wnt signaling (110). While Miller et al suggest that canonical Wnt signaling may not be important for the pathogenesis of PKD, a disruption in the switch between canonical and non-canonical Wnt signaling could be involved in cystogenesis (108).
Cell proliferation and fluid secretion
Epithelial cell proliferation and fluid secretion are two hallmark features in ADPKD (111). Even the gross inspection of polycystic kidneys show an increased epithelial cell proliferation phenotype evidenced by the increase in the circumference of the kidney tubules that form cysts. Renal tubules normally fall within a range of a few micrometers in diameter. However, in ADPKD, renal tubules considerably increase in size so as to fall within a size range of millimeters to centimeters when they become cysts. Microscopic examination of polycystic kidneys shows the presence of large numbers of cells in the cyst wall epithelium (112). Several growth-stimulating factors such as epidermal growth factor (EGF), transforming growth factor α (TGF α), and the EGF receptor (EGFR) promote cell proliferation in PKD. Overexpression and mislocalization of EGFR to the apical side of the cystic epithelium has been reported in several animal models and in human PKD patients. Transgenic animals overexpressing TGF α also developed PKD (13).
An important factor which leads to the proliferative phenotype in ADPKD is the second messenger adenosine 3’, 5’ cyclic monophosphate (cAMP). cAMP is an intracellular mediator of adenylyl cyclase signaling and has been shown to stimulate proliferation of cyst-lining cells isolated from human ADPKD kidneys. The mammalian kidney is a target of several hormones such as arginine vasopressin (AVP), parathyroid hormone, secretin, and vasoactive intestinal peptide as well as of autacoids such as prostaglandins and adenosine, all of which lead to the elevation of the intracellular concentration of cAMP. In normal human kidneys, cAMP stimulation leads to a non-mitogenic response. However, ADPKD kidneys show a proliferative response to cAMP stimulation. Moreover, normal human kidney (NHK) cells behave like ADPKD cells with a cAMP stimulatory phenotype when treated with calcium channel blockers. In contrast, the mitogenic response to cAMP was reversed in ADPKD cells when they were treated with calcium channel activators or calcium ionophores. Additional studies have shown that cAMP mediates its effect in PKD cells by activating the B-Raf/MEK/ERK pathway (23, 88, 113–115).
During the early stages of cystogenesis, cysts are attached to their parental renal tubules and a derivative of the glomerular filtrate enters the cysts. Once these cysts expand to approximately 2mm in diameter, the cyst closes off from its parental tubule and after that fluid can only enter the cysts through transepithelial secretion. A transporter present in the basolateral membrane of cyst-lining cells, the Na-K-2Cl transporter NKCC1, mediates the entry of chloride into the cytoplasm. A chloride transporter present on the apical membrane of cystic epithelial cells, the cystic fibrosis transmembrane conductance regulator (CFTR), mediates the entry of chloride into the cyst lumen (1, 13). The activity of CFTR increases in ADPKD possibly due to secondary effects from the increased concentration of cAMP (1). It has also been shown that patients who have both cystic fibrosis, and therefore a mutation in CFTR, and PKD have a milder cystic disease (116).
Other molecular abnormalities in ADPKD
An increase in apoptotic DNA fragmentation and in-situ labeling of apoptotic cells was described in PKD patients and in two mouse models of PKD. Apoptotic cells were present in both cystic and non-cystic tubules as well as in glomeruli. The progressive loss of renal function seen in PKD may be explained by this increase in apoptosis. Moreover, homozygous deletion of the anti-apoptotic gene, Bcl2, results in PKD in mice (9, 117). The loss of Bcl2 also results in increased cell proliferation, which may also contribute to cystogenesis (118). ADPKD tissues have also shown an increase in the expression of c-Myc. Moreover, kidney specific overexpression of c-Myc in mice results in renal cysts characterized by both an increase in proliferation and apoptosis (13, 119).
Abnormalities in the innate immune system
Abnormalities in the innate immune system are another hallmark feature in both ADPKD and ARPKD. In ADPKD, the accelerated production of monocyte chemotactic protein-1 (MCP-1) leads to an increase in mononuclear cells in the renal parenchyma. Increased urinary excretion of MCP-1 can be detected in the early stages of cystic disease in ADPKD patients, indicating that the immune system pathway is disregulated early in the disease process. Recent studies have shown activated macrophages surrounding cysts in both ADPKD and ARPKD patients (120–121). Moreover, depletion of macrophages in both Pkd1 and cpk mouse models of polycystic kidney disease reduced cyst progression, demonstrating a role for activated macrophages in cyst growth and disease progression (120–121). Analysis of the cpk mouse model, which resembles human ARPKD, showed that ~ 60 monocyte/macrophage markers were overexpressed in cpk mice, and genome wide transcription profiling in the cpk mouse model and a rat model of PKD, Han:SPRD, showed an overexpression of macrophage markers and other innate immune factors. The expression pattern of one of these overexpressed macrophage markers, CD14, was analyzed in cpk mice, as well as in ADPKD and ARPKD patients. In the cpk mice, CD14 expression increased with increasing age in controls, however, there was a significant increase in cystic kidneys compared to age-matched littermate controls. While the number of CD14 positive mononuclear cells could not account for the increase in CD14 expression, histological analysis revealed CD14 in the proximal tubules and principal cells of collecting ducts, and in the cysts derived from these segments. Although CD14 is a membrane-anchored receptor, once activated, soluble CD14 can be shed from the membrane. Both ARPKD and ADPKD patients showed an abnormal increase in the shedding of CD14. In ADPKD kidneys, these shed soluble forms of CD14 were washed out into the urine, where they may be an indicator of PKD progression (122–123).
