Abstract
Pancreas development is a complex and dynamic process orchestrated by cellular and molecular events, including morphogenesis and cell differentiation. As a result of recent explorations into possible cell-therapy-based treatments for diabetes, researchers have made significant progress in deciphering the developmental program of pancreas formation. In vitro pancreas organ culture systems provide a valuable tool for exploring the mechanisms of gene regulation, cellular behaviors, and cell differentiation. In this chapter, we review three common techniques for culturing embryonic pancreas explants. Each technique is suitable for different applications. Specifically, culturing embryonic pancreas on culture inserts provides an excellent platform to test the effects of chemical compounds. Conversely, when the embryonic pancreas is cultured in fibronectin-coated glass microwells, the system provides unique culture conditions to monitor organ growth and cellular dynamic events. Lastly, when the embryonic pancreas is embedded in Matrigel, organogenesis can be studied in a three-dimensional environment instead of limiting the analysis to one plane.
Keywords: Pancreas, Development, Explants, Differentiation, Morphogenesis
1 Introduction
The pancreas is an essential organ for nutrient metabolism, with both endocrine and exocrine features. The main function of the endocrine pancreas is to regulate blood glucose homeostasis; the exocrine pancreas, conversely, is primarily digestive. Malformation of either the exocrine or the endocrine cells, or both, leads to diseases such as diabetes, pancreatitis, and pancreatic cancer.
Both exocrine and endocrine cells arise from a common pancreatic progenitor cell pool during pancreas development. In mice, pancreas organogenesis starts at embryonic day (E) 8.5–E9.0, when the first epithelial endoderm-derived pancreatic progenitor cells invaginate into surrounding mesenchymal tissues. Over the next 3 days, pancreatic progenitor cells proliferate and the majority of the progenitor cells remain pluripotent. During these early stages, the pancreas expands significantly in size, but overall morphology initially undergoes little change [1]. By E12.5, pancreas morphogenesis is initiated and cell differentiation begins [1, 2]. From this stage onwards, pancreas development is marked by the development of a separate endocrine and exocrine compartment. At birth, cell differentiation is completed and the pancreas has assumed the morphology and cytoarchitecture of the mature organ.
The last two decades have provided significant insight into the molecular mechanisms controlling pancreatic cell lineage choices and cell differentiation. Recent investigations have also explored how pancreas organogenesis is coordinated with endocrine and exocrine cell differentiation (for review, see refs. 3, 4). Our knowledge of pancreas development is mainly based on in vivo genetic studies using animal models (primarily transgenic mouse models). However, there are significant limitations to generating and analyzing transgenic mouse models—specifically, the inherently slow nature of genetic research in genetic mouse models. Moreover, due to the highly dynamic nature of pancreas morphogenesis, studies using end-stage analysis often fail to provide sufficient information to understand the cellular events that occur throughout the developmental process.
To overcome these difficulties, numerous studies have established different culture conditions and strategies for studying pancreas development ex vivo [5, 6]. These techniques now allow the monitoring of pancreas morphological changes and cell differentiation in real time. Ex vivo culture systems also allow for testing the effects of compounds on pancreatic development.
2 Materials
All reagents are to be prepared under a cell culture hood; only cell culture grade reagents are to be used. All hardware and reagents are to be handled with standard sterile techniques.
2.1 Cell Culture Supplies
- Glass-bottom culture dish:
- Dish diameter: 35 mm.
- Coating: Uncoated.
- Glass thickness: No. 0–1.5.
- Cover slip (choose a thickness appropriate to the lens used, see Note 1): Glass diameter: 10 mm.
4-Well × 1 mL MultiDish cell culture dish with round wells and lid, cell-culture-treated polystyrene, sterile.
Nuclepore Track-Etched Membranes (13 mm, 1.0 μm pore).
Tissue culture dishes (10 mm).
2.2 Reagents
DPBS (Dulbecco's phosphate-buffered saline).
-
Pancreas culture medium.
The culture medium containing DMEM/F12 medium with HEPES, l-glutamine, and without phenol red, supplemented with 10 % FBS (fetal bovine serum), and antibiotic/antimycotic agents (100 U/mL penicillin, 100 μg/mL streptomycin) (see Note 2).
