Abstract
Objective
Evaluate the impact of near-term delivery on neurodevelopmental (ND) outcomes in children with congenital heart disease (CHD).
Methods
Secondary analysis of data from a study of genetic polymorphisms and ND outcomes after cardiac surgery in infants. The effect of gestational age (GA) as a continuous variable on ND outcomes was evaluated using general linear regression models. GA was also evaluated as a categorical variable to seek a threshold for better outcomes. ND domains tested at 4 years of age included cognition, language skills, attention, impulsivity, memory, executive function, social competence, visual-motor, and fine-motor skills.
Results
ND outcomes and GA were available for 378 infants. Median GA was 39 weeks (range, 28–42 weeks) with 351 born at 36 weeks or more (near-term/term). In univariate analysis of the near-term/term subgroup, older GA predicted better performance for cognition, visual-motor, and fine-motor skills. After covariate adjustment, older GA predicted better performance for fine-motor skills (P = .018). Performance for cognition, language, executive function, social skills, visual-motor, and fine-motor skills was better for those born at 39 to 40 weeks of GA or more versus those born at less than 39 weeks (all P<.05).
Conclusions
These findings are consistent with the hypothesis that delivery before 39 to 40 weeks of GA is associated with worse outcomes in patients with CHD. Early delivery of a child with CHD is often indicated because of maternal or fetal health issues. In the absence of these concerns, these data suggest that elective (or spontaneous) delivery at 39 to 40 weeks of GA is associated with better ND outcomes.
Congenital heart disease (CHD) is the most common birth defect with an estimated prevalence of 8 per 1000 live births. Each year, more than 30,000 infants undergo cardiac surgery. With improvements in survival, increased focus has been directed toward neurodevelopmental (ND) outcomes. Over 50% of survivors of cardiac surgery during infancy are identified with learning disabilities and behavioral issues once they reach school age.1 These neurobehavioral problems ultimately affect their academic achievements and employment opportunities. Unfortunately, most known risk factors for adverse ND outcomes are patient-related factors such as prematurity, low birth weight, associated genetic anomalies, and lower socioeconomic status, not management factors.2,3 Identification of modifiable factors as targets of therapeutic interventions is essential to improve ND outcomes.
The risks of premature birth have been well described. There is increasing evidence that even late preterm delivery (34–37 weeks’ gestational age [GA]) is associated with an increased morbidity and mortality in children without CHD. Late preterm birth is associated with an increased incidence of respiratory distress syndrome, hypoglycemia, hypothermia, feeding difficulties, and apnea.4,5 In addition, recent data suggest that near-term (37–38 weeks’ GA) birth may also affect long-term ND outcomes.6 The American College of Obstetricians and Gynecologists recommends that elective delivery should not be performed before a GA of 39 weeks.7
The optimal GA for delivery of a fetus with CHD is not known. Early delivery is often indicated for maternal or fetal health issues, such as pre-eclampsia, placental insufficiency, or fetal distress. However, increasing use of prenatal diagnosis of CHD has led to an increased incidence of elective delivery, often before 39 weeks’ GA. Infants with a prenatal diagnosis of CHD have greater use of induction of labor and younger GA at delivery than do those in whom the diagnosis is made after birth.8 Recently, late preterm delivery of infants with CHD has been shown to be associated with increased morbidity and mortality in the cardiac intensive care unit.9,10 To date, there are no data to determine whether near-term delivery affects ND outcomes in children with CHD. We investigated the impact of GA on ND outcomes at 4 years of age after cardiac surgery during infancy.
PATIENTS AND METHODS
Study Population
This is a secondary analysis of data from a prospective study of the role of apolipoprotein E (APOE) gene polymorphisms on ND outcomes after cardiac surgery during infancy.2 Patients were eligible if they underwent surgical intervention in the first 6 months of life for CHD with cardiopulmonary bypass (CPB) with or without deep hypothermic circulatory arrest (DHCA). Patients with multiple congenital anomalies, a recognizable genetic or phenotypic syndrome other than chromosome 22q11 deletion syndrome, or language other than English spoken in the home were excluded from participation. The current study used data from a detailed ND evaluation performed at 4 years of age. The impact of GA on neurodevelopment was assessed for all patients who had ND testing at 4 years of age. To evaluate the impact of late preterm/near-term delivery on ND outcomes, we performed a subanalysis of those patients born at a GA of 36 weeks or older. The Institutional Review Board at The Children’s Hospital of Philadelphia approved the study, and the parent or guardian provided informed consent.
Operative Management
Operations were performed by 5 cardiac surgeons with a team of cardiac anesthesiologists. Alpha-stat blood gas management was used. Pump flow rates were not standardized for this study. In general, during normothermic or mild hypothermic CPB (nasopharyngeal [NP] temperature > 28°C), the pump flow rate was maintained at 100 to 150 mL · kg−1 · min−1 to achieve a mean arterial pressure of 30 to 55 mm Hg. When moderate hypothermia (NP temperature 22°C–28°C) was used, the pump flow rate was maintained at100mL· kg−1· min−1 with a target mean arterial pressure greater than 30 mm Hg. For deep hypothermic continuous CPB (NP temperature <22°C), a pump flow rate of 25 to 50 mL · kg−1 · min−1 was used. These were general guidelines that were modified according to the clinical situation. DHCA was selectively used at the surgeon’s discretion, not according to a predetermined protocol. Before DHCA, patients underwent core and surface cooling, with topical hypothermia of the head, to an NP temperature of 18°C. Modified ultrafiltration was performed in all patients after CPB. Postoperatively, patients were managed in the cardiac intensive care unit by a team of cardiologists, intensivists, nurses, and surgeons.
