Elevated atmospheric CO2 concentrations inhibit nitrate assimilation at night and decrease dark respiration in plants dependent on nitrate as a nitrogen source.
Abstract
A major contributor to the global carbon cycle is plant respiration. Elevated atmospheric CO2 concentrations may either accelerate or decelerate plant respiration for reasons that have been uncertain. We recently established that elevated CO2 during the daytime decreases plant mitochondrial respiration in the light and protein concentration because CO2 slows the daytime conversion of nitrate (NO3−) into protein. This derives in part from the inhibitory effect of CO2 on photorespiration and the dependence of shoot NO3− assimilation on photorespiration. Elevated CO2 also inhibits the translocation of nitrite into the chloroplast, a response that influences shoot NO3− assimilation during both day and night. Here, we exposed Arabidopsis (Arabidopsis thaliana) and wheat (Triticum aestivum) plants to daytime or nighttime elevated CO2 and supplied them with NO3− or ammonium as a sole nitrogen (N) source. Six independent measures (plant biomass, shoot NO3−, shoot organic N, 15N isotope fractionation, 15NO3− assimilation, and the ratio of shoot CO2 evolution to O2 consumption) indicated that elevated CO2 at night slowed NO3− assimilation and thus decreased dark respiration in the plants reliant on NO3−. These results provide a straightforward explanation for the diverse responses of plants to elevated CO2 at night and suggest that soil N source will have an increasing influence on the capacity of plants to mitigate human greenhouse gas emissions.
The CO2 concentration in Earth’s atmosphere has increased from about 270 to 400 µmol mol–1 since 1800, and may double before the end of the century (Intergovernmental Panel on Climate Change, 2013). Plant responses to such increases are highly variable, but plant nitrogen (N) concentrations generally decline under elevated CO2 (Cotrufo et al., 1998; Long et al., 2004). One explanation for this decline is that CO2 inhibits nitrate (NO3−) assimilation into protein in the shoots of C3 plants during the daytime (Bloom et al., 2002, 2010, 2012, 2014; Cheng et al., 2012; Pleijel and Uddling, 2012; Myers et al., 2014; Easlon et al., 2015; Pleijel and Högy, 2015). This derives in part from the inhibitory effect of CO2 on photorespiration (Foyer et al., 2009) and the dependence of shoot NO3− assimilation on photorespiration (Rachmilevitch et al., 2004; Bloom, 2015).
A key factor in global carbon budgets is plant respiration at night (Amthor, 1991; Farrar and Williams, 1991; Drake et al., 1999; Leakey et al., 2009). Nighttime elevated CO2 may inhibit, have a negligible effect on, or stimulate dark respiration, depending on the plant species (Bunce, 2001, 2003; Wang and Curtis, 2002), plant development stage (Wang et al., 2001; Li et al., 2013), experimental approach (Griffin et al., 1999; Baker et al., 2000; Hamilton et al., 2001; Bruhn et al., 2002; Jahnke and Krewitt, 2002; Bunce, 2004), and total N supply (Markelz et al., 2014). The current study is, to our knowledge, the first to examine the influence of N source, NO3− versus ammonium (NH4+), on plant dark respiration at elevated CO2 during the night.
Plant organic N compounds account for less than 5% of the total dry weight of a plant, but conversion of NO3− into organic N expends about 25% of the total energy in shoots (Bloom et al., 1989) and roots (Bloom et al., 1992). During the day, photorespiration supplies a portion of the energy (Rachmilevitch et al., 2004; Foyer et al., 2009), but at night, this energetic cost is borne entirely by the respiration of C substrates (Amthor, 1995) and may divert a substantial amount of reductant from the mitochondrial electron transport chain (Cousins and Bloom, 2004). The relative importance of NO3− assimilation at night versus the day, however, is still a matter of intense debate (Nunes-Nesi et al., 2010). Here, we estimated NO3− assimilation using several independent methods and show in Arabidopsis (Arabidopsis thaliana) and wheat (Triticum aestivum), two diverse C3 plants, that NO3− assimilation at night can be substantial, and that elevated CO2 at night inhibits this process.