-
Purified human serum fibronectin (hFN).
Prepare stock hFN solution (1 mg/mL) by reconstituting 5 mg lyophilzed hFN1 in 5 mL sterile pure water. Let the solution sit on ice for 5 min; do not agitate or swirl it, as this will cause the fibronectin to precipitate out of solution. Prepare aliquots of 100 μL each, and store at −80 °C. After thawing, keep the hFN solution at 4 °C for no more than 2 weeks.
-
Matrigel™.
BD growth factor-reduced (GFR), phenol red-free Matrigel™.
Thaw BD Matrigel™ Matrix GFR phenol red free by submerging the vial in ice in a 4 °C refrigerator overnight. Swirl vial to ensure that Matrigel™ is evenly dispersed. Keep Matrigel™ on ice at all times to prevent solidification.
2.3 Preparation of hFN-Coated Glass-Bottomed Culture Dish
Thaw the hFN stock solution (1 mg/mL) slowly on ice. Do not agitate or swirl it. If the fibronectin has aggregated into insoluble clumps or transparent filaments, discard the aliquot and use a new one.
Further dilute stock hFN solution to 0.1 mg/mL with sterile pure water.
For each glass-bottomed dish (with 10 mm glass slips), add 100 μl 0.1 mg/mL hFN solution onto the glass slips. Make sure that the solution covers entire surface of the glass slips.
Let the hFN solution dry completely at room temperature in the cell culture hood; allow 2 h for drying. After drying, the hFN-coated plates can be stored at 4 °C for no more than 4 weeks.
3 Methods
3.1 Mouse Embryos, Pancreas Dissection, and Tissue Preparation
Chill sterile DPBS and DMEM/F12 medium on ice. Sterilize dissecting area by spraying with 70 % ethanol.
E0.5 is defined as midday on the day of emergence of the vaginal plug. By E11.5 (see Note 3), sacrafice pregnant dams with CO2 inhalation, followed by cervical dislocation. The uteri are subsequently isolated.
Extract the string of embryos, and place intact in a 10-cm petri dish containing ice-cold DPBS.
Dissect each embryo by opening its abdominal wall using fine forceps under a dissecting stereomicroscope. The stomach and liver are identified first; the dorsal pancreas is located on the left ventral side of the stomach (Fig. 1a).
-
Separate the stomach and liver tissues from the embryonic pancreas and duodenum, which are left attached to each other; transfer the embyonic pancreas and duodenum into ice-cold DMEM/F12 medium using a pre-rinsed pipetman tip (see Note 4). The dissected tissue can be stored in this condition for no more than 4 h before culturing.
All three-culture techniques described herein use the same tissue preparation procedure.
Fig. 1.
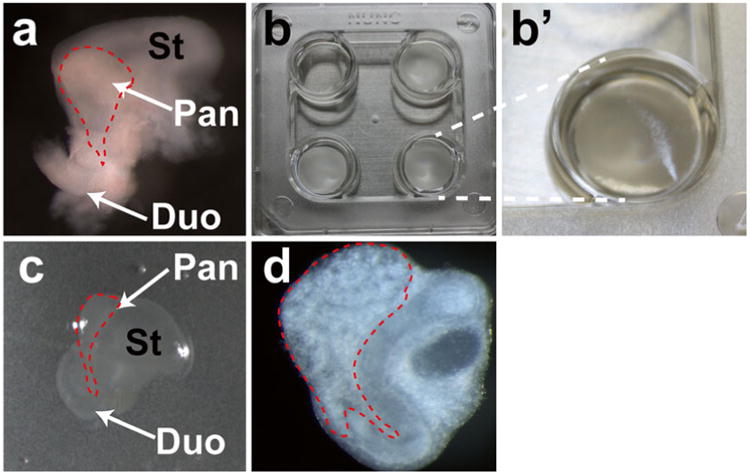
Illustration of pancreas explants on cell culture inserts. (a) Example of a dissected E11.5 pancreas. The stomach and duodenum are still attached to the pancreas. Note the red dashes outlining the dorsal pancreas for illustrative purposes. (b) Nuclepore Membrane inserts floating on top of the liquid medium. For comparison purposes, the well in the upper left corner has no insert. (b′) Zoomed-in image of right lower corner, showing that filter is floating. Note membrane orientation (reflective side up). (c) Explant tissue, shown growing at air/medium interface. Stomach and duodenum are still attached to pancreas, for illustrative purposes. (d) After 3-day culturing, the dissected E11.5 pancreas explants expand, and develop branching structures. St stomach, Pan pancreas, Duo duodenum. (Color figure online)
3.2 Pancreas Explants on Cell Culture Inserts
This method is particularly useful for studying the effects of chemical compounds on pancreatic development. Pancreatic buds can be cultured in this condition to up to 7 days, thus allowing quantitative exploration of cell proliferation and gene expression. Due to the potential for inconsistencies in experimental conditions, it is advisable to incorporate multiple experimental replicates, so as to allow statistical analysis and reproducible results.
