Abstract
The transcription factor NF-κB is critical for T cell activation and survival. We have shown that mice expressing a T cell-restricted NF-κB superrepressor (IκBαΔN-Tg) permanently accept heart but not skin allografts. Overexpression of the prosurvival factor Bcl-xL in T cells restored heart rejection, suggesting that graft acceptance in IκBαΔN-Tg mice was due to deletion of alloreactive T cells. In vitro, the increased death of IκBαΔN-Tg T cells upon TCR stimulation when compared to wildtype T cells was mostly due to Fas/FasL interaction. Similarly, Fas played a key role in cardiac allograft acceptance by IκBαΔN-Tg mice as both genetic and antibody-mediated inhibition of Fas signaling restored cardiac allograft rejection. Rejection correlated with graft infiltration by T cells and splenic production of IFN-γ upon allostimulation. These results indicate that T cell inhibition of NF-κB results in cardiac allograft acceptance because of increased susceptibility to Fas-mediated cell death.
Keywords: Apoptosis, Fas, Mouse, T cells, Tolerance, Transplantation, NF-κB
Introduction
T cells play a major role in acute allograft rejection. NF-κB is a critical transcription factor for T cell activation, proliferation and survival (1). Regulation of NF-κB activation is determined by the interaction between the NF-κB dimers and their inhibitors, members of the IκB family. Phosphorylation of the IκBs induces their degradation in an ubiquitin-proteasome-dependent manner. Overexpression of a non-phosphorylatable form of the inhibitor IκBα in T cells leads to decreased NF-κB activity, therefore impairing NF-κB-mediated T cell proliferation, IL-2 and IFN-γ production, and cell survival (2–4). The latter is mostly due to the inability of NF-κB-impaired T cells to upregulate the prosurvival factors Bcl-xL and c-FLIP (5). In contrast, Th2 responses remain unaffected in NF-κB-impaired T cells (3). Mice overexpressing the non-degradable form of IκBα selectively in their T cells (IκBαΔN-Tg mice) do not develop Th1-mediated diseases, such as collagen-induced arthritis, and are also incapable of mounting an anti-parasite response against Toxoplasma gondii (6, 7).
We have previously observed that cardiac allografts are permanently accepted in IκBαΔN-Tg mice, although these animals retain the capacity to reject non-vascularized skin allografts (8). Cardiac allograft rejection in IκBαΔN-Tg mice could be restored by the overexpression in T cells of the pro-survival factor Bcl-xL (9). This result suggested that cardiac alloantigens were promoting apoptosis of alloreactive NF-κB-impaired T cells, while skin allografts could surmount the NF-κB deficiency and trigger T cell activation. Indeed, we have found that donor Langerhans cells from the skin can significantly activate NF-κB-impaired T cells and are sufficient to drive acute rejection of heart allografts in IκBαΔN-Tg mice (10). However, the mechanism of NF-κB-impaired T cell apoptosis following cardiac transplantation remained to be established.
T cells can undergo apoptosis following interactions between Fas (CD95) and FasL, TNF and TNFR1, or DR5 and TRAIL (11). Fas mediates its effects through the activation of caspases 3 and 9, and Fas-dependent apoptosis can be prevented by expression of cFLIP (12). TCR stimulation results in proliferation and death of normal T cells, but susceptibility to TCR-mediated apoptosis is significantly increased in IκBαΔN-Tg T cells (5). This is likely due to the fact that Fas engagement results in activation of both death-inducing caspases and death-protecting NF-κB, such that Fas-mediated NF-κB activity limits Fas-dependent apoptosis (13). Expression of a non functional form of Fas in IκBαΔN-Tg T cells (Lpr/ IκBαΔN) has been reported to protect IκBαΔN-Tg T cells from TCR-mediated apoptosis in vitro (5). These results prompted us to hypothesize that cardiac allograft acceptance in IκBαΔN-Tg mice may be due to unopposed Fas-mediated T cell death among NF-κB-impaired T cells. Our results indicate that pharmacological or genetic inhibition of Fas restored the capacity of IκBαΔN-Tg mice to reject cardiac allografts, promoted cardiac allograft infiltration by CD4+ and CD8+ T cells and resulted in donor-specific IFN-γ production. Therefore, Fas-mediated cell death of alloreactive T cells is necessary for cardiac allograft acceptance in mice with impaired NF-κB signaling in T cells. These data point to T cell-intrinsic NF-κB and Fas as potential therapeutic targets to promote transplantation tolerance.
Materials and Methods
Animals
Six to eight weeks-old C57Bl/6 (B6, H-2b) and BALB/c (H-2d), mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice transgenic for a T cell-restricted IκBα super-repressor, IκBαΔN-Tg mice, were obtained from Mark Boothby (Vanderbilt University, TE) and backcrossed to the B6 background for over 10 generations (2). Mice deficient in Fas signaling, Lpr (B6 background), were backcrossed to IκBαΔN-Tg mice. Animals were housed in individually ventilated cages in a specific pathogen-free animal facility. Groups of transplanted mice were treated on days 0, 2, 4, and 8 after transplantation with 250 µg/injection of anti-FasL mAb (Clone MF4L) or control rat IgG (Jackson Immunoresearch Laboratories Inc., PA). Experiments were performed in agreement with our Institutional Animal Care and Use Committee and according to the NIH guidelines for animal use.