RESULTS
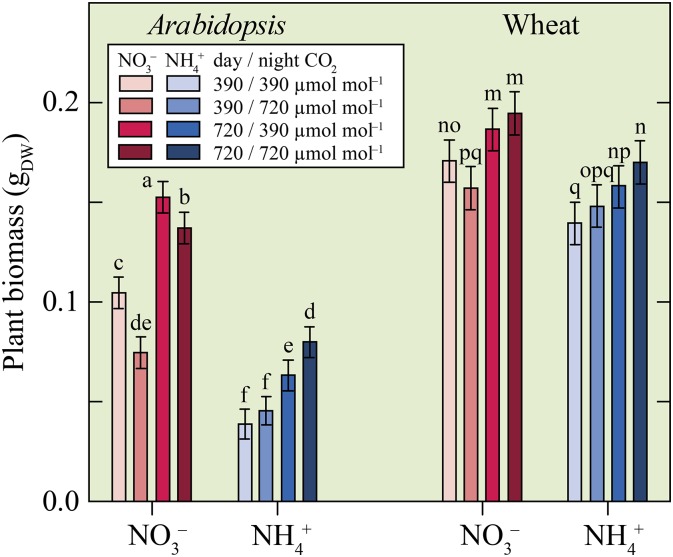
Exposure to elevated CO2 only at night decreased biomass accumulation for Arabidopsis and wheat under NO3− nutrition (Fig. 1). For Arabidopsis, exposure to elevated CO2 only during the day also increased biomass accumulation under NO3− nutrition (Fig. 1). Under NH4+ nutrition, plants exposed to elevated CO2 day and night accumulated more biomass than those exposed to ambient CO2 during the day or night (Fig. 1). Both species accumulated more biomass when they received NO3− as an N source rather than NH4+, but this was statistically significant in wheat only when all of the CO2 treatments were treated as a group (Table I). These results are similar to those of previous experiments (Bloom et al., 2012) and may derive from a cation-anion imbalance that develops in plants receiving NH4+ as a sole N source (Epstein and Bloom, 2005). For example, plants under NH4+ nutrition may become potassium deficient or may accumulate too much molybdenum (Smart and Bloom, 1993).
Figure 1.
Total biomass (grams dry weight [gDW]) for Arabidopsis (left) and wheat (right) receiving NO3− or NH4+ and exposed to one of four CO2 treatments: ambient during the day and night (390/390 µmol mol−1), ambient during the day and elevated at night (390/720 µmol mol−1), elevated during the day and ambient at night (720/390 µmol mol−1), or elevated during the day and night (720/720 µmol mol−1). Shown are the mean ± se (n = 11–15). Bars for one species labeled with different letters differed by P < 0.10.
Table I. A mixed-model ANOVA on the effects of N source (NO3− or NH4+) and atmospheric CO2 concentration regime (ambient day and night, 390/390 µmol mol−1; ambient during the day and elevated at night, 390/720 µmol mol−1; elevated during the day and ambient at night, 720/390 µmol mol−1; or elevated day and night, 720/720 µmol mol−1) on various parameters of Arabidopsis and wheat (n = 10–16).
Dash, No data; ns, nonsignificant differences; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
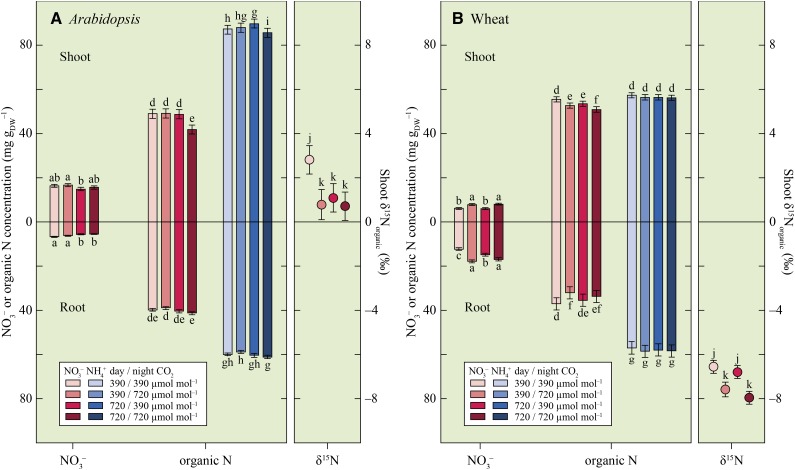
Elevated CO2 at night increased free NO3− concentrations in shoots under NO3− nutrition (Fig. 2), but in Arabidopsis, this was statistically significant only when all of the CO2 treatments were treated as a group (Table I). In plants receiving NO3− nutrition, elevated CO2 both day and night decreased shoot organic N concentration (Fig. 2). We observed similar trends in earlier experiments (Bloom et al., 2002, 2010, 2012). Plants receiving NO3− nutrition had lower shoot and root organic N concentrations than those receiving NH4+ nutrition (Fig. 2), although P was less than 0.25 for wheat at ambient CO2 day and night. Total organic N per shoot or root was similar under both N forms, however (data not shown), because of the biomass differences under the two forms (Fig. 1).