CD14 is a potent stimulator of TNFα secretion, which can lead to a cystogenic pathway. The shed form of CD14 is also a mediator for renal endotoxin-induced tubulointerstitial injury. An injury related CD14 overexpression is also seen in renal ischemia reperfusion injury models. CD14 is a major ligand for Toll-like receptor-4 (TLR4), through which it may transactivate cystogenic pathways. The Wnt pathway, the AP-1 pathway, and the PI3K pathway are all modulated by TLR4, and these pathways have also been linked to cyst formation (122).
Abnormalities in extracellular matrix (ECM)
PC1 has been shown to be associated with the ECM components and is thought to have a role in cell-extracellular matrix interactions. Perhaps because of this role, alterations in the tubular basement membrane (TBM) including its thickening, splitting, fraying and multi-layering have been seen in human PKD. Cystic kidneys from several animal models of PKD also show BM abnormalities, including thickened and laminated basement membranes, as well as increased expression of α1 type IV collagen and laminins β1 and β2 (124). A hypomorphic laminin α5 mouse model has also been shown to develop PKD. Speculation of PC1 working as a receptor for extracellular matrix molecules and its expression in the basolateral surfaces may explain the PKD phenotype seen in the hypomorphic laminin α5 mutant. Moreover, recombinant PC1 peptides have also been shown to bind laminin in vitro. Increased EGF expression, an abnormality observed in PKD, may also be indirectly involved in modulating the effects of laminin since many of the laminin chains consists of multiple EGF- like repeats, some of which even bind to the EGF receptor signaling downstream via this pathway (125).
Other matricellular proteins that have been shown to contribute to cyst progression include periostin (126–127). Perisotin is highly expressed in cells isolated from both ADPKD and ARPKD patients, where it contributes to cell proliferation and fibrosis (126). Moreover, deletion of perisotin in pcy/pcy mice reduced mTOR activity, suggesting a periostin-ILK-AKT-mTOR signaling pathway that contributes to disease progression (127).
Abnormalities in mechanosensation
Polycystins, by virtue of their location on the primary cilia of renal epithelial cells, are thought to play a role in mechanosensation. In response to fluid flow and bending of the cilia, polycystins present on the cilia mediate the entry of calcium into cells. Pkd1−/− renal epithelial cells have normal primary cilia, but lack the flow-induced calcium response of normal cells (88). Mutations in any of the polycystins, as it occurs in ADPKD, lead to abnormalities in the fluid-flow sensing mechanism, which may contribute to cyst development. The kidney specific inactivation of Kif3a, a subunit of kinesin-II, essential for cilia formation, prevents renal ciliogenesis and results in the development of PKD in mice (128).
Abnormalities in OCD
Defective planar cell polarity is another hallmark feature seen in PKD. This phenotype can also be attributed to the ciliary hypothesis in PKD. It has been proposed that cilia in the developing kidney are responsible for the OCD along the length of a renal tubule. Loss of OCD was observed during advanced stages of cystogenesis in the pck rat, an orthologous model of ARPKD. Measurement of mitotic angles in the pck rat showed that the loss of OCD preceded tubular dilation in these animals. The kidney specific inactivation of Hnf1β, a transcription factor involved in the regulation of expression of Pkd2 and Pkhd1 (the gene mutated in ARPKD), also resulted in PKD as a result of severe mitotic angle distortion and loss of OCD seen even at birth (109). However, recent results with the kidney specific inactivation of Pkd1, Pkd2 and Pkhd1 mouse models show that loss of OCD is not required for cystogenesis. Precystic tubules from conditional Pkd1 or Pkd2 mice did not show the loss of OCD. Once the tubules dilated to form cysts, loss of OCD was seen. Moreover, the conditional deletion of Pkhd1 in the kidneys resulted in the loss of OCD, however, these mice did not develop PKD (129).
Modifiers of polycystic kidney disease progression
ADPKD is a fully penetrant trait in that virtually 100% of individuals who inherit one of the mutated polycystin genes will develop PKD in their lifetime. The disease presentation and severity, however, vary among ADPKD patients, even within family members (2). This phenotypic variability associated with disease presentation led to the investigation of modifying loci and environmental factors that may influence disease progression. Although ADPKD is a single-gene disorder, the disease phenotype is a complex trait. In single-gene disorders with complex traits, the primary mutant gene product is embedded in a complex system that includes genetic modifiers and modulating environmental factors (130). Belibi et al tested the effects of caffeine on normal and ADPKD cultured cells. Caffeine inhibits phosphodiesterases (PDE), the enzymes that hydrolyze cAMP, leading to the accumulation of this second messenger. Increased cAMP can lead to a mitotic response in the ADPKD cells, as described above. This study found that caffeine, and other PDE inhibitors such IBMX and rolipram, increased intracellular cAMP levels in ADPKD cells (131). Therefore, caffeine is an important environmental influence in the progression of ADPKD that patients should avoid.
An early onset of PKD is seen in patients with microdeletions in the PKD1 and TSC2 genes, which are adjacent to each other on the same chromosome (132–133). Bilineal inheritance of heterozygous PKD1 and PKD2 mutations also leads to severe renal disease in some patients (133). Transheterozygous mutations in the Pkd1 and Pkd2 genes in mice have also been reported to have greater than additive renal disease phenotype (sibs twins modifier). Moreover, patients who have cystic fibrosis (therefore a mutation in CFTR) and PKD have a milder disease (116). The possible existence of modifier genes in ADPKD patients was also confirmed from an analysis carried out in monozygotic (MZ) twin siblings. Large intrafamilial variability was observed in the progression of renal disease in these MZ twins (134). More recently, a number of gene networks have been identified (135–136). Fedeles et al showed that a network of five genes, including two gene products involved in translocation and quality control in the ER, were necessary for the PC1/PC2 complex (135). These genes, PRKCSH and SEC63, which encode glucosidase IIb and Sec63p, respectively, interact with PKD1 and PKD2 to establish a functional PC1/PC2 complex. The same study demonstrated that PKHD1 could be modified by PKD1, showing that, within this network, PC1 is the rate-limiting factor in the progression of PKD. These results suggest that the inability to correctly traffic a normal polycystin complex may contribute to PKD, and support a model of gene dosage effects (137–139). This was further supported by studies showing reduced polycystin2 in the cilia of induced pluripotent stem cells generated from PKD patients with PKD1 mutations (136).