Antibodies
The antibodies anti-CD3 (clone 145-2C11), anti-CD28 (clone PV-1), anti-FasL (clone MFL-4), anti-TNF (clone MP6-XT22) and anti-TRAIL (clone N2B2) were purified from supernatants of hybridomas following elution on a protein G column.
Cell death assays
WT or IκBαΔN-Tg splenocytes were stimulated in the presence of 1 µg/ml of anti-CD3 mAb for 24, 48 or 72h in the presence or absence of 10 µg/ml each of anti-FasL, anti-TNF, anti-TRAIL, a combination of these or rat or hamster IgG. Cell death was determined by DAPI inclusion by flow cytometry.
Cytokine production assay
Splenocytes from wildtype (WT) or IκBαΔN-Tg mice were stimulated with soluble anti-CD3 mAb (1 µg/ml, clone 145-2C11). For IFN-γ ELISA, supernatants were harvested on day 5 post-stimulation, and analyzed by ELISA using Ab pairs, as instructed by the manufacturer (BD PharMingen).
Cardiac allograft
Abdominal heterotopic cardiac transplantation was performed using a technique adapted from that originally described by Corry et al (14). Briefly, cardiac allografts were transplanted in the abdominal cavity by anastomosing the aorta and pulmonary artery of the graft end-to-side to the recipient's aorta and vena cava, respectively. The day of rejection was defined as the last day of a detectable heartbeat.
IFN-γ ELISPOTs
Splenocytes (106/well) from mice transplanted 21 days earlier with BALB/c hearts and treated with rat IgG or anti-FasL mAb were stimulated with irradiated (2000 rads) B6 or BALB/c splenocytes (4×105/well) and incubated for 18 h in a 7% CO2 incubator. The ELISPOT assay was conducted according to the instructions of the manufacturer (BD Biosciences), and the numbers of IFN-γ-producing spots per well were calculated using the ImmunoSpot Analyzer (CTL Analyzers LLC).
Immunohistochemistry
Grafts were removed at different time-points following transplantation, embedded with OCT (Tissue-Tek Miles Inc, Elkhart), and immediately frozen in liquid nitrogen. The samples were sliced into 6-µm-thick sections at −20°C and stained with anti-CD8 rat IgG supernatant (neat) or anti-CD4 purified rat IgG antibody as previously described (8). Slides were evaluated under light microscopy by a pathologist blinded to the clinical rejection status of the heart. The number of CD4+ and CD8+ cells was counted in 3–4 randomly chosen high-powered visual fields per section (× 400 magnifications, approximately 254 mm2).
Statistical analysis
Cardiac graft mean survival time (MST), standard deviation, and p-values were calculated using Kaplan-Meier/log rank test methods. Comparisons of means were performed using the Student’s t test or the Tukey test for multiple comparisons, as appropriate.
Results
TNF, TRAIL and Fas participate in AICD of IκBαΔN-Tg T cells in vitro
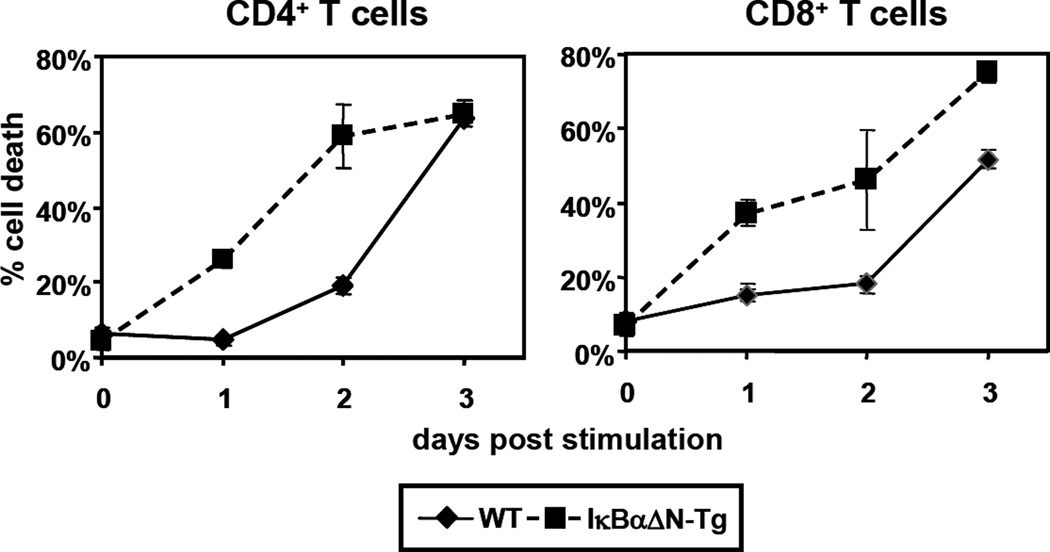
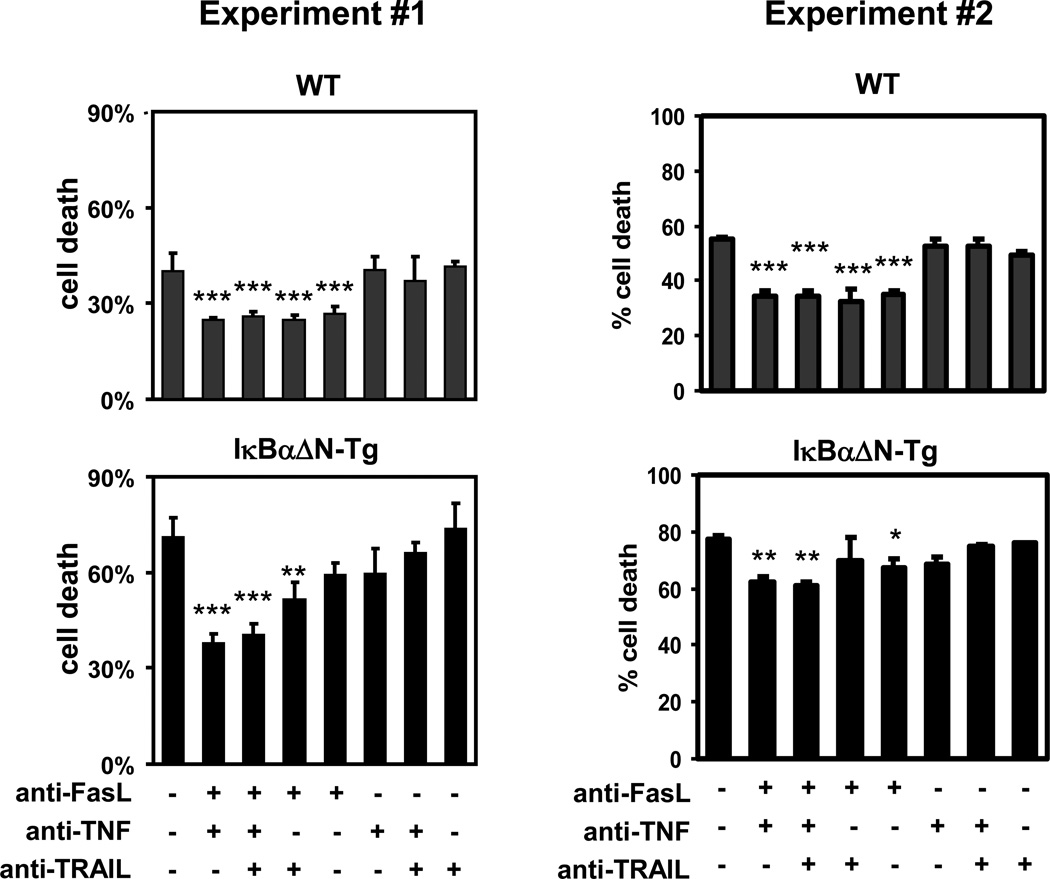
