Abstract
Obesity continues to be a major public health problem in the United States and worldwide. While recent statistics have demonstrated that obesity rates have begun to plateau, more severe classes of obesity are accelerating at a faster pace with important implications in regards to treatment. Bariatric surgery has a profound and durable effect on weight loss, being to date one of the most successful interventions for obesity.
Objective
To provide updates to the possible role of gut hormones in post bariatric surgery weight loss and weight loss maintenance.
Design and Methods
The current review examines the changes in gastro-intestinal hormones with bariatric surgery and the potential mechanisms by which these changes could result in decreased weight and adiposity.
Results
The mechanism by which bariatric surgery results in body weight changes is incompletely elucidated, but it clearly goes beyond caloric restriction and malabsorption.
Conclusion
Changes in gastro-intestinal hormones, including increases in GLP-1, PYY, and oxyntomodulin, decreases in GIP and ghrelin, or the combined action of all these hormones might play a role in induction and long-term maintenance of weight loss.
Introduction
Obesity has reached epidemic proportions in the United States, where 66% of the population is overweight or obese, and worldwide. In 2009, about 2.4 million more adults in the US were obese than in 2007, and it is estimated that if the current trends are extrapolated, by 2048 all Americans will be overweight and obese. Moreover, the prevalence of more severe classes of obesity is accelerating at a much faster rate than less severe classes of obesity. Between 1998 and 2000, the prevalence of BMI of 40 or greater quadrupled and prevalence of BMI 50 or greater increased five-fold while obesity, as defined by BMI of 30 or greater doubled during this period. The health risks of obesity are numerous and grave: type 2 diabetes, cardiovascular disease, cancer, osteoarthritis, non-alcoholic fatty liver disease, polycystic ovary syndrome, sleep apnea, depression, and reduced life expectancy. The costs of obesity were estimated to be 147 billion (dollars) in 2008, and expected to increase (1).
Obesity has a multifactorial etiology and involves powerful energy homeostasis control mechanisms. As a result, obesity therapy is a complex intervention and the maintenance of weight loss is one of its challenges. Medical management of obesity includes calorie restriction, exercise, behavioral changes, and pharmacotherapy. Lifestyle interventions (diet, exercise, and behavioral changes) result in modest weight loss of 5-10% of initial body weight, and a high percentage of patients regain the weight at 1-2 years. Drug treatment of obesity in United States is limited to several drugs. Phentermine, a sympatomimetic that inhibits catecholamine re-uptake in the Central nervous system (CNS), is approved for short-term use and results in 6.3 kg weight loss in studies lasting up to 9 months (placebo-subtracted weight loss, PSWL, 3.6 kg) (2). Orlistat (a lipase inhibitor) for long-term use has been shown to result in about 10% weight loss at 1 year (PSWL 2.08 kg) (2). Two new anti-obesity drugs were recently approved by FDA. The first is a combination of phentermine and topiramate (an anticonvulsivant drug whose weight loss mechanism is not fully known), and has demonstrated weight loss ranging 4-11% at one year (PSWL 4.1-10.9 kg or 3.5-9.4% for low and full dose, respectively) (3). The second is a selective serotonin 2c receptor agonist, lorcaserin hydrochloride, which has demonstrated 5.8% weight loss at one year (PSWL 3.6 kg) (3). Contrave® (naltrexone SR/bupropion SR) is an investigational medication for the treatment of obesity. It combines two well-established drugs, naltrexone (stimulates proopiomelanocortin (POMC) neurons) and bupropion (prevents inhibition of POMC neurons by blocking the action of β-endorphin), in a sustained release formulation. The PSWL with Contrave was 4.2-5.2 kg at 1 year. While drug treated patients are more likely to maintain weight losses, weight regain often occurs when medication is stopped.
Bariatric surgery is currently the most effective treatment option for obesity. While weight loss varies with the type of surgery, it is significant and durable. A recent study showed that at 10 years the average weight loss was 16.1% (range 13-25% among various surgical procedures) (4). Obesity-related comorbidities improve significantly, and in particular type 2 diabetes, resulting in a more recent alternative name—metabolic surgery.
Bariatric surgery began with the observation that patients with short bowel syndrome (due to removal of portions of intestine for other diseases) or patients with gastrectomy experience significant weight loss. The first bariatric surgeries in the 1950s did just that—it removed portions of the intestine and the upper and lower intestine were linked together; in the 1960s the stomach was bypassed (5). Gastric volume reduction and intestinal re-routing resulting in caloric restriction and/or malabsorption, respectively, were long considered to be the main factors responsible for the weight loss. It seems obvious that restriction of movement of food through the gastrointestinal tract might limit food intake and reduce fat storage, at least in the short-term. However, often the weight loss exceeds that expected by the reduction in food intake due to stomach restriction alone (6). Recently, there has been a growing consensus that gastro-intestinal hormones play an important role in the bariatric surgery-induced weight loss.
Bariatric Surgery Procedures
Laparoscopic adjustable gastric banding
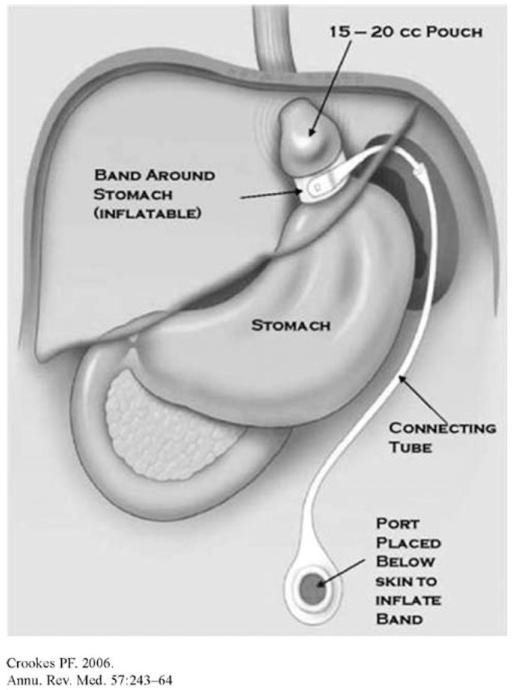
Laparoscopic adjustable gastric banding (LAGB) is the most commonly performed bariatric surgery procedure in the world. Together with the second most common, laparoscopic Roux-en-Y gastric bypass (LRYGB), they accounted for 82% of the bariatric surgeries performed worldwide in 2008 (7). In the US, LAGB occupies second place after gastric bypass, and represents 29% of the bariatric surgeries (8). In LAGB, an adjustable silicone band is placed around the stomach immediately below the gastroesophageal junction, physically reducing gastric size and resulting in a pouch with an initial volume of about 15 ml. The lumen of the band is connected via tubing to a subcutaneous port and injection of saline allows the band to be adjusted. Inflation of the balloon functionally tightens the band and thereby increases weight loss, while deflation of the balloon loosens the band and reduces weight loss (Figure 1) (5). It is generally believed that the major mechanism through which LAGB results in weight loss is via restriction of food intake; there is no evidence that LAGB affects gastric emptying (9). The average excess weight loss (EWL, calculated as (preoperative weight – follow-up weight)/(preoperative weight – ideal body weight) × 100)) LAGB, according to a meta-analysis is 42.6% post EWL at 1 year, 50.3% at 2 years, and 55.2% at >3 years postsurgery (10). Buchwald et al. showed a weight loss of 27 kg (9.6 kg/m2 BMI) at assessments <2 years and 38 kg (12.0 kg/m2 BMI) > 2 years (11) (Table 1).
FIGURE 1.
Laparoscopic adjustable gastric banding (LAGB); (reproduced with permission from ref. (5)).
