Abstract
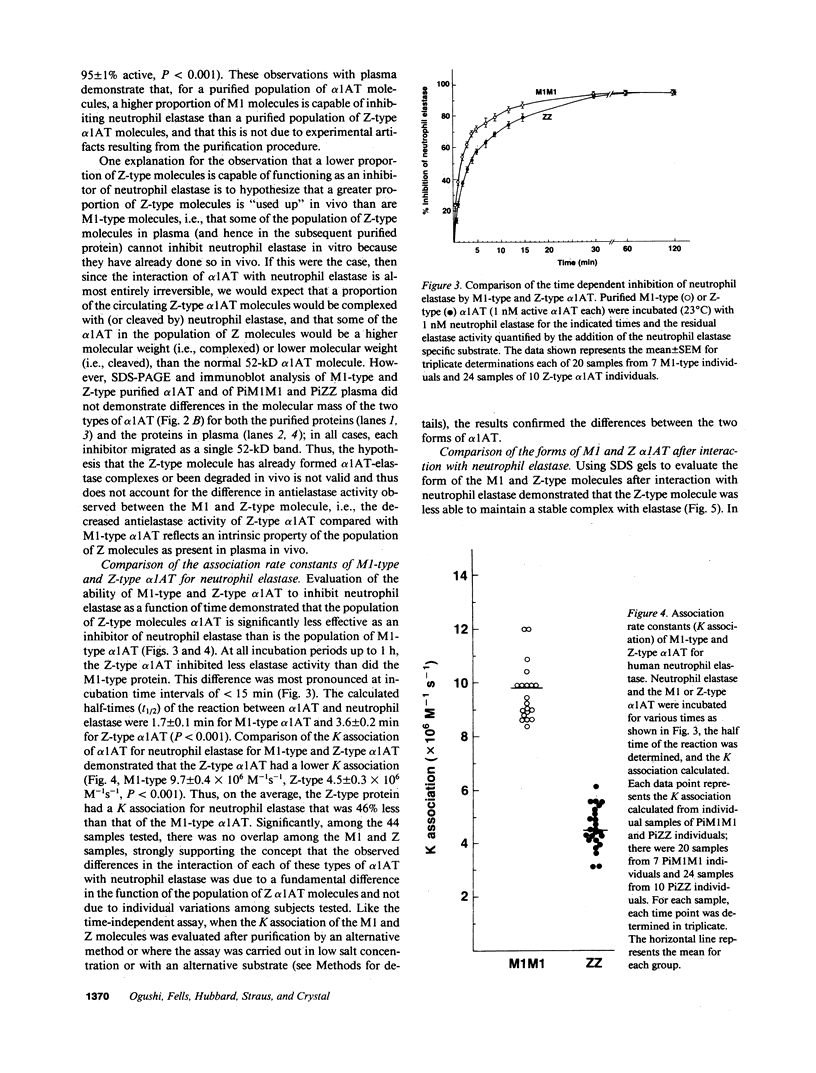
Alpha 1-antitrypsin (alpha 1AT) deficiency resulting from homozygous inheritance of the Z-type alpha 1AT gene is associated with serum alpha 1AT levels of less than 50 mg/dl and the development of emphysema in the third to fourth decades. Despite the overwhelming evidence that the emphysema of PiZZ individuals develops because of a "deficiency" of alpha 1AT and hence an insufficient antineutrophil elastase defense of the lung, epidemiologic evidence has shown that levels of alpha 1AT of only 80 mg/dl protect the lung from an increased risk of emphysema. With this background, we hypothesized that homozygous inheritance of the Z-type may confer an added risk beyond a simple deficiency of alpha 1AT by virtue of an inability of the Z-type alpha 1AT molecule to inhibit neutrophil elastase as effectively as the common M1-type molecule. To evaluate this hypothesis, the functional status of alpha 1AT from PiZZ individuals (n = 10) was compared with that of alpha 1AT from PiM1M1 individuals (n = 7) for its ability to inhibit neutrophil elastase (percent inhibition) as well as its association rate constant for neutrophil elastase (K association). Plasma alpha 1AT concentration, measured by radial immunodiffusion, was 34 +/- 1 mg/dl in PiZZ patients vs. 237 +/- 14 mg/dl for PiM1M1 plasma, a sevenfold difference. When titrated against neutrophil elastase, the present inhibition of PiZZ plasma was significantly less than Pi M1M1 plasma (ZZ 78 +/- 1% vs. M1M1 95 +/- 1%, P less than 0.001) as was purified Z type alpha 1AT (ZZ, 63 +/- 2% vs. M1M1 86 +/- 2%, P less than 0.001). Sodium dodecyl sulfate (SDS) gel comparisons of the complexes formed with M1-type alpha 1AT and Z-type alpha 1AT with elastase demonstrated the Z alpha 1AT-elastase complexes were less stable than the M1 alpha 1AT-elastase complexes, thus releasing some of the enzyme to continue to function as a protease. Consistent with these observations, the K association of purified Z-type alpha 1AT for neutrophil elastase was lower than that of M1-type alpha 1AT (ZZ 4.5 +/- 0.3 X 10(6) M-1s-1 vs. M1M1 9.7 +/- 0.4 X 10(6) M-1s-1, P less than 0.001), suggesting that for the population of alpha 1AT molecules, the active Z-type molecules take more than twice as long as the active M1-type alpha 1AT to inhibit neutrophil elastase. Consequently, not only is there less alpha1AT in PiZZ individuals, but the population of Z-type alpha1AT molecules is less competent as an inhibitor of neutrophil elastase than M1-type alpha1AT molecules. This combination of defects suggests that PiZZ individuals have far less functional antielastase protection than suggested by the reduced concentrations of alpha1AT alone, further explaining their profound risk for development of emphysema.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams W. R., Weinbaum G., Weissbach L., Weissbach H., Brot N. Enzymatic reduction of oxidized alpha-1-proteinase inhibitor restores biological activity. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7483–7486. doi: 10.1073/pnas.78.12.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathurst I. C., Travis J., George P. M., Carrell R. W. Structural and functional characterization of the abnormal Z alpha 1-antitrypsin isolated from human liver. FEBS Lett. 1984 Nov 19;177(2):179–183. doi: 10.1016/0014-5793(84)81279-x. [DOI] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Beatty K., Matheson N., Travis J. Kinetic and chemical evidence for the inability of oxidized alpha 1-proteinase inhibitor to protect lung elastin from elastolytic degradation. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):731–736. doi: 10.1515/bchm2.1984.365.2.731. [DOI] [PubMed] [Google Scholar]
