Abstract
Herpesviruses have been implicated as etiologic factors in the pathogenesis of human arteriosclerosis. We have examined the pathobiological effects of human herpes simplex virus (HSV-1) infection in influencing lipid accumulation and metabolism in human and bovine arterial smooth muscle cells (SMC). Significantly greater amounts of saturated cholesteryl esters (CE) and triacylglycerols (TG) accumulate in HSV-1-infected human and bovine arterial SMC than uninfected cells. This CE accumulation results, in part, from decreased CE hydrolysis. Furthermore, arachidonate-stimulated, HSV-1-infected arterial SMC have a reduced capacity to produce prostacyclin (an agonist of intracellular CE hydrolytic activity) than uninfected, stimulated SMC. It appears that HSV-1 may induce lipid accumulation in arterial SMC similar, in part, to the lipid accumulation observed in vivo during human atherogenesis. Thus, herpesviruses may contribute to lipid accumulation, which is a characteristic feature of atherosclerosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow N. R., Rose F. B., Whalen R. A. Organ culture of human aorta: prolonged survival with support of viral replication. J Infect Dis. 1975 May;131(5):575–578. doi: 10.1093/infdis/131.5.575. [DOI] [PubMed] [Google Scholar]
- Castelli W. P. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984 Feb 27;76(2A):4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
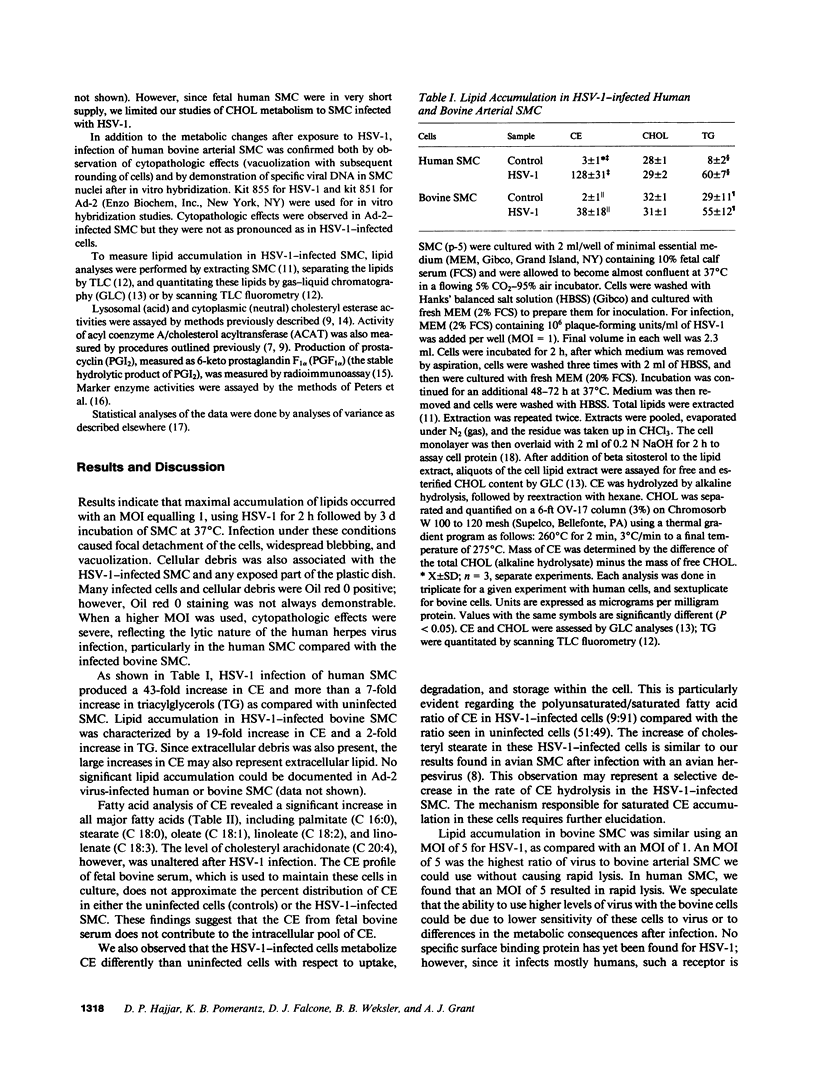