Figure 2.
NO3− and organic N concentration (milligrams per grams dry weight [mg gDW–1]) in the shoot (top left) and root (bottom left) and shoot δ15N (‰) of organic N (right) for Arabidopsis (A) and wheat (B) receiving NO3− or NH4+ and exposed to one of four CO2 treatments: ambient during the day and night (390/390 µmol mol−1), ambient during the day and elevated at night (390/720 µmol mol−1), elevated during the day and ambient at night (720/390 µmol mol−1), or elevated during the day and night (720/720 µmol mol−1). Shown are the mean ± se (n = 8–10). Bars and symbols for one species labeled with different letters differed by P < 0.10.
Ambient CO2 during the day and night increased the δ15N (‰) of organic N in shoots (Fig. 2). If NO3− availability does not limit assimilation, shoots preferentially assimilate 14N-NO3− (Carlisle et al., 2014). Therefore, the higher shoot δ15Norganic signatures under ambient rather than elevated CO2 indicate that the availability of free NO3− in the shoot was more limiting under ambient than elevated CO2 because shoot NO3− assimilation was faster (Bloom et al., 2010, 2014).
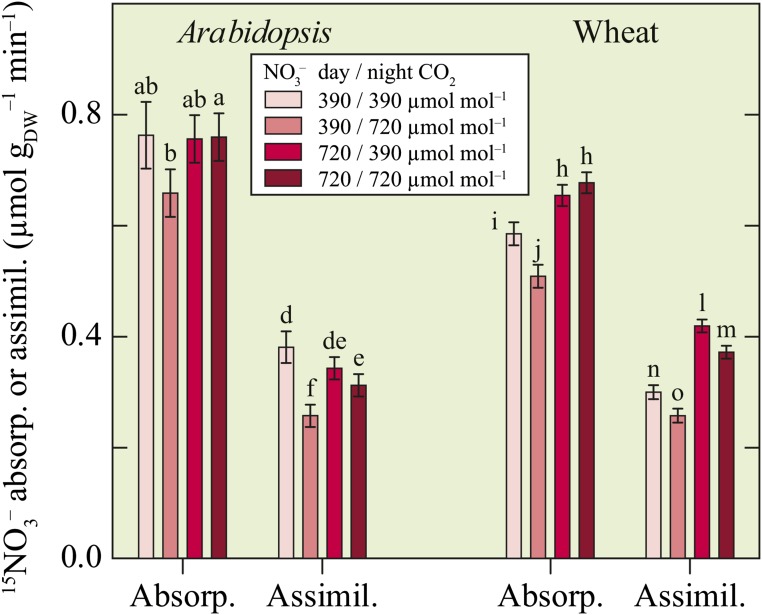
About one-half of the 15NO3− absorbed was assimilated during the night (Fig. 3). Elevated CO2 at night decreased plant 15NO3− assimilation, although P was less than 0.30 for Arabidopsis receiving elevated CO2 during the day (Fig. 3). The rates of 15NO3− assimilation at night were about two-thirds of the daytime rates that we reported previously (Bloom et al., 2010).
Figure 3.
Whole-plant 15NO3− absorption and assimilation (µmol NO3− gDW–1 [for grams per dry weight] min–1) in the dark for Arabidopsis and wheat exposed to one of four CO2 treatments: ambient during the day and night (390/390 µmol mol–1), ambient during the day and elevated at night (390/720 µmol mol–1), elevated during the day and ambient at night (720/390 µmol mol–1), or elevated during the day and night (720/720 µmol mol–1). Shown are the means ± se (n = 5–14). Bars for one species labeled with different letters differed by P < 0.10.