Pax2 is a developmentally regulated gene that is required for the differentiation and proliferation of the renal epithelium in both mice and human. Expression of Pax2 is seen in the nephric duct, ureteric bud and induced mesenchyme in the normal developing kidney. Pax2 expression is seen in the nephrogenic zone of the kidney from E11 to postnatal day 10 in mice (140). Pax2 null mutant mice lacked the urogenital system including absent kidneys and ureters while the overexpression of Pax2 led to multicystic disease (141–143). Human juvenile cystic kidneys, ADPKD kidneys and cpk (a mouse model which resembles ARPKD) mouse kidneys showed persistent expression of Pax2 (140).
The pattern of expression of Pax2 during kidney development and in cystic kidneys prompted two independent groups of investigators to ask whether Pax2 can regulate cystogenesis. Ostrom et al showed that reduced gene dosage of Pax2 modified the cystic phenotype in the cpk mouse model of PKD. Mice homozygous for the cpk mutation and heterozygous for the Pax2 gene, had reduced cystic severity and a longer life span (140). The role of Pax2 in cystogenesis was also investigated in an ADPKD mouse model, Pkd1del34. Fetal kidneys (E18.5) from the homozygous cystic mice, Pkd1del34/del34, showed immunoreactivity to Pax2 in the cystic epithelium, bifurcating ureteric buds, and also in the condensing mesenchyme. Double mutant mice, which were homozygous for the Pkd1del34 mutation and heterozygous for the Pax2 mutation (Pkd1−/−;Pax2+/−), showed a marked reduction in kidney mass and cyst size when compared to Pkd1−/−;Pax2+/+ mice (141). Recently a signaling cascade has been elucidated in PKD showing that increased activity of c-Met results in increased activity of NF-kB, leading to increased expression of Wnt7a and Wnt7b, which finally leads to increased expression of Pax2 in cyst lining cells (142).
The role of another developmentally regulated gene, Cux1, was also investigated in PKD. Cux1 is a homeodomain protein that regulates the cell cycle by repressing the expression of the p21 and p27 cyclin kinase inhibitors (143). Cux1 is highly expressed during renal development, but sharply down regulated upon nephron maturation. Transgenic mice ectopically expressing Cux1 develop multiorgan hyperplasia, including renal hyperplasia, similar to p27 knockout mice (144–147). Cux1 is ectopically expressed in several mouse models of PKD, as well as in human ADPKD kidneys (148–150).
An ARPKD mouse model carrying a targeted mutation in the Cux1 gene was generated to determine the role of Cux1 in the progression of PKD. The cpk mouse model, which resembles human ARPKD, was crossed to Cux1tm1Ejn. This Cux1 mutant mouse carries an in-frame deletion of Cux1 that spanned exons 15 and 16. The phenotype of Cux1tm1Ejn includes wavy hair and curly vibrissae, and mutant females have a lactation defect and are unable to support pups. However, the kidneys of these mice are phenotypically normal. The Cux1tm1Ejn phenotype was similar to the mutations in the EGFR pathway. The genetic or pharmacologic inhibition of EGFR activity results in decreased cyst formation and improved kidney function. The Cux1tm1Ejn mice were crossed with Cys1cpk mice anticipating a similar alteration in cyst progression. The double mutant mice indeed showed a modification in cyst progression. However, the mutation in Cux1 did not reduce the cyst progression as expected, rather the Cux1 mutation accelerated disease progression in these animals. This phenotype was explained by the ectopic expression of Cux1ΔCR1 (the truncated form of Cux1) protein in the double knockout mice, which in turn repressed p21 and p27 thereby increasing cell proliferation and resulting in a rapid progression of the disease (150).
Additional genes that have been shown to modify PKD progression include Dicer. Dicer is an enzyme involved in the generation of mature microRNAs. When Dicer was ablated in mouse kidneys, glomerular and tubular cysts developed, resulting from an increased expression of Pkd1. The inactivation of Dicer was associated with the down regulation of miR-200, a kidney enriched microRNA family that targets PKD1 (151). Conversely, the microRNA cluster miR-17~92 is upregulated in mouse models of PKD and initiates cyst development when expressed in transgenic mice (152). Thus, either the upregulation of Pkd1 or the down regulation of Pkd1 by miRNAs results in cystogenesis (153).
Conversely, a comprehensive systems biology analysis of mice in which Pkd1 was conditionally knocked out between postnatal day 5 and 9 and analyzed at postnatal days 12 and 14 did not show any differences in microRNAs between wild type and mutant mice. Rather, a fundamental role for metabolic pathways in cystogenesis was demonstrated. In particular, a central role for HNF4α, a transcription factor that regulates several metabolic pathways, in protecting Pkd1 mutant kidneys from severe cystic disease was indicated (154).
Conclusion
Remarkable progress has been made in the study of PKD since the identification of the genes that are mutated to give rise to ARPKD and ADPKD. However, there is much that is still unknown about the proteins these genes encode. Questions remain regarding the function of these proteins in regulating cell proliferation. There is increasing evidence that PKD is a developmental disorder, however, there is still much to learn regarding the role these proteins play in normal renal development (155). Although these proteins have been shown to regulate numerous signaling pathways, a clear-cut link between the disruption of these pathways and the genesis and progression of cystic disease has yet to be identified (155). Answering these questions holds the promise for the development of effective therapeutic strategies for the treatment of PKD patients.
Figure 5. Potential mechanisms of cystogenesis and disease progression in ADPKD.
