Abstract
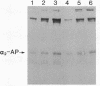
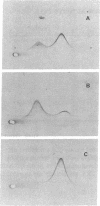
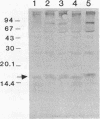
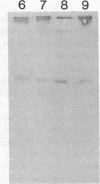
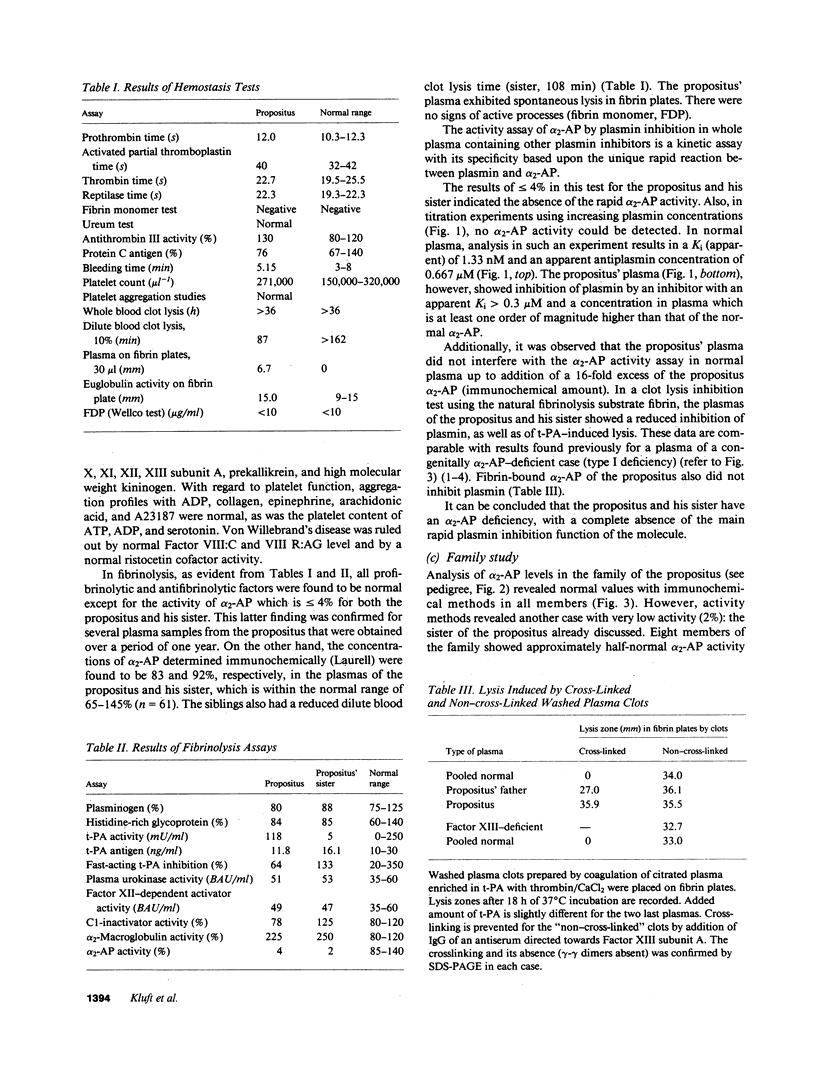
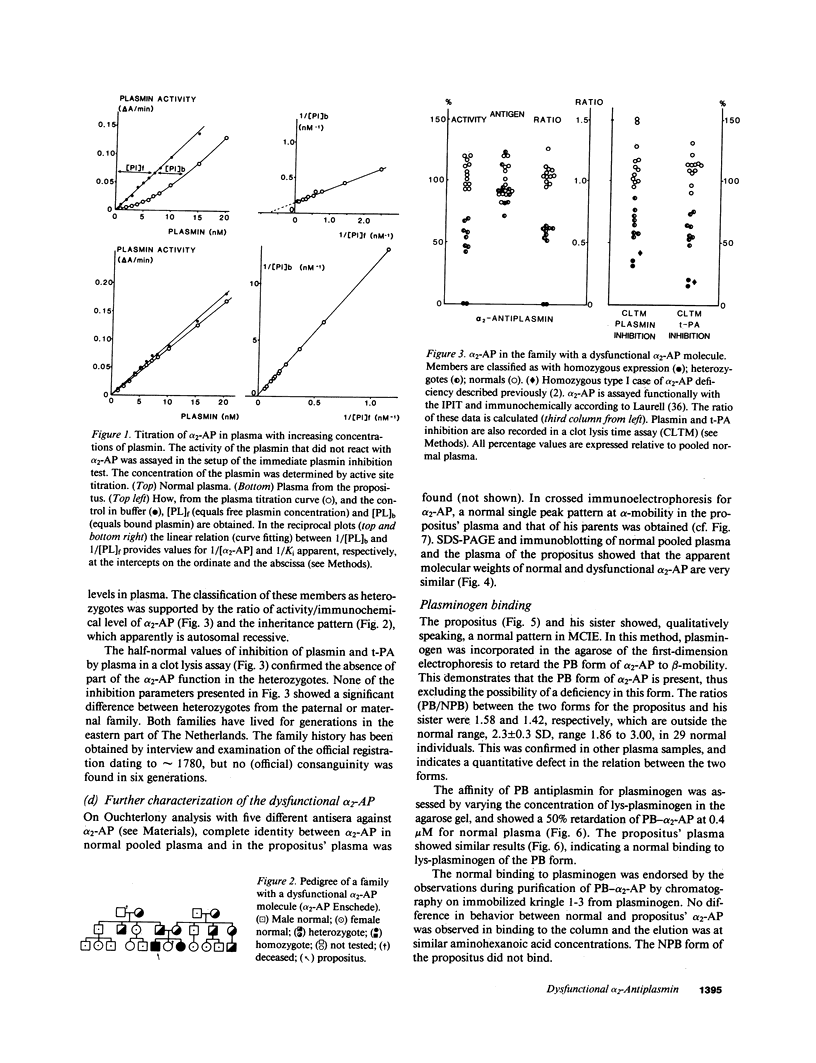
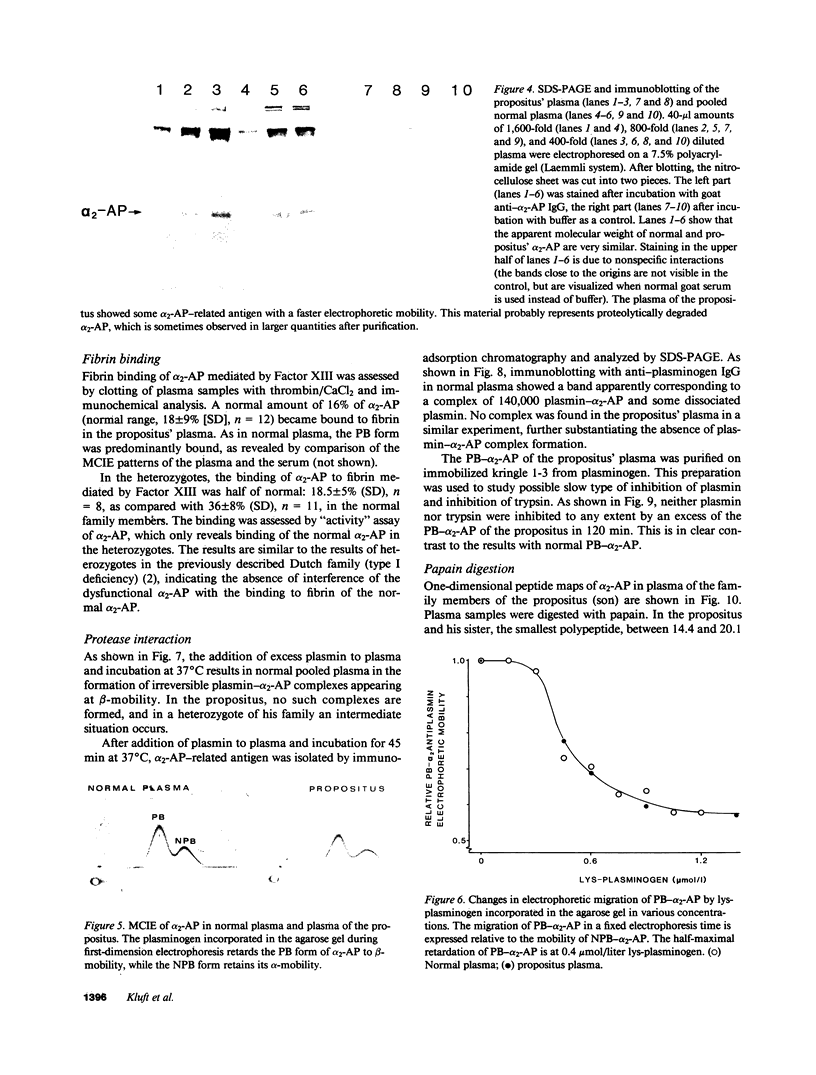
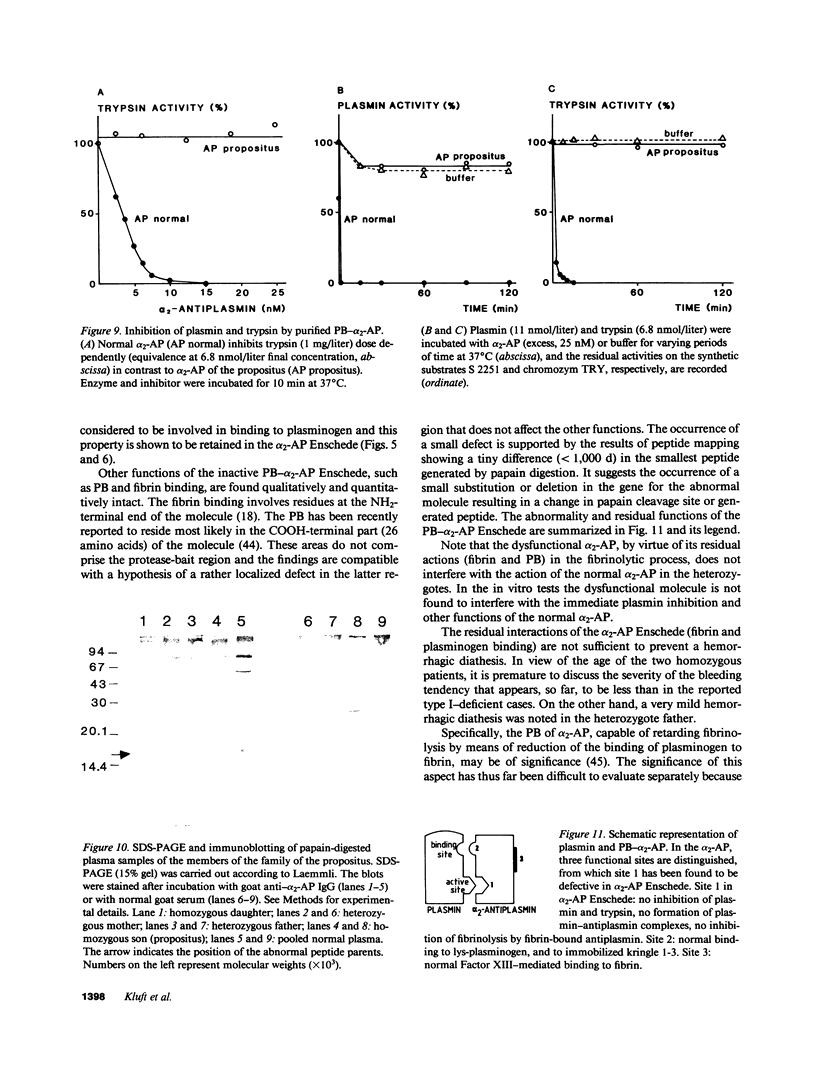
alpha 2-Antiplasmin (alpha 2-AP) is a major fibrinolysis inhibitor, whose complete, congenital absence has been found to be associated with a distinct hemorrhagic diathesis. We studied a 15-yr-old male with a hemorrhagic diathesis after trauma from early childhood on. This bleeding tendency was associated with a minimal alpha 2-AP level recorded functionally in the immediate plasmin inhibition test: less than or equal to 4% of normal. However, a normal plasma concentration of alpha 2-AP antigen (83%) was found. His sister (5 yr old) showed similar results (2 and 92%). In their family, eight heterozygotes could be identified by half-normal activity results and normal antigen concentrations. The inheritance pattern is autosomal recessive. On analysis, the alpha 2-AP of the propositus was homogeneous in all respects tested, suggesting a homozygous defect. We designated the abnormal alpha 2-AP as alpha 2-AP Enschede. alpha 2-AP Enschede showed the following characteristics: (a) complete immunological identity with normal alpha 2-AP; (b) normal molecular weight (sodium dodecyl sulfate-polyacrylamide gel electrophoresis); (c) normal alpha-electrophoretic mobility; (d) presence in plasma of both molecular forms excluding an excessive conversion to the less reactive non-plasminogen-binding form; (e) quantitatively normal binding to lys-plasminogen and to immobilized plasminogen kringle 1-3; and (f) normal Factor XIII-mediated binding to fibrin. Functional abnormalities were found in: (i) no inhibition of amidolytic activities of plasmin and trypsin, even on prolonged incubation; (ii) no formation of plasmin-antiplasmin