Abstract
Objectives
Chronic kidney disease (CKD) is more prevalent among women and is associated with adverse cardiovascular events. Among women with symptoms and signs of ischemia enrolled in the Women’s Ischemia Syndrome Evaluation (WISE), a relatively high mortality rate was observed in those with no obstructive coronary artery disease. Coronary microvascular dysfunction or reduced coronary flow reserve (CFR) was a strong and independent predictor of adverse outcomes. The objective of this analysis was to determine if renal function was associated with coronary microvascular dysfunction in women with signs and symptoms of ischemia.
Methods
The WISE was a multicenter, prospective, cohort study of women undergoing coronary angiography for suspected ischemia. Among 198 women with additional measurements of CFR, we determined the estimated glomerular filtration rate (eGFR) with the CKD-EPI equation. We tested the association between eGFR and CFR with regression analysis.
Results
The median eGFR was 89 ml/min. The eGFR correlated with CFR (r = 0.22; P = 0.002). This association persisted even after covariate adjustment. Each 10-unit decrease in eGFR was associated with a 0.04-unit decrease in CFR (P = 0.04).There was a strong interaction between eGFR and age (P = 0.006): in those ≥60 years old, GFR was strongly correlated with CFR (r = 0.55; P<0.0001). No significant correlation was noted in those <60 years old.
Conclusions
Reduced renal function was significantly associated with lower CFR in women with symptoms and signs of ischemia. Coronary microvascular dysfunction warrants additional study as a mechanism contributing to increased risk of cardiovascular events in CKD.
Introduction
Chronic kidney disease (CKD) is associated with adverse cardiac events and excessive cardiovascular (CV) mortality[1]. Traditional risk factors for atherosclerosis do not account for the majority of risk of CV events associated with CKD [2]. Moreover, revascularization[3] or aggressive treatment of CV risk factors[4,5] have had little, or at best modest, success in preventing adverse cardiac events particularly among dialysis patients, suggesting that pathogenic mechanisms other than obstructive coronary artery disease (CAD) may contribute to excess CV mortality in those with CKD. We previously reported that in women with symptoms or signs of ischemia, even mild CKD is associated with coronary atherosclerosis[6]. In a cohort of these women, coronary microvascular dysfunction (CMD), as evidenced by a decreased coronary flow reserve (CFR) to adenosine, is a strong and independent predictor of adverse outcomes over 5.4 years of follow-up[7].
CKD is associated with inflammation, and serum levels of the acute phase reactant C reactive protein (CRP), interleukin-6 (IL-6), and serum amlyoid A (SAA) correlate with level of renal function[8] and all-cause as well as cardiovascular mortality[9]. However, the association between inflammation and microvascular disease is unclear. The pro-inflammatory cytokine IL-6 has been shown to be elevated in obese patients with decreased CFR[10]. However, in women with chest pain and no obstructive CAD, studies have not demonstrated an association between CFR and IL-6 or other inflammatory markers[11].
Interestingly, CKD is associated with microvascular dysfunction in other organ systems such as the cerebral and retinal circulation[12,13]. The role of CMD in CKD is also supported by animal models, which show that uremic mice have reduced myocardial capillary density[14]. In the setting of left ventricular hypertrophy, which commonly accompanies CKD, obstructive CAD can potentially impair vasodilatation. Pharmacological stress tests, which rely on adenosine, have decreased sensitivity in dialysis patients compared with the general population, suggesting the possibility of an impaired vasodilator response to adenosine in CKD [15].
We hypothesized that reduced renal function, determined by estimated glomerular filtration rate (eGFR), would be independently associated with a reduced CFR.