- Bruce R. M., Cohen B. H., Diamond E. L., Fallat R. J., Knudson R. J., Lebowitz M. D., Mittman C., Patterson C. D., Tockman M. S. Collaborative study to assess risk of lung disease in Pi MZ phenotype subjects. Am Rev Respir Dis. 1984 Sep;130(3):386–390. doi: 10.1164/arrd.1984.130.3.386. [DOI] [PubMed] [Google Scholar]
- Callea F., Fevery J., Massi G., Lievens C., de Groote J., Desmet V. J. Alpha-1-antitrypsin (AAT) and its stimulation in the liver of PiMZ phenotype individuals. A "recruitment-secretory block" ("R-SB") phenomenon. Liver. 1984 Oct;4(5):325–337. [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Vaughan L., Brennan S. O., Owen M. C., Boswell D. R. Human alpha 1-antitrypsin: carbohydrate attachment and sequence homology. FEBS Lett. 1981 Dec 7;135(2):301–303. doi: 10.1016/0014-5793(81)80805-8. [DOI] [PubMed] [Google Scholar]
- Castillo M. J., Nakajima K., Zimmerman M., Powers J. C. Sensitive substrates for human leukocyte and porcine pancreatic elastase: a study of the merits of various chromophoric and fluorogenic leaving groups in assays for serine proteases. Anal Biochem. 1979 Oct 15;99(1):53–64. doi: 10.1016/0003-2697(79)90043-5. [DOI] [PubMed] [Google Scholar]
- Constans J., Viau M., Gouaillard C. Pi M4: an additional Pi M subtype. Hum Genet. 1980;55(1):119–121. doi: 10.1007/BF00329137. [DOI] [PubMed] [Google Scholar]
- Courtney M., Jallat S., Tessier L. H., Benavente A., Crystal R. G., Lecocq J. P. Synthesis in E. coli of alpha 1-antitrypsin variants of therapeutic potential for emphysema and thrombosis. Nature. 1985 Jan 10;313(5998):149–151. doi: 10.1038/313149a0. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Larsson C. Purification and partial characterization of pas-positive inclusion bodies from the liver in alpha 1-antitrypsin deficiency. N Engl J Med. 1975 Jan 23;292(4):176–180. doi: 10.1056/NEJM197501232920403. [DOI] [PubMed] [Google Scholar]
- Fagerhol M. K., Cox D. W. The Pi polymorphism: genetic, biochemical, and clinical aspects of human alpha 1-antitrypsin. Adv Hum Genet. 1981;11:1-62, 371-2. [PubMed] [Google Scholar]
- Fulmer J. D., Roberts W. C., von Gal E. R., Grystal R. G. Small airways in idiopathic pulmonary fibrosis. Comparison of morphologic and physiologic observations. J Clin Invest. 1977 Sep;60(3):595–610. doi: 10.1172/JCI108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS P., PFITZER E. A., TOLKER E., BABYAK M. A., KASCHAK M. EXPERIMENTAL EMPHYSEMA: ITS PRODUCTION WITH PAPAIN IN NORMAL AND SILICOTIC RATS. Arch Environ Health. 1965 Jul;11:50–58. doi: 10.1080/00039896.1965.10664169. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Northemann W., Schmelzer E., Gross V., Gauthier F., Heinrich P. C. Synthesis of alpha 1-antitrypsin in rat-liver hepatocytes and in a cell-free system. Eur J Biochem. 1982 Aug;126(1):189–195. doi: 10.1111/j.1432-1033.1982.tb06765.x. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Cook R. R., Roberts C. J., McLaughlin B. J., Powers J. C. Active site mapping of the serine proteases human leukocyte elastase, cathepsin G, porcine pancreatic elastase, rat mast cell proteases I and II. Bovine chymotrypsin A alpha, and Staphylococcus aureus protease V-8 using tripeptide thiobenzyl ester substrates. Biochemistry. 1984 Jun 19;23(13):2995–3002. doi: 10.1021/bi00308a023. [DOI] [PubMed] [Google Scholar]