Acknowledgements
The authors thank Drs. Dale Abrahamson, Gustavo Blanco, Brenda Rongish, Doug Wright and members of The Kidney Institute at KUMC for many helpful suggestions and discussions. This work was funded by NIH grants DK58377 and DK57301.
References
- 1.Grantham JJ. Polycystic kidney disease: huge kidneys, huge problems, huge progress. Trans Am Clin Climatol Assoc. 1997;108:165–170. discussion 70-2. [PMC free article] [PubMed] [Google Scholar]
- 2.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002 Sep;13(9):2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 3.Harris PC, Torres VE. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest. 2014;124:2315–2324. doi: 10.1172/JCI72272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher AR, Germino GG, Somlo S. Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:118–130. doi: 10.1053/j.ackd.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D'Eustachio P, et al. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest. 2002 Feb;109(4):533–540. doi: 10.1172/JCI14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekser B, Rigotti P. Images in clinical medicine. Autosomal dominant polycystic kidney disease. N Engl J Med. 2010 Jul 1;363(1):71. doi: 10.1056/NEJMicm0905399. [DOI] [PubMed] [Google Scholar]
- 7.Grantham JJ. Polycystic kidney disease: a predominance of giant nephrons. Am J Physiol. 1983 Jan;244(1):F3–F10. doi: 10.1152/ajprenal.1983.244.1.F3. [DOI] [PubMed] [Google Scholar]
- 8.Brown JA. Images in clinical medicine. End-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2002 Nov 7;347(19):1504. doi: 10.1056/NEJMicm020402. [DOI] [PubMed] [Google Scholar]
- 9.Woo D. Apoptosis and loss of renal tissue in polycystic kidney diseases. N Engl J Med. 1995 Jul 6;333(1):18–25. doi: 10.1056/NEJM199507063330104. [DOI] [PubMed] [Google Scholar]
- 10.Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics. 2003 May;111(5 Pt 1):1072–1080. doi: 10.1542/peds.111.5.1072. [DOI] [PubMed] [Google Scholar]
- 11.Parfrey PS. Autosomal-recessive polycystic kidney disease. Kidney Int. 2005 Apr;67(4):1638–1648. doi: 10.1111/j.1523-1755.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 12.Guay-Woodford LM. Renal cystic diseases: diverse phenotypes converge on the cilium/centrosome complex. Pediatr Nephrol. 2006 Oct;21(10):1369–1376. doi: 10.1007/s00467-006-0164-9. [DOI] [PubMed] [Google Scholar]
- 13.Ibraghimov-Beskrovnaya O, Bukanov N. Polycystic kidney diseases: from molecular discoveries to targeted therapeutic strategies. Cell Mol Life Sci. 2008 Feb;65(4):605–619. doi: 10.1007/s00018-007-7362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993 Jul 29;329(5):332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 15.Reed BY, McFann K, Bekheirnia MR, Nobakhthaghighi N, Masoumi A, Johnson AM, Shamshirsaz AA, Kelleher CL, Schrier RW. Variation in age at ESRD in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2008 Feb;51(2):173–183. doi: 10.1053/j.ajkd.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamshirsaz AA, Reza Bekheirnia M, Kamgar M, Johnson AM, McFann K, Cadnapaphornchai M, Nobakhthaghighi N, Schrier RW. Autosomal-dominant polycystic kidney disease in infancy and childhood: progression and outcome. Kidney Int. 2005 Nov;68(5):2218–2224. doi: 10.1111/j.1523-1755.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002 Oct;13(10):2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 18.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995 Jun;10(2):151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 19.Martinez JR, Grantham JJ. Polycystic kidney disease: etiology, pathogenesis, and treatment. Dis Mon. 1995 Nov;41(11):693–765. doi: 10.1016/s0011-5029(05)80007-0. [DOI] [PubMed] [Google Scholar]
- 20.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992 May;41(5):1311–1319. doi: 10.1038/ki.1992.195. [DOI] [PubMed] [Google Scholar]
- 21.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009 Jul;76(2):149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinman TI. Polycystic kidney disease: a new perspective from the beginning. Kidney Int. 2005 Nov;68(5):2398–2399. doi: 10.1111/j.1523-1755.2005.00711.x. [DOI] [PubMed] [Google Scholar]
- 23.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007 Apr 14;369(9569):1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 24.Ong AC, Harris PC. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 2005 Apr;67(4):1234–1247. doi: 10.1111/j.1523-1755.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 25.Paul BM, Consugar MB, Ryan Lee M, Sundsbak JL, Heyer CM, Rossetti S, Kubly VJ, Hopp K, Torres VE, Coto E, Clementi M, Bogdanova N, de Almeida E, Bichet DG, Harris PC. Evidence of a third ADPKD locus is not supported by re-analysis of designated PKD3 families. Kidney Int. 2014;85:383–392. doi: 10.1038/ki.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT. Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol. 2010 May;5(5):889–896. doi: 10.2215/CJN.00550110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shokeir MH. Expression of "adult" polycystic renal disease in the fetus and newborn. Clin Genet. 1978 Aug;14(2):61–72. doi: 10.1111/j.1399-0004.1978.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 28.Bae K, Park B, Sun H, Wang J, Tao C, Chapman AB, Torres VE, Grantham JJ, Mrug M, Bennett WM, Flessner MF, Landsittel DP, Bae KT. Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP). Segmentation of individual renal cysts from MR images in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2013;8:1089–1097. doi: 10.2215/CJN.10561012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxen L. In: Oraganogenesis of the Kidney. Barlow PBG PW, Wylie CC, editors. Cambridge University Press; 1987. [Google Scholar]
- 30.Bouchard M. Transcriptional control of kidney development. Differentiation. 2004 Sep;72(7):295–306. doi: 10.1111/j.1432-0436.2004.07207001.x. [DOI] [PubMed] [Google Scholar]