Materials and Methods
The Women’s Ischemia Syndrome Evaluation (WISE) (clinicaltrials.gov NCT00000554), a prospective cohort study initiated by the National Heart, Lung and Blood Institute to improve the understanding of pathological mechanisms and diagnostic evaluation of ischemic heart disease in women, enrolled women with symptoms and signs of ischemia referred for coronary angiography. Details of the WISE study design have been described previously[16]. Briefly, in addition to coronary angiography, key demographic and laboratory variables were collected at baseline including assessment of CAD risk factors, blood chemistries, lipid levels, inflammatory markers, and functional capacity. Serum creatinine was measured using standard technique in the clinical laboratory at each of the four sites. Creatinine clearance (ml/min) was estimated with use of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation after decreasing creatinine levels by 5%, as recommended[17] for calibration. Lipids were measured in fasting blood plasma at the WISE lipid core laboratory (Cedars-Sinai Medical Center, Los Angeles, CA). Qualitative and quantitative CAD measures were made at the angiographic core laboratory masked to other clinical data[18]. Women with at least one ≥50% diameter stenosis were classified as having significant CAD, those with maximum stenosis ≥20% but <50% as minimal CAD, and women with <20% stenosis in all coronary arteries as no CAD. A CAD severity score was calculated by a modified Gensini index termed the WISE CAD severity score. All patients provided written informed consent, and the protocol was approved in accordance with the ethical principles outlined in the 1975 Declaration of Helsinki by the institutional review boards at each center (University of Florida, Gainesville, FL; University of Pittsburgh, Pittsburgh, PA; Cedars-Sinai Medical Center, Los Angeles, CA; and Rhode Island Hospital, Providence, RI).
Coronary reactivity testing protocol
Coronary reactivity testing was performed in a stenosis free area of the left anterior descending coronary artery when possible, with the left circumflex artery as a secondary choice[19]. A Doppler-tipped guidewire (0.014-inch FloWire, JOMED/Cardiometrics, Mountain View, CA, now Volcano Corporation, San Diego, CA) was advanced through the diagnostic catheter, and when a stable velocity signal was obtained, baseline recordings were made. Intracoronary bolus injections of 18 mcg of adenosine (Adenocard, Fujisawa USA, Deerfield, IL), a predominantly non–endothelium-dependent microvascular dilator, was administered into the left main coronary artery. A dose of 18 mcg was chosen, since in the WISE cohort, we have previously found that CFR measurements were identical regardless of if we used low (18 mcg) or high (36 mcg) dose of adenosine[20]. At least 3 injections were done to assure a stable average peak blood flow velocity was obtained, with return to baseline velocity documented before each bolus. Pulsed-wave Doppler flow spectra were used to calculate time-averaged peak velocity (APV). Recordings were analyzed at the WISE CFR Core Lab (University of Florida) masked to all other data, and CFR was defined as the ratio of APV after adenosine to average baseline velocity just before adenosine. In prior WISE studies, this measure correlated closely (r = 0.87, P<0.001) with volumetric flow[18].
Statistical analysis
There were 198 patients with measurements available for CFR and eGFR. Clinical characteristics were stratified by median renal function (eGFR ≥89 or <89 ml/min/1.73 m2) and CFR (≥2.5 or <2.5). Data are presented as either mean ± standard deviation for continuous variables or n(%) for categorical variables. Skewed variables are reported as medians (IQR) and transformed to log base 2 to satisfy the assumptions of homogeneity, linearity, and Gaussian distribution of errors. General linear regression was applied to compute P-values for continuous variables, and logistic regression for categorical variables. All P-values for baseline variables, except age, are age-adjusted. Normality of log2CFR and eGFR were tested using the Kolmogorov-Smirnov test. Pearson’s correlation was used to check univariate correlation between log2CFR, eGFR, and demographic factors. Multivariable linear regression analysis was conducted to identify potential predictors of CFR including history of diabetes, hypertension, dyslipidemia, metabolic syndrome, body mass index (BMI), CAD severity score, double product (heart rate X systolic blood pressure), hemoglobin, ever smoker, CRP, IL-6, SAA, age, current hormone replacement therapy, and eGFR (in unit of 10). A backward selection was employed, and covariates with P-value >0.2 were sequentially removed from the model. History of diabetes, hypertension, and dyslipidemia were forced into the model. All statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC).