Pre-warm explant medium; allow 1 h in 37 °C water bath.
Add 1 mL pre-warmed explant medium into each well of the MultiDish cell-culture dish. Keep the liquid surface level; avoid introducing air bubbles.
Using forceps, extract one Nuclepore Track-Etched Membrane from separator paper inserts (see Note 5); identify the membrane orientation and find the reflective side (Fig. 1b, b′).
Keeping the reflective side up, place the membrane on the surface of the explant medium without disrupting the surface tension. If the membrane sinks into the medium, remove it with forceps, dispose of it, and then repeat the procedure with a new membrane.
Use a 20-μl pipette with a pre-rinsed pipetman tip to transfer the pancreatic tissue from its storage in ice-cold DMEM/F12, to the top of the Nuclepore Membrane. The tissue should grow at the air/medium interface (Fig. 1c). During the transfer, avoid introducing air bubbles or excessive medium on the surface of the membrane.
Add 20 μL of explant medium onto the air/medium interface, and reorient the tissue. The dorsal pancreas must be ventral side up, and the tissue must not be folded. This is critical to ensure consistency of tissue morphology at the end of the culture period. Place the cultures in a tissue-culture incubator (37 °C; 5 % CO2).
The pancreatic tissue will flatten out in the culture medium; allow an hour for this to occur. After 24 h, the branches of the pancreas will have developed enough to be visually identifiable (Fig. 1c, d).
Change the explant medium daily; at each change, add 20 μL of fresh pre-warmed medium to the air/medium interface to reduce contamination.
3.3 Pancreas Explants on hFN-Coated Glass- Bottomed Dishes
This method, when combined with real-time microscopy and fluorescence labeling, is particularly useful for the study of pancreatic branching morphogenesis and behavior of individual cells [6, 7]. Many recent studies have used genetic strategies to label different cell types with either membrane-associated or nuclear fluorescent proteins, thereby allowing the tracking of individual cell behaviors. Single-cell imaging techniques, using four-dimensional live imaging, have been established for the early developmental stages of both the pancreas and kidney [6, 8]. Because the hFN substrate causes the pancreatic tissue to flatten over time and thereby become thinner (Fig. 2a, b), the tissue poses less of an obstruction to the light from the microscope, which facilitates imaging.
Fig. 2.