TABLE 1. Summary of bariatric procedures effect on weight loss, gastro-intestinal hormones and vagal activity.
| Procedure | Weight loss (avg %EWLa) |
GI hormones | Vagal activity |
|---|---|---|---|
| LAGB | 42.6–55.2 | GLP-1, PYY no change GIP no change Ghrelin ↑, ↓ or no change |
Band adjustment affects vagal activity? |
| SG | 53.3–77.5 | GLP-1 ↑, PYY ↑ GIP ↑(?) Ghrelin ↓ |
Preservation or sectioning of vagus might affect food intake |
| RYGBP | 59.3–63.2 | GLP-1 ↑↑, PYY ↑↑, oxyntomodulin ↑ GIP ↓ or no change Ghrelin ↑, ↓ or no change |
Preservation or sectioning of vagus might affect food intake |
| BPD | 56.0–73.7 | GLP-1 ↑↑, PYY ↑↑ GIP ↓ Ghrelin ↑, ↓ or no change |
Preservation or sectioning of vagus might affect food intake |
Sleeve gastrectomy
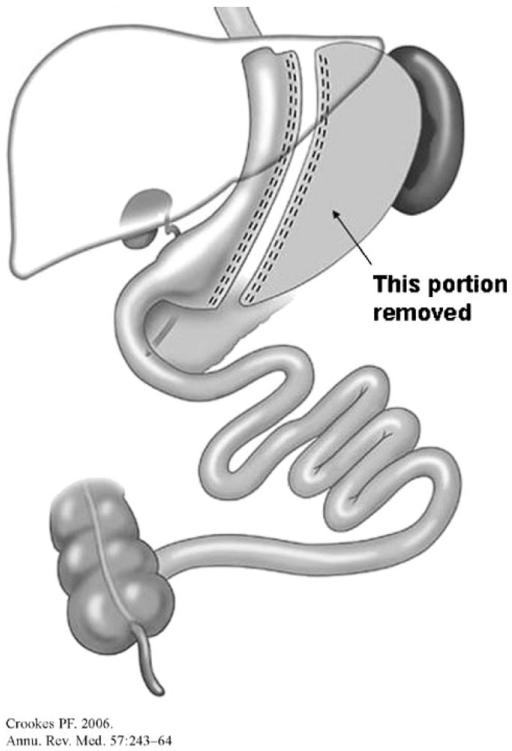
Sleeve gastrectomy (SG) is a left partial gastrectomy of the fundus and body, to create a tubular stomach that resembles the size and shape of a banana (Figure 2). Generally considered a restrictive procedure, there is now growing consensus that the mechanism of weight loss and changes in energy homeostasis with SG also involves neurohormonal changes, due to stomach resection and expedited delivery of nutrients into the small intestine (via accelerated gastric emptying) (12). As a result of technical efficiency and good EWL (77.5% or 13.3 kg/m2 BMI at 3 years, and 53.3% EWL or 8.8 kg/m2 BMI at 6+ years) (13), the number of SG performed has an upward trend: the percentage of SG performed worldwide has increased from none in 2003 to 5.4% in 2008 (7). Recently, The American Society for Metabolic and Bariatric Surgery recognized SG as an acceptable option as a primary bariatric procedure (it was initially introduced as the first step of the duodenal switch procedure) with a risk/benefit profile between RYGB and LAGB.
FIGURE 2.
Sleeve gastrectomy (SG); reproduced with permission from ref. (5).
Gastric bypass
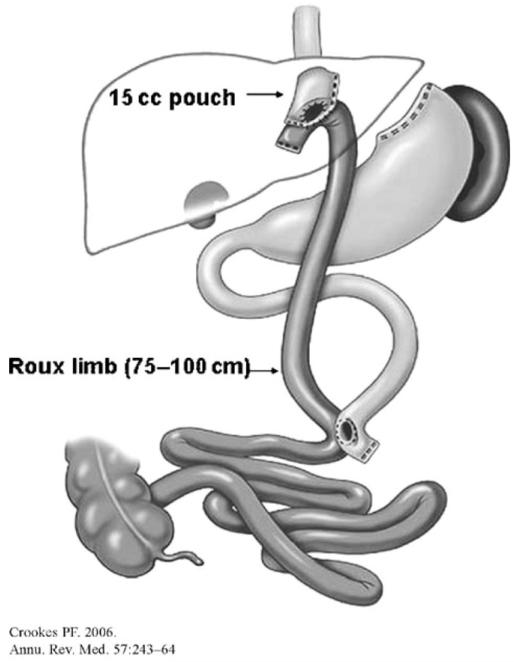
Gastric bypass (GB) was first introduced more than 40 years ago by Mason. Since then there have been numerous advances in the technique, access, surgical equipment, and short-term and long-term outcome. The most common version, the laparoscopic Roux-en-Y-gastric bypass (RYGB), is the second most frequently performed bariatric surgery procedure, accounting for 39.7% of the 344,221 bariatric surgeries performed worldwide in 2008 (7). Of these, 86,138 were in the US, where GB is the most frequently performed procedure, accounting for 69% of bariatric surgeries (8). In RYGB, the gastric volume is restricted by creating a 15-30 ml gastric pouch, while the nutrient flow is rerouted from the stomach directly into the proximal jejunum through a gastrojejunal anastomosis, resulting in three limbs: a biliopancreatic limb (from ligament of Treitz to jejuno-jejunostomy, transmits bile and pancreatic juices to the jejuno-jejunostomy), a 100-150 cm alimentary limb (jejunal Roux-en-Y limb anastomosed to the stomach), and a common channel (from entroenterostomy to ileocecal valve) (5) (Figure 3). The mechanism of GB effect is not completely elucidated, but includes a combination of stomach reduction, changes in GI motility (increased gastric emptying), and hormonal and neural changes due to nutrient rerouting and anatomical rearrangement (5). In a meta-analysis of weight loss post-bariatric surgery, Buchwald et al. showed that GB resulted in an average weight loss of 45.3 kg (16.3 kg/m2 BMI) or about 59.3% EWL for assessments at less than 2 years post-surgery and 41.4 kg (16.1 kg/m2 BMI) or 63.2% EWL for assessments at more than 2 years (11).
FIGURE 3.
Roux-en-Y gastric bypass (RYGB); reproduced with permission from ref. (5).
Biliopancreatic diversion
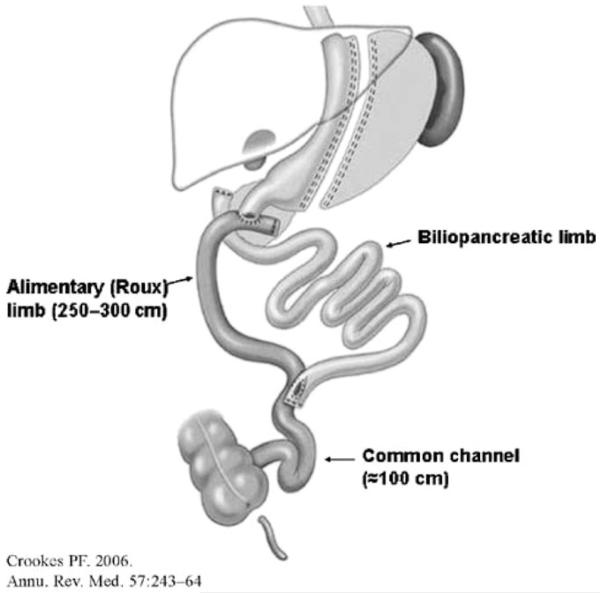
Devised by Scopinaro, biliopancreatic diversion (BPD) consists of a distal gastrectomy with a long Roux-en-Y reconstruction where the enteroenterostomy is placed at a distal ileal level. Thus, the bypassed portion of the duodenum includes the point of entry for biliary and pancreatic secretions, leading to a delayed mixing of food with bilio-pancreatic secretions. More frequently performed than the Scopinaro procedure is BPD with duodenal switch (BPD-DS), a version in which instead of distal gastrectomy a sleeve type gastrectomy is performed and a Roux limb is anastomosed not to the stomach but to the duodenum, thus preserving the pylorus (5) (Figure 4). BPD results in impressive weight loss of ~70% EWL, maintained at long-term follow ups (<2 years, loss of 38 kg or 20.0 kg/m2 BMI; >2 years 49.8 kg or 17.5 kg/m2 BMI) (11). However, it is not often performed, because of its technical complexity and because of long-term significant nutrient deficiencies. As such it represents only 2% of total worldwide bariatric surgery procedures (7).
FIGURE 4.
Bilio-pancreatic diversion with duodenal switch (BPD-DS); reproduced with permission from ref. (5).