- Hayes J. A., Korthy A., Snider G. L. The pathology of elastase-induced panacinar emphysema in hamsters. J Pathol. 1975 Sep;117(1):1–14. doi: 10.1002/path.1711170102. [DOI] [PubMed] [Google Scholar]
- Hercz A., Barton M. The purification and properties of human alpha1-antitrypsin (alpha1-antiprotease), variant Z. Eur J Biochem. 1977 Apr 15;74(3):603–610. doi: 10.1111/j.1432-1033.1977.tb11429.x. [DOI] [PubMed] [Google Scholar]
- Hodges L. C., Chan S. K. Locations of oligosaccharide chains in human alpha 1-protease inhibitor and oligosaccharide structures at each site. Biochemistry. 1982 May 25;21(11):2805–2810. doi: 10.1021/bi00540a036. [DOI] [PubMed] [Google Scholar]
- Hutchison D. C., Tobin M. J., Cook P. J. Alpha 1 antitrypsin deficiency: clinical and physiological features in heterozygotes of Pi type SZ. A survey by the British Thoracic Association. Br J Dis Chest. 1983 Jan;77(1):28–34. doi: 10.1016/0007-0971(83)90003-7. [DOI] [PubMed] [Google Scholar]
- Jallat S., Carvallo D., Tessier L. H., Roecklin D., Roitsch C., Ogushi F., Crystal R. G., Courtney M. Altered specificities of genetically engineered alpha 1 antitrypsin variants. Protein Eng. 1986 Oct-Nov;1(1):29–35. doi: 10.1093/protein/1.1.29. [DOI] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Janoff A., George-Nascimento C., Rosenberg S. A genetically engineered, mutant human alpha-1-proteinase inhibitor is more resistant than the normal inhibitor to oxidative inactivation by chemicals, enzymes, cells, and cigarette smoke. Am Rev Respir Dis. 1986 Mar;133(3):353–356. doi: 10.1164/arrd.1986.133.3.353. [DOI] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Janoff A., White R., Carp H., Harel S., Dearing R., Lee D. Lung injury induced by leukocytic proteases. Am J Pathol. 1979 Oct;97(1):111–136. [PMC free article] [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Fagerhol M. Properties of isolated human alpha1-antitrypsins of Pi types M, S and Z. Eur J Biochem. 1978 Feb 1;83(1):143–153. doi: 10.1111/j.1432-1033.1978.tb12078.x. [DOI] [PubMed] [Google Scholar]
- Johnson D., Travis J. Structural evidence for methionine at the reactive site of human alpha-1-proteinase inhibitor. J Biol Chem. 1978 Oct 25;253(20):7142–7144. [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
- Karlinsky J. B., Snider G. L. Animal models of emphysema. Am Rev Respir Dis. 1978 Jun;117(6):1109–1133. doi: 10.1164/arrd.1978.117.6.1109. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Black L. F. Alpha1-antitrypsin and its deficiency. Am Rev Respir Dis. 1974 Aug;110(2):176–194. doi: 10.1164/arrd.1974.110.2.176. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Fallat R. J. Alpha-1-antitrypsin deficiency: a defect in protein synthesis. Clin Chim Acta. 1969 Jun;24(3):401–403. doi: 10.1016/0009-8981(69)90111-9. [DOI] [PubMed] [Google Scholar]
- Larsson C., Dirksen H., Sundström G., Eriksson S. Lung function studies in asymptomatic individuals with moderately (Pi SZ) and severely (Pi Z) reduced levels of alpha1-antitrypsin. Scand J Respir Dis. 1976;57(6):267–280. [PubMed] [Google Scholar]