TCR stimulation induces T cells to enter the cell cycle, produce cytokines and ultimately undergo apoptosis (15). The transcription factor NF-κB plays a critical role in these events and particularly in cell survival, by triggering the upregulation of the pro-survival proteins IAP1/IAP2, XIAP (16) Bcl-2, Bcl-xL, and survivin (16–19). Consistent with the importance of NF-κB in cell survival, TCR stimulation of IκBαΔN-Tg splenocytes in vitro resulted in increased death of both CD4+ and CD8+ T cells when compared with WT T cells, as determined by DAPI staining by flow cytometry (Figure 1). Reduced survival of IκBαΔN-Tg T cells was likely due to apoptosis as analysis of cell cycle progression by intracellular propidium iodide staining revealed an increased percentage of sub-diploid cells compared to wildtype T cells (data not shown). To determine the mechanism of cell death in IκBαΔN T cells, WT and IκBαΔN-Tg splenocytes were stimulated for 3 days in the presence of blocking antibodies to FasL, TNF, or TRAIL. As shown in Figure 2, single blockade of Fas/FasL interactions resulted in a significant reduction of TCR-induced apoptosis in WT CD4+ T cells and blockade of TNF or TRAIL did not have additional effects. In contrast, IκBαΔN-Tg CD4+ T cells were not or weakly significantly protected from apoptosis by blockade of Fas/FasL engagement alone. However, concomitant blockade of FasL with that of TNF and TRAIL significantly reduced TCR-induced cell death of NF-κB-impaired CD4+ T cells to levels similar to those observed in WT T cells, whereas no protection from cell death occurred in the absence of FasL blockade (Figure 2). Similar data were obtained with CD8+ T cells (data not shown). This result suggests that Fas, TNF and TRAIL all contribute to apoptosis of NF-κB-impaired T cells following TCR stimulation, although the role of Fas may be more important since Fas engagement can still trigger apoptosis when both TNF and TRAIL pathways are blocked. Thus, Fas may play a dominant role in apoptosis of NF-κB-impaired T cells in vitro, but TNF and TRAIL also participate.
Figure 1. NF-κB-impaired T cells have increased susceptibility to apoptosis post TCR stimulation.
WT or IκBαΔN-Tg splenocytes were stimulated with 1 µg/ml of soluble anti-CD3 mAb, and percent death within CD4+ and CD8+ T cells was assessed over time using DAPI incorporation and flow cytometry analysis. The plot is representative of more than 4 independent experiments.
Figure 2. Blockade of Fas, TNF and TRAIL reduces TCR-induced cell death of NF-κB-impaired T cells.
WT and IκBαΔN-Tg splenocytes were stimulated in the presence of 1 µg/ml of soluble anti-CD3 mAb, in the presence or absence of anti-FasL, anti-TNF, or anti-TRAIL mAbs (10 µg/ml each). Three days later cell death was assessed by DAPI incorporation in CD4+ cells. Two independent experiments are shown. *p<0.05; **p<0.01; ***p<0.001 when compared to the untreated samples in each plot.