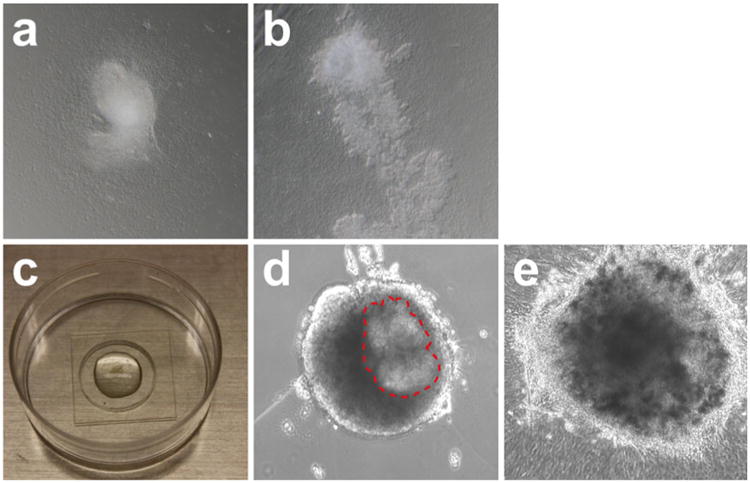
Pancreas explants on hFN-coated glass-bottomed dish and embedded in three-dimensional Matrigel™. (a) E11.5 pancreas explants grow on hFN-coated glass-bottomed dish for 24 h. Note that the tissue has attached to the glass bottom and flattened out at the edges. (b) After 5 days in culture, pancreas explants further spread out and develop branching structures. (c) E11.5 pancreas explants embedded in solidified Matrigel™ on glass microwells of MatTek™ dishes. (d) After 24-h culturing, the dissected E11.5 pancreas explants retain round morphology. Note that the pancreas epithelium can be identified as outlined with red dash lines. At this stage, the explants start to undergo morphological changes. (e) After 3 days in culture, the edge of the explants spreads out while the whole tissue remains encapsulated in the Matrigel™. The branched tip structures can be readily identified
Pre-warm explant medium in 37 °C water bath; allow 1 h for warming.
Add 200 μL of pre-warmed explant medium directly into the hFN-coated glass slips of MatTek™ dishes (see Note 6). Incubate at room temperature for 20 min and then aspirate the medium. Add 100 μL of fresh pre-warmed explant medium into the hFN-coated glass microwells of MatTek™ dishes.
Using a 20-μL pipette with a pre-rinsed pipetman tip, transfer the pancreas explants onto the hFN-coated glass microwells so that the tissue can settle to the bottom.
Reorient the tissue by gently pipetting the medium; avoid introducing air bubbles. The dorsal pancreas must be ventral side up, and the tissue must not be folded.
Slowly aspirate the medium using a 200-μL pipetman until the explant tissue flattens out in the glass microwells. Add 10 μL of fresh explant medium onto the glass to keep the tissue wet.
In order to keep the tissue moisturized inside the dishes, slowly add 1 mL explant medium to the edge of the MatTek™ dishes; ensure that no medium overflows into the microwells.
Place the cultures in a tissue-culture incubator (37 °C; 5 % CO2); allow 45 min for the explants to attach to the glass-bottomed microwells.
To verify whether the tissue has properly attached to the glass-bottomed microwells, slowly add 1 mL of explant medium into the dish, ensuring that the medium covers the entire surface. If the explants detach from the glass-bottomed microwells, the procedure has failed; aspirate all medium and start again from step 2 of Subheading 3.3.
Place the cultures in a tissue-culture incubator (37 °C; 5 % CO2) and change the explant medium daily.
3.4 Pancreas Explants Embedded in Three- Dimensional Matrigel™
This method is particularly useful for the three-dimensional study of cell/extracellular matrix (ECM) interactions. In this section we demonstrate the use of Matrigel™, a proprietary combination of (primarily) laminin and collagen. Recent studies have successfully used hydrogel supplements with different assortments of ECM molecules to generate culture environments in which both pancreatic progenitor cells [9] and adult pancreatic ductal progenitor cells [10] can be maintained. When combining with live-imaging techniques, this method can also serve as a platform for investigating branching morphogenesis in 3-D [11].
Thaw Matrigel™ as previously explained. Chill DMEM/F12 medium, Eppendorf tubes, and pipetman tips on ice. Pre-warm explant medium in 37 °C water bath; allow 1 h for warming. Use only ice-chilled pipetman tips and Eppendorf tubes to handle Matrigel™.
Transfer pancreas explants with 100 μL ice-cold DMEM/F12 into an ice-chilled Eppendorf tube. Pipette and mix 100 μL Matrigel™ with the explant with medium (50 % dilution). Avoid introducing excessive air bubbles in this step.
Place the Matrigel™/explant mix on ice so that air bubbles can escape; allow 10 min for this step.
Transfer 200 μL of Matrigel™/explant mix into uncoated glass microwells of MatTek™ dishes. Avoid introducing air bubbles and overflow.