Less frequently used and historical procedures
Vertical banded gastroplasty (VBG) reduces the stomach size by using an undivided staple line to create a narrow stomach pouch and an added fixed, non-adjustable band to decrease the emptying rate of the pouch. The pouch has a small opening through which food can enter the rest of the stomach. VBG was introduced in 1980 and in its original form had relatively higher complication rate and lower success rates in weight loss than other procedures. MacLean’s modification of the standard open Mason procedure resulted in a decrease in complication rate and a good weight loss, but the procedure is infrequently used (5). In 2008 VBG accounted for 1.1% of total bariatric surgeries performed worldwide (7).
Jejuno-ileal bypass (JIB), a procedure in which proximal jejunum is anastomosed to the terminal ileum was the first surgical approach to obesity treatment. The JIB induced a state of malabsorption by bypassing most of the intestines while keeping the stomach intact. JIB is not used anymore because of the numerous and sometimes severe side-effects, such as protein malnutrition, vitamin and mineral deficiencies (with night blindness due to vitamin A deficiency and osteoporosis due to vitamin D deficiency), renal failure, liver disease, and even death (due to bacterial overgrowth in the bypassed intestine) (5).
New and experimental procedures
Endoluminal sleeve—as the name implies, these are endoluminal devices designed to “coat” a portion of the digestive tract with an impermeable surface. They can be secured at the gastro–esophageal junction and bypass the stomach, the duodenum and part of the jejunum, or secured at the gastro–duodenal junction—duodenojejunal sleeve. Endoluminal treatments for obesity are promising; they are less invasive than other bariatric surgery procedures and result in good weight loss: a recent trial of 24 patients with gastrojejunal sleeve reported 39.7% EWL after 12 weeks (14).
Duodeno-jejunal bypass (DJB)—though rapidly gaining ground as metabolic surgery, DJB is not a weight loss procedure, since it is designed to mimic the nutrient re-routing of the RYGB without gastric restriction. DJB was devised in an attempt to improve glucose metabolism in patients with type 2 diabetes with non-severe obesity. The results of early trials indicate significant reductions in HbA1 and in glycemia in the first 3-6 months after surgery. Recently, Cohen et al. reported 40% diabetes remission at 12 months in type 2 diabetic patients with low BMI (15).
Mechanisms of Weight Loss
Malabsorption
Malabsorption is a plausible weight loss mechanism especially in the types of bariatric surgery in which considerable portions of the small intestine are excluded from contact with nutrients, such as RYGB and BPD. Biliary and pancreatic secretions are also diverted to distal portions of the intestine, such that a limited area of the intestinal mucosa is exposed to the mix of nutrients, bile salts, and enzymes. Kumar et al. reported increases in fecal fat 6 months (126%) and 12 months (87%) after RYGB (16); similar increases were reported after BPD. Odstrcil et al. found that the coefficient of fat absorption was reduced post long-limb RYGB (72% at 5 months and 68% at 14 months) (17). There were no significant reductions in protein and carbohydrate absorption. Overall, malabsorption accounted for a modest 6-11% of the total reduction in combustible energy absorption. It is important to mention that the long-limb RYGB is not the standard procedure.
Caloric restriction
There has been considerable debate whether the mechanism of weight loss in bariatric surgery is limited to reduction in caloric intake. Caloric intake is dramatically reduced immediately after bariatric surgery (to 200-300 kcal/day) and it is likely that it is primarily responsible for the initial post-surgical weight loss. Isbell et al. showed that placing subjects for 4 days on a diet identical to the first 4 days post GB diet results in similar weight loss (1.4 and 2.2 kg) (18). In a study by Campos et al., in which participants with and without GB were assigned to a low (800 kcal/day) diet for 14 days, the weight loss was not significantly different between the two groups (19).
However, it was also noticed that the time to lose a specific amount of weight is different in bariatric surgery versus lifestyle intervention, and between various bariatric surgery procedures. Olivan et al. showed that it took obese subjects with GB 30 days to lose 10 kg, vs. 55 days for obese subjects on a low calorie diet (~1,000 kcal/day) (20). Recently, Rodriguez et al. showed that compared to a SG group, pair fed rats had more body weight and more adiposity (21).
Caloric restriction thus may play an important role in the initial post-surgical weight loss. In the long-term, as other studies suggested, additional mechanisms may come into play. Indeed, in both restrictive and bypass procedures, but especially in the bypass type, changes in the gastro-intestinal hormones and in enteral neural connections occur, and these changes may play important roles in weight loss.
Gastro-Intestinal Hormone Changes After Bariatric Surgery
L-cell derived peptides: GLP-1, PYY, and oxyntomodulin
Glucagon-like peptide-1
Glucagon-like peptide-1 (GLP-1) is a 30 amino acid peptide released from the L-cells in response to meal ingestion. L-cell stimulation increases not only GLP-1 but also GLP-1 related peptides (all derived from the same proglucagon molecule: glicentin, oxyntomodulin, intervening peptide-2 and GLP-2), as well as PYY. GLP-1 has multiple effects on glucose homeostatic regulation and on energy homeostasis via effects on food intake and satiety. Together with glucose-dependent insulinotropic polypeptide (GIP), GLP-1 is a major insulinotropic hormone responsible for the incretin effect (the enhancement of insulin secretion by oral glucose vs. an isoglycemic intravenous load). In animal models, it has been shown that GLP-1 stimulates beta-cell growth and proliferation and inhibits apoptosis, which results in an increased beta-cell mass. In the pancreas GLP-1 also inhibits glucagon secretion, an important mechanism in improving glycemic tolerance. By slowing gastric emptying and intestinal motility, GLP-1 participates in the “ileal break,” the phenomenon whereby the presence of nutrients in the distal gut results in a decrease in gastro-intestinal motility, thus contributing to a feeling of fullness and reduces hunger and food intake (22). GLP-1 inhibits food and water intake and promotes satiety. In the brain, GLP-1 is synthesized by a population of neurons in the nucleus of the solitary tract; their fibers project to other areas of the brain, in particular the paraventricular and arcuate nucleus of the hypothalamus. It is possible that peripheral GLP-1 also acts in the central nervous system, by binding to GLP-1 receptors that are abundant in the so called “leaky” areas of the blood–brain barrier (subfornical organ, area postrema, median eminence, and pituitary) by crossing the blood--brain barrier, or by activating peripheral sensors that in turn communicate with brain areas involved in satiety and food intake regulation (23). Postprandial GLP-1 is increased as early as 2 days after RYGB (24) and 1 week after BPD (25). The mechanism for GLP-1 increase after bariatric surgery is not yet fully elucidated. Weight loss via non-surgical methods results in no changes in GLP-1 or GLP-1 decreases, not increases (23). In addition, Isbell et al. showed that when the postprandial levels of GLP-1 were compared after the same amount of weight loss via caloric restriction or RYGB only the surgical group had increased GLP-1 levels (18).
One hypothesis is that direct delivery of nutrients to distal gut post GB results in higher GLP-1 secretion from the L-cells (26). This hypothesis is supported by the finding that procedures with intestinal rearrangement (RYGB, BPD) produce larger increases than procedures without (27). In humans, McLaughlin et al. report the case of a patient with hyperinsulinemic hypoglycemia and increased plasma GLP-1 levels after RYGB. Insertion of a gastrostomy tube in the remnant stomach and thus a change in nutrient delivery from the distal gut to the more physiological proximal gut resulted in reversal of abnormal GLP-1 and insulin secretion (28). Similar results of increased GLP-1 in peroral vs. gastric remnant feeding were reported by Dirksen et al. (29).
GLP-1 is increased after SG (a procedure without intestinal rearrangement), though less than after RYGB. Peterli et al., in a longitudinal study looking at GLP-1 increases after RYGB or SG, found that post-prandial plasma GLP-1 was higher in the RYGB group than in the SG group at 1 week post-surgery. However, by 3 months the groups had similar levels of GLP-1 (30). In spite of the absence of an anatomical “shortcut,” an expedite delivery of nutrients could also be the mechanism behind increased GLP-1 secretion post SG, as it has been shown that gastric emptying is accelerated after SG (12).