Results
Baseline characteristics
The mean age was 55±10 years, and most women were menopausal (74%) and had multiple risk factors for CAD including hypertension (56%), hyperlipidemia (51%), metabolic syndrome (45%), diabetes (22%), and smoking (20%). The baseline characteristics categorized by renal function (eGFR ≥89 or <89 ml/min/1.73 m2) and CFR (≥2.5 or <2.5) are summarized in Table 1.
Table 1. Baseline characteristics of the study population, categorized by glomerular filtration rate (eGFR) and coronary flow reserve (CFR).
| Baseline Characteristic | Total | CFR<2.5 eGFR<89 (n = 54) | CFR≥2.5 eGFR<89 (n = 48) | CFR<2.5 eGFR≥89 (n = 45) | CFR≥2.5 eGFR≥89 (n = 51) | P-Value* |
|---|---|---|---|---|---|---|
| CKD-EPI eGFR, Mean ± SD | 85±19 | 67±17 | 74±10 | 101±8 | 100±9 | <0.0001 |
| Demographics and Medical History | ||||||
| Age, years | 55±10 | 62±10 | 56±9 | 50±9 | 50±8 | <0.001 |
| Post-menopausal % | 74 | 89 | 77 | 64 | 65 | 0.86 |
| Non-white % | 18 | 13 | 15 | 16 | 27 | 0.14 |
| Current HRT use % | 46 | 36 | 54 | 47 | 47 | 0.15 |
| SBP, mmHg | 135±21 | 143±22 | 131±19 | 133±23 | 133±19 | 0.30 |
| DBP, mmHg | 77±11 | 77±10 | 74±12 | 77±8 | 78±12 | 0.79 |
| Pulse pressure, mm Hg | 59±18 | 66±20 | 56±17 | 56±20 | 55±14 | 0.17 |
| Pulse, beats/min | 73±12 | 72±11 | 70±13 | 79±11 | 72±11 | 0.81 |
| HTN % | 56 | 67 | 42 | 53 | 62 | 0.33 |
| Current anti-HTN Rx % | 61 | 70 | 50 | 62 | 59 | 0.97 |
| Dyslipidemia % | 51 | 62 | 48 | 53 | 42 | 0.97 |
| Diabetes % | 22 | 28 | 6 | 24 | 28 | 0.22 |
| Metabolic syndrome % | 45 | 57 | 39 | 36 | 46 | 0.17 |
| Family history of CAD % | 70 | 74 | 65 | 71 | 70 | 0.55 |
| Current smoker % | 20 | 13 | 19 | 24 | 26 | 0.94 |
| Ever smoker % | 57 | 63 | 50 | 53 | 62 | 0.66 |
| DASI, medians (Q1,Q3) | 14(7,25) | 10(5,16) | 24(11,32) | 13(7,24) | 12(7,25) | 0.38 |
| Laboratory Data | ||||||
| Total cholesterol, mg/dl | 188±45 | 194±48 | 183±42 | 184±39 | 189±49 | 0.94 |
| Triglycerides, mg/dl | 144±140 | 153±115 | 133±105 | 130±94 | 160±209 | 0.98 |
| HDL, mg/dl | 51±13 | 52±11 | 50±12 | 52±12 | 51±15 | 0.77 |
| LDL, mg/dl (calculated) | 111±39 | 119±45 | 106±34 | 109±35 | 111±42 | 0.57 |
| Hemoglobin, g/dl | 13.0±1.4 | 13.0±1.7 | 13.4±1.1 | 12.9±1.1 | 12.6±1.4 | 0.15 |
| Creatinine, mg/dl | 0.79±0.22 | 0.96±0.30 | 0.87±0.11 | 0.64±0.10 | 0.66±0.10 | <0.0001 |
| Inflammatory Markers | ||||||
| Hs-CRP, mg/dl, medians (Q1, Q3) | (0.15,0.88) | (0.20,0.80) | (011,0.63) | (0.20,0.98) | (0.16,0.95) | 0.19 |
| IL6, pg/ml, medians (Q1, Q3) | (1.71,5.26) | (1.69,5.75) | (1.87,4.84) | (1.82,4.65) | (1.65,4.51) | 0.94 |
| SAA, mg/dl, medians (Q1, Q3) | (0.30,0.94) | (0.33, 1.20) | (0.24,0.58) | (0.33,0.95) | (0.35,0.90) | 0.73 |