complexes in plasma with plasmin added in excess; and (iii) no inhibition of fibrinolysis by fibrin-bound alpha 2-AP. In the heterozygotes, the presence of abnormal alpha 2-AP did not interfere with several functions of the residual normal alpha 2-AP. One-dimensional peptide mapping showed an abnormal pattern of papain digestion. We conclude that in this family, abnormal antiplasmin molecules, defective in plasmin inhibition but with normal plasminogen-binding properties, have been inherited. The residual plasminogen-binding properties do not protect against a hemorrhagic diathesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Saito H., Kamiya T., Koie K., Sakata Y., Kobakura M. Congenital deficiency of alpha 2-plasmin inhibitor associated with severe hemorrhagic tendency. J Clin Invest. 1979 May;63(5):877–884. doi: 10.1172/JCI109387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Chohan I. S., Vermylen J., Singh I., Balakrishnan K., Verstraete M. Sodium acetate buffer: a diluent of choice in the clot lysis time technique. Thromb Diath Haemorrh. 1975 Apr 30;33(2):226–229. [PubMed] [Google Scholar]
- Clemmensen I., Thorsen S., Müllertz S., Petersen L. C. Properties of three different molecular forms of the alpha 2 plasmin inhibitor. Eur J Biochem. 1981 Nov;120(1):105–112. doi: 10.1111/j.1432-1033.1981.tb05675.x. [DOI] [PubMed] [Google Scholar]
- Collen D., Wiman B. Fast-acting plasmin inhibitor in human plasma. Blood. 1978 Apr;51(4):563–569. [PubMed] [Google Scholar]
- Holmsen H., Storm E., Day H. J. Determination of ATP and ADP in blood platelets: a modification of the firefly luciferase assay for plasma. Anal Biochem. 1972 Apr;46(2):489–501. doi: 10.1016/0003-2697(72)90323-5. [DOI] [PubMed] [Google Scholar]
- Ichinose A., Mimuro J., Koide T., Aoki N. Histidine-rich glycoprotein and alpha 2-plasmin inhibitor in inhibition of plasminogen binding to fibrin. Thromb Res. 1984 Feb 15;33(4):401–407. doi: 10.1016/0049-3848(84)90079-3. [DOI] [PubMed] [Google Scholar]