- 31.Lipschutz JH. Molecular development of the kidney: a review of the results of gene disruption studies. Am J Kidney Dis. 1998 Mar;31(3):383–397. doi: 10.1053/ajkd.1998.v31.pm9506676. [DOI] [PubMed] [Google Scholar]
- 32.Schedl A, Hastie ND. Cross-talk in kidney development. Curr Opin Genet Dev. 2000 Oct;10(5):543–549. doi: 10.1016/s0959-437x(00)00125-8. [DOI] [PubMed] [Google Scholar]
- 33.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 34.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009 Dec;136(23):3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechner MS, Dressler GR. The molecular basis of embryonic kidney development. Mech Dev. 1997 Mar;62(2):105–120. doi: 10.1016/s0925-4773(97)00667-9. [DOI] [PubMed] [Google Scholar]
- 36.Potter SS, Brunskill EW, Patterson LT. Defining the genetic blueprint of kidney development. Pediatr Nephrol. 2011;26:1469–1478. doi: 10.1007/s00467-011-1807-z. [DOI] [PubMed] [Google Scholar]
- 37.Bernhardt WM, Schmitt R, Rosenberger C, Munchenhagen PM, Grone HJ, Frei U, et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006 Jan;69(1):114–122. doi: 10.1038/sj.ki.5000062. [DOI] [PubMed] [Google Scholar]
- 38.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- 40.Grieshammer U, Le Ma, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- 41.Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–1395. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- 42.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol. 2006;299:466–477. doi: 10.1016/j.ydbio.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 44.Michos O, Gonçalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development. 2007;134:2397–2405. doi: 10.1242/dev.02861. [DOI] [PubMed] [Google Scholar]
- 45.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 46.Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 2002;16:1423–1432. doi: 10.1101/gad.993302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong KQ, Yallowitz AR, Sun H, Dressler GR, Wellik DM. A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol Cell Biol. 2007;27:7661–7668. doi: 10.1128/MCB.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Brien LL, McMahon AP. Progenitor programming in mammalian nephrogenesis. Nephrology (Carlton) 2013;18:177–179. doi: 10.1111/nep.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel SR, Dressler GR. The genetics and epigenetics of kidney development. Semin Nephrol. 2013;33:314–326. doi: 10.1016/j.semnephrol.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parnell SC, Magenheimer BS, Maser RL, Zien CA, Frischauf AM, Calvet JP. Polycystin-1 activation of c-Jun N-terminal kinase and AP-1 is mediated by heterotrimeric G proteins. J Biol Chem. 2002 May 31;277(22):19566–19572. doi: 10.1074/jbc.M201875200. [DOI] [PubMed] [Google Scholar]
- 51.Moy GW, Mendoza LM, Schulz JR, Swanson WJ, Glabe CG, Vacquier VD. The sea urchin sperm receptor for egg jelly is a modular protein with extensive homology to the human polycystic kidney disease protein, PKD1. J Cell Biol. 1996 May;133(4):809–817. doi: 10.1083/jcb.133.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward CJ, Turley H, Ong AC, Comley M, Biddolph S, Chetty R, et al. Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1524–1528. doi: 10.1073/pnas.93.4.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bateman A, Sandford R. The PLAT domain: a new piece in the PKD1 puzzle. Curr Biol. 1999 Aug 26;9(16):R588–R590. doi: 10.1016/s0960-9822(99)80380-7. [DOI] [PubMed] [Google Scholar]
- 54.Chauvet V, Qian F, Boute N, Cai Y, Phakdeekitacharoen B, Onuchic LF, et al. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am J Pathol. 2002 Mar;160(3):973–983. doi: 10.1016/S0002-9440(10)64919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chapin HC, Caplan MJ. The cell biology of polycystic kidney disease. J Cell Biol. 2010 Nov 15;191(4):701–710. doi: 10.1083/jcb.201006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest. 2004 Nov;114(10):1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 58.Knepper MA, Pisitkun T. Exosomes in urine: who would have thought…? Kidney Int. 2007 Nov;72(9):1043–1045. doi: 10.1038/sj.ki.5002510. [DOI] [PubMed] [Google Scholar]
- 59.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009 Feb;20(2):278–288. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semmo M, Köttgen M, Hofherr A. The TRPP subfamily and polycystin-1 proteins. Handb Exp Pharmacol. 2014;222:675–711. doi: 10.1007/978-3-642-54215-2_27. [DOI] [PubMed] [Google Scholar]
- 61.Ong AC, Ward CJ, Butler RJ, Biddolph S, Bowker C, Torra R, et al. Coordinate expression of the autosomal dominant polycystic kidney disease proteins, polycystin-2 and polycystin-1, in normal and cystic tissue. Am J Pathol. 1999 Jun;154(6):1721–1729. doi: 10.1016/S0002-9440(10)65428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geng L, Segal Y, Pavlova A, Barros EJ, Lohning C, Lu W, et al. Distribution and developmentally regulated expression of murine polycystin. Am J Physiol. 1997 Apr;272(4 Pt 2):F451–F459. doi: 10.1152/ajprenal.1997.272.4.F451. [DOI] [PubMed] [Google Scholar]
- 63.Markowitz GS, Cai Y, Li L, Wu G, Ward LC, Somlo S, et al. Polycystin-2 expression is developmentally regulated. Am J Physiol. 1999 Jul;277(1 Pt 2):F17–F25. doi: 10.1152/ajprenal.1999.277.1.F17. [DOI] [PubMed] [Google Scholar]
- 64.Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996 Dec 13;87(6):979–987. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- 65.Pei Y. A "two-hit" model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med. 2001;7:151–156. doi: 10.1016/s1471-4914(01)01953-0. [DOI] [PubMed] [Google Scholar]
- 66.Harris PC. What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol. 2010;21:1073–1076. doi: 10.1681/ASN.2010030328. [DOI] [PubMed] [Google Scholar]