Place the cultures in a tissue-culture incubator (37 °C; 5 % CO2) so that the Matrigel™ will solidify completely; allow 1 h (Fig. 2c).
Slowly add 2 mL of the explant medium into the MatTek™ dishes from the side. Ensure that the solidified Matrigel™ is not disrupted and that the medium covers the entire surface.
Place the cultures in a tissue-culture incubator (37 °C; 5 % CO2) and change the explant medium daily (Fig. 2d, e).
4 Notes
-
Coverslip number and thickness index:
Coverslip No. Thickness (mm) 0 0.085–0.13 1.0 0.13–0.16 1.5 0.16–0.19 2.0 0.19–0.23 For explants with fluorescent tags, we recommend the use of the F12/DMEM medium without phenol red, to avoid quenching effect.
In this chapter we only show procedures using E11.5 embryos. Earlier or later stages of embryonic pancreatic development can also be successfully cultured using the same conditions. We have tested all three culture procedures using embryonic pancreas dissected from E9.5 to E15.5 with success. However, pancreases of earlier stages than E11.5 are more difficult to orient properly, and the end results may show greater variation.
Pre-rinse the 200-μL pipetman tip with explant medium to lubricate the tip wall and prevent tissue sticking to the tip. If the tissue size is larger than the opening, cut the opening to enlarge the holes.
Here we used Nuclepore Track-Etched Membranes (diameter: 13 mm; pore size: 1 μm). This is the only pore size recommended: larger pore sizes may lead to the membrane sinking below the surface of the liquid, and smaller pore sizes may compromise liquid exchange efficiency and cultured tissue morphology.
To avoid overflow, add no more than 200 μl of medium. This step is to wash off excessive salt and impurities deposited on glass slips during hFN coating procedure.
Acknowledgments
This work was supported by the NIH/NIDDK awards to M.S. (R01-DK078803 and R01-DK68471), and JDRF postdoctoral fellowships H.P.S. (3-2009-161).
References
- 1.Villasenor A, Chong DC, Henkemeyer M, Cleaver O. Epithelial dynamics of pancreatic branching morphogenesis. Development. 2010;137:4295–4305. doi: 10.1242/dev.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesavan G, Sand FW, Greiner TU, Johansson JK, Kobberup S, et al. Cdc42-mediated tubulogenesis controls cell specification. Cell. 2009;139:791–801. doi: 10.1016/j.cell.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 3.Shih HP, Wang A, Sander M. Pancreas organogenesis: from lineage determination to morphogenesis. Annu Rev Cell Dev Biol. 2013;29:81–105. doi: 10.1146/annurev-cellbio-101512-122405. [DOI] [PubMed] [Google Scholar]
- 4.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 5.Esni F, Miyamoto Y, Leach SD, Ghosh B. Primary explant cultures of adult and embryonic pancreas. Methods Mol Med. 2005;103:259–271. doi: 10.1385/1-59259-780-7:259. [DOI] [PubMed] [Google Scholar]
- 6.Petzold KM, Spagnoli FM. A system for ex vivo culturing of embryonic pancreas. J Vis Exp. 2012;66:e3979. doi: 10.3791/3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke ZD, Li WC, Slack JM, Tosh D. Isolation and culture of embryonic pancreas and liver. Methods Mol Biol. 2010;633:91–99. doi: 10.1007/978-1-59745-019-5_7. [DOI] [PubMed] [Google Scholar]
- 8.Packard A, Georgas K, Michos O, Riccio P, Cebrian C, et al. Luminal mitosis drives epithelial cell dispersal within the branching ureteric bud. Dev Cell. 2013;27:319–330. doi: 10.1016/j.devcel.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013;140:4452–4462. doi: 10.1242/dev.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin L, Feng T, Shih HP, Zerda R, Luo A, et al. Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci U S A. 2013;110:3907–3912. doi: 10.1073/pnas.1301889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri S, Hebrok M. Dynamics of embryonic pancreas development using real-time imaging. Dev Biol. 2007;306:82–93. doi: 10.1016/j.ydbio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