- Kluft C., Los N. Demonstration of two forms of alpha 2-antiplasmin in plasma by modified crossed immunoelectrophoresis. Thromb Res. 1981 Jan 1;21(1-2):65–71. doi: 10.1016/0049-3848(84)90033-1. [DOI] [PubMed] [Google Scholar]
- Kluft C., Los P., Jie A. F. The molecular form of alpha 2-antiplasmin with affinity for plasminogen is selectively bound to fibrin by factor XIII. Thromb Res. 1984 Feb 15;33(4):419–425. doi: 10.1016/0049-3848(84)90081-1. [DOI] [PubMed] [Google Scholar]
- Kluft C., Los P., Jie A. F., van Hinsbergh V. W., Vellenga E., Jespersen J., Henny C. P. The mutual relationship between the two molecular forms of the major fibrinolysis inhibitor alpha-2-antiplasmin in blood. Blood. 1986 Mar;67(3):616–622. [PubMed] [Google Scholar]
- Kluft C., Traas D. W., Jie A. F., Hoegee-de Nobel E. The suitability of various plasmin preparations for the functional assay of alpha 2-antiplasmin in plasma. Thromb Haemost. 1982 Dec 27;48(3):320–324. [PubMed] [Google Scholar]
- Kluft C., Vellenga E., Brommer E. J., Wijngaards G. A familial hemorrhagic diathesis in a Dutch family: an inherited deficiency of alpha 2-antiplasmin. Blood. 1982 Jun;59(6):1169–1180. [PubMed] [Google Scholar]
- Kluft C., Wijngaards G., Jie A. F. Intrinsic plasma fibrinolysis: involvement of urokinase-related activity in the factor XII-independent plasminogen proactivator pathway. J Lab Clin Med. 1984 Mar;103(3):408–419. [PubMed] [Google Scholar]
- Knot E. A., ten Cate J. W., Lamping R. J., Gie L. K. Alpha 2-antiplasmin: functional characterization and metabolism in a heterozygote deficient patient. Thromb Haemost. 1986 Jun 30;55(3):375–378. [PubMed] [Google Scholar]
- Kordich L., Feldman L., Porterie P., Lago O. Severe hemorrhagic tendency in heterozygous alpha 2-antiplasmin deficiency. Thromb Res. 1985 Dec 1;40(5):645–651. doi: 10.1016/0049-3848(85)90302-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mielke C. H., Jr, Kaneshiro M. M., Maher I. A., Weiner J. M., Rapaport S. I. The standardized normal Ivy bleeding time and its prolongation by aspirin. Blood. 1969 Aug;34(2):204–215. [PubMed] [Google Scholar]