No increases in postprandial GLP-1 were found after gastric banding (9,31), suggesting that only procedures with intestinal rearrangement or possibly those that expedited nutrient delivery to the distal intestine result in increases in GLP-1. However, this assumption is challenged by a recent study by Kampe et al. Using a rat model of adjustable gastric banding, they found that band inflation resulted in weight loss, decreased food intake, and increased plasma levels of GLP-1 and PYY following a liquid meal. To explain the GLP-1 and PYY increases they suggested that band inflation activates stretch receptors at the gastro-esophageal junction and they communicate via neural connections with the hindgut (32).
An alternative mechanism for GLP-1 increase is dipeptidyl peptidase-IV (DPP-IV) inhibition. This ubiquitous enzyme rapidly degrades GLP-1, leading to a short in vivo half-life of 1.5-2 min (22). In patients with type 2 diabetes, Alam et al. showed that DPP-IV activity was significantly decreased after GB but not after caloric restriction (33). Interestingly, the increased peak GLP-1 and GIP response to oral glucose after GBP did not correlate with DPP-4 activity. In contrast, Lugari et al. reported increased plasma DPP-IV activity post BPD. Again, this was not correlated with plasma GLP-1 levels, which were increased (34). Based on this evidence, it is unlikely that DPP-IV inhibition is a major mechanism for GLP-1 increase post bariatric surgery.
Does increased GLP-1 result in increased weight loss after certain types of bariatric surgery? Procedures with greater increases in plasma GLP-1 (RYGB, BPD) also have greater weight loss (27). However, when patients post-BPD and post-VBG were compared at 6 months, despite significantly increased plasma levels of GLP-1 (19 times) the weight loss in the two groups was the same, suggesting that GLP-1 plays only a modest role in the weight loss (35).
The role of GLP-1 on post-surgical weight loss might be better quantified by examining the impact on food intake, hunger, and satiety. Falken et al. found that GLP-1 increases progressively at 3 days, 2 months, and 1 year after GB and that the increase was associated with dramatic changes in hunger and fullness (36). Similarly, Borg et al. reported increases in postprandial plasma GLP-1 at 1, 3, and 6 months after RYGB, as well as reduction of hunger and increases in satiety, but no changes in nausea or aversion to food (37). A more in-depth analysis was done by Leroux and coworkers in a prospective study looking at postprandial levels of enteral hormones at 2, 4, 7, and 42 days after RYGB. The authors also measured hunger and satiety in “responders” (patients with significant weight loss post surgery) and “non-responders” (patients with poor weight loss or weight regain). In all subjects plasma GLP-1 increased immediately after surgery and the GLP-1 increases were significantly correlated with decreases in hunger score and increases in fullness score. Suboptimal responses to GLP-1 were associated with patients who had poor weight loss. Blockade of gut hormone release with somatostatin during a meal increased food intake in a the GB group but not in a weight loss matched gastric banding group, suggesting that the GLP-1 response contributes to changes in food intake in the GB group, but not in the banding group (24). It is important to mention, however, that somatostatin suppresses other gut hormones, such as PYY, GLP-2, oxyntomodulin, etc and that any of these other hormones could contribute to the observed effects during the blockade. Interestingly, in a subsequent longitudinal study the same group found increased satiety at 18 and 24 months post-RYGB without significant increases in plasma GLP-1 (38).
While direct evidence that GLP-1 is responsible for post-bariatric surgery weight loss is lacking, at least in the intestinal rearrangement type of procedures, the hormone is consistently shown to be increased. GLP-1 is a potent food intake inhibitor, and it is very likely that, together with PYY, contributes to at least some of the weight loss observed after bariatric surgery. More research, with direct blockade of GLP-1 increase or signaling during bariatric surgery, will probably reveal in detail the role of this pluripotent hormone.
Peptide YY
PYY is a 36 amino acid released by the L-cells of the gastro-intestinal tract (the same cells that secrete GLP-1), as well as by the brain. PYY is co-secreted with GLP-1 in response to meal stimulation (in proportion to the calorie content of the meal) and degraded by DPP-IV. PYY inhibits gastric emptying and intestinal motility, being part of the “ileal brake” together with GLP-1. Its active form PYY 3-36 inhibits food intake by binding to Y-2 neuronal receptors and inhibiting the release of Neuropeptide Y (NPY) (39).
Obese subjects have lower fasting and meal-stimulated PYY levels than normal subjects; PYY levels were found increased 2 days after RYGB (24) and 1 week after SG (30) and they appear to increase progressively to 6 months (37) and are still increased 2 years after surgery (38). It is very likely that the increase in PYY after bariatric surgery is a result of the procedure, not of the weight loss, since most studies indicate that weight loss in the absence of bariatric surgery does not result in PYY increases (40).
PYY levels increase after GB (RYGB and BPD (6,24,27,38)) and SG (30,41,40), but not after gastric banding (42). This suggests that, similar to the mechanism presented for GLP-1, rapid delivery of nutrients to the distal gut after RYGB and BPD could stimulate the L-cells with the resulting increases in PYY (26). As for SG, it has been shown that it increases gastric emptying, with the resulting increased nutrient delivery and stimulation of distal intestinal cells (12). Another hypothesis is that SG is associated with incomplete digestion due to decreased gastric acid secretion and that delivery to the duodenum of higher pH undigested chyme could result in increased PYY (41).
To explore the role of PYY in surgery-induced weight loss, a number of studies investigated the correlation between the levels of PYY and the weight loss achieved, or the correlation of PYY levels with eating behavior. Surgeries that result in higher plasma PYY levels, such as RYGB and BPD, are associated with greater weight loss (11). In a cross-sectional study comparing non-diabetic lean, post RYGB, post gastric banding, and obese weight matched subjects, Korner et al. reported postprandial increases in plasma total PYY and PYY 3-36 that were 2-4 times higher in RYGB than in all the other groups. The RYGB group also reported greater satiety (6). Their data are consistent with the concept that the PYY rise promotes increased satiety and earlier meal termination and results in weight loss and maintenance of weight loss. Subsequently, in a prospective study, the same group showed that at 1 year there was greater weight loss in RYGB compared to gastric banding (30% vs. 15%) and that the plasma PYY area under the curve (AUC) was greater in the GB than in the banding group. The weight loss percentage was not correlated with the PYY AUC, but this could be explained by other hormones such as GLP-1, ghrelin, etc. also contributing to weight loss (27). In a study by Bose et al., gut hormones including PYY were measured post RYGB and post gastric banding at two time points: after the same amount of weight was lost (12 kg) and 1 year after surgery. At the first time point patients with RYGB had a more favorable gut hormone plasma profile, which possibly explained the greater weight loss at 1 year (42). Morinigo et al. found significantly increased postprandial plasma PYY 6 weeks after RYGB compared both with baseline and weight-matched obese subjects. Surprisingly, the change in PYY (or GLP-1) did not correlate with changes in eating behavior parameters (43). However, in a follow-up study looking at hormone levels 1 year after surgery, the authors found that a large plasma PYY response to a meal predicted a better weight loss outcome (44). In a study by Peterli et al., PYY was similarly increased post RYGB and SG, and this was associated with similar weight loss (30). Valderas et al. found that the PYY AUC was also correlated with satiety (40). In a prospective study, LeRoux et al. showed that suboptimal PYY responses were associated with patients who had poor weight loss or weight regain at 25 months after RYGB. Infusion of blocking agent somatostatin reduced hormonal response in post-RYGB patients and resulted in a doubling of the food intake the day of infusion. Interestingly, the same effect was not observed in gastric banding patients, suggesting that gut hormones do not play a role in weight loss after these types of operations (24). A recent animal study may shed some light into this issue: when GB surgery was performed in diet-induced obesity wild type and PYYKO mice, there was no difference in weight loss between surgery and sham-operated mice in the PYYKO mice, though the wild type lost weight with bariatric surgery (45).
There is thus convincing evidence that PYY is one of the major hormonal contributors to post-bariatric surgery weight loss. Increased PYY resulting from bariatric surgery (via increased direct delivery of nutrients to the L-cells, decreased transit time, or high pH of undigested chyme) results in satiety, decreased food intake and possibly changes in energy expenditure, leading to weight loss both in the early phase and over long-term.