- Laurell C. B., Pierce J., Persson U., Thulin E. Purification of alpha1-antitrypsin from plasma through thiol-disulfide interchange. Eur J Biochem. 1975 Sep 1;57(1):107–113. doi: 10.1111/j.1432-1033.1975.tb02281.x. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Marco V., Mass B., Meranze D. R., Weinbaum G., Kimbel P. Induction of experimental emphysema in dogs using leukocyte homogenates. Am Rev Respir Dis. 1971 Oct;104(4):595–598. doi: 10.1164/arrd.1971.104.4.595. [DOI] [PubMed] [Google Scholar]
- McRae B., Nakajima K., Travis J., Powers J. C. Studies on reactivity of human leukocyte elastase, cathepsin G, and porcine pancreatic elastase toward peptides including sequences related to the reactive site of alpha 1-protease inhibitor (alpha 1-antitrypsin). Biochemistry. 1980 Aug 19;19(17):3973–3978. doi: 10.1021/bi00558a013. [DOI] [PubMed] [Google Scholar]
- Mega T., Lujan E., Yoshida A. Studies on the oligosaccharide chains of human alpha 1-protease inhibitor. I. Isolation of glycopeptides. J Biol Chem. 1980 May 10;255(9):4053–4056. [PubMed] [Google Scholar]
- Meyer J. F., Bieth J., Metais P. On the inhibition of elastase by serum. Some distinguishing properties of alpha1-antitrypsin and alpha2-macroglobulin. Clin Chim Acta. 1975 Jul 9;62(1):43–53. doi: 10.1016/0009-8981(75)90278-8. [DOI] [PubMed] [Google Scholar]
- Miller R. R., Kuhlenschmidt M. S., Coffee C. J., Kuo I., Glew R. H. Comparison of the chemical, physical, and survival properties of normal and Z-variant alpha-1-antitrypsins. J Biol Chem. 1976 Aug 10;251(15):4751–4757. [PubMed] [Google Scholar]
- Mornex J. F., Chytil-Weir A., Martinet Y., Courtney M., LeCocq J. P., Crystal R. G. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J Clin Invest. 1986 Jun;77(6):1952–1961. doi: 10.1172/JCI112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse J. O., Lebowitz M. D., Knudson R. J., Burrows B. A community study of the relation of alpha1-antitrypsin levels to obstructive lung diseases. N Engl J Med. 1975 Feb 6;292(6):278–281. doi: 10.1056/NEJM197502062920602. [DOI] [PubMed] [Google Scholar]
- Morse J. O., Lebowitz M. D., Knudson R. J., Burrows B. Relation of protease inhibitor phenotypes to obstructive lung diseases in a community. N Engl J Med. 1977 May 26;296(21):1190–1194. doi: 10.1056/NEJM197705262962102. [DOI] [PubMed] [Google Scholar]
- Morse J. O. alpha1-antitrypsin deficiency (first of two parts). N Engl J Med. 1978 Nov 9;299(19):1045–1048. doi: 10.1056/NEJM197811092991905. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Powers J. C., Ashe B. M., Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979 May 25;254(10):4027–4032. [PubMed] [Google Scholar]
- Nukiwa T., Brantly M., Garver R., Paul L., Courtney M., LeCocq J. P., Crystal R. G. Evaluation of "at risk" alpha 1-antitrypsin genotype SZ with synthetic oligonucleotide gene probes. J Clin Invest. 1986 Feb;77(2):528–537. doi: 10.1172/JCI112333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukiwa T., Satoh K., Brantly M. L., Ogushi F., Fells G. A., Courtney M., Crystal R. G. Identification of a second mutation in the protein-coding sequence of the Z type alpha 1-antitrypsin gene. J Biol Chem. 1986 Dec 5;261(34):15989–15994. [PubMed] [Google Scholar]
- Oakeshott J. G., Muir A., Clark P., Martin N. G., Wilson S. R., Whitfield J. B. Effects of the protease inhibitor (Pi) polymorphism on alpha-1-antitrypsin concentration and elastase inhibitory capacity in human serum. Ann Hum Biol. 1985 Mar-Apr;12(2):149–160. doi: 10.1080/03014468500007641. [DOI] [PubMed] [Google Scholar]