Data Collection
Preoperative factors that might independently affect the neurobehavioral outcomes including GA and genetic anomalies were obtained from hospital records. For the majority of the patients, GA was determined from the maternal records. In a small number of cases, GA was determined from neonatal records. Weight and age at operation were recorded for the initial operation and subsequent operations with CPB. Operative variables including duration of CPB and DHCA, lowest NP temperature, and hematocrit after hemodilution were recorded. Prenatal diagnosis was noted.
Four-Year ND Examination
The ND examination was performed between the fourth and fifth birthdays and growth measurements (ie, weight, length, head circumference) were recorded. Maternal education, socioeconomic status using the Hollingshead method, and ethnicity were determined by parental report. A health history was obtained focusing on the incidence of interim illness: rehospitalizations, neurologic events or interim evaluations, current medications, and parental concerns about health.
Patients were evaluated by a genetic dysmorphologist at the 1- and/or 4-year evaluations. Additional genetic analyses were performed if indicated. Neonatal recognition of dysmorphic features can be difficult; therefore, some patients were enrolled for whom the diagnosis of a genetic syndrome was not made until a later evaluation. Patients were classified as having no definite genetic syndrome or chromosomal abnormality (normal), suspected genetic syndrome (suspect), or definite genetic or chromosomal abnormality (abnormal).
ND Testing
Multiple ND domains were tested including cognition, language, speech, memory, executive function, and visual-motor, fine-motor, and academic skills. Cognitive skills were assessed using the Full-Scale Intelligence Quotient from the Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III).11 Core language skills were assessed using the Preschool Language Scale-4 Total Language Score (PLS-4 TLS).12 Attention and impulsivity were assessed through parental report by the Attention Deficit/Hyperactivity Disorder (ADHD) Rating Scale-IV, Preschool Version. Social skills were evaluated using the Preschool and Kindergarten Behavior Scales (PKBS). Executive function and memory were assessed using the Neuro-PSYchology (NEPSY) core domain scores for attention/executive function and memory.13 Visualmotor integration was assessed with the developmental test of Visual-Motor Integration (VMI), a simple copying task that assesses the child’s fine-motor and visual-motor coordination skills.14 Fine-motor skills were tested using the Wide Range Assessment of Visual-Motor Abilities pegboard (WRAVMA), a manipulative dexterity test.15 All the instruments, except the ADHD-IV and the PKBS, have an expected mean of 100 with a standard deviation of 15. Not every child was able to complete every test. If a child was judged to be too developmentally impaired to complete the tasks, a score was imputed by assigning them the lowest possible score for the specific test. If the child was unable to complete the tasks for other reasons, the child was excluded from the analysis for that domain. Additional details concerning the specific tests are provided in Appendix 1.
Statistical Methods
Type of CHD was coded according to a previously described classification incorporating cardiac anatomy and perioperative physiology, which has been shown to be predictive of perioperative mortality. Class I is 2 ventricles with no aortic arch obstruction, class II is 2 ventricles with aortic arch obstruction, class III is a single ventricle with no arch obstruction, and class IV is a single ventricle with arch obstruction. Patients with tetralogy of Fallot and transposition of the great arteries are included in class I, whereas patients with hypoplastic left heart syndrome and variants are included in class IV.
The ND outcomes were analyzed as continuous outcomes, including (1) WPPSI Full-Scale Intelligence Quotient, (2) PLS-4, (3) impulsivity score, (4) inattentive score, (5) NEPSY core domain score for memory, (6) NEPSY core domain score for attention/executive function, (7) PKBS Social Skill Total Score, (8) VMI score, and (9) WRAVMA Fine Motor Score. A descriptive analysis was conducted to explore the distribution of each ND outcome using mean, standard deviation, median, minimum, and maximum, as appropriate. The assumptions of the linear model were tested for each outcome. If the assumptions were not valid, log transformation was used. General linear models were used to evaluate the effect of GA on each ND outcome without adjustment of other risk factors and with other risk factors, including maternal education, APOE genotype, severity of CHD, hematocrit at first operation (percent), and socioeconomic status. Because most elective deliveries occur after 36 weeks’ GA, outcomes were assessed in the total cohort (n = 378) and the late preterm/term subgroup with GA of 36 weeks or more (n = 351). To determine whether there was a GA threshold associated with better ND outcomes in the late preterm/term subgroup, we compared outcomes based on week of GA, for example, less than 37 weeks versus 37 weeks or more, less than 38 weeks versus 38 weeks or more, and so on. The threshold of GA on each ND outcome was assessed with the general linear model. All statistical analyses are conducted using the statistical software package SAS version 9 (SAS Institute, Inc, Cary, NC).
RESULTS
Between September 1998 and April 2003, a total of 675 eligible infants underwent cardiac surgery. Of these 675 infants, 23 died before consent could be obtained and parents of 102 patients declined participation, resulting in a total enrollment of 550 (81%). Twenty-one deaths occurred during the initial hospitalization and an additional 43 patients died before 5 years of age. Characteristics of the 486 patients who were eligible for ND testing at 4 years of age have been described previously.2 Of the surviving patients, 381 completed testing and 105 did not return. The only significant difference in the baseline characteristics between returning and nonreturning patients was underrepresentation of African Americans in the returning patients (21% vs 29%).