The defect in Th1 differentiation by IκBαΔN-Tg T cells is in part Fas-dependent
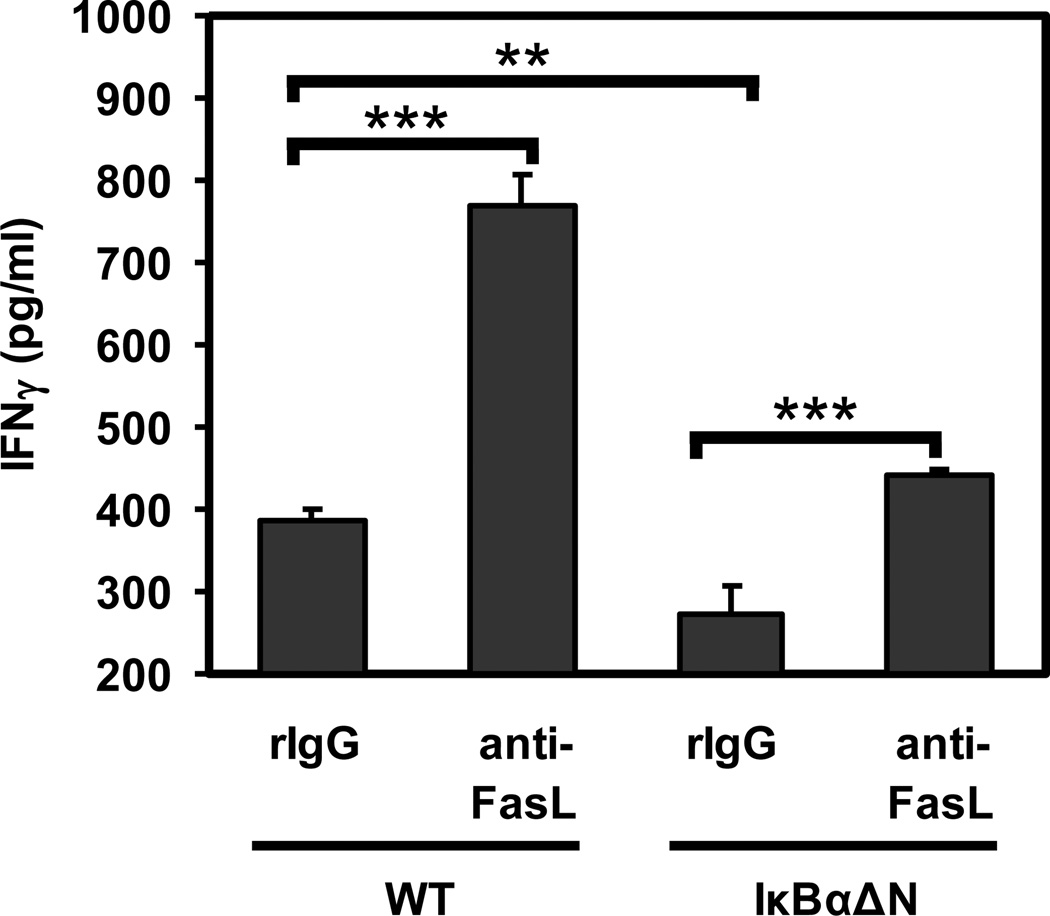
NF-κB is required for the differentiation of naïve T cells into IFN-γ producing Th1 cells (3), and IFN-γ expression usually correlates with cardiac allograft rejection. In order to address whether Fas-mediated signals were preventing the generation of IFN-γ-producing IκBαΔN T cells, WT or IκBαΔN-Tg CD4+ T cells were stimulated and allowed to differentiate for 4 days in the presence of anti-FasL mAb or control IgG prior to restimulation with PMA and ionomycin. Upon restimulation, IκBαΔN T cells that had differentiated in the presence of anti-FasL were able to produce more IFN-γ compared to IgG-treated control cells, although at lower levels than anti-FasL-treated wildtype T cells (Figure 3). This result suggests that NF-κB-impaired T cells can progress through the type 1 differentiation pathway when Fas-mediated signals are blocked.
Figure 3. Blockade of Fas enhances IFN-γ production by IκBαΔN-Tg T cells in vitro.
WT and IκBαΔN-Tg splenocytes were stimulated in the presence of soluble anti-CD3 mAb (1 µg/ml), in the presence or absence of isotype control or anti-FasL mAb (10 µg/ml). Supernatants were collected at 5 days and assayed for concentration of IFN-γ by ELISA. **p<0.01; ***p<0.001.