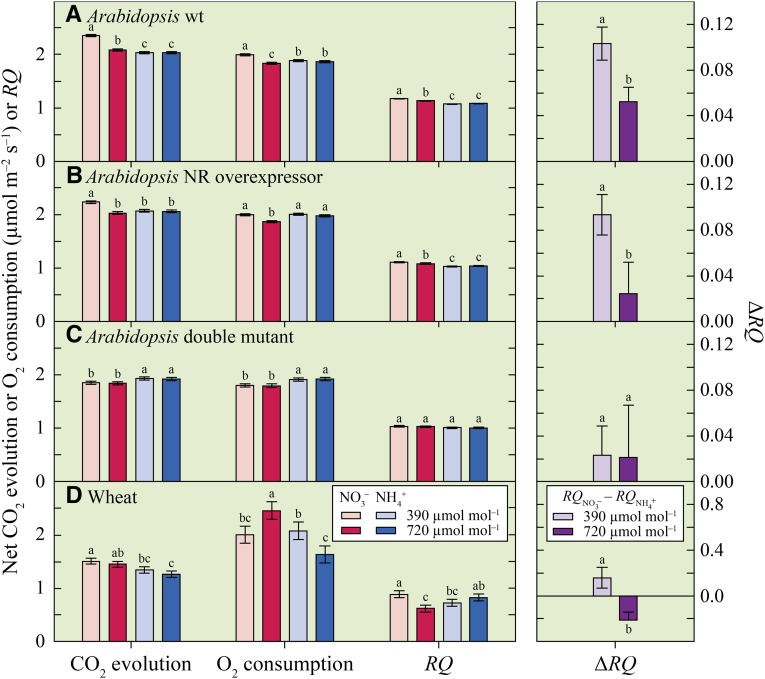
In Arabidopsis and wheat receiving NH4+ as their sole N source, neither net CO2 evolution nor net O2 consumption changed significantly with CO2 treatment (Fig. 4). Under NO3− nutrition, elevated CO2 decreased net CO2 evolution and net O2 consumption in Arabidopsis genotypes with the capacity to assimilate NO3− and increased net O2 consumption in wheat (Fig. 4, A, B, and D), but had no effect in an Arabidopsis double mutant with limited NO3− assimilation (Fig. 4C). The change in respiratory quotient (RQ) with a shift from NO3− to NH4+ nutrition (ΔRQ; where RQ is the ratio of net CO2 evolution to net O2 consumption) was insensitive to the CO2 treatment in the Arabidopsis double mutant with a limited capacity to assimilate NO3− (Fig. 4C), but decreased at elevated CO2 in Arabidopsis genotypes with the capacity to assimilate NO3− and in wheat (Fig. 4, A, B, and D).
Figure 4.
Shoot net CO2 evolution, net O2 consumption, and RQ at night under ambient (390 µmol mol−1) or elevated (720 µmol mol−1) CO2 atmosphere for three Arabidopsis genotypes and wheat receiving NO3− or NH4+ (left). The effect was measured in the Arabidobsis wild type (wt), a transformant overexpressing nitrate reductase (NR overexpressor), and a double mutant lacking detectable NR activity (double mutant). Shown are the mean ± se (n = 6–14). Changes in the shoot RQ (ΔRQ) with the shift from NO3− to NH4+ as an N source (right). For each parameter within one genotype, bars labeled with different letters differed by P < 0.10.
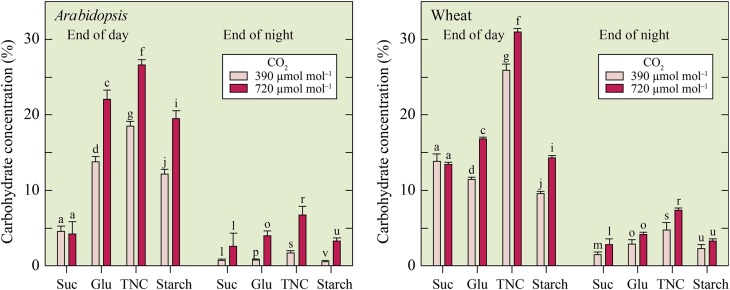
Carbohydrate concentrations at the end of the day, except for those of Suc, were higher in the elevated CO2 treatment than the ambient CO2 treatment (Fig. 5). At the end of the night, the carbohydrate concentrations were lower than at the end of the day (Fig. 5). Carbohydrate concentrations at the end of the night were generally higher in the elevated CO2 treatment than the ambient CO2 treatment (Fig. 5).
Figure 5.
Free Suc, total Glc (Glu), total nonstructural carbohydrates (TNC), and starch (Starch) concentrations in shoots of Arabidopsis and wheat at the end of the day and at the end of the night in plants exposed to ambient (390 µmol mol–1) or elevated (720 µmol mol–1) CO2 and NO3− nutrition. Concentrations are in percentage (w/w). Shown are the mean ± se (n = 3–4). For each parameter within one species, bars labeled with different letters differed by P < 0.05.
DISCUSSION
Here, six independent measures, plant biomass accumulation under NO3− nutrition (Fig. 1), shoot free NO3− concentration (Fig. 2), shoot organic N concentrations (Fig. 2), shoot δ15N in the organic fraction (Fig. 2), plant 15N-NO3− assimilation (Fig. 3), and ΔRQ (Fig. 4), indicated that elevated CO2 at night inhibited nighttime NO3− assimilation in Arabidopsis and wheat. Plants that received NH4+ as their sole N source or had mutations that limited NO3− assimilation did not respond to elevated CO2 at night (Figs. 1, 2, and 4).