Oxyntomodulin
Oxyntomodulin is a 39 amino acid peptide co-secreted with GLP-1 and PYY from the intestinal L-cells in response to food ingestion. Cut from the larger proglucagon molecule, oxyntomodulin contains the entire glucagon sequence and a C-terminal extension. Oxyntomodulin is degraded by DPP-IV, with a half-life of 12 min, and binds to the GLP-1 receptor but with a much lower affinity, about 50 times lower than GLP-1. Like GLP-1 and PYY, it is an anorectic hormone; it also inhibits gastric acid secretion and motility, and has positive effects on glucose homeostasis. Oxyntomodulin administration reduces food intake in both lean and obese individuals and may also increase energy expenditure (46).
In a study by Laferrere et al., oxyntomodulin plasma levels in response to OGTT increased twofold one month after GBP but not after an equivalent amount of weight was lost via diet (47). The changes in oxyntomodulin correlated with changes in GLP-1 and PYY, so it is difficult to distinguish its effect from the effects of these other two hormones. Falken et al. measured changes in enteroglucagon after RYGB (enteroglucagon contains oxyntomodulin and glicentin). There was a progressive rise in enteroglucagon 3 days, 2 months, and 1 year after RYGB, suggesting a role for oxyntomodulin in the consequent weight loss (36).
After bariatric surgery, especially after GB, the increases in GLP-1, PYY, and oxyntomodulin, all hormones with powerful anorectic actions, would contribute significantly to the weight loss. At present, the possible involvement of this “L-cell triumvirate” is the most compelling hypothesis explaining the effects of bariatric surgery on weight reduction. Preliminary data pertaining to the combined effect of GLP-1 and PYY on appetite and food intake supports this hypothesis. De Silva et al. showed that administration of PYY3-36 and GLP-17-36 amide to fasting human subjects resulted in similar energy intake and brain activity (as assessed by fMRI) to the one observed after feeding (48).
Glucose-dependent insulinotropic polypeptide
GIP is a 42-amino acid peptide secreted from the intestinal K-cells, located mainly in the duodenum and proximal jejunum. GIP secretion is increased by nutrient ingestion via luminal nutrients and possibly via neural pathways. The hormone is degraded by the same enzyme that cleaves GLP-1, DPP-IV, with a plasma half-life of 7.3 min. GIP is involved not only in glucose metabolism, via its incretin role, but also in lipid metabolism. GIP increases lipogenesis by stimulating lipoprotein lipase activity, enhancing fatty acid synthesis and incorporation into triglycerides, and downregulating glucagon-stimulated lipolysis, all of which promote fat deposition. In animal models, antagonism of GIP receptor was able to prevent or reverse obesity and reduce hepatic and muscle lipid stores. In humans, acute infusion of GIP increased abdominal subcutaneous adipose tissue blood flow, free fatty acids (FFA) re-esterification, and triacylglycerol (TAG) deposition (49).
Several human studies have reported a decrease in postprandial GIP secretion after BPD and RYGB but not after gastric banding (31,49). This suggests that bypassing the upper portion of the small intestine, by eliminating nutrient contact with GIP secreting K-cells, is responsible for the reduction in GIP secretion. Salinari et al. showed that 1 month after BPD in obese type 2 diabetics, plasma GIP AUC during an OGTT was decreased four times and was not significantly different from that of a lean control group (50). A similar finding was reported by Guidone et al., who measured GIP in type 2 diabetic patients 1 week after BPD (25). Interestingly, when circadian GIP AUC was measured post-BPD in non-diabetic vs. diabetic patients, the AUC was only reduced in patients with diabetes (51). The hypothesis that was advanced to explain these results is that in patients with type 2 diabetes, improvement of glucose homeostasis post-bariatric surgery exerts an inhibitory effect on GIP secretion. The different effect of the same procedure in normal glucose tolerant and in type 2 diabetic patients requires further investigation.
Post-RYGB, the results of GIP measurement are inconsistent. GIP has been found to be decreased, not changed or increased (49). These discrepancies could be explained by the differences in the intestinal limb length, by the temporal pattern of GIP and differences in sampling time point, or by differences in the content of the meal stimulus.
GIP might play role in maintenance of weight loss after GB surgery. Exclusion of the upper portion of the intestine, where the GIP producing K-cells are located, would result in decreased exposure to nutrients of the K cells, resulting in decreased levels of GIP. Lower GIP could contribute to less fat accumulation and result in weight loss or long-term weight loss maintenance. In a cross-sectional study, Korner et al. found that postprandial levels of GIP were reduced in RYGB compared to AGB or overweight controls, suggesting that a comparatively lower GIP could account for greater weight loss with RYGB (31). However, the fact than in a procedure which consistently induces weight loss, such as RYGB, the levels of GIP are found to be increased, decreased, or not changed casts doubts that GIP plays more than a marginal role in the induction and/or maintenance of weight loss.
Ghrelin
Ghrelin’s name is derived from its property to increase growth hormone secretion (Growth Hormone RELeasing peptide). A 28 amino acid peptide secreted by the X/A-like cells in the oxyntic glands of the stomach fundus (and in smaller amounts by the duodenum, jejunum, and ileum), ghrelin is involved in short-term (meal to meal) and long-term regulation of food intake. Ghrelin is acylated in the serine in position 3 by the action of the enzyme ghrelin O-acyltransferase (GOAT), resulting in the active form, acylated ghrelin. Though most of ghrelin effects on energy balance have been shown for the acylated ghrelin, the non-acylated form, des-acyl ghrelin, has been shown to have independent biological activity. Thus, part of the difficulty in assessing the role of ghrelin in bariatric surgery in weight loss comes from the different assays employed to measure ghrelin, i.e. whether they measured total, acylated or des-acylated ghrelin. Ghrelin is an orexigenic hormone: total ghrelin increases preprandially and decreases after food intake; administration of ghrelin increases food intake and adiposity. The mechanism of appetite stimulation by ghrelin involves actions in the NPY/AgRP neurons in the arcuate nucleus of hypothalamus. Ghrelin levels are decreased in obesity, but obese people have increased hunger and take longer to reach satiety. An attenuated suppression of ghrelin in the obese has been reported, suggesting that the lack of suppression in the obese may contribute to a lack of satiety following smaller meals (52). The role of ghrelin in the etiology of obesity is still unclear, and so is the role of ghrelin in bariatric surgery-induced weight loss.
Based on the location of ghrelin producing-cells, the restrictive component of bariatric surgery procedures, by reducing access of nutrients to ghrelin producing-cells should decrease ghrelin levels and presumably result in satiety. Indeed, Cummings et al. measured 24 h plasma profile of ghrelin 9-31 months post-RYGB and compared them to BMI-matched obese subjects who lost weight via dieting and with lean volunteers. After surgery, fasting, postprandial, and inter-prandial plasma ghrelin was lower compared to the obese or to lean subjects; 77% lower than in normal weight controls and 72% lower than in obese weight matched controls. There were no meal related fluctuations and no diurnal fluctuations. However, further studies failed to observe consistent changes in ghrelin after RYGB or BPD, as summarized in several excellent reviews (53,54). Plasma ghrelin levels (fasting and/or postprandial) are either increased, not changed, or decreased, with large variability in the type of study (prospective or cross-sectional), time of measurement after surgery, type of surgery (within procedures and by procedure type), type of ghrelin assay used and diabetes status of the patient population (normal glucose tolerance or type 2 diabetes). Some investigators set out to explore whether differences in surgical technique might contribute to these disparate results, and thus offer a unified hypothesis regarding the role of ghrelin in bariatric surgery-induced weight loss.
Fruhbeck and coworkers have conducted a series of studies in which the changes in ghrelin were investigated when the same amount of weight was lost via RYGB, AGB, BPD, or diet (55). They found that surgeries that conserve the contact of nutrients with the stomach fundus (AGB, BPD) or weight loss via diet, do not result in fasting plasma ghrelin decreases, while those that do not conserve the fundus (RYGB, gastrectomy) lead to a decrease in fasting plasma ghrelin. To test the hypothesis that nutrient contact with stomach fundus affects plasma ghrelin levels and the outcome of bariatric surgery, Perez-Romero et al. performed a prospective longitudinal 2 year study comparing patients who underwent either of two surgical procedures: RYGB that preserves food contact with stomach fundus (ringed RYGB) and one that does not (modified RYGB). There was no significant difference between groups, indicating that the ghrelin increase does not depend exclusively on the contact with gastric fundus (56).