Cardiac allograft acceptance in IκBαΔN-Tg mice is Fas-dependent
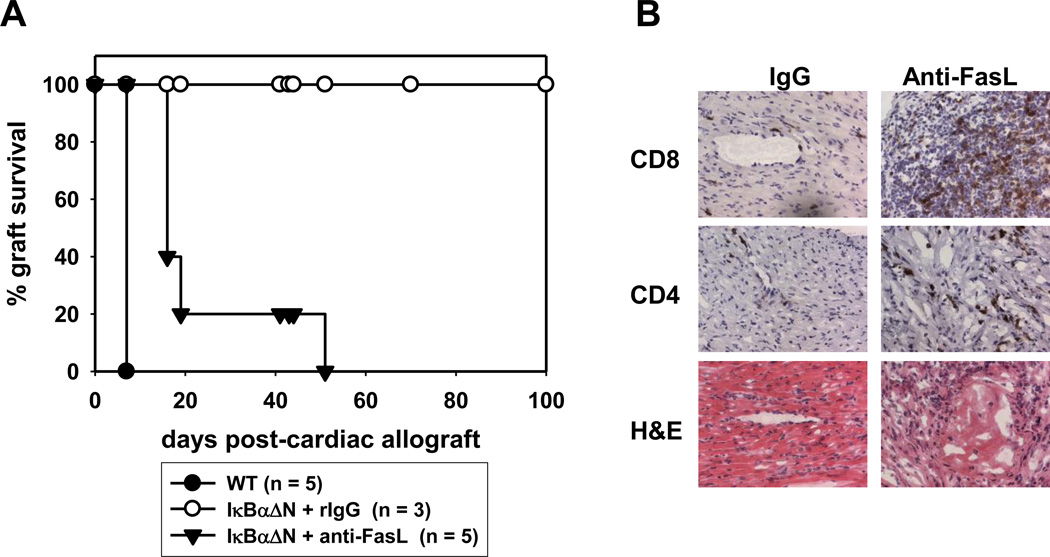
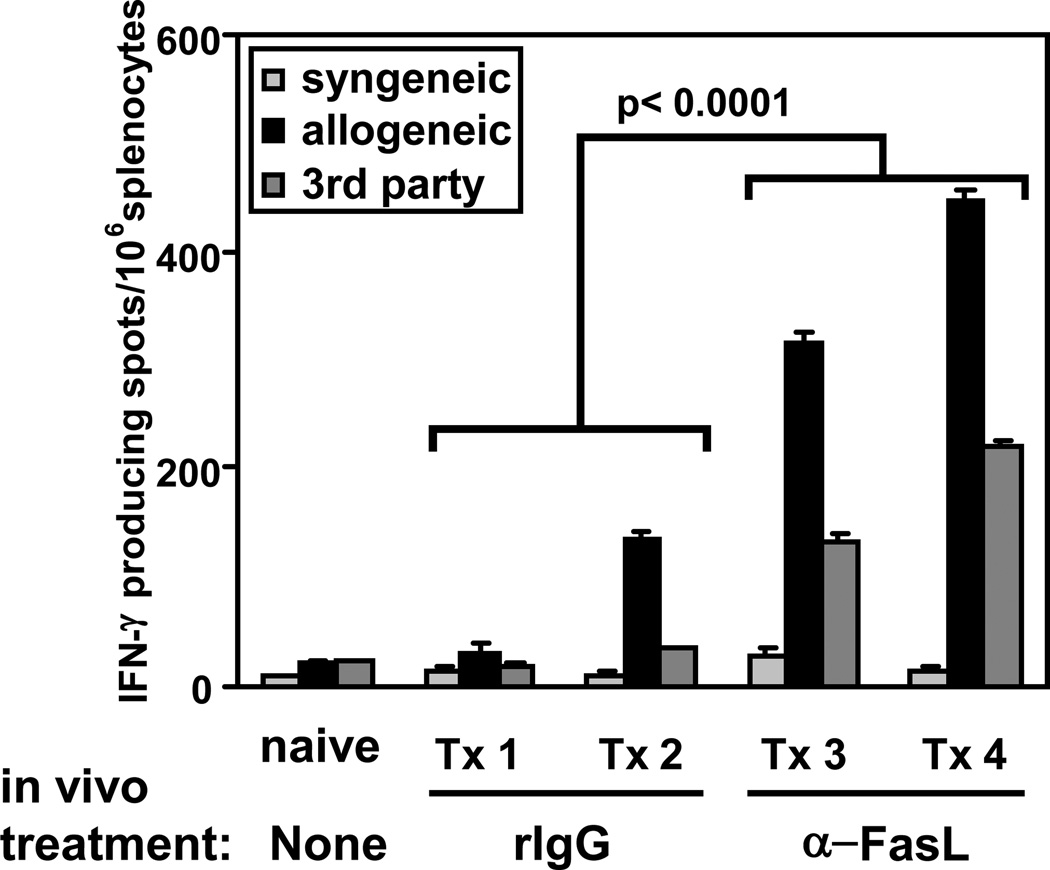
We have previously reported that IκBαΔN mice accept cardiac allograft long term (8). We proposed that graft acceptance was likely due to deletion of alloreactive T cells, as cardiac allograft rejection was restored in IκBαΔN mice that also expressed the anti-apoptotic protein Bcl-xL in T cells (9). In order to test whether IκBαΔN alloreactive cells were deleted through a Fas-mediated mechanism in vivo, we transplanted BALB/c (H2d) hearts into B6 (H2b) WT, IκBαΔN or IκBαΔNxLpr recipient mice. Whereas all IκBαΔN mice accepted the allogeneic hearts long term, IκBαΔNxLpr mice successfully rejected cardiac allografts, although with delayed kinetics compared to WT mice (Figure 4). To exclude that rejection in IκBαΔNxLpr mice was due to enhanced immune responses or autoimmunity triggered by the genetic absence in Fas signaling, IκBαΔN mice were treated with blocking anti-FasL mAb at the time of cardiac transplantation. As shown in Figure 5A, anti-FasL treatment, but not administration of an irrelevant IgG, triggered the rejection of cardiac allografts by IκBαΔN mice, similarly to untreated WT mice, although with slightly slower kinetics. In contrast, blockade of TNF was less effective and blockade of TRAIL showed no effect (data not shown). The anti-FasL-facilitated rejection correlated with a massive recruitment of CD4+ and CD8+ T cells into the allogeneic hearts (Figure 5B), as well as with an increase in the number of IFN-γ+ cells upon restimulation of splenocytes with donor APCs (Figure 6). These results suggest that cardiac allograft acceptance in IκBαΔN-Tg mice is due to apoptosis of alloreactive T cells in a Fas-dependent manner, which prevents development of IFN-γ-producing type 1 T cells.