| Angiographic Data | ||||||
| CAD(≥ 50% stenosis)% | 18 | 36 | 17 | 11 | 14 | 0.08 |
| CAD severity score, medians (Q1, Q3) | 6(5,9) | 9(5,14) | 5(5,8) | 5(5,8) | 5(5,9) | 0.16 |
| CFR, medians (Q1, Q3) | 2.5(2.1,3) | 2.0(1.7,2.3) | 3.0(2.6,3.4) | 2.2(1.9,2.3) | 2.9(2.6,3.3) | <0.0001 |
CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration equation, eGFR = estimated glomerular filtration rate, HRT = hormone replacement therapy, SBP = systolic blood pressure, DBP = diastolic blood pressure, HTN = hypertension, CAD = coronary artery disease, DASI = Duke Activity Status Index, HDL = high density lipoprotein, LDL = low density lipoprotein, Hs-CRP = high-sensitivity C-reactive protein, Q1 = 25th percentile, Q3 = 75th percentile, IL-6 = interleukin 6, SAA = serum amyloid A. P-values represent the comparison between four GFR/CFR combination groups. P-values for all variables except age were adjusted for age.
Renal function and CFR
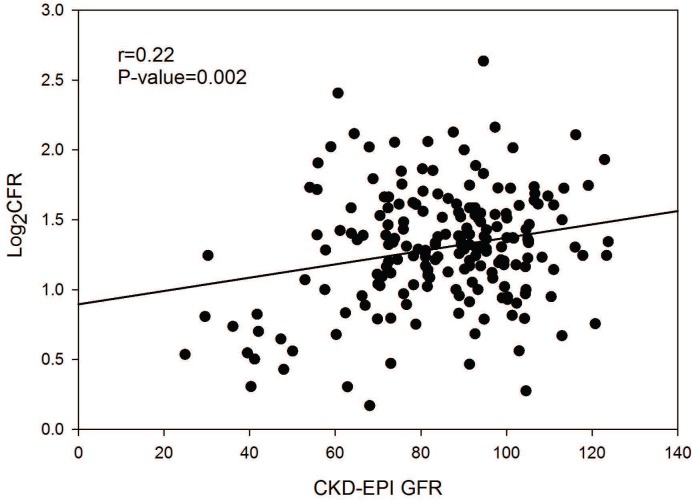
In univariate analysis, renal function, eGFR, was significantly correlated with CFR (r = 0.22, P = 0.002), as shown in Fig 1. The association persisted even when adjusted for age, diabetes, hypertension, dyslipidemia, double product, BMI, severity of obstructive CAD, and current hormone replacement therapy (P = 0.0003, model R2 = 0.18). No other variables were significant independent predictors of CFR. Results of the multivariable regression are summarized in Table 2.
Fig 1. Linear Regression of CFR and eGFR.
Coronary flow reserve (CFR) was log transformed. Estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Table 2. Independent predictors of coronary flow reserve (CFR).
| Model 1 (R2 = 0.18) | Model 2 (R2 = 0.22) | |||
|---|---|---|---|---|
| β (SE) | P-value (add in order) | β (SE) | P-value (add in order) | |
| Age | -0.05(0.04) | 0.0003 | 0.03(0.05) | 0.0003 |
| Diabetes | 0.02(0.09) | 0.52 | -0.004(0.09) | 0.52 |
| Hypertension | -0.08(0.07) | 0.31 | -0.08(0.07) | 0.29 |
| Dyslipidemia | -0.04(0.07) | 0.52 | -0.04(0.07) | 0.51 |
| HR×SBP | -0.04(0.02) | 0.041 | -0.03(0.02) | 0.037 |
| BMI | 0.008(0.005) | 0.15 | 0.006(0.005) | 0.14 |
| CAD score | -0.009(0.005) | 0.06 | -0.01(0.005) | 0.05 |
| Current HRT | 0.13(0.06) | 0.047 | 0.12(0.06) | 0.042 |
| eGFR | 0.04(0.02) | 0.040 | 0.01(0.02) | 0.036 |
| Interaction of eGFR and Age | - | - | 0.05(0.02) | 0.006 |
SE = standard error, HR = heart rate, SBP = systolic blood pressure, BMI = body mass index, CAD = coronary artery disease, HRT = hormone replacement therapy, eGFR = glomerular filtration rate ml/min/per 1.73 m2. Age and eGFR were divided by 10 and double product by 1000 for consistency.