- 67.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, et al. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet. 1997 Oct;17(2):179–181. doi: 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- 68.Wu G, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998 Apr 17;93(2):177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 69.Pritchard L, Sloane-Stanley JA, Sharpe JA, Aspinwall R, Lu W, Buckle V, et al. A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype. Hum Mol Genet. 2000 Nov 1;9(18):2617–2627. doi: 10.1093/hmg/9.18.2617. [DOI] [PubMed] [Google Scholar]
- 70.Thivierge C, Kurbegovic A, Couillard M, Guillaume R, Cote O, Trudel M. Overexpression of PKD1 causes polycystic kidney disease. Mol Cell Biol. 2006 Feb;26(4):1538–1548. doi: 10.1128/MCB.26.4.1538-1548.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004 Dec 15;13(24):3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 72.Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, van't Hoff WG, Niaudet P, Torres VE, Harris PC. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009;75:848–855. doi: 10.1038/ki.2008.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vujic M, Heyer CM, Ars E, Hopp K, Markoff A, Orndal C, Rudenhed B, Nasr SH, Torres VE, Torra R, Bogdanova N, Harris PC. Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol. 2010;21:1097–1102. doi: 10.1681/ASN.2009101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest. 2012;122:4257–4273. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007 Dec;13(12):1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, Breuning MH, de Heer E, Peters DJ. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet. 2007 Dec 15;16(24):3188–3196. doi: 10.1093/hmg/ddm299. [DOI] [PubMed] [Google Scholar]
- 77.Takakura A, Contrino L, Beck AW, Zhou J. Pkd1 inactivation induced in adulthood produces focal cystic disease. J Am Soc Nephrol. 2008 Dec;19(12):2351–2363. doi: 10.1681/ASN.2007101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet. 2009 Jul 15;18(14):2523–2531. doi: 10.1093/hmg/ddp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bell PD, Fitzgibbon W, Sas K, Stenbit AE, Amria M, Houston A, et al. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol. 2011 May;22(5):839–848. doi: 10.1681/ASN.2010050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weimbs T. Third-hit signaling in renal cyst formation. J Am Soc Nephrol. 2011 May;22(5):793–795. doi: 10.1681/ASN.2011030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weimbs T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol. 2007;293:F1423–F1432. doi: 10.1152/ajprenal.00275.2007. [DOI] [PubMed] [Google Scholar]
- 82.Prasad S, McDaid JP, Tam FW, Haylor JL, Ong AC. Pkd2 dosage influences cellular repair responses following ischemia-reperfusion injury. Am J Pathol. 2009;175:1493–1503. doi: 10.2353/ajpath.2009.090227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008 Jun 1;17(11):1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma N, Malarkey EB, Berbari NF, O'Connor AK, Vanden Heuvel GB, Mrug M, Yoder BK. Proximal tubule proliferation is insufficient to induce rapid cyst formation after cilia disruption. J Am Soc Nephrol. 2013;24:456–464. doi: 10.1681/ASN.2012020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005 Dec;6(12):928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 88.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003 Feb;33(2):129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 89.Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006 Jan;17(1):178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 90.Arnould T, Kim E, Tsiokas L, Jochimsen F, Gruning W, Chang JD, et al. The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J Biol Chem. 1998 Mar 13;273(11):6013–6018. doi: 10.1074/jbc.273.11.6013. [DOI] [PubMed] [Google Scholar]
- 91.Le NH, van der Wal A, van der Bent P, Lantinga-van Leeuwen IS, Breuning MH, van Dam H, et al. Increased activity of activator protein-1 transcription factor components ATF2, c-Jun, and c-Fos in human and mouse autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2005 Sep;16(9):2724–2731. doi: 10.1681/ASN.2004110913. [DOI] [PubMed] [Google Scholar]
- 92.Puri S, Magenheimer BS, Maser RL, Ryan EM, Zien CA, Walker DD, et al. Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem. 2004 Dec 31;279(53):55455–55464. doi: 10.1074/jbc.M402905200. [DOI] [PubMed] [Google Scholar]
- 93.Lanoix J, D'Agati V, Szabolcs M, Trudel M. Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD) Oncogene. 1996;13:1153–1160. [PubMed] [Google Scholar]
- 94.Weimbs T, Olsan EE, Talbot JJ, editors. Regulation of STATs by polycystin-1 and their role in polycystic kidney disease. JAKSTAT. 2013;2:e23650-1–e23650-9. doi: 10.4161/jkst.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002 Apr 19;109(2):157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 96.Felekkis KN, Koupepidou P, Kastanos E, Witzgall R, Bai CX, Li L, Tsiokas L, Gretz N, Deltas C. Mutant polycystin-2 induces proliferation in primary rat tubular epithelial cells in a STAT-1/p21-independent fashion accompanied instead by alterations in expression of p57KIP2 and Cdk2. BMC Nephrol. 2008 Aug 25;9:10. doi: 10.1186/1471-2369-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X, Luo Y, Starremans PG, McNamara CA, Pei Y, Zhou J. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat Cell Biol. 2005 Dec;7(12):1202–1212. doi: 10.1038/ncb1326. [DOI] [PubMed] [Google Scholar]