The integrity of vagal fibers might also explain different ghrelin levels in different studies. Sundbom et al. measured fasting plasma ghrelin post-RYGB and plasma levels of pancreatic polypeptide (PP), an indicator of vagal functionality. They found a remarkable correlation between variations in the two peptides, suggesting that certain surgical procedures that compromise vagus integrity might have an effect on ghrelin levels (57). However, Perathoner et al. showed that when patients with RYGB either did or did not have the anterior vagal trunk transected during surgery, there was no difference in postoperative weight loss, parameters of satiety assessment or plasma ghrelin between the two groups, discounting vagal involvement (58).
To assess the possible involvement of ghrelin in post-surgical weight loss, Couce et al. compared fasting plasma ghrelin and weight loss in patients after laparoscopic RYGB or after other laparoscopic gastro-intestinal surgery. Plasma ghrelin fell in both groups 2 h after surgery, and continued to be lower 10 days after surgery in the RYGB group but not in the control. However, by 6 months plasma ghrelin levels were no longer low in RYGB or in the control group, in spite of significant weight loss (body weight increased in the control) (59). Busetto et al. investigated whether fasting plasma ghrelin before LAGB was a predictor of weight loss 2 years after surgery. The patients were divided in higher ghrelin levels than expected based on BMI and normal ghrelin levels. Both groups had similar %EWL after surgery without any significant differences in band management (60). Other investigators have looked at the changes in ghrelin and how they correlate with changes in eating behavior. Dixon et al. performed an experiment in which plasma ghrelin and satiety visual analog scales were compared in AGB patients who attended blind crossover breakfast tests, one with optimal band restriction and one with reduced restriction. Patients with optimal restriction experienced greater satiety, but there was no difference in ghrelin levels between the two groups (61). In RYGB patients, Christou et al. looked at fasting plasma ghrelin in those who achieved successful weight loss (EWL 72%) and less than ideal weight loss (EWL 27%) 3 years after surgery. There was no difference between fasting or postprandial levels in the two groups and no correlation between ghrelin levels and VAS score (62).
From these data it appears that, despite the initial enthusiasm, the role of ghrelin in bariatric surgery induced weight loss might be marginal. Certainly, procedures in which ghrelin is decreased might benefit from the additional inhibition of feeding resulting from less ghrelin action. However, it is unlikely that ghrelin plays a central role in the induction and maintenance of weight loss.
Role of the Vagus Nerve
The neural connections between the intestine and the brain, and especially the vagus nerve, might play an important role in the weight loss post-bariatric surgery. Many gastrointestinal hormones are released in response to neural as well as nutrient signals, and hormonal effects could be mediated via a neural pathway. The vagus nerve innervates most of the gastrointestinal tract, so afferent sensory fibers are in close proximity to the gastric fundus cells that produce ghrelin, L-cells that produce GLP-1, PYY, and oxyntomodulin and other endocrine cells (63). At least part of the effects of GLP-1, PYY, CCK, and ghrelin appear to be mediated via a vagal pathway. GLP-1 might act via vagal fibers to inhibit stomach motility, and to reduce spontaneous meal size (22). The gastric vagal afferents are a major pathway for ghrelin’s effects on feeding (52) and PYY acts through vagal receptors as well (39).
Vagotomy has been shown to block ghrelin’s effects on food intake, so it is possible that sectioning the branch of vagus nerve that innervates stomach fundus during bariatric procedures results in weight loss via a lack of ghrelin action (52). In a rodent study, Bueter et al. showed that preservation of the para-esophageal bundle of the vagus nerve during GB resulted in lower body weight and reduced food intake. GLP-1 and PYY levels were increased after surgery, though they were not different with or without vagal preservation (64), suggesting that perhaps the hormones’ action via the vagal pathway might mediate the weight loss. A recent study by Shin et al. showed that vagal innervation of the hepatic portal vein and liver is not necessary for GB-induced weight loss in obese rats. The authors suggest that the celiac (that innervate the distal duodenum, jejunum, ileum, cecum, and colon) and gastric vagal branches are more likely the ones involved in the GB effects (63).
So far, the human studies investigating the benefits of vagotomy to increase the bariatric surgery weight loss have proved disappointing. In an open-label case–controlled study conducted at Central Carolina Surgery, Martin et al. found that gastric banding with or without vagotomy had similar EWL at 3 years, 38% and 36%, respectively (65). Similar results were reported by Angrisani et al. in patients with gastric banding (66) and by Perathoner et al. after RYGB with and without vagotomy (58). The only report of improved weight loss with vagotomy is the study of Kral et al., who found that adding truncal vagotomy to VBG had a beneficial effect on weight loss (51% vs. 34% EWL) (67).
Bile Acids and Bariatric Surgery
Bile acids, synthesized in the hepatocytes and released in the bile, are important regulators of energy balance through nuclear receptor farnesoid X receptor (FXR) and the G-protein-coupled membrane receptor TGR5. Activation of FXR by bile acids after a meal induces synthesis of the intestinal peptide hormone FGF19, and triggers a cascade that controls fed-fasted state metabolism. Bile acids could also promote weight loss by increasing energy expenditure in brown adipose via TGR5. Administration of bile acids to mice increased energy expenditure in brown adipose tissue by induction of the cAMP-dependent thyroid hormone activating enzyme type 2 iodothyronine deiodinase (68).
Bile acids might be involved in bariatric surgery-induced changes in energy homeostasis by two mechanisms: (a) increased secretion of bile acids with direct effects on energy balance and (b) increased delivery to distal intestine due to nutrient and bile re-routing, with increased stimulation of L-cell production and release of hormones with attenuating effects on satiety.
Patti et al. showed that total serum bile acid concentrations were higher in RYGB post-surgery than in obese patients (69), while Pournaras et al. (70) found increased fasting FGF19 and increased fasting total bile acids at 4 and 42 days post-surgery in GB but not in gastric banding patients. However, in a study by Nakatani et al. serum bile acids and serum incretins (GLP-1 and GIP) were increased in patients after both malabsorptive procedures (RYGB and SG-DJB) and restrictive procedures (SG, gastric banding) (71). The mechanism of increased bile acids post-bariatric surgery is not known. It has been suggested that malabsorptive procedures might decrease the enterohepatic circulation of bile acids, followed by increased conversion of cholesterol to bile acids. In the restrictive type of procedures, reduced cholesterol intake might increase cholesterol biosynthesis and bile acid secretion. Other post-surgical changes, such as changes in diet, gut microflora, intestinal motility, etc could contribute to increased postprandial bile acid absorption.
GB surgery increases not only delivery of nutrients, but also of bile acids to the distal intestine. Katsuma et al. showed that bile acids promote GLP-1 secretion through TGR5 in a murine enteroendocrine cell (72). Bile acids might activate L-cell secretion, with subsequent release of PYY, GLP-1, and oxyntomodulin, hormones involved in satiety and decreased appetite.
Long-Term Effects and Weight Regain After Bariatric Surgery
Bariatric surgery is undeniably very successful in producing short- and medium-term weight loss. However, as more and more bariatric surgery procedures are performed, the question arises of what is the long-term effect of these surgeries on body weight, how often weight is regained, and do gut hormones play a role in the process.
In the prospective, controlled Swedish Obese Subjects Study, at 10 years after surgery the absolute weight loss was 16% in the bariatric surgery group (25% for the GB, 16% for VBG, and 13% for gastric banding). Approximately, 20% of the patients regained all their lost weight within 1-3 years (4). In an early study, Camerini et al. showed that there was good short-term weight loss after gastric banding (at 1 year BMI was 79% of preop) but BMI tended to increase over time, by approximately 0.42 BMI units/year; at 13 years postoperatively 60% of bands had to be removed (73). After RYGB, Freire et al. found that weight regain was seen in 56% of the patients (29% regained over 10% of the nadir weight). Weight regain increased significantly with time after surgery, reaching 84.8% for over 5 years (74). Weight loss was more sustained after BPD with duodenal switch than RYGB in superobese patients. At 3 years post-surgery, the %EWL was 63% for RYGB and 84% for BPD-DS. Weight loss success (defined as %EWL >50%) was achieved by 83% of the RYGB and 98% of the BPD-DS patients (75).