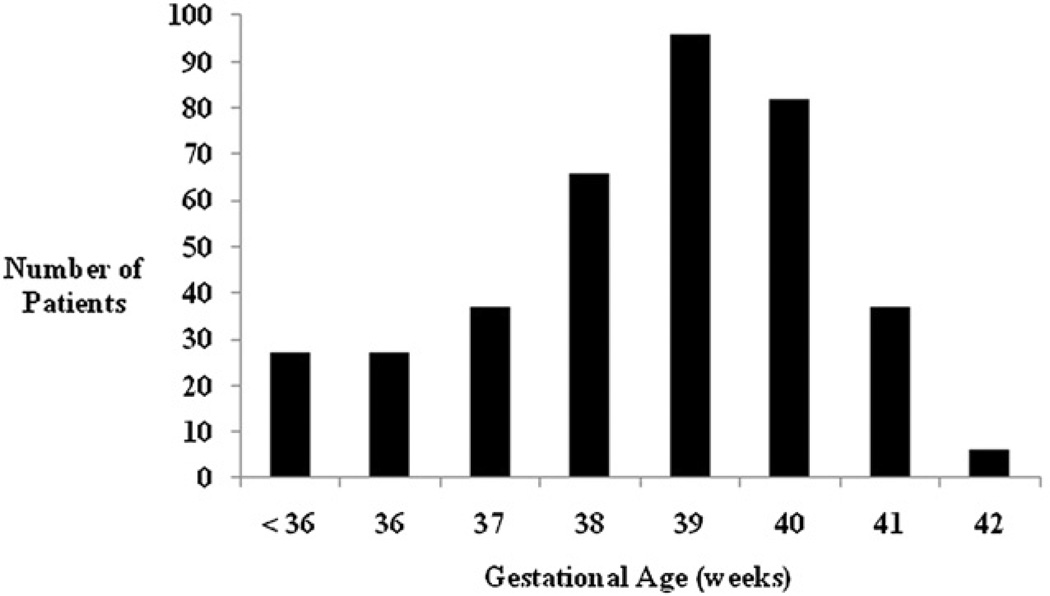
Of the 381 patients who returned for 4-year ND testing, 378 had GA available. Baseline patient characteristics for both the total cohort (n = 378) and the subgroup with GA of 36 weeks or more (n = 351) are reported in Table 1. The distribution by GA is shown in Figure 1. A prenatal diagnosis of CHD had been made in only 124 (33%) of the 378 patients and had not been made in 242 (64%). It could not be determined whether a prenatal diagnosis had been made in 12 (3%) patients. Indications for delivery in this cohort could not be reliably determined from available data. For the entire cohort, median GA was 39 weeks (range, 28–42 weeks) with a mean birth weight of 3.1 ± 0.6 kg. The median GA was 38 weeks for patients with a prenatal diagnosis and 39 weeks for those with a postnatal diagnosis. Mean scores for each ND domain tested are shown in Table 2.
TABLE 1.
Patient characteristics
| Total cohort (n = 378) |
Gestational age ≥ 36 wk (n = 351) |
|
|---|---|---|
| Gender | ||
| Female | 164 (43%) | 152 (43%) |
| Male | 214 (57%) | 199 (57%) |
| Ethnicity | ||
| White | 253 (66%) | 235 (67%) |
| African American | 80 (21%) | 75 (21%) |
| Asian | 15 (4%) | 15 (4%) |
| Other | 30 (9%) | 26 (9%) |
| Gestational age (wk) | 38 ± 2.1 | 39 ± 1.4 |
| Birth weight (kg) | 3.1 ± 0.63 | 3.2 ± 0.53 |
| Genetic anomaly | ||
| Suspected/confirmed | 84 (22%) | 74 (21%) |
| Congenital heart disease | ||
| Two ventricles/no arch obstruction | 199 (53%) | 179 (51%) |
| Two ventricles/arch obstruction | 47 (13%) | 47 (13%) |
| One ventricle/no arch obstruction | 36 (9%) | 33 (9%) |
| One ventricle/arch obstruction | 96 (25%) | 92 (26%) |
| Operative characteristics (first operation) | ||
| Median age (d) | 8 (1–188) | 7 (1–188) |
| Median weight (kg) | 3.6 (1.5–7.8) | 3.6 (1.7–7.8) |
| Total CPB time (min) | 49 (14–242) | 49 (14–242) |
| DHCA use | 215 (57%) | 201 (57%) |
| Total DHCA time (min) | 21 (0–102) | 23 (0–102) |
| Hematocrit after hemodilution (%) | 28 ±4 | 28 ±4 |
| Postoperative length of stay (d) | 8.5 (2–106) | 8 (2–106) |
| Additional operations before | ||
| 4-year testing | ||
| None | 223 (59%) | 208 (59%) |
| 1–2 | 145 (38%) | 133 (38%) |
| >2 | 10 (3 %) | 10 (3%) |
CPB, Cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest.
FIGURE 1.
Distribution of patients by gestational age.
TABLE 2.
Mean scores for neurodevelopmental outcomes
| Domain | Total cohort (n = 378) |
Gestational age ≥ 36 wk (n = 351) |
|---|---|---|
| Cognition | 95.1 ± 19.1 | 96.0 ± 18.5 |
| Language | 97.1 ± 18.7 | 97.9 ± 18.0 |
| Attention | 6.3 ± 5.4 | 6.1 ± 5.3 |
| Impulsivity | 7.2 ± 5.6 | 7.1 ± 5.6 |
| Executive function | 98.4 ± 14.9 | 98.6 ± 14.6 |
| Memory | 92.6 ± 17.0 | 93.3 ± 16.8 |
| Social skills | 106.0 ± 13.0 | 106.2 ± 12.8 |
| Visual motor | 92.3 ± 18.3 | 93.1 ± 17.9 |
| Fine motor | 94.3 ± 18.7 | 95.4 ± 18.0 |
The assumptions of the linear model were valid for the FSIQ, PLS-4, NEPSY Core Domains for attention/executive function and memory, VMI, and WRAVMA. Log transformation remedied the violations for inattention and impulsivity. Unadjusted outcomes for the entire cohort (n = 378) demonstrated that lower GA was associated with worse outcomes for almost every ND domain tested: cognition, language, inattention, executive function, memory, visual-motor and fine-motor skills, and social skills (P <.03). After adjustment for confounders, younger GA at delivery predicted worse performance for cognition, language, inattention, visual-motor skills, and fine-motor skills (P<.01) (Table 3).