Inflammatory markers, eGFR, and CFR
When stratified by eGFR and CFR, in women with renal dysfunction, CRP, IL-6, and SAA were higher in those with low CFR compared with those with normal CFR. However, analysis of variance adjusted for age showed no significant differences between groups (Table 1). In the multivariable regression model, inflammatory markers CRP, IL-6, and SAA did not contribute significantly to the model.
Age, renal function, and CFR
When age was included in the model, eGFR remained an independent predictor of CFR (r = 0.22, P = 0.0001) even though age and renal function showed a significant interaction (P = 0.006). When age was dichotomized as < or ≥60 years, 42% of women with eGFR<89 ml/min/1.73 m2 and 48% of those with eGFR ≥89 ml/min/1.73 m2 who were <60 years old had a low CFR (<2.5). However, in those ≥60 years old, 63% of those with eGFR <89 ml/min/1.73 m2 had a low CFR compared with only 36% of those with eGFR ≥89 ml/min/1.73 m2. eGFR and CFR showed a linear correlation among those ≥60 years old (r = 0.55, P<0.0001), while there was no significant correlation in those <60 years old (r = 0.02, P = 0.81) (Fig 2). The interaction of age and renal function persisted even when hormone replacement therapy was included as a covariate.
Fig 2. Scatter plot of eGFR and Log2CFR in women (A) <60 years of age (n = 135) and (B) ≥60 years of age (n = 63).
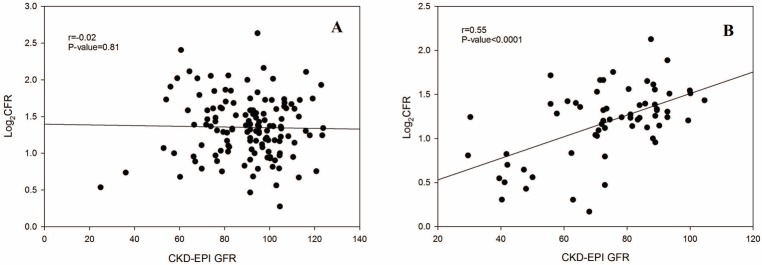
C oronary flow reserve (CFR) was log transformed. Estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Discussion
Studies examining the association of CFR with renal function have shown conflicting results. A study of 22 non-diabetic subjects found no significant differences in CFR between individuals with moderate to severe CKD and 10 healthy controls[21]. However, this was a relatively small sample of mostly men (66%), and even the apparently healthy controls had an average eGFR of 76±5 ml/min/1.73 m2, which is below normal. Others have found an association between CFR and renal function. Charytan et al. assessed CFR by positron emission tomography in 435 non-diabetic individuals. Although baseline CFR was significantly associated with eGFR, no significant association was found after adjusting for age and hypertension status, but on longitudinal follow up a decline in eGFR was a strong and independent predictor of a decrease in CFR[22]. However, Charytan et al. estimated CFR using intravenous adenosine with positron imaging, and coronary angiographic data were not available. Although overall the patients were mostly male (74%), there were significant sex differences in the subgroups, with 51% women in the severe CKD group but only 27% in mild CKD and 16% in healthy controls. Thus it is not clear how their results apply to women, particularly those without significant obstructive CAD.
