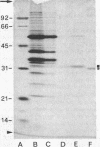
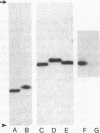
Abstract
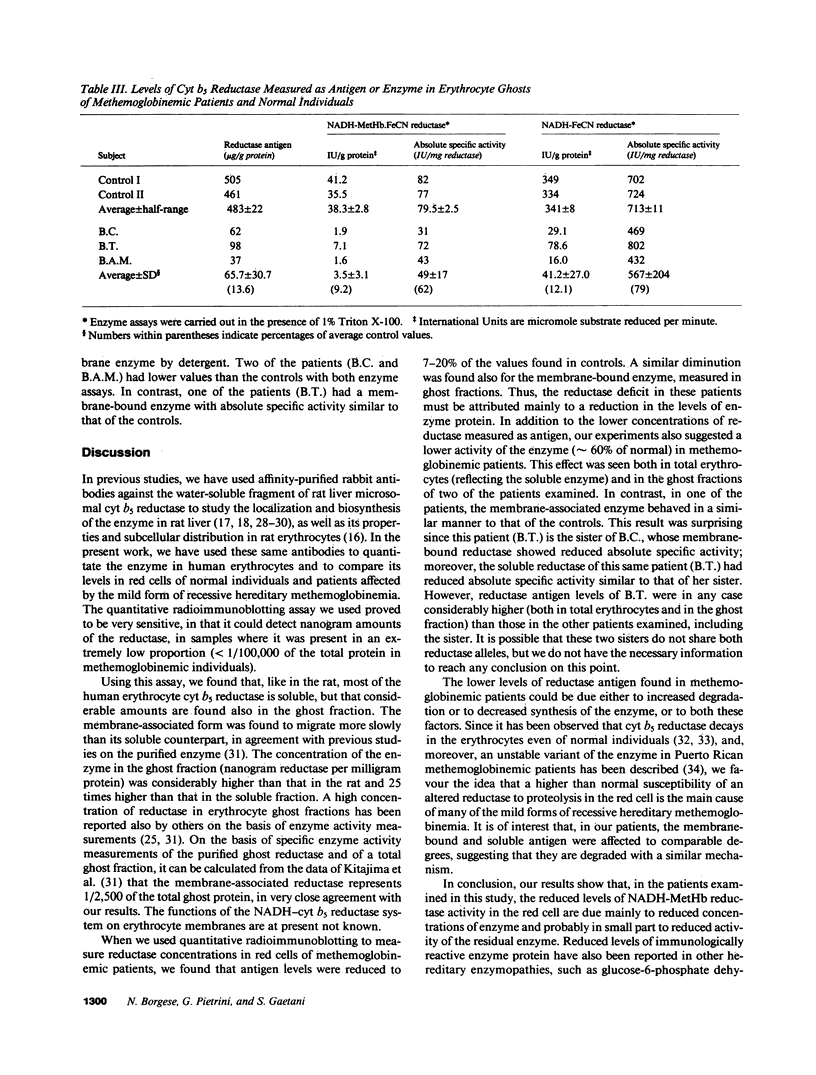
The activity of NADH-cytochrome b5 reductase (NADH-methemoglobin reductase) is generally reduced in red cells of patients with recessive hereditary methemoglobinemia. To determine whether this lower activity is due to reduced concentration of an enzyme with normal catalytic properties or to reduced activity of an enzyme present at normal concentration, we measured erythrocyte reductase concentrations with a quantitative radioimmunoblotting method, using affinity-purified polyclonal antibodies against rat liver microsomal reductase as probe. In five patients with the "mild" form of recessive hereditary methemoglobinemia, in which the activity of erythrocyte reductase was 4-13% of controls, concentrations of the enzyme, measured as antigen, were also reduced to 7-20% of the control values. The concentration of membrane-bound reductase antigen, measured in the ghost fraction, was similarly reduced. Thus, in these patients, the reductase deficit is caused mainly by a reduction in NADH-cytochrome b5 reductase concentration, although altered catalytic properties of the enzyme may also contribute to the reduced enzyme activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E. Selectivity of proteases as a basis for tissue distribution of enzymes in hereditary deficiencies. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3767–3768. doi: 10.1073/pnas.80.12.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Borgese N., Gaetani S. Site of synthesis of rat liver NADH--cytochrome b5 reductase, an integral membrane protein. FEBS Lett. 1980 Apr 7;112(2):216–220. doi: 10.1016/0014-5793(80)80183-9. [DOI] [PubMed] [Google Scholar]
- Borgese N., Macconi D., Parola L., Pietrini G. Rat erythrocyte NADH-cytochrome b5 reductase. Quantitation and comparison between the membrane-bound and soluble forms using an antibody against the rat liver enzyme. J Biol Chem. 1982 Nov 25;257(22):13854–13861. [PubMed] [Google Scholar]
- Borgese N., Meldolesi J. Localization and biosynthesis of NADH-cytochrome b5 reductase, an integral membrane protein, in rat liver cells. I. Distribution of the enzyme activity in microsomes, mitochondria, and golgi complex. J Cell Biol. 1980 Jun;85(3):501–515. doi: 10.1083/jcb.85.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N., Pietrini G. Distribution of the integral membrane protein NADH-cytochrome b5 reductase in rat liver cells, studied with a quantitative radioimmunoblotting assay. Biochem J. 1986 Oct 15;239(2):393–403. doi: 10.1042/bj2390393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N., Pietrini G., Meldolesi J. Localization and biosynthesis of NADH-cytochrome b5 reductase, an iontegral membrane protein, in rat liver cells. III. Evidence for the independent insertion and turnover the enzyme in various subcellular compartments. J Cell Biol. 1980 Jul;86(1):38–45. doi: 10.1083/jcb.86.