TABLE 3.
Multivariable analysis
| Total cohort (n = 378) |
Gestational age ≥ 36 wk (n = 351) |
|||
|---|---|---|---|---|
| Domain | Estimate | P | Estimate | P |
| Cognition | 1.32 | .0005 | 0.71 | .2 |
| Language | 1.05 | .0046 | 0.14 | .8 |
| Attention | 0.33 | .0086 | −0.05 | .8 |
| Impulsivity | −0.10 | .5 | 0.14 | .5 |
| Executive function | 0.50 | .1 | 0.2 | .7 |
| Memory | 0.74 | .056 | 0.02 | 1.0 |
| Social skills | 0.49 | .1 | 0.52 | .3 |
| Visual motor | 1.37 | .0005 | 1.0 | .1 |
| Fine motor | 2.06 | <.0001 | 1.42 | .018 |
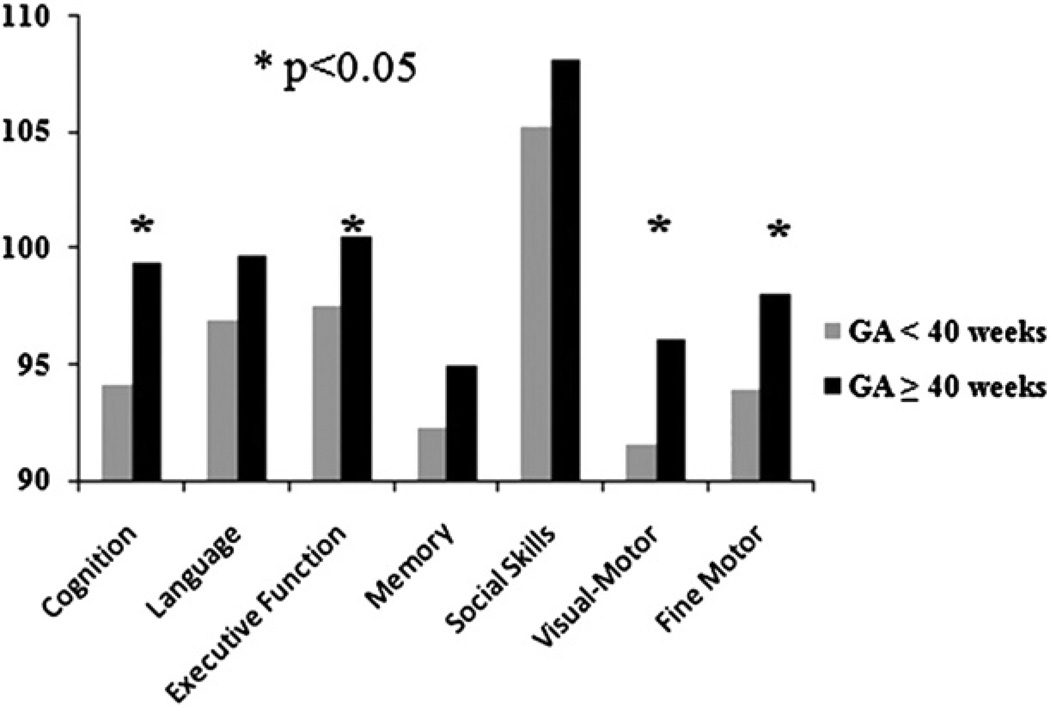
It is widely known that significant prematurity adversely affects ND outcomes. To assess the impact of late preterm/ near-term delivery on ND outcomes without the confounding effects of very young GA, we analyzed the subgroup of patients born at 36 weeks’ GA or more (n = 351). In this subgroup, older GA predicted better performance for cognition, visual-motor, and fine-motor skills. Adjusting for patient and operative management confounders, older GA still predicted better performance for fine-motor skills (P = .018) (Table 3). When compared by week of GA, outcomes for cognition, language skills, executive function, social skills, visual-motor, and fine-motor skills were all better for infants born at 39 to 40 weeks compared with those born at younger GAs (all P < .05) (Table 4 and Figure 2).
TABLE 4.
Comparison of neurodevelopmental outcomes by gestational age
| Gestational age threshold (wk) |
Cognition |
Language |
Executive function |
Social skills |
Visual motor |
Fine motor |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | |
| <37 vs ≥37 | 3.626 | .1646 | 3.063 | .2261 | −0.626 | .7747 | 3.444 | .0546 | 5.882 | .0177 | 8.864 | .0004 |
| <38 vs ≥38 | 1.634 | .4286 | 0.272 | .8929 | 0.312 | .8523 | 1.496 | .2946 | 1.337 | .5002 | 3.449 | .0834 |
| <39 vs ≥39 | 5.238 | .0112 | 2.786 | .1716 | 2.915 | .0798 | 2.927 | .0409 | 4.503 | .0239 | 4.147 | .0388 |
| <40 vs ≥40 | 10.472 | .0005 | 5.973 | .0439 | 4.930 | .0382 | 0.673 | .7474 | 9.392 | .0012 | 7.838 | .0072 |
| <41 vs ≥41 | 12.562 | .0990 | 9.405 | .2052 | 13.604 | .0231 | 2.970 | .5736 | 10.035 | .1726 | 13.831 | .0615 |
No threshold identified for inattention, impulsivity, or memory.