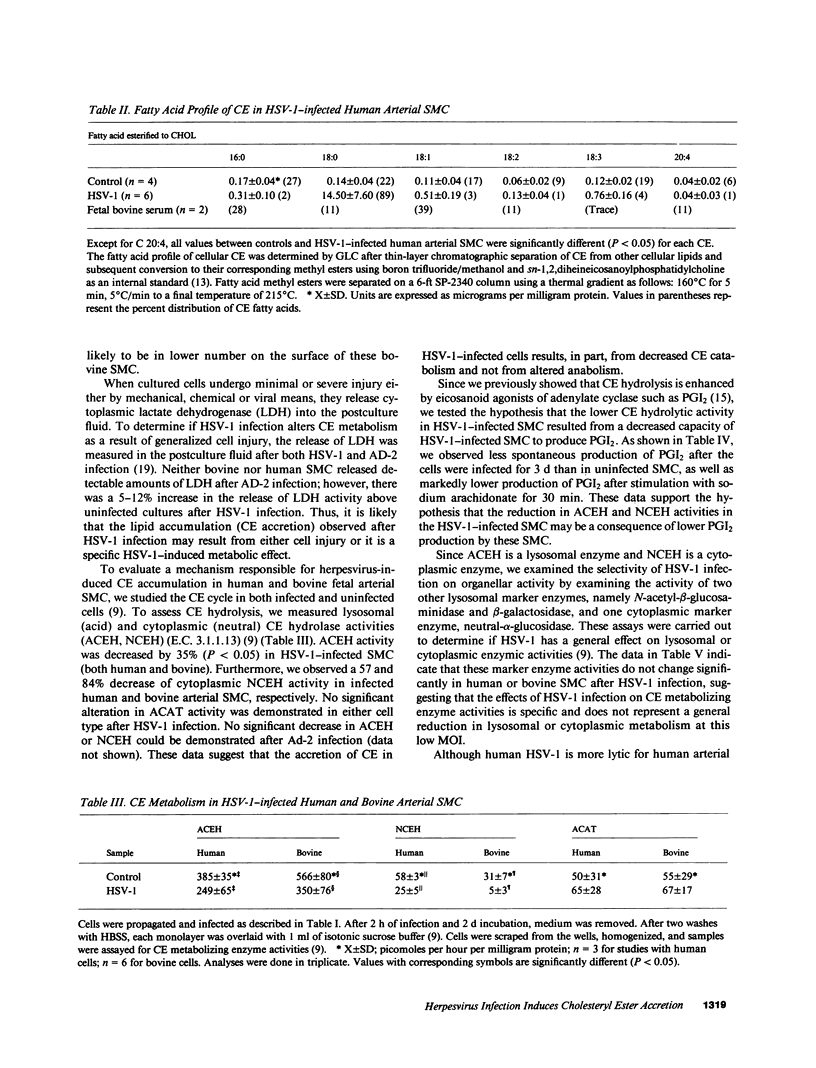
- Fabricant C. G., Hajjar D. P., Minick C. R., Fabricant J. Herpesvirus infection enhances cholesterol and cholesteryl ester accumulation in cultured arterial smooth muscle cells. Am J Pathol. 1981 Nov;105(2):176–184. [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Garcia-Palmieri M. R., Kagan A., Kannel W. B., Schiffman J. Differences in coronary heart disease in Framingham, Honolulu and Puerto Rico. J Chronic Dis. 1974 Sep;27(7-8):329–344. doi: 10.1016/0021-9681(74)90013-7. [DOI] [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Guinn G. A., Gyorkey P., DeBakey M. E. Herpesviridae in the endothelial and smooth muscle cells of the proximal aorta in arteriosclerotic patients. Exp Mol Pathol. 1984 Jun;40(3):328–339. doi: 10.1016/0014-4800(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Fabricant C. G., Minick C. R., Fabricant J. Virus-induced atherosclerosis. Herpesvirus infection alters aortic cholesterol metabolism and accumulation. Am J Pathol. 1986 Jan;122(1):62–70. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Falcone D. J., Fabricant C. G., Fabricant J. Altered cholesteryl ester cycle is associated with lipid accumulation in herpesvirus-infected arterial smooth muscle cells. J Biol Chem. 1985 May 25;260(10):6124–6128. [PubMed] [Google Scholar]
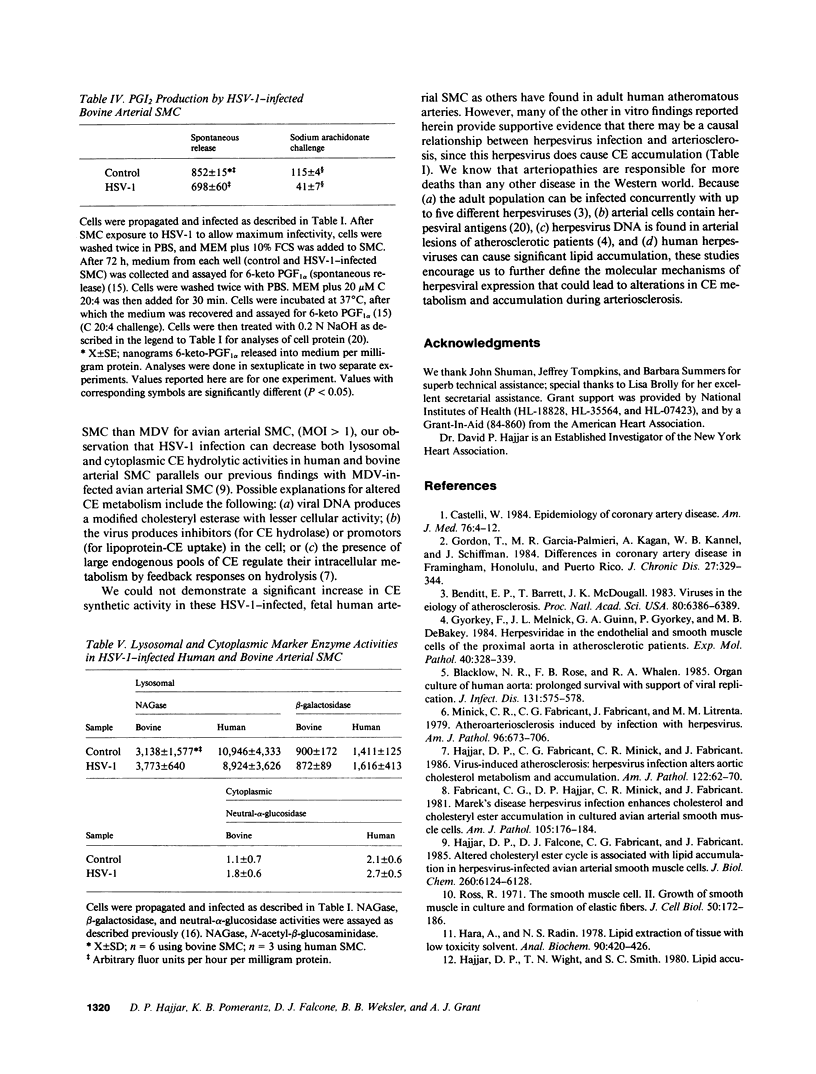