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choury D., Leroux A., Kaplan J. C. Membrane-bound cytochrome b5 reductase (methemoglobin reductase) in human erythrocytes. Study in normal and methemoglobinemic subjects. J Clin Invest. 1981 Jan;67(1):149–155. doi: 10.1172/JCI110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddona P. E., Frohman M. A., Kelley W. N. Radioimmunochemical quantitation of human adenosine deaminase. J Clin Invest. 1979 Sep;64(3):798–803. doi: 10.1172/JCI109526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H. The reduction of methaemoglobin in red blood cells and studies on the cause of idiopathic methaemoglobinaemia. Biochem J. 1948;42(1):13–23. doi: 10.1042/bj0420013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto-Tamura R., Takesue Y., Takesue S. Immunological similarity between NADH-cytochrome b5 reductase of erythrocytes and liver microsomes. Biochim Biophys Acta. 1976 Feb 16;423(2):293–302. doi: 10.1016/0005-2728(76)90186-9. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hegesh E., Calmanovici N., Avron M. New method for determining ferrihemoglobin reductase (NADH-methemoglobin reductase) in erythrocytes. J Lab Clin Med. 1968 Aug;72(2):339–344. [PubMed] [Google Scholar]
- Hegesh E., Hegesh J., Kaftory A. Congenital methemoglobinemia with a deficiency of cytochrome b5. N Engl J Med. 1986 Mar 20;314(12):757–761. doi: 10.1056/NEJM198603203141206. [DOI] [PubMed] [Google Scholar]
- Hultquist D. E., Passon P. G. Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nat New Biol. 1971 Feb 24;229(8):252–254. doi: 10.1038/newbio229252a0. [DOI] [PubMed] [Google Scholar]
- Kitajima S., Yasukochi Y., Minakami S. Purification and properties of human erythrocyte membrane NADH-cytochrome b5 reductase. Arch Biochem Biophys. 1981 Aug;210(1):330–339. doi: 10.1016/0003-9861(81)90196-x. [DOI] [PubMed] [Google Scholar]
- Kuma F., Inomata H. Studies on methemoglobin reductase. II. The purification and molecular properties of reduced nicotinamide adenine dinucleotide-dependent methemoglobin reductase. J Biol Chem. 1972 Jan 25;247(2):556–560. [PubMed] [Google Scholar]
- Kuma F., Ishizawa S., Hirayama K., Nakajima H. Studies on methemoglobin reductase. I. Comparative studies of diaphorases from normal and methemoglobinemic erythrocytes. J Biol Chem. 1972 Jan 25;247(2):550–555. [PubMed] [Google Scholar]
- Kuma F., Prough R. A., Masters B. S. Studies on methemoglobin reductase. Immunochemical similarity of soluble methemoglobin reductase and cytochrome b5 of human erythrocytes with NADH-cytochrome b5 reductase and cytochrome b5 of rat liver microsomes. Arch Biochem Biophys. 1976 Feb;172(2):600–607. doi: 10.1016/0003-9861(76)90113-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leroux A., Junien C., Kaplan J., Bamberger J. Generalised deficiency of cytochrome b5 reductase in congenital methaemoglobinaemia with mental retardation. Nature. 1975 Dec 18;258(5536):619–620. doi: 10.1038/258619a0. [DOI] [PubMed] [Google Scholar]