FIGURE 2.
Infants born at 40 weeks’ gestational age (GA) or more had better scores for cognition, executive function, and visual and fine-motor skills than those born at less than 40 weeks.
DISCUSSION
In this cohort of survivors of cardiac surgery in infancy, older GA predicted better performance for multiple ND domains at 4 years of age. In the subgroup of infants born at late preterm/near term (GA ≥ 36 weeks), ND outcomes were significantly better for those born at a GA of 39 to 40 weeks or more than for those born at 36 to 38 weeks. These data are consistent with the increasing evidence of the adverse effects of late preterm/near-term delivery (before 39 weeks) in normal infants and those with CHD. Early delivery of a child with CHD is often indicated because of maternal or fetal health issues. In the absence of these concerns, these data suggest that elective delivery before 39 to 40 weeks of GA should be avoided.
In near-term infants, with or without CHD, elective induction of labor or cesarean delivery may be scheduled to accommodate patient and physician convenience, and there is a risk that it may be performed earlier than is appropriate. Timing of elective delivery is based on an arbitrarily selected time point at which the fetus is presumed to have reached physiologic maturity to allow successful transition to the extrauterine environment. The most recent definition of term gestation at 37 weeks has its roots from the 1970s when a cutoff point between preterm and term gestation was determined during a meeting of the Second European Congress of Perinatal Medicine.16 Over the past decade, there has been a growing recognition of the increased incidence of adverse outcomes in neonates without birth defects born at 37 to 38 weeks’ gestation compared with those born at more than 39 weeks.17 As a result, the recent guidelines of the American College of Obstetricians and Gynecologists recommend a GA of 39 weeks for elective deliveries and cesarean sections.7 In addition, the recognition that timing of delivery affects neonatal outcomes led the National Quality Forum and the Joint Commission to standardize perinatal care, instituting mandatory reporting of indications for delivery for all singleton pregnancies delivered before 39 weeks.16
Approximately 15% of neonates with CHD are born prematurely and/or with low birth weight.18,19 In many cases, maternal, fetal, or placental issues contribute to early delivery, including preterm premature rupture of membranes, maternal pre-eclampsia/eclampsia, oligohydramnios, intrauterine growth restriction, nonreassuring fetal status, hydrops, and placental abnormalities.20 However, elective delivery before 39 to 40 weeks’ GA in a cohort of CHD patients was approximately 20%. This recent study evaluated the impact of GA on mortality and intensive care unit morbidity in 971 infants with CHD. Compared with the group of neonates who were delivered at 39 to 40 weeks’ GA, neonates born at 37 to 38 weeks had increased mortality and morbidity rates and tended to require a longer duration of mechanical ventilation. For the entire cohort, the indications for delivery were maternal or fetal health issues in 27%, spontaneous labor in 44%, elective induction in 20%, and unknown in 9%. For infants born before 37 weeks’ GA, the indications for delivery were most commonly spontaneous labor or maternal/fetal health concerns. However, for infants born at 38 weeks’ GA, the timing of delivery was elective in 20%. A recent study evaluated the impact of late preterm delivery on mortality for infants with CHD using National Health Statistics–linked birth/ death files from 2000 to 2003. Mortality decreased with each increasing week of GA. There was a significant negative linear relationship between mortality and GA up to 40 weeks.9
Our data provide further evidence of the impact of late preterm/near-term delivery in infants with CHD by demonstrating improved 4-year ND outcomes in those born at 39 to 40 weeks or more. Even in children without birth defects, the risk for developmental delay and disabilities is 36% higher in those born late preterm compared with term. Significant brain maturation occurs from 34 weeks to term. By 34 weeks, the late preterm brain has only reached 65% of the volume of a term brain with a significant amount of growth and maturation occurring during the remaining 6 weeks of gestation.21 In children with CHD, late preterm/near-term birth may have an even greater impact on brain development and resultant ND outcome owing to the delayed brain maturation that is already observed in this population.22 Our group has shown that neonates with CHD born at more than 38 weeks of gestation have delayed brain development by approximately 4 weeks.22 Fetal brain magnetic resonance images performed during the third trimester in patients with complex CHD have provided evidence of impaired brain growth in utero compared with gestational controls.23 Delayed fetal brain maturation and development is consistent with postnatal data demonstrating smaller head circumferences and structurally immature brains in term gestation neonates with hypoplastic left heart syndrome and dextrotransposition of the great arteries compared with normal term neonates.22,24,25 Delayed brain maturation has been associated with an increased risk for periventricular leukomalacia in patients with complex CHD.25 Therefore, late preterm or early near-term delivery in conjunction with brain immaturity may increase risk for periventricular leukomalacia with a potential result of worse ND outcomes.
For ND outcomes to be improved for infants with CHD, it is essential to identify modifiable risk factors that can be targets for therapeutic interventions. Several previous studies have shown that patient factors, such as socioeconomic status and genetic anomalies, are more significant determinants of ND outcomes than are management strategies. Unfortunately, most identified risk factors cannot be modified. However, timing of elective delivery for late preterm/ near-term infants with CHD can be avoided. This study and previous reports demonstrate that delivery before 39 to 40 weeks’ GA is associated with increased mortality, early morbidity, and worse ND outcomes.