The causes of weight regain after bariatric surgery remain to be understood, but it is possible that differences in gastro-intestinal hormone responses to surgery might contribute to differences between subjects. Using a rodent model of RYGB, Meguid et al. saw a dramatic increase in PYY:leptin ratio of animals that had successful maintenance of weight loss. On the contrary, the rats that were unable to develop a high PYY:leptin concentration ratio were not able to maintain RYGB-induced appetite suppression and weight loss (76). In obese patients, Bohdjalian et al. found that 5 years after SG %EWL was 55% at 5 years; and 19% of patients had weight regain of > 10 kg from nadir. Some of the weight regain patients were converted to GB. Plasma ghrelin levels were lower in the non-converted patients and higher in the weight regain patients (77).
Bariatric Surgery and Type 2 Diabetes Remission
In 1992, Pories et al. published the results of a 10-year follow-up on the effects of GB in type 2 diabetes: they showed diabetes reverted to normal in 141 of 163 patients (86%) (78). This finding, confirmed by numerous other studies, spurred a wave of research into the mechanism of diabetes remission or improvement with bariatric (now also called metabolic) surgery.
Since all bariatric surgery procedures result in significant weight loss accompanied by a decrease in fat mass, it was postulated that improved insulin sensitivity is a significant contributor to diabetes amelioration (19). However, it soon became apparent that improved beta cell function also plays a significant role in the glucose homeostatic improvement (25). Moreover, diabetes remission frequently occurs immediately after surgery, in the absence of significant weight loss, suggesting that weight-loss independent mechanisms come into play. A new type of surgery, DJB, in which stomach size is not reduced and the weight loss is minimal, could contribute in elucidating some of these mechanisms. Cohen et al. found 40% diabetes remission with DJB (15), while Klein et al. showed that DJB improved beta cell function, but not insulin resistance, and only moderately improved glucose homeostasis in overweight and class I obese patients with T2DM (79).
Among the many factors contributing to glucose homeostatic improvement, changes in gastro-intestinal hormones probably play a significant role. The “upper” and “lower” gut theories propose that the bypass of the upper intestine (with the bypass of a hypothetical “diabetogenic “factor), or the enhanced delivery of nutrients to the lower intestine (with release of GLP-1, PYY and other hormones favorable to glucose homeostasis) are responsible for the diabetes improvement (26,80). Of course, the question is not whether it’s exclusively one or the other of the mechanisms (it’s probably both) but the proportional importance of each. It seems plausible that a combination of enhanced lower gut hormones and decreased upper gut hormones are mediators of glycemic improvement. In the same time, it is very tempting to believe that a “diabetogenic factor” released by the upper gut—maybe gut glucagon or, more exciting, a yet undiscovered hormone evolutionarily evolved to defend against hypoglycemia—is a major contributor to diabetes remission.
Conclusions
Obesity continues to be a major health problem both in the United States and the world at large. Human longevity is for the first time showing signs of decreasing in industrialized countries—due in large part to an equivalent epidemic in the comorbidities arising from obesity. Perhaps most concerning is the proportion of the population now entering into the super morbidly obese range or those who are 150 pounds overweight. The new challenge is losing vast amounts of weight and keeping it off for decades. To date, bariatric surgery remains the only intervention which allows for significant and durable weight loss which has been shown to translate into improvement or resolution of comorbidities and subsequently improvement of mortality (4).
Understanding the mechanisms by which the current weight loss surgeries act is paramount in translating this knowledge into less or noninvasive therapies. Evidence shows that bariatric surgery works by more than just restriction of gastric volume or even re-routing intestinal secretions to cause malabsorption. Fifty years after the first bariatric surgery we are finally understanding it is the alteration of gut hormones which are likely responsible for the significant and durable changes in weight after surgery, not just the physical limitations imposed by surgery. Indeed, these new realizations refocus the perception of the stomach and small intestine from simple vehicles for nutrients and absorption into powerful yet misunderstood endocrine organs. The possible involvement of L-cell products in the increased effectiveness of metabolic surgery has been proposed, and the overwhelming evidence points towards GLP-1 and PYY as the likely contributors to the weight loss after bariatric surgery. Other gastro-intestinal hormones, such as GIP, oxyntomodulin, GLP-2 and ghrelin may also play individual roles, but the evidence for these factors is less clear. What is clear is that additional studies on the effect of metabolic surgery on weight loss are needed. Once the mechanisms are elucidated, it can be expected that new therapies, less invasive than metabolic surgery itself, will emerge.
Acknowledgments
RNB is supported by NIH grants R37 DK 27619 and RO1 DK 29867.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008 Oct;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 2.Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord. 2002;26:262–273. doi: 10.1038/sj.ijo.0801889. [DOI] [PubMed] [Google Scholar]
- 3.Heal DJ, Gosden J, Smith SL. A review of late-stage CNS drug candidates for the treatment of obesity. Int J Obes (Lond) 2013;37:107–117. doi: 10.1038/ijo.2012.26. [DOI] [PubMed] [Google Scholar]
- 4.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 5.Crookes PF. Surgical treatment of morbid obesity. Annu Rev Med. 2006;57:243–264. doi: 10.1146/annurev.med.56.062904.144928. [DOI] [PubMed] [Google Scholar]
- 6.Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg. 2011;213:261–266. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Usinger L, Hansen KB, Kristiansen VB, Larsen S, Holst JJ, Knop FK. Gastric emptying of orally administered glucose solutions and incretin hormone responses are unaffected by laparoscopic adjustable gastric banding. Obes Surg. 2011;21:625–632. doi: 10.1007/s11695-011-0362-9. [DOI] [PubMed] [Google Scholar]
- 10.Garb J, Welch G, Zagarins S, Kuhn J, Romanelli J. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes Surg. 2009;19:1447–1455. doi: 10.1007/s11695-009-9927-2. [DOI] [PubMed] [Google Scholar]
- 11.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Melissas J, Koukouraki S, Askoxylakis J, et al. Sleeve gastrectomy: a restrictive procedure? Obes Surg. 2007;17:57–62. doi: 10.1007/s11695-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 13.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319–324. doi: 10.1097/SLA.0b013e3181e90b31. [DOI] [PubMed] [Google Scholar]
- 14.Sandler BJ, Rumbaut R, Swain CP, et al. Human experience with an endoluminal, endoscopic, gastrojejunal bypass sleeve. Surg Endosc. 2011;25:3028–3033. doi: 10.1007/s00464-011-1665-6. [DOI] [PubMed] [Google Scholar]
- 15.Cohen R, Caravatto PP, Correa JL, et al. Glycemic control after stomach-sparing duodenal-jejunal bypass surgery in diabetic patients with low body mass index. Surg Obes Relat Dis. 2012;8:375–380. doi: 10.1016/j.soard.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Lieske JC, Collazo-Clavell ML, et al. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 2011;149:654–661. doi: 10.1016/j.surg.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odstrcil EA, Martinez JG, Santa Ana CA, et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92:704–713. doi: 10.3945/ajcn.2010.29870. [DOI] [PubMed] [Google Scholar]
- 18.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliván B, Teixeira J, Bose M, et al. Effect of weight loss by diet or gastric bypass surgery on peptide YY3-36 levels. Ann Surg. 2009;249:948–953. doi: 10.1097/SLA.0b013e3181a6cdb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez A, Becerril S, Valentí V, et al. Short-term effects of sleeve gastrectomy and caloric restriction on blood pressure in diet-induced obese rats. Obes Surg. 2012;22:1481–1490. doi: 10.1007/s11695-012-0702-4. [DOI] [PubMed] [Google Scholar]