- 98.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, et al. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006 Jan;10(1):57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 99.Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G, Song X, Pei Y, Weimbs T. Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci U S A. 2011;108:18067–18072. doi: 10.1073/pnas.1111966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, et al. Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci U S A. 2011 May 10;108(19):7985–7990. doi: 10.1073/pnas.1103816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006 Apr 4;103(14):5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dere R, Wilson PD, Sandford RN, Walker CL. Carboxy terminal tail of polycystin-1 regulates localization of TSC2 to repress mTOR. PLoS One. 2010;5(2):e9239. doi: 10.1371/journal.pone.0009239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huber TB, Walz G, Kuehn EW. mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int. 2011;79:502–511. doi: 10.1038/ki.2010.457. [DOI] [PubMed] [Google Scholar]
- 104.Watnick T, Germino GG. mTOR inhibitors in polycystic kidney disease. N Engl J Med. 2010;363:879–881. doi: 10.1056/NEJMe1006925. [DOI] [PubMed] [Google Scholar]
- 105.Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, et al. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999 Feb 19;274(8):4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- 106.Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND, et al. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet. 2008 Oct 15;17(20):3105–3117. doi: 10.1093/hmg/ddn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001 Sep 20;20(42):5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 108.Miller MM, Iglesias DM, Zhang Z, Corsini R, Chu L, Murawski I, Gupta I, Somlo S, Germino GG, Goodyer PR. T-cell factor/β-catenin activity is suppressed in two different models of autosomal dominant polycystic kidney disease. Kidney Int. 2011;80:146–153. doi: 10.1038/ki.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006 Jan;38(1):21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 110.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005 May;37(5):537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, et al. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 2004 Sep;66(3):964–973. doi: 10.1111/j.1523-1755.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 112.Calvet JP. Polycystic kidney disease: primary extracellular matrix abnormality or defective cellular differentiation? Kidney Int. 1993 Jan;43(1):101–108. doi: 10.1038/ki.1993.17. [DOI] [PubMed] [Google Scholar]
- 113.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003 Jun;63(6):1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 114.Calvet JP. MEK inhibition holds promise for polycystic kidney disease. J Am Soc Nephrol. 2006;17:1498–1500. doi: 10.1681/ASN.2006040353. [DOI] [PubMed] [Google Scholar]
- 115.Park EY, Sung YH, Yang MH, Noh JY, Park SY, Lee TY, Yook YJ, Yoo KH, Roh KJ, Kim I, Hwang YH, Oh GT, Seong JK, Ahn C, Lee HW, Park JH. Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J Biol Chem. 2009;284:7214–7222. doi: 10.1074/jbc.M805890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, et al. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl(−) Co-transporter-dependent cystic dilation. J Am Soc Nephrol. 2006 Dec;17(12):3424–3437. doi: 10.1681/ASN.2006030295. [DOI] [PubMed] [Google Scholar]
- 117.Sorenson CM, Padanilam BJ, Hammerman MR. Abnormal postpartum renal development and cystogenesis in the bcl-2 (−/−) mouse. Am J Physiol. 1996 Jul;271(1 Pt 2):F184–F193. doi: 10.1152/ajprenal.1996.271.1.F184. [DOI] [PubMed] [Google Scholar]
- 118.Hoetelmans RW, Vahrmeijer AL, van Vlierberghe RL, Keijzer R, van de Velde CJ, Mulder GJ, Van Dierendonck JH. The role of various Bcl-2 domains in the anti-proliferative effect and modulation of cellular glutathione levels: a prominent role for the BH4 domain. Cell Prolif. 2003;36:35–44. doi: 10.1046/j.1365-2184.2003.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trudel M, D'Agati V, Costantini F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991 Apr;39(4):665–671. doi: 10.1038/ki.1991.80. [DOI] [PubMed] [Google Scholar]
- 120.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2011;22:1809–1814. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Swenson-Fields KI, Vivian CJ, Salah SM, Peda JD, Davis BM, van Rooijen N, Wallace DP, Fields TA. Macrophages promote polycystic kidney disease progression. Kidney Int. 2013;83:855–864. doi: 10.1038/ki.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou J, Ouyang X, Cui X, Schoeb TR, Smythies LE, Johnson MR, et al. Renal CD14 expression correlates with the progression of cystic kidney disease. Kidney Int. 2010 Sep;78(6):550–560. doi: 10.1038/ki.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vanden Heuvel GB. CD14 : a candidate biomarker for the prognosis of polycystic kidney disease. Kidney Int. 2010 Sep;78(6):537–538. doi: 10.1038/ki.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Katz SK, Hakki A, Miller AS, Finkelstein SD. Ultrastructural tubular basement membrane lesions in adult polycystic kidney disease. Ann Clin Lab Sci. 1989 Sep-Oct;19(5):352–359. [PubMed] [Google Scholar]
- 125.Shannon MB, Patton BL, Harvey SJ, Miner JH. A hypomorphic mutation in the mouse laminin alpha5 gene causes polycystic kidney disease. J Am Soc Nephrol. 2006 Jul;17(7):1913–1922. doi: 10.1681/ASN.2005121298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wallace DP, Quante MT, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol. 2008;295:F1463–F1471. doi: 10.1152/ajprenal.90266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wallace DP, White C, Savinkova L, Nivens E, Reif GA, Pinto CS, Raman A, Parnell SC, Conway SJ, Fields TA. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int. 2014;85:845–854. doi: 10.1038/ki.2013.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, et al. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol. 2010 Feb;21(2):295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guay-Woodford LM. Murine models of polycystic kidney disease: molecular and therapeutic insights. Am J Physiol Renal Physiol. 2003 Dec;285(6):F1034–F1049. doi: 10.1152/ajprenal.00195.2003. [DOI] [PubMed] [Google Scholar]