- Pannell R., Johnson D., Travis J. Isolation and properties of human plasma alpha-1-proteinase inhibitor. Biochemistry. 1974 Dec 17;13(26):5439–5445. doi: 10.1021/bi00723a031. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S., Barr P. J., Najarian R. C., Hallewell R. A. Synthesis in yeast of a functional oxidation-resistant mutant of human alpha-antitrypsin. Nature. 1984 Nov 1;312(5989):77–80. doi: 10.1038/312077a0. [DOI] [PubMed] [Google Scholar]
- Rowley P. T., Sevilla M. L., Schwartz H. Serm alpha-antitrypsin types: elastase inhibition versus trypsin inhibition. Hum Hered. 1974;24(5-6):472–481. doi: 10.1159/000152685. [DOI] [PubMed] [Google Scholar]
- Rowley P. T., Sevilla M. L., Schwartz R. H. Pathogenesis of deficient serum alpha1-antitrypsin in the type ZZ homozygote. Biochem Genet. 1974 Sep;12(3):235–242. doi: 10.1007/BF00486092. [DOI] [PubMed] [Google Scholar]
- Scott C. F., Carrell R. W., Glaser C. B., Kueppers F., Lewis J. H., Colman R. W. Alpha-1-antitrypsin-Pittsburgh. A potent inhibitor of human plasma factor XIa, kallikrein, and factor XIIf. J Clin Invest. 1986 Feb;77(2):631–634. doi: 10.1172/JCI112346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Tegner H., Kuhn C., Ohlsson K., Starcher B. C., Pierce J. A. The induction of pulmonary emphysema with human leukocyte elastase. Am Rev Respir Dis. 1977 Sep;116(3):469–475. doi: 10.1164/arrd.1977.116.3.469. [DOI] [PubMed] [Google Scholar]
- Shigeoka J. W., Hall W. J., Hyde R. W., Schwartz R. H., Mudholkar G. S., Speers D. M., Lin C. C. The prevalence of alpha-antitrypsin heterozygotes (Pi MZ) in patients with obstructive pulmonary disease. Am Rev Respir Dis. 1976 Dec;114(6):1077–1084. doi: 10.1164/arrd.1976.114.6.1077. [DOI] [PubMed] [Google Scholar]
- Stone P. J. The elastase-antielastase hypothesis of the pathogenesis of emphysema. Clin Chest Med. 1983 Sep;4(3):405–412. [PubMed] [Google Scholar]
- Straus S. D., Fells G. A., Wewers M. D., Courtney M., Tessier L. H., Tolstoshev P., Lecocq J. P., Crystal R. G. Evaluation of recombinant DNA-directed E.coli produced alpha 1-antitrypsin as an anti-neutrophil elastase for potential use as replacement therapy of alpha 1-antitrypsin deficiency. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1177–1184. doi: 10.1016/0006-291x(85)91739-5. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Hayakawa S., Adachi T., Ito Y., Hirano K., Sawaki S. A simple one-step purification of human alpha 1-proteinase inhibitor by immunoadsorbent column chromatography. J Biochem Biophys Methods. 1981 Dec;5(5):243–249. doi: 10.1016/0165-022x(81)90034-8. [DOI] [PubMed] [Google Scholar]
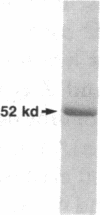
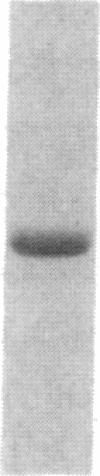
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Owen M., George P., Carrell R., Rosenberg S., Hallewell R. A., Barr P. J. Isolation and properties of recombinant DNA produced variants of human alpha 1-proteinase inhibitor. J Biol Chem. 1985 Apr 10;260(7):4384–4389. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Vercaigne D., Morcamp C., Martin J. P., Raoult J. P. Tryptic and elastolytic inhibitory capacities of serum from various Pi phenotypes. Clin Chim Acta. 1979 Apr 2;93(1):71–83. doi: 10.1016/0009-8981(79)90246-8. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]