Figure 4. Genetic impairment of Fas enables heart rejection in IκBαΔN-Tg mice.
IκBαΔN-Tg, Lpr or LprxIκBαΔN-Tg mice (all H-2b) were transplanted with heterotopic BALB/c allogeneic hearts (H-2d). Graft survival was assessed over time by palpation of the beating heart. MST: lpr 24±8; LprxIκBαΔN-Tg 67±20; IκBαΔN-Tg >100.
Figure 5. Blockade of Fas precipitates cardiac allograft rejection in lκBαΔN-Tg mice.
Wildtype or IκBαΔN-Tg mice (H-2b) were transplanted with BALB/c hearts (H-2d) and treated with control IgG or anti-FasL mAb (500 µg i.v. on day 0 and 250 µg on days 2, 4 and 8). A. Graft survival was assessed over time. MST anti-FasL mAb: 32±18, rIgG >100. B. Immunohistochemistry performed on the allografts from A harvested after rejection (control IgG) or after at least 60 days (anti-FasL mAb). Presence of CD8+ (upper panel) or CD4+ (middle panel) cells was assessed in parallel to hematoxylin-eosin staining (H&E).
Figure 6. Blockade of Fas restores donor-specific IFN-γ production by in transplanted IκBαΔN-Tg mice.
IκBαΔN-Tg mice were transplanted with BALB/c cardiac allografts and treated with anti-FasL or rat IgG as for Figure 5. On day 21 post-transplantation (Tx), splenocytes were harvested and stimulated with syngeneic (B6), allogeneic (BALB/c) or third party (C3H, H-2k) irradiated splenocytes for 24h. The frequency of IFN-g-producing cells was assessed by ELISPOT. Tx1-4 correspond to individual animals.
Discussion
We have previously shown that mice with a selective impairment in T cell-intrinsic NF-κB activity accept cardiac allografts long term and develop donor-specific tolerance (8). We had argued that lack of allograft rejection was likely due to deletion of alloreactive T cells because over-expression of the anti-apoptotic molecule Bcl-xL in T cells restored rejection of heart allografts (9). However, the mechanism by which NF-κB-impaired alloreactive T cells underwent apoptosis following cardiac transplantation remained to be demonstrated. Our current results suggest that apoptosis of NF-κB-impaired T cells in transplanted mice is Fas-dependent.
The importance of T cell death as a mechanism to enable transplantation tolerance has long been recognized. This was first demonstrated by Sir Peter Medawar who showed that neonatal exposure to donor alloantigens can result in clonal deletion of alloreactive T cells and permanent acceptance of skin allografts (20). Death of alloreactive T cells can also be achieved using myeloablation and bone marrow reconstitution to create chimerism and transplantation tolerance (21). Conversely, mice with defects in the ability of T cells to undergo apoptosis, have been shown to be resistant to the induction of tolerance via costimulation-targeting therapies or rapamycin (22), although not in all models (23).
The role of Fas in mediating apoptosis of antigen-specific T cells was first identified following discovery of expression of FasL by parenchymal cells from immune privileged sites such as the anterior chamber of the eye or the testes (24). Several groups have attempted to mimic this physiological situation for therapeutic purposes, by genetically engineering tissues to express FasL and thus destroy infiltrating T cells that express active Fas. In pancreatic islets, this was counterproductive as it resulted in β-cell destruction by fratricide because β cells also express Fas (25). However, this approach has proven more successful upon transplantation of FasL-transduced allogeneic chondrocytes in pigs (26) or using injection of donor splenocytes engineered to express FasL in a model of cardiac transplantation in rats (27). Another recent approach has been to generate killer artificial APCs, using beads coated with anti-Fas antibody together with HLA-A2-Ig dimers. These beads result in the deletion of HLA-A2-specific human T cells in a Fas-dependent manner (28). Our results indicate that selective inactivation of T cell-intrinsic NF-κB also facilitates Fas-dependent apoptosis of alloreactive T cells.
In addition to Fas, other death domain-containing receptors have been shown to play a role in T cell apoptosis. These include TNFR and TRAIL (29, 30). The TRAIL/DR5 pathway in particular has recently been identified as a critical mechanism by which CD4+FoxP3+ regulatory T cells (Tregs) mediate apoptosis of CD4+ conventional T cells and Tregs failed to enhance survival of skin allografts in the presence of blocking anti-DR5 antibody (31). However, blockade of TNF and TRAIL had little impact on cardiac allograft acceptance by IκBαΔN-Tg mice (data not shown). This may be because Tregs do not appear to play a role in transplantation tolerance in IκBαΔN-Tg mice, as these mice did not display increased numbers of Tregs, increased suppressor function by Tregs or increased susceptibility of conventional T cells to suppression by Tregs (9).