There are limitations to this study. Because this is a retrospective review of medical records, in most cases we were successful in obtaining the GA from the maternal records. However, in some cases GA was obtained from neonatal records inasmuch as this was the only available record. The indications for delivery could not be reliably determined from the medical records for most patients. A prenatal diagnosis was made in only 33% of the patients and thus the findings may not reflect current practice.
CONCLUSION
There is increasing evidence that delivery before 39 to 40 weeks of GA is associated with worse short- and intermediate-term outcomes, both for normal infants and for those with CHD. This study demonstrates that younger GA is associated with worse ND outcomes for infants with CHD. There are multiple reasons for early delivery of a fetus with CHD. Many of these, such as pre-eclampsia/ eclampsia, placental insufficiency, and fetal distress, cannot be currently modified. However, a significant number of near-term fetuses are delivered early because of concerns over spontaneous labor outside of the hospital or for convenience of scheduling. In the absence of maternal or fetal indications requiring early delivery, these data are consistent with the hypothesis that elective (or spontaneous) delivery of infants with CHD at 39 to 40 weeks of GA is associated with better ND outcomes. Further studies are necessary to confirm that delivery at a GA of 39 to 40 weeks, when appropriate, will indeed lead to better ND outcomes.
Acknowledgments
Supported by an American Heart Association National Grant-in-Aid (9950480N), grant HL071834 from the National Institutes of Health, grant T32HL-0791543 from the National Heart, Lung, and Blood Institute, and the Daniel M. Tabas Endowed Chair in Pediatric Cardiothoracic Surgery.
Abbreviations and Acronyms
- ADHD
attention deficit hyperactivity disorder
- CHD
congenital heart disease
- CPB
cardiopulmonary bypass
- DHCA
deep hypothermic circulatory arrest
- GA
gestational age
- ND
neurodevelopmental
- NEPSY
Neuro-PSYchology
- NP
nasopharyngeal
- PKBS
Preschool and Kindergarten Behavior Scales
- PLS-4 TLS
Preschool Language Scale-4 Total Language Score
- VMI
Visual-Motor Integration
- WPPSI-III
Wechsler Preschool and Primary Scale of Intelligence, third edition
- WRAVMA
Wide Range Assessment of Visualmotor Abilities (pegboard);
APPENDIX 1
The Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III), is a standardized test of intelligence for children from 3.5 to 7 years of age.11 It is commonly used in both clinical settings and in research. It takes approximately 45 minutes to administer and yields 3 summary scores and 12 subtest scores. Scores include Full-Scale Intelligence Quotient, Verbal Intelligence Quotient, and Performance Intelligence Quotient with means of 100 and standard deviations of 15 points. It covers a wide range of cognitive tasks. There is a large body of data that explains the meaning of test findings. The WPPSI-R has proven to have moderate to strong reliability (coefficients for Verbal, Performance, Processing Speed, Full, and General Language were .92, .87, .93, .92, and .90 respectively) and validity (correlation with other cognitive tests in the positive and significant range [.74 to .90]) in a variety of studies.
The Preschool Language Test-4 (PLS-4) is a general test of early language skills.12 It provides a measure of language comprehension and expressive communication. Standard scores are derived on the basis of age and performance. The Total Language Score (mean of 100 with a standard deviation of 15) is derived on the basis of performance on the receptive and expressive sections.
The ADHD Rating Scale-IV, Preschool Version, is an 18-item questionnaire that requires parents to rate the frequency of occurrence of ADHD symptoms, as defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).9 The respondent rates each item on a Likert scale of 0 (not at all) to 3 (very often). This scale was developed specifically for children 3 to 6 years of age. Normative data were collected from a stratified sample of 907 children. Mean scores are provided for inattention and hyperactivity/impulsivity. Percentile rankings were used to identify children rated by their parents as scoring significantly high in inattention or hyperactivity.
The Preschool and Kindergarten Behavior Scales (PKBS) provide 3 scores for social skills, including social cooperation, social interaction, and social independence, and 5 scores for problem behaviors, including self-centered/explosive, attention problems/overactive, antisocial/aggressive and social withdrawal, anxiety/somatic, and total.10 Normative data were obtained from 2855 preschool-aged children (3–6 years of age). Reliability was reported to range from 0.81 to 0.97 in internal consistency and in the moderate to high range (0.58–0.87) in test–retest reliability. Predictive validity studies suggested that PKBS scores were able to predict need for special education services. Validity studies comparing the PKBS with other tests of social skills indicated strong correlations.
The Developmental Test of Visual Motor Integration (VMI) is a copying task that will be used to assess the child’s fine-motor and visual-motor coordination skills.14 It takes 10 minutes to complete and yields standard scores with a mean of 100 and a standard deviation of 15. It is a widely recognized test, and the new edition was published in 1989. Handedness will also be noted on the VMI. Interrater reliability had a median of 0.93. Generally, researchers found the VMI to be a valuable predictor when used in combination with other measures. Positive correlation with other tests of visual skills and motor skills were documented in the manual ranging from 0.72 to 0.76.
The NEPSY (Neuro-PSYchology) is a developmental neuropsychologic assessment tool that was published in 1998.13 The NEPSY Statue subtest assesses inhibition and motor persistence. The NEPSY subtests yield scale scores with a mean of 10 and standard deviation of 3. Reliability ranges from 0.50 to 0.81. Validity studies indicate that there is a low correlation between the attention/executive function subtests and tests of general intelligence. The use of the NEPSY with clinical populations of children diagnosed with ADHD indicates that identified children score significantly poorer on the tests of attention.