Chade et al. assessed CFR using intracoronary adenosine in 605 patients without significant obstructive CAD[23]. Patients with eGFR ≥60 ml/min/1.73 m2 had higher CFR compared to those with eGFR <60 ml/min/1.73 m2. CFR was lower in women, the elderly, and those with hypertension. Multiple logistic regression analysis adjusted for age, sex, and hypertension showed that the association of renal function and CFR persisted. But when subjects were dichotomized into groups with eGFR ≥60 and <60 ml/min/1.73 m2, there was no discernible effect of milder degrees of renal failure on CFR, particularly in women. Bezante et al. suggested that an eGFR of <60 ml/min/1.73 m2 conferred a seven-fold higher risk of having an impaired CFR[24], but there were only 7 women with CKD.
Our study, the only one focusing solely on women, contained the largest number of women with coronary angiographic data, and had a prospectively defined protocol requiring that angiograms and CFR recordings be read by core labs masked to all other patient data. Determination of CMD was by direct coronary blood flow measurements in response to intracoronary adenosine to measure CFR. We found that even among women with only mild renal dysfunction, CFR is linearly associated with eGFR, with each 10-unit decrease in eGFR being associated with a 0.02-unit decrease in CFR (P = 0.004). We have previously shown in the WISE cohort that clinical and demographic variables explain only 16% of the variance in CFR and are not independently associated with CFR. eGFR on the other hand is significantly associated with CFR, even after controlling for multiple covariates including diabetes, hypertension, dyslipidemia and severity of obstructive coronary artery disease.
An important confounder is that CKD is more prevalent with aging[25]. Likewise CMD is more frequent in older individuals, particularly those ≥60 years old[25,26]. As expected, when age is included as a covariate in models where GFR is estimated using equations as opposed to directly measured, the association between GFR and cardiovascular events is greatly reduced[27]. Importantly, in our model eGFR was associated with CFR even when controlled for age, validating the strength of the association. There was a significant interaction between age and renal function—in those ≥60 years old there was a strong linear correlation between eGFR and CFR which was not present in those <60 years old. Further mechanistic studies are critical to determine whether coronary microvascular and renal dysfunction are due to aging, per se, or due to a common, as yet unidentified, pathological process.
A low CFR has been associated with inflammation. In discordant male twins, Vaccarino et al. found that reduced CFR on PET scan was associated with elevated levels of inflammatory markers[28]. A decreased CFR has also been demonstrated in inflammatory disorders such as rheumatoid arthritis[29] and lupus[30]. Interestingly, in our exclusively female cohort inflammatory markers such as CRP, IL-6, and SAA were not associated with renal function and did not add to the ability of eGFR to predict CFR. This suggests that in women with CMD, CKD and decreased CFR are not a consequence of inflammation. CKD is often associated with hypertension and left ventricular hypertrophy (LVH). Decreased CFR has been demonstrated in conditions accompanied by LVH such as aortic stenosis[31] and Fabry’s disease[32]. In the WISE cohort, characterized by mild renal dysfunction and very little ventricular hypertrophy, we have previously shown that LVH does not predict CFR.
Study limitations
Although our study showed a statistically significant relation between CFR and GFR, renal function only explains 4% of the variance in CFR overall (r = 0.21) and 30% in those over the age of 60 years (r = 0.55). This is at least in part because the WISE cohort included women who were referred for coronary angiography and thus excluded those with severe renal dysfunction. This limits generalization of results to other populations. We did not assess proteinuria at baseline or at follow up. Recent data suggest that presence of albuminuria would better stratify those at risk for vascular events, especially among those with milder degrees of CKD[33]. We did not have longitudinal assessment of renal function. This is particularly relevant since some studies have suggested that CFR correlates with changes in renal function[22]. Although our limited numbers precluded subgroup and outcome analysis, we have previously shown that CFR was the most important predictor of major adverse outcomes in a larger WISE cohort with 5.4 years follow-up[7]. This also held true among the women without obstructive CAD. The CFR significantly improved prediction of adverse outcomes over angiographic CAD severity and other risk conditions. Recent studies have suggested that CFR might predict those at high risk of CAD events in patients with CKD[34,35].