Our results show that blockade of Fas resulted in increased recovery of IFN-γ-producing cells in vitro and increased frequency of IFN-γ-secreting alloreactive IκBαΔN-Tg T cells in vivo. This is consistent with the fact that Th1 cells that make IFN-γ and IL-2 are more susceptible to Fas-mediated apoptosis than Th2 cells that produce IL-4 (32). It is interesting to note that IκBαΔN-Tg T cells are known to have defects in Th1 differentiation (3), clonal expansion and IFN-γ gene activation (33). Our results of recovered IFN-γ production by IκBαΔN-Tg T cells upon Fas/FasL blockade suggest that one of the main reasons why NF-κB-impaired T cells fail to become Th1 cells in vivo is Fas signaling. It is noteworthy that blockade of the Fas/FasL pathway resulted in as much IFN-γ production by IκBαΔN-Tg T cells as by isotype control-treated wildtype cells (see Figure 3) despite increased apoptosis of the anti-FasL-treated NF-κB-impaired T cells compared to the IgG-treated wildtype cells (see Figure 2). This may be because of a slight increase in IFN-γ production on a per cell basis as determined by intracellular staining (data not shown) or to the ability of the NF-κB-impaired T cells to differentiate and produce IFN-γ prior to their apoptosis.
Histological analysis of rejecting cardiac allografts from IκBαΔN-Tg mice treated with anti-FasL mAb revealed infiltration by both CD4+ and CD8+ T cells suggesting a role for both T cell subsets in acute rejection of donor hearts. Although cardiomyocytes do not express MHC class II, donor dendritic cells that express class II have been recently identified in cardiac valves and aortic root (34). Furthermore, expression of class II by donor hearts but not recipient mice is known to be sufficient for rejection of cardiac allografts by adoptively transferred CD4+ T cells (35). These data suggest that direct recognition of alloantigen by a subset of CD4+ T cells can mediate acute rejection of heart allografts despite low MHC class II expression. Although CD4+ but not CD8+ T cells are necessary and sufficient for rejection of cardiac allografts, CD8+ T cells are usually more abundantly found in rejecting heart transplants and become essential for cardiac allograft rejection if the function of CD4+ T cells is impaired such as in CD28-deficient mice (36). Whether CD8+ T cells are essential for rejection in anti-FasL-treated IκBαΔN-Tg mice in which T cell function is also reduced remains to be demonstrated.
In addition to its role in mediating apoptosis of T cells, Fas has been shown to play a role in allograft cells’ death. For instance, expression of both perforin and FasL by CD8+ T cells was recently shown to be important for the rejection of islet allografts (37). However, blockade of Fas/FasL interactions in our model promoted rather than prevented rejection of cardiac allografts in IκBαΔN-Tg mice, suggesting that other cytotoxic pathways can mediate destruction of cardiac allografts in the absence of Fas. This is consistent with a previous report demonstrating that cardiac allograft acceptance could still occur in gld mice that lack expression of FasL in all recipient cells and therefore can not engage Fas on graft cells (38).
In summary, our data show that the mechanism by which inhibition of T cell-intrinsic NF-κB activation leads to tolerance following cardiac transplantation is via Fas-mediated apoptosis of NF-κB-impaired T cells. Our results support development of T cell-specific NF-κB inhibitors or of T cell-specific Fas-agonistic drugs for use in clinical transplantation.
Acknowledgements
We thank the technical help of Terry Li (University of Chicago Immunohistochemistry Facility) and Shirley Bond (University of Chicago Light Microscopy Facility).
Funding: This work was supported by NIH/NIAID RO1 #AI052352 (MLA)
Abbreviations
- B6
C57Bl/6
- IκBαΔN-Tg
mice transgenic for an IκBα super-repressor
- mAb
monoclonal antibody
- MST
mean survival time
- NF-κB
nuclear factor κ B
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- TRAIL
TNF-related apoptosis-inducing ligand
- Tregs
regulatory T cells
- WT
wildtype
Footnotes
Authorship: LL Molinero co-designed, performed or supervised all experiments and co-wrote manuscript, Y Wang and P Zhou transplanted the allografts, H. Yagita contributed important reagents, ML Alegre co-designed the experiments and co-wrote the manuscript.