- 22.Nauck MA. Unraveling the science of incretin biology. Eur J Intern Med. 2009;20(Suppl 2):S303–S308. doi: 10.1016/j.ejim.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Burcelin R, Serino M, Cabou C. A role for the gut-to-brain GLP-1-dependent axis in the control of metabolism. Curr Opin Pharmacol. 2009;9:744–752. doi: 10.1016/j.coph.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 24.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 25.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 26.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 27.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95:1851–1855. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 29.Dirksen C, Hansen DL, Madsbad S, et al. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care. 2010;33:375–377. doi: 10.2337/dc09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterli R, Wölnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 31.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kampe J, Stefanidis A, Lockie SH, et al. Neural and humoral changes associated with the adjustable gastric band: insights from a rodent model. Int J Obes (Lond) 2012;36:1403–1411. doi: 10.1038/ijo.2012.25. [DOI] [PubMed] [Google Scholar]
- 33.Alam ML, Van der Schueren BJ, Ahren B, et al. Gastric bypass surgery, but not caloric restriction, decreases dipeptidyl peptidase-4 activity in obese patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:378–381. doi: 10.1111/j.1463-1326.2011.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lugari R, Dei Cas A, Ugolotti D, et al. Glucagon-like peptide 1 (GLP-1) secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreatic diversion. Horm Metab Res. 2004;36:111–115. doi: 10.1055/s-2004-814222. [DOI] [PubMed] [Google Scholar]
- 35.Valverde I, Puente J, Martín-Duce A, et al. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg. 2005;15:387–397. doi: 10.1381/0960892053576613. [DOI] [PubMed] [Google Scholar]
- 36.Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 37.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 38.Pournaras DJ, Osborne A, Hawkins SC, et al. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes Surg. 2010;20:56–60. doi: 10.1007/s11695-009-9989-1. [DOI] [PubMed] [Google Scholar]
- 39.Ballantyne GH. Peptide YY(1-36) and peptide YY(3-36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 40.Valderas JP, Irribarra V, Boza C, et al. Medical and surgical treatments for obesity have opposite effects on peptide YY and appetite: a prospective study controlled for weight loss. J Clin Endocrinol Metab. 2010;95:1069–1075. doi: 10.1210/jc.2009-0983. [DOI] [PubMed] [Google Scholar]
- 41.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 42.Bose M, Machineni S, Oliván B, et al. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring) 2010;18:1085–1091. doi: 10.1038/oby.2009.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morínigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–1601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 44.Morínigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–275. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- 45.Chandarana K, Gelegen C, Karra E, et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes. 2011;60:810–818. doi: 10.2337/db10-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zac-Varghese S, Tan T, Bloom SR. Hormonal interactions between gut and brain. Discov Med. 2010;10:543–552. [PubMed] [Google Scholar]
- 47.Laferrère B, Swerdlow N, Bawa B, et al. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95:4072–4076. doi: 10.1210/jc.2009-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Silva A, Salem V, Long CJ, et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14:700–706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao RS, Kini S. GIP and bariatric surgery. Obes Surg. 2011;21:244–252. doi: 10.1007/s11695-010-0305-x. [DOI] [PubMed] [Google Scholar]
- 50.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–380. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mingrone G, Nolfe G, Gissey GC, et al. Circadian rhythms of GIP and GLP1 in glucose-tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia. 2009;52:873–881. doi: 10.1007/s00125-009-1288-9. [DOI] [PubMed] [Google Scholar]
- 52.Castãneda TR, Tong J, Datta R, Culler M, Tschöp MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Cummings DE, Shannon MH. Ghrelin and gastric bypass: is there a hormonal contribution to surgical weight loss? J Clin Endocrinol Metab. 2003;88:2999–3002. doi: 10.1210/jc.2003-030705. [DOI] [PubMed] [Google Scholar]
- 54.Lee H, Te C, Koshy S, Teixeira JA, Pi-Sunyer FX, Laferrère B. Does ghrelin really matter after bariatric surgery? Surg Obes Relat Dis. 2006;2:538–548. doi: 10.1016/j.soard.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Frühbeck G, Diez-Caballero A, Gil MJ, et al. The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes Surg. 2004;14:606–612. doi: 10.1381/096089204323093363. [DOI] [PubMed] [Google Scholar]
- 56.Pérez-Romero N, Serra A, Granada ML, et al. Effects of two variants of Roux-en-Y Gastric bypass on metabolism behaviour: focus on plasma ghrelin concentrations over a 2-year follow-up. Obes Surg. 2010;20:600–609. doi: 10.1007/s11695-009-0035-0. [DOI] [PubMed] [Google Scholar]
- 57.Sundbom M, Holdstock C, Engström BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 58.Perathoner A, Weiss H, Santner W, et al. Vagal nerve dissection during pouch formation in laparoscopic Roux-Y-gastric bypass for technical simplification: does it matter? Obes Surg. 2009;19:412–417. doi: 10.1007/s11695-008-9657-x. [DOI] [PubMed] [Google Scholar]
- 59.Couce ME, Cottam D, Esplen J, Schauer P, Burguera B. Is ghrelin the culprit for weight loss after gastric bypass surgery? A negative answer. Obes Surg. 2006;16:870–878. doi: 10.1381/096089206777822151. [DOI] [PubMed] [Google Scholar]
- 60.Busetto L, Segato G, De Luca M, et al. High ghrelin concentration is not a predictor of less weight loss in morbidly obese women treated with laparoscopic adjustable gastric banding. Obes Surg. 2006;16:1068–1074. doi: 10.1381/096089206778026307. [DOI] [PubMed] [Google Scholar]
- 61.Dixon AF, Dixon JB, O’Brien PE. Laparoscopic adjustable gastric banding induces prolonged satiety: a randomized blind crossover study. J Clin Endocrinol Metab. 2005;90:813–819. doi: 10.1210/jc.2004-1546. [DOI] [PubMed] [Google Scholar]
- 62.Christou NV, Look D, McLean AP. Pre- and post-prandial plasma ghrelin levels do not correlate with satiety or failure to achieve a successful outcome after Roux-en-Y gastric bypass. Obes Surg. 2005;15:1017–1023. doi: 10.1381/0960892054621071. [DOI] [PubMed] [Google Scholar]
- 63.Shin AC, Zheng H, Berthoud HR. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg. 2012;255:294–301. doi: 10.1097/SLA.0b013e31823e71b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bueter M, Löwenstein C, Ashrafian H, et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20:616–622. doi: 10.1007/s11695-010-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin MB, Earle KR. Laparoscopic adjustable gastric banding with truncal vagotomy: any increased weight loss? Surg Endosc. 2011;25:2522–2525. doi: 10.1007/s00464-011-1580-x. [DOI] [PubMed] [Google Scholar]
- 66.Angrisani L, Cutolo PP, Ciciriello MB, et al. Laparoscopic adjustable gastric banding with truncal vagotomy versus laparoscopic adjustable gastric banding alone: interim results of a prospective randomized trial. Surg Obes Relat Dis. 2009;5:435–438. doi: 10.1016/j.soard.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 67.Kral JG, Görtz L, Hermansson G, Wallin GS. Gastroplasty for obesity: long-term weight loss improved by vagotomy. World J Surg. 1993;17:75–78. doi: 10.1007/BF01655710. discussion 79. [DOI] [PubMed] [Google Scholar]
- 68.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25:1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pournaras DJ, Glicksman C, Vincent RP, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 73.Camerini G, Adami G, Marinari GM, et al. Thirteen years of follow-up in patients with adjustable silicone gastric banding for obesity: weight loss and constant rate of late specific complications. Obes Surg. 2004;14:1343–1348. doi: 10.1381/0960892042584049. [DOI] [PubMed] [Google Scholar]
- 74.Freire RH, Borges MC, Alvarez-Leite JI, Toulson Davisson Correia MI. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition. 2012;28:53–58. doi: 10.1016/j.nut.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Topart P, Becouarn G, Ritz P. Weight loss is more sustained after biliopancreatic diversion with duodenal switch than Roux-en-Y gastric bypass in superobese patients. Surg Obes Relat Dis. 2002 Mar 3; doi: 10.1016/j.soard.2012.02.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 76.Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: a significant 20% complication related to PYY. Nutrition. 2008;24:832–842. doi: 10.1016/j.nut.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 77.Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20:535–540. doi: 10.1007/s11695-009-0066-6. [DOI] [PubMed] [Google Scholar]
- 78.Pories WJ, MacDonald KG, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55(2 Suppl):582S–585S. doi: 10.1093/ajcn/55.2.582s. [DOI] [PubMed] [Google Scholar]
- 79.Klein S, Fabbrini E, Patterson BW, et al. Moderate effect of duodenal-jejunal bypass surgery on glucose homeostasis in patients with type 2 diabetes. Obesity (Silver Spring) 2012;20:1266–1272. doi: 10.1038/oby.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]