Conclusion
Our results demonstrate that renal function estimated by eGFR is significantly associated with CFR, and even mild decline in renal function is associated with CMD. Treatment modalities directed at traditional risk factors for atherosclerosis have had little impact in reducing mortality and morbidity in patients with CKD. CMD deserves additional study as a contributor to increased cardiovascular morbidity and mortality associated with CKD. Understanding the mechanistic basis of CMD in women with CKD has the potential to identify targets for novel therapeutics and to improve prognosis in a high-risk cohort for whom conventional therapies have been uniformly disappointing.
Acknowledgments
Disclaimer: This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Data Availability
The fully anonymized WISE I dataset is available to the public via the Biologic Specimen and Data Repository Information Coordinating Center web site at https://biolincc.nhlbi.nih.gov/studies/wise/?q=WISE (NHLBI project official Sean Coady, coadys@nih.gov).
Funding Statement
This work was supported by contracts from the National Heart, Lung and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, CA, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, CA, The Society for Women’s Health Research (SWHR), Washington, D.C., and the Linda Joy Pollin Women’s Heart Health Program, Los Angeles, CA. Dr. Pepine receives funding from the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064. No funders or sponsors had a role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 2. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, et al. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50: 217–224. [DOI] [PubMed] [Google Scholar]
- 3. Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation. 2002;106: 2207–2211. [DOI] [PubMed] [Google Scholar]
- 4. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353: 238–248. [DOI] [PubMed] [Google Scholar]
- 5. Holdaas H, Holme I, Schmieder RE, Jardine AG, Zannad F, Norby GE, et al. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22: 1335–1341. 10.1681/ASN.2010090987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reis SE, Olson MB, Fried L, Reeser V, Mankad S, Pepine CJ, et al. Mild renal insufficiency is associated with angiographic coronary artery disease in women. Circulation. 2002;105: 2826–2829. [DOI] [PubMed] [Google Scholar]
- 7. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55: 2825–2832. 10.1016/j.jacc.2010.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nerpin E, Helmersson-Karlqvist J, Riserus U, Sundstrom J, Larsson A, Jobs E, et al. Inflammation, oxidative stress, glomerular filtration rate, and albuminuria in elderly men: a cross-sectional study. BMC Res Notes. 2012;5: 537 10.1186/1756-0500-5-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68: 766–772. [DOI] [PubMed] [Google Scholar]
- 10. Tona F, Serra R, Di Ascenzo L, Osto E, Scarda A, Fabris R, et al. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr Metab Cardiovasc Dis. 2014;24: 447–453. 10.1016/j.numecd.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 11. Marroquin OC, Kip KE, Mulukutla SR, Ridker PM, Pepine CJ, Tjandrawan T, et al. Inflammation, endothelial cell activation, and coronary microvascular dysfunction in women with chest pain and no obstructive coronary artery disease. Am Heart J. 2005;150: 109–115. [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi M, Hirawa N, Yatsu K, Kobayashi Y, Yamamoto Y, Saka S, et al. Relationship between silent brain infarction and chronic kidney disease. Nephrol Dial Transplant. 2009;24: 201–207. 10.1093/ndt/gfn419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ooi QL, Tow FK, Deva R, Alias MA, Kawasaki R, Wong TY, et al. The microvasculature in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6: 1872–1878. 10.2215/CJN.10291110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amann K, Wiest G, Zimmer G, Gretz N, Ritz E, Mall G. Reduced capillary density in the myocardium of uremic rats–a stereological study. Kidney Int. 1992;42: 1079–1085. [DOI] [PubMed] [Google Scholar]
- 15. Marwick TH, Steinmuller DR, Underwood DA, Hobbs RE, Go RT, Swift C, et al. Ineffectiveness of dipyridamole SPECT thallium imaging as a screening technique for coronary artery disease in patients with end-stage renal failure. Transplantation. 1990;49: 100–103. [DOI] [PubMed] [Google Scholar]