The Wide Range Assessment of Visual Motor Abilities (WRAVMA) pegboard is a manipulative dexterity test.15 The child inserts as many pegs as possible within 90 seconds using a nearly square pegboard. The pegboard is “waffled” to add to its fine-motor demands as well as to increase its aesthetic appeal. First the test is completed with the dominant hand and then the nondominant hand. This test was chosen from the collection of pegboard tasks (such as the Purdue Pegboard and the Grooved Pegboard) because it is the only one that was designed and standardized for a 4-year-old population. The scores that are provided are standard scores and percentiles that were published in 1995. There is strong reliability and validity for the proposed age group.
Discussion
Dr Frank A. Pigula (Boston, Mass). Dr Goff and her colleagues have looked at the 4-year neurodevelopmental (ND) outcomes of children who underwent surgery in infancy, and their primary variable of interest was their gestational age (GA) at birth. I have a few comments and then 2 questions.
Your manuscript suggests that the children with congenital heart disease (CHD) born between 39 and 40 weeks’ gestation are somehow privileged with respect to neurologic development. I found it notable in your manuscript that despite controlling for patient and operative characteristics, this was a general effect affecting not only the most severe forms of CHD but also those that we would deem less severe.
I think this study is valuable in that it extends the time horizon to 4 years regarding ND outcomes and it also further informs some of the findings that have been presented by Dr John Costello and some of my colleagues at Boston Children’s Hospital, which you have referred to in your presentation. They identified that patients born at or before 38 weeks experience significantly higher mortality risk with a greater likelihood of serious morbidity. Thus the effect between that study and your study is a magnification of this effect. The child that is lucky enough to survive surgery is at greater risk for further ND problems in the future.
I think this is all the more important when one realizes, as you had alluded to, that some of these deliveries, what you would call near-term deliveries, are elective and therefore discretionary. In Dr Costello’s review of that, it was up to 20% to 25% of the deliveries. That is one area in which some discretion and judgment can be used.
I have 3 questions for you.
While the discussion focused on the GA and its relationship to outcomes, did you examine the effect of the corrected GA, in other words, the period of time after birth but before surgery? For instance, if a child was born at 38 weeks but operated on at 40 weeks, did the child acquire any benefit?
Dr Goff. We did not look at corrected GA at surgery, but we did adjust for GA at birth with the multivariate analysis, which adjusted for both patient and operative confounders.
Dr Pigula. Is it correct to say that, by your analysis, it would not be a reasonable approach to delay surgery to allow further development of the baby?
Dr Goff. Correct.
Dr Pigula. Can you speculate as to why the near-term brain seems to be at an ND disadvantage as opposed to the brain at a term delivery at 40 weeks?
Dr Goff. Dr Hannah Kinney, from Children’s Hospital Boston, published an article in Seminars in Perinatology discussing how much brain growth occurs in utero during the third trimester. At 34 weeks, the fetal brain has only reached 65% of the brain volume and maturation of a term brain at 39 to 40 weeks. There is an incredible amount of brain maturation that occurs from 34 to 39 weeks gestation. So a baby born late preterm/near term may be more vulnerable to white matter injury.
Dr Pigula. Presumably the environment plays a large role in what happens to these babies.
Dr Goff. The in utero environment?
Dr Pigula. Well, yes, in between the in utero and postnatal environment.
Dr Goff. Yes, both the in utero and postnatal environment play a role in the risk for white matter injury, which may lead to later ND issues.
Dr Pigula. Given the consonance of your findings with Costello’s, how do you suggest we proceed? It seems to me that this information has to make its way to the decision makers, and those would be the obstetricians that are involved with the prenatal care for these babies. Do you have any suggestions for us about how to approach this kind of care delivery aspect?
Dr Goff. These data provide further evidence of the importance of timing of elective delivery at 39 weeks. Even in infants without CHD, The American College of Obstetricians and Gynecologists has made a concerted effort to push delivery toward 39 weeks. In conjunction with the data from both Costello and Cnota, we have increasing evidence in our population of children with CHD that timing of delivery is incredibly important and impacts outcome. For those who have institutions where there is a multidisciplinary approach, an organized plan for timing delivery can be determined for a woman, for example, carrying a baby with hypoplastic left heart syndrome. If there are no maternal or fetal issues, a multidisciplinary meeting (fetal cardiologists, maternal fetal medicine specialists, and cardiac surgeons) could take place after 34 weeks to organize timing of delivery at 39 to 40 weeks. Of course, this plan for elective delivery is dependent on continued assessment for new maternal or fetal health issues.
Dr Pigula. One observation is that sometimes these families come from a distance away and these deliveries are scheduled on a fairly elective basis. However, schedules and travel are built around them. I think that is one of the logistical items that we are going to have to learn to be flexible with, not only us but also the obstetricians.
Dr Shunji Sano (Okayama, Japan). Is there any difference in ND outcome between the infant with hypoplastic left heart syndrome and the other groups? Infants with hypoplastic left heart syndrome have much less blood flow to the brain during fetal life. Is there any difference?
Dr Goff. When we looked at the whole cohort, we adjusted for the type of CHD in the analyses. We specifically removed complexity of disease from the picture.
Dr Gaynor. I think I can make a comment. One of the things to obviously look at is: Is there an interaction of GA with cardiac diagnosis or other factors? Given the sample size, we did not look for interaction terms. We did adjust for diagnosis. You could say that, maybe the more severe defects will have more in utero pertubations, there is going to be an even magnified effect of younger GA. There may be an interaction between certain types of CHD or other factors with GA. We have not looked at that in this sample, but I think it is an area for future research.