- 131.Belibi FA, Wallace DP, Yamaguchi T, Christensen M, Reif G, Grantham JJ. The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002 Nov;13(11):2723–2729. doi: 10.1097/01.asn.0000025282.48298.7b. [DOI] [PubMed] [Google Scholar]
- 132.Laass MW, Spiegel M, Jauch A, Hahn G, Rupprecht E, Vogelberg C, et al. Tuberous sclerosis and polycystic kidney disease in a 3-month-old infant. Pediatr Nephrol. 2004 Jun;19(6):602–608. doi: 10.1007/s00467-004-1442-z. [DOI] [PubMed] [Google Scholar]
- 133.Pei Y. Nature and nurture on phenotypic variability of autosomal dominant polycystic kidney disease. Kidney Int. 2005 Apr;67(4):1630–1631. doi: 10.1111/j.1523-1755.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 134.Persu A, Duyme M, Pirson Y, Lens XM, Messiaen T, Breuning MH, et al. Comparison between siblings and twins supports a role for modifier genes in ADPKD. Kidney Int. 2004 Dec;66(6):2132–2136. doi: 10.1111/j.1523-1755.2004.66003.x. [DOI] [PubMed] [Google Scholar]
- 135.Fedeles SV, Tian X, Gallagher AR, Mitobe M, Nishio S, Lee SH, Cai Y, Geng L, Crews CM, Somlo S. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet. 2011;43:639–647. doi: 10.1038/ng.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Freedman BS, Lam AQ, Sundsbak JL, Iatrino R, Su X, Koon SJ, Wu M, Daheron L, Harris PC, Zhou J, Bonventre JV. Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J Am Soc Nephrol. 2013;24:1571–1586. doi: 10.1681/ASN.2012111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pei Y, Lan Z, Wang K, Garcia-Gonzalez M, He N, Dicks E, Parfrey P, Germino G, Watnick T. A missense mutation in PKD1 attenuates the severity of renal disease. Kidney Int. 2012;81:412–417. doi: 10.1038/ki.2011.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Happé H, van der Wal AM, Salvatori DC, Leonhard WN, Breuning MH, de Heer E, Peters DJ. Cyst expansion and regression in a mouse model of polycystic kidney disease. Kidney Int. 2013;83:1099–1108. doi: 10.1038/ki.2013.13. [DOI] [PubMed] [Google Scholar]
- 139.Eccles MR, Stayner CA. Polycystic kidney disease - where gene dosage counts. F1000Prime Rep. 2014;6:24. doi: 10.12703/P6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ostrom L, Tang MJ, Gruss P, Dressler GR. Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev Biol. 2000 Mar 15;219(2):250–258. doi: 10.1006/dbio.2000.9618. [DOI] [PubMed] [Google Scholar]
- 141.Stayner C, Iglesias DM, Goodyer PR, Ellis L, Germino G, Zhou J, et al. Pax2 gene dosage influences cystogenesis in autosomal dominant polycystic kidney disease. Hum Mol Genet. 2006 Dec 15;15(24):3520–3528. doi: 10.1093/hmg/ddl428. [DOI] [PubMed] [Google Scholar]
- 142.Qin S, Taglienti M, Cai L, Zhou J, Kreidberg JA. c-Met and NF-κB-dependent overexpression of Wnt7a and-7b and Pax2 promotes cystogenesis in polycystic kidney disease. J Am Soc Nephrol. 2012;23:1309–1318. doi: 10.1681/ASN.2011030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Coqueret O, Bérubé G, Nepveu A. The mammalian Cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J. 1998 Aug 17;17(16):4680–4694. doi: 10.1093/emboj/17.16.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ledford AW, Brantley JG, Kemeny G, Foreman TL, Quaggin SE, Igarashi P, et al. Deregulated expression of the homeobox gene Cux-1 in transgenic mice results in downregulation of p27(kip1) expression during nephrogenesis, glomerular abnormalities, and multiorgan hyperplasia. Dev Biol. 2002 May 1;245(1):157–171. doi: 10.1006/dbio.2002.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996 May 31;85(5):733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 146.Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 147.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 148.Vanden Heuvel GB, Bodmer R, McConnell KR, Nagami GT, Igarashi P. Expression of a cut-related homeobox gene in developing and polycystic mouse kidney. Kidney Int. 1996 Aug;50(2):453–461. doi: 10.1038/ki.1996.336. [DOI] [PubMed] [Google Scholar]
- 149.Sharma M, Brantley JG, Alcalay NI, Zhou J, Heystek E, Maser RL, et al. Differential expression of Cux-1 and p21 in polycystic kidneys from Pkd1 null and cpk mice. Kidney Int. 2005 Feb;67(2):432–442. doi: 10.1111/j.1523-1755.2005.67099.x. [DOI] [PubMed] [Google Scholar]
- 150.Alcalay NI, Sharma M, Vassmer D, Chapman B, Paul B, Zhou J, et al. Acceleration of polycystic kidney disease progression in cpk mice carrying a deletion in the homeodomain protein Cux1. Am J Physiol Renal Physiol. 2008 Dec;295(6):F1725–F1734. doi: 10.1152/ajprenal.90420.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patel V, Hajarnis S, Williams D, Hunter R, Huynh D, Igarashi P. MicroRNAs regulate renal tubule maturation through modulation of Pkd1. J Am Soc Nephrol. 2012;23:1941–1948. doi: 10.1681/ASN.2012030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Patel V, Williams D, Hajarnis S, Hunter R, Pontoglio M, Somlo S, Igarashi P. miR-17~92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc Natl Acad Sci U S A. 2013;110:10765–10770. doi: 10.1073/pnas.1301693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang E, Hsieh-Li HM, Chiou YY, Chien YL, Ho HH, Chin HJ, Wang CK, Liang SC, Jiang ST. Progressive renal distortion by multiple cysts in transgenic mice expressing artificial microRNAs against Pkd1. J Pathol. 2010;222:238–248. doi: 10.1002/path.2765. [DOI] [PubMed] [Google Scholar]
- 154.Menezes LF, Zhou F, Patterson AD, Piontek KB, Krausz KW, Gonzalez FJ, Germino GG. Network analysis of a Pkd1-mouse model of autosomal dominant polycystic kidney disease identifies HNF4α as a disease modifier. PLoS Genet. 2012;8:e1003053. doi: 10.1371/journal.pgen.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tran PV, Sharma M, Li X, Calvet JP. Developmental signaling: Does it bridge the gap between cilia dysfunction and renal cystogenesis? Birth Defects Res C Embryo Today. 2014;102:159–173. doi: 10.1002/bdrc.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]