- 16. Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, et al. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33: 1453–1461. [DOI] [PubMed] [Google Scholar]
- 17. Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011;162: 548–554. 10.1016/j.ahj.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 18. Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33: 1469–1475. [DOI] [PubMed] [Google Scholar]
- 19. Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5: 646–653. 10.1016/j.jcin.2012.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petersen JW, Mehta PK, Kenkre TS, Anderson RD, Johnson BD, Shufelt C, et al. Comparison of low and high dose intracoronary adenosine and acetylcholine in women undergoing coronary reactivity testing: results from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE). Int J Cardiol. 2014;172: e114–115. 10.1016/j.ijcard.2013.12.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koivuviita N, Tertti R, Jarvisalo M, Pietila M, Hannukainen J, Sundell J, et al. Increased basal myocardial perfusion in patients with chronic kidney disease without symptomatic coronary artery disease. Nephrol Dial Transplant. 2009;24: 2773–2779. 10.1093/ndt/gfp175 [DOI] [PubMed] [Google Scholar]
- 22. Charytan DM, Shelbert HR, Di Carli MF. Coronary microvascular function in early chronic kidney disease. Circ Cardiovasc Imaging. 2010;3: 663–671. 10.1161/CIRCIMAGING.110.957761 [DOI] [PubMed] [Google Scholar]
- 23. Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int. 2006;69: 266–271. [DOI] [PubMed] [Google Scholar]
- 24. Bezante GP, Viazzi F, Leoncini G, Ratto E, Conti N, Balbi M, et al. Coronary flow reserve is impaired in hypertensive patients with subclinical renal damage. Am J Hypertens. 2009;22: 191–196. 10.1038/ajh.2008.351 [DOI] [PubMed] [Google Scholar]
- 25. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33: 278–285. [DOI] [PubMed] [Google Scholar]
- 26. Chareonthaitawee P, Kaufmann PA, Rimoldi O, Camici PG. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50: 151–161. [DOI] [PubMed] [Google Scholar]
- 27. van der Velde M, Bakker SJ, de Jong PE, Gansevoort RT. Influence of age and measure of eGFR on the association between renal function and cardiovascular events. Clin J Am Soc Nephrol. 2010;5: 2053–2059. 10.2215/CJN.08851209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaccarino V, Khan D, Votaw J, Faber T, Veledar E, Jones DP, et al. Inflammation is related to coronary flow reserve detected by positron emission tomography in asymptomatic male twins. J Am Coll Cardiol. 2011;57: 1271–1279. 10.1016/j.jacc.2010.09.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turiel M, Atzeni F, Tomasoni L, de Portu S, Delfino L, Bodini BD, et al. Non-invasive assessment of coronary flow reserve and ADMA levels: a case-control study of early rheumatoid arthritis patients. Rheumatology (Oxford). 2009;48: 834–839. 10.1093/rheumatology/kep082 [DOI] [PubMed] [Google Scholar]
- 30. Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30: 1837–1843. 10.1093/eurheartj/ehp205 [DOI] [PubMed] [Google Scholar]
- 31. Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL. Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med. 1982;307: 1362–1366. [DOI] [PubMed] [Google Scholar]
- 32. Kalliokoski RJ, Kalliokoski KK, Sundell J, Engblom E, Penttinen M, Kantola I, et al. Impaired myocardial perfusion reserve but preserved peripheral endothelial function in patients with Fabry disease. J Inherit Metab Dis. 2005;28: 563–573. [DOI] [PubMed] [Google Scholar]
- 33. Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303: 423–429. 10.1001/jama.2010.39 [DOI] [PubMed] [Google Scholar]
- 34. Nakanishi K, Fukuda S, Shimada K, Miyazaki C, Otsuka K, Kawarabayashi T, et al. Prognostic value of coronary flow reserve on long-term cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol. 2013;112: 928–932. 10.1016/j.amjcard.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 35. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Dorbala S, et al. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging. 2012;5: 1025–1034. 10.1016/j.jcmg.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The fully anonymized WISE I dataset is available to the public via the Biologic Specimen and Data Repository Information Coordinating Center web site at https://biolincc.nhlbi.nih.gov/studies/wise/?q=WISE (NHLBI project official Sean Coady, coadys@nih.gov).