Dr Sano. Also, you just analyzed after 36 weeks’ gestation. Was there any patient with less than 36 weeks’ gestation?
Dr Goff. In the total cohort, there were 27 patients out of the 378 patients.
Dr Sano. Many papers said that infants born at less than 32 or 34 weeks of GA are likely to have a brain hemorrhage after bypass or some other complication. I wonder whether any other problem, including ND outcome or this abnormality, is prevalent at even less than 34 to 36 weeks of gestation.
Dr Goff. I think we know that premature birth is certainly a risk factor for ND problems. We also know that a significant amount of brain growth occurs during the third trimester. This is important because Dr Dan Licht from our institution published data showing that the brain maturation of a baby with CHD born at 38 to 39 weeks is delayed around 4 weeks. If a baby with CHD is born at 36 weeks, then that baby’s brain is similar to a baby born at 32 weeks. As a result, this baby has an increased risk for white matter injury which has been associated with later ND problems.
Footnotes
Disclosures: Authors have nothing to disclose with regard to commercial support.
Read at the 91st Annual Meeting of The American Association for Thoracic Surgery, Philadelphia, Pennsylvania, May 7-11, 2011.
References
- 1.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–e767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 2.Gaynor JW, Nord AS, Wernovsky G, Bernbaum J, Solot CB, Burnham N, et al. Apolipoprotein E genotype modifies the risk of behavior problems after infant cardiac surgery. Pediatrics. 2009;124:241–250. doi: 10.1542/peds.2008-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133:1344–1353. doi: 10.1016/j.jtcvs.2006.10.087. 53 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118:1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 5.Tomashek KM, Shapiro-Mendoza CK, Davidoff MJ, Petrini JR. Differences in mortality between late-preterm and term singleton infants in the United States, 1995–2002. J Pediatr. 2007;151:450–456. doi: 10.1016/j.jpeds.2007.05.002. 6 e1. [DOI] [PubMed] [Google Scholar]
- 6.Morse SB, Zheng H, Tang Y, Roth J. Early school-age outcomes of late preterm infants. Pediatrics. 2009;123:e622–e629. doi: 10.1542/peds.2008-1405. [DOI] [PubMed] [Google Scholar]
- 7.ACOG. Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114(2 Pt 1):386–397. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett JM, Wypij D, Bellinger DC, Rappaport LA, Heffner LJ, Jonas RA, et al. Effect of prenatal diagnosis on outcomes in D-transposition of the great arteries. Pediatrics. 2004;113:e335–e340. doi: 10.1542/peds.113.4.e335. [DOI] [PubMed] [Google Scholar]
- 9.Cnota JF, Gupta R, Michelfelder EC, Ittenbach RF. Gestational age below 40 weeks is associated with increase death rates in infants with congenital heart disease. Circulation. 2009;120:S585. [Google Scholar]
- 10.Costello JM, Polito A, Brown DW, McElrath TF, Graham DA, Thiagarajan RR, et al. Birth before 39 weeks’ gestation is associated with worse outcomes in neonates with heart disease. Pediatrics. 2010;126:277–284. doi: 10.1542/peds.2009-3640. [DOI] [PubMed] [Google Scholar]
- 11.Wechsler D. Preschool and primary scale of intelligence. 3rd ed. San Antonio (TX): Harcourt Assessment Co; 2002. [Google Scholar]
- 12.Zimmerman I, Steiner VP, Evatt R. Preschool language scale-IV. 4th ed. San Antonio (TX): Harcourt Assessment; 2002. [Google Scholar]
- 13.Korkman M, Kirk U, Kemp S. NEPSY. 2nd ed. San Antonio (TX): Psychcorp/Harcourt Assessment; 2007. [Google Scholar]
- 14.Beery K. The VMI: developmental test of visual motor integration—administration, scoring and testing manual. Cleveland (OH): Modern Circulation Press; 1989. [Google Scholar]
- 15.Adams D, Sheslow D. Wide range assessment of visual motor abilities. Wilmington (DE): Wide Range; 1995. [Google Scholar]
- 16.Fleischman AR, Oinuma M, Clark SL. Rethinking the definition of “term pregnancy.”. Obstet Gynecol. 2010;116:136–139. doi: 10.1097/AOG.0b013e3181e24f28. [DOI] [PubMed] [Google Scholar]
- 17.Engle WA, Kominiarek MA. Late preterm infants, early term infants, and timing of elective deliveries. Clin Perinatol. 2008;35:325–341. doi: 10.1016/j.clp.2008.03.003. vi. [DOI] [PubMed] [Google Scholar]
- 18.Ades A, Johnson BA, Berger S. Management of low birth weight infants with congenital heart disease. Clin Perinatol. 2005;32:999–1015. doi: 10.1016/j.clp.2005.09.001. x–xi. [DOI] [PubMed] [Google Scholar]
- 19.Williams RV, Ravishankar C, Zak V, Evans F, Atz AM, Border WL, et al. Birth weight and prematurity in infants with single ventricle physiology: pediatric Heart Network Infant Single Ventricle Trial screened population. Congenit Heart Dis. 2010;5:96–103. doi: 10.1111/j.1747-0803.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loftin RW, Habli M, Snyder CC, Cormier CM, Lewis DF, Defranco EA. Late preterm birth. Rev Obstet Gynecol. 2010 Winter;3:10–19. [PMC free article] [PubMed] [Google Scholar]
- 21.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006;30:81–88. doi: 10.1053/j.semperi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. discussion 36–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 25.Andropoulos DB, Hunter JV, Nelson DP, Stayer SA, Stark AR, McKenzie ED, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. Epub 2009 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]