- Hajjar D. P., Weksler B. B., Falcone D. J., Hefton J. M., Tack-Goldman K., Minick C. R. Prostacyclin modulates cholesteryl ester hydrolytic activity by its effect on cyclic adenosine monophosphate in rabbit aortic smooth muscle cells. J Clin Invest. 1982 Sep;70(3):479–488. doi: 10.1172/JCI110639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Wight T. N., Smith S. C. Lipid accumulation and ultrastructural change within the aortic wall during early spontaneous atherogenesis. Am J Pathol. 1980 Sep;100(3):683–705. [PMC free article] [PubMed] [Google Scholar]
- Haley N. J., Fowler S., de Duve C. Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells. J Lipid Res. 1980 Nov;21(8):961–969. [PubMed] [Google Scholar]
- Hara A., Radin N. S. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978 Oct 1;90(1):420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Melnick J. L., Petrie B. L., Dreesman G. R., Burek J., McCollum C. H., DeBakey M. E. Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet. 1983 Sep 17;2(8351):644–647. doi: 10.1016/s0140-6736(83)92529-1. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Fabricant C. G., Fabricant J., Litrenta M. M. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979 Sep;96(3):673–706. [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz K. B., Fleisher L. N., Tall A. R., Cannon P. J. Enrichment of endothelial cell arachidonate by lipid transfer from high density lipoproteins: relationship to prostaglandin I2 synthesis. J Lipid Res. 1985 Oct;26(10):1269–1276. [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]