- Miles L. A., Plow E. F., Donnelly K. J., Hougie C., Griffin J. H. A bleeding disorder due to deficiency of alpha 2-antiplasmin. Blood. 1982 Jun;59(6):1246–1251. [PubMed] [Google Scholar]
- Petersen L. C., Clemmensen I. Kinetics of plasmin inhibition in the presence of a synthetic tripeptide substrate. The reaction with pancreatic trypsin inhibitor and two forms of alpha 2-plasmin inhibitor. Biochem J. 1981 Oct 1;199(1):121–127. doi: 10.1042/bj1990121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G. H., White J. G., Jachimowicz A. A., Witkop C. J. An improved method for the extraction of endogenous platelet serotonin. J Lab Clin Med. 1976 Jan;87(1):129–137. [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Zaal-de Jong M., Welbergen J. Purification and partial characterization of plasminogen activator from human uterine tissue. Biochim Biophys Acta. 1979 Sep 29;580(1):140–153. doi: 10.1016/0005-2795(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., van Hinsbergh V. W., Sens E. H. Quantitation of tissue-type plasminogen activator in human endothelial cell cultures by use of an enzyme immunoassay. Thromb Res. 1984 Jan 15;33(2):145–153. doi: 10.1016/0049-3848(84)90175-0. [DOI] [PubMed] [Google Scholar]
- Saito H., Goodnough L. T., Knowles B. B., Aden D. P. Synthesis and secretion of alpha 2-plasmin inhibitor by established human liver cell lines. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5684–5687. doi: 10.1073/pnas.79.18.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y., Aoki N. Cross-linking of alpha 2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J Clin Invest. 1980 Feb;65(2):290–297. doi: 10.1172/JCI109671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Morita T., Iwanaga S. Identification of the plasminogen-binding site of human alpha 2-plasmin inhibitor. J Biochem. 1986 Jun;99(6):1699–1705. doi: 10.1093/oxfordjournals.jbchem.a135645. [DOI] [PubMed] [Google Scholar]