References
- 1.Alegre ML. Targeting NF-kB in the immune system to prevent acute allograft rejection. Current Opinions in Organ Transplantation. 2004;9:252. [Google Scholar]
- 2.Boothby MR, Mora AL, Scherer DC, et al. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J.Exp.Med. 1997;185:1897. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronica MA, Mora AL, Mitchell DB, et al. Preferential role for NF-kappa B/Rel signaling in the type 1 but not type 2 T cell-dependent immune response in vivo. J Immunol. 1999;163:5116. [PubMed] [Google Scholar]
- 4.Hettmann T, DiDonato J, Karin M, et al. An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis. J Exp Med. 1999;189:145. doi: 10.1084/jem.189.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora AL, Corn RA, Stanic AK, et al. Antiapoptotic function of NF-kappaB in T lymphocytes is influenced by their differentiation status: roles of Fas, c-FLIP, and Bcl-xL. Cell Death Differ. 2003;10:1032. doi: 10.1038/sj.cdd.4401257. [DOI] [PubMed] [Google Scholar]
- 6.Seetharaman R, Mora AL, Nabozny G, et al. Essential role of T cell NF-kappa B activation in collagen-induced arthritis. J Immunol. 1999;163:1577. [PubMed] [Google Scholar]
- 7.Tato CM, Villarino A, Caamano JH, et al. Inhibition of NF-kappa B activity in T and NK cells results in defective effector cell expansion and production of IFN-gamma required for resistance to Toxoplasma gondii. J Immunol. 2003;170:3139. doi: 10.4049/jimmunol.170.6.3139. [DOI] [PubMed] [Google Scholar]
- 8.Zhou P, Hwang KW, Palucki DA, et al. Impaired NF-kB activation in T cells permits tolerance to primary heart allografts and to secondary donor skin grafts. Amer. J. Transplantation. 2003;3:139. doi: 10.1034/j.1600-6143.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou P, Balin SJ, Mashayekhi M, et al. Transplantation tolerance in NF-kappaB-impaired mice is not due to regulation but is prevented by transgenic expression of Bcl-xL. J Immunol. 2005;174:3447. doi: 10.4049/jimmunol.174.6.3447. [DOI] [PubMed] [Google Scholar]
- 10.Molinero L, Zhou P, Wang Y, et al. Epidermal Langerhans cells play a major role in skin allograft rejection in mice with NF-kB-impaired T cells. Am J Transplant. 2008;8:21. doi: 10.1111/j.1600-6143.2007.02038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou T, Mountz JD, Kimberly RP. Immunobiology of tumor necrosis factor receptor superfamily. Immunol Res. 2002;26:323. doi: 10.1385/IR:26:1-3:323. [DOI] [PubMed] [Google Scholar]
- 12.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Legembre P, Barnhart BC, Zheng L, et al. Induction of apoptosis and activation of NF-kappaB by CD95 require different signalling thresholds. EMBO Rep. 2004;5:1084. doi: 10.1038/sj.embor.7400280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Sica A, Dorman L, Viggiano V, et al. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272:30412. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- 16.Stehlik C, de Martin R, Kumabashiri I, et al. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu ZL, McKinsey TA, Liu L, et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci U S A. 1997;94:10057. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamatani M, Che YH, Matsuzaki H, et al. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 19.Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol. 2008;180:7240. doi: 10.4049/jimmunol.180.11.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billingham RE, Brent L, Medawar PB. Activity acquired tolerance to foreign cells. Nature. 1953;172:602. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 21.Wekerle T, Sykes M. Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation. 1999;68:459. doi: 10.1097/00007890-199908270-00001. [DOI] [PubMed] [Google Scholar]
- 22.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 23.Lehnert AM, Murray-Segal L, Cowan PJ, et al. Blockade of the passive cell death pathway does not prevent tolerance induction to islet grafts. Transplantation. 2007;83:653. doi: 10.1097/01.tp.0000255592.09784.ba. [DOI] [PubMed] [Google Scholar]
- 24.Griffith TS, Brunner T, Fletcher SM, et al. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 25.Kang SM, Schneider DB, Lin Z, et al. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997;3:738. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 26.Xie GH, Wang SJ, Wang Y, et al. Fas Ligand gene transfer enhances the survival of tissue-engineered chondrocyte allografts in mini-pigs. Transpl Immunol. 2008;19:145. doi: 10.1016/j.trim.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Yolcu ES, Gu X, Lacelle C, et al. Induction of tolerance to cardiac allografts using donor splenocytes engineered to display on their surface an exogenous fas ligand protein. J Immunol. 2008;181:931. doi: 10.4049/jimmunol.181.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schutz C, Fleck M, Mackensen A, et al. Killer artificial antigen-presenting cells: a novel strategy to delete specific T cells. Blood. 2008;111:3546. doi: 10.1182/blood-2007-09-113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtzman MJ, Green JM, Jayaraman S, et al. Regulation of T cell apoptosis. Apoptosis. 2000;5:459. doi: 10.1023/a:1009657321461. [DOI] [PubMed] [Google Scholar]
- 30.Li X, McKinstry KK, Swain SL, et al. IFN-gamma acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptotic signals. J Immunol. 2007;179:939. doi: 10.4049/jimmunol.179.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren X, Ye F, Jiang Z, et al. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4(+)CD25(+) regulatory T cells. Cell Death Differ. 2007;14:2076. doi: 10.1038/sj.cdd.4402220. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Brunner T, Carter L, et al. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J.Exp.Med. 1997;185:1837. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corn RA, Aronica MA, Zhang F, et al. T cell-intrinsic requirement for NF-kappa B induction in postdifferentiation IFN-gamma production and clonal expansion in a Th1 response. J Immunol. 2003;171:1816. doi: 10.4049/jimmunol.171.4.1816. [DOI] [PubMed] [Google Scholar]
- 34.Choi JH, Do Y, Cheong C, et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;16:16. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pietra BA, Wiseman A, Bolwerk A, et al. CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. J Clin Invest. 2000;106:1003. doi: 10.1172/JCI10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szot GL, Zhou P, Rulifson I, et al. Different mechanisms of cardiac allograft rejection in wildtype and CD28-deficient mice. Am J Transplant. 2001;1:38. doi: 10.1034/j.1600-6143.2001.010108.x. [DOI] [PubMed] [Google Scholar]
- 37.Sleater M, Diamond AS, Gill RG. Islet allograft rejection by contact-dependent CD8+ T cells: perforin and FasL play alternate but obligatory roles. Am J Transplant. 2007;7:1927. doi: 10.1111/j.1600-6143.2007.01889.x. [DOI] [PubMed] [Google Scholar]
- 38.Wagener ME, Konieczny BT, Dai Z, et al. Alloantigen-driven T cell death mediated by Fas ligand and tumor necrosis factor-alpha is not essential for the induction of allograft acceptance. Transplantation. 2000;69:2428. doi: 10.1097/00007890-200006150-00037. [DOI] [PubMed] [Google Scholar]