- Lostanlen D., Lenoir G., Kaplan J. C. NADH cytochrome b5 reductase activity in lymphoid cell lines. Expression of the defect in epstein Barr virus transformed lymphoblastoid cell lines from patients with recessive congenital methemoglobinemia. J Clin Invest. 1981 Jul;68(1):279–285. doi: 10.1172/JCI110244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki T., Tamura M., Takeshita M., Yoneyama Y. Age-dependent decay of cytochrome b5 and cytochrome b5 reductase in human erythrocytes. Biochem J. 1981 Jan 15;194(1):327–330. doi: 10.1042/bj1940327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Corte G., Pietrini G., Borgese N. Localization and biosynthesis of NADH-cytochrome b5 reductase, an integral membrane protein, in rat liver cells. II. Evidence that a single enzyme accounts for the activity in its various subcellular locations. J Cell Biol. 1980 Jun;85(3):516–526. doi: 10.1083/jcb.85.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli A., Benatti U., Gaetani G. F., De Flora A. Biochemical mechanisms of glucose-6-phosphate dehydrogenase deficiency. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1979–1983. doi: 10.1073/pnas.75.4.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J., Korza G., Heinemann F. S., Hediger M. A., Strittmatter P. Complete amino acid sequence of steer liver microsomal NADH-cytochrome b5 reductase. J Biol Chem. 1985 Oct 5;260(22):11953–11961. [PubMed] [Google Scholar]
- Passon P. G., Hultquist D. E. Soluble cytochrome b 5 reductase from human erythrocytes. Biochim Biophys Acta. 1972 Jul 12;275(1):62–73. doi: 10.1016/0005-2728(72)90024-2. [DOI] [PubMed] [Google Scholar]
- SCOTT E. M. The relation of diaphorase of human erythrocytes to inheritance of methemoglobinemia. J Clin Invest. 1960 Jul;39:1176–1179. doi: 10.1172/JCI104131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. M., Paress P. S., Ross J. M., DiPillo F., Rizek R. Unstable variant of NADH methemoglobin reductase in Puerto Ricans with hereditary methemoglobinemia. J Clin Invest. 1972 Jun;51(6):1594–1601. doi: 10.1172/JCI106957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Tamura M., Yubisui T., Yoneyama Y. Exponential decay of cytochrome b5 and cytochrome b5 reductase during senescence of erythrocytes: relation to the increased methemoglobin content. J Biochem. 1983 Mar;93(3):931–934. doi: 10.1093/jb/93.3.931. [DOI] [PubMed] [Google Scholar]
- Tanishima K., Tanimoto K., Tomoda A., Mawatari K., Matsukawa S., Yoneyama Y., Ohkuwa H., Takazakura E. Hereditary methemoglobinemia due to cytochrome b5 reductase deficiency in blood cells without associated neurologic and mental disorders. Blood. 1985 Dec;66(6):1288–1291. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Baugher B. W., Landa L., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Purification and characterization of mutant forms of the enzyme. J Biol Chem. 1981 Oct 25;256(20):10306–10312. [PubMed] [Google Scholar]
- Yubisui T., Miyata T., Iwanaga S., Tamura M., Yoshida S., Takeshita M., Nakajima H. Amino acid sequence of NADH-cytochrome b5 reductase of human erythrocytes. J Biochem. 1984 Aug;96(2):579–582. doi: 10.1093/oxfordjournals.jbchem.a134871. [DOI] [PubMed] [Google Scholar]