- Tamaki T., Aoki N. Cross-linking of alpha 2-plasmin inhibitor to fibrin catalyzed by activated fibrin-stabilizing factor. J Biol Chem. 1982 Dec 25;257(24):14767–14772. [PubMed] [Google Scholar]
- Verheijen J. H., Chang G. T., Kluft C. Evidence for the occurrence of a fast-acting inhibitor for tissue-type plasminogen activator in human plasma. Thromb Haemost. 1984 Jul 29;51(3):392–395. [PubMed] [Google Scholar]
- Verheijen J. H., Mullaart E., Chang G. T., Kluft C., Wijngaards G. A simple, sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurements in plasma. Thromb Haemost. 1982 Dec 27;48(3):266–269. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wiman B. Affinity-chromatographic purification of human alpha 2-antiplasmin. Biochem J. 1980 Oct 1;191(1):229–232. doi: 10.1042/bj1910229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman B., Boman L., Collen D. On the kinetics of the reaction between human antiplasmin and a low-molecular-weight form of plasmin. Eur J Biochem. 1978 Jun 1;87(1):143–146. doi: 10.1111/j.1432-1033.1978.tb12360.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Collen D. On the kinetics of the reaction between human antiplasmin and plasmin. Eur J Biochem. 1978 Mar 15;84(2):573–578. doi: 10.1111/j.1432-1033.1978.tb12200.x. [DOI] [PubMed] [Google Scholar]
- Wiman B., Nilsson T., Cedergren B. Studies on a form of alpha 2-antiplasmin in plasma which does not interact with the lysine-binding sites in plasminogen. Thromb Res. 1982 Oct 15;28(2):193–199. doi: 10.1016/0049-3848(82)90261-4. [DOI] [PubMed] [Google Scholar]
- Yoshioka A., Kamitsuji H., Takase T., Iida Y., Tsukada S., Mikami S., Fukui H. Congenital deficiency of alpha 2-plasmin inhibitor in three sisters. Haemostasis. 1982;11(3):176–184. doi: 10.1159/000214659. [DOI] [PubMed] [Google Scholar]