Several physiological mechanisms could be responsible for these responses. Elevated CO2 inhibited the activity of the mitochondrial enzymes succinate dehydrogenase and cytochrome c oxidase (Gonzalez-Meler et al., 1996; Drake et al., 1999; Gonzàlez-Meler and Siedow, 1999). These may interfere with carbohydrate catabolism and decrease the energy available for NO3− assimilation. Elevated CO2 also inhibited the translocation of nitrite from the cytoplasm into the chloroplast, the site where the subsequent conversion into amino acids occurs (Bloom et al., 2002).
Our finding that elevated CO2 inhibited nighttime NO3− assimilation and that this inhibition could impede the growth of plants exposed to elevated CO2 at night is consistent with several other studies. In soybean (Glycine max) receiving both NH4+ and NO3− as N sources, elevated CO2 inhibited nighttime respiration, carbohydrate translocation, and NO3− assimilation as monitored from the disappearance of NO3− from leaf discs (Bunce, 2004). This most likely explains the slower growth of soybean exposed to elevated CO2 at night (Bunce, 2003). Plants may compensate to some extent for elevated CO2 during the day or night by increasing the proportion of NO3− assimilated in the roots (Kruse et al., 2002, 2003) because root NO3− assimilation is relatively insensitive to CO2 concentration (Bloom et al., 2010, 2014). Nonetheless, roots did not fully compensate for slower shoot NO3− assimilation in the current study because plant biomass (Fig. 1) and shoot organic N (Fig. 2) both tended to decline when nighttime CO2 was elevated.
The relative dependence of a plant on NO3− versus NH4+ varies with species, physiological state of the plant, and availability of each N form from the medium. The availability of each N form depends on many factors in the rhizosphere, including microbial activity, water status, pH, and cation exchange capacity (Epstein and Bloom, 2005). The wide range in plant responses to nighttime elevated CO2 that others have encountered (e.g. Bunce, 2003), therefore, may derive from differences in the relative dependence of plants on NO3− and NH4+.
The relative dependence of Arabidopsis and wheat on NO3− and NH4+ was not a factor in the current experiments because plants received either NO3− or NH4+ as a sole N source. Indeed, Arabidopsis and wheat showed similar differential responses to the N source: they both grew larger when receiving NO3− rather than NH4+ as an N source (Fig. 1), both had lower organic N concentrations when receiving NO3− rather than NH4+ as an N source (Fig. 2), and both respired more CO2 when assimilating NO3− at relatively high rates (ambient CO2, NO3− nutrition) than when assimilating NH4+ (Fig. 4).
Dark respiration, both net CO2 evolution and net O2 consumption, decreased at elevated CO2 under NO3− nutrition and did not change under NH4+ nutrition (Fig. 4). Assimilation of NO3− expends about 25% of carbon catabolism, whereas that of NH4+ expends about 3% (Bloom et al., 1992; Cousins and Bloom, 2004). Therefore, it is expected that CO2 inhibition of NO3− assimilation would produce observable changes in respiratory gas fluxes.
Our gas-exchange system sealed plants into shoot and root cuvettes with a stopper around the stem, so the surface area of the seal was minimal. Any leaks that might have occurred would have equally influenced the gas fluxes of plants receiving the two N forms and the various genotypes. Therefore, the differences in gas fluxes that we observed among N forms and genotypes did not derive from the measurement artifacts that others have encountered (e.g. Jahnke and Krewitt, 2002).
The RQ, ratio of net CO2 evolved to net O2 consumed, was generally higher in Arabidopsis than wheat (Fig. 4). This suggests that Arabidopsis and wheat were catabolizing different substrates. For example, catabolism of malic acid results in an RQ of 1.33, whereas that of carbohydrates results in an RQ of 1.0, that of lipids results in an RQ of 0.70, and that of ketones results in an RQ of 0.66 (Stiles, 1994). Differences between Arabidopsis and wheat in the substrates supporting dark respiration will require further examination, but carbohydrate concentrations showed similar changes overnight in both species (Fig. 5).
Plant carbohydrate concentrations can influence the concentrations of other plant constituents such as NO3− and organic N. Nonetheless, plant material for the NO3− and organic N analyses was collected in the morning when the differences in carbohydrates between the CO2 treatments were relatively small, only a few percent of dry mass (Fig. 5). This could decrease the difference in NO3− between the CO2 treatments and contribute to the increase in organic N, but would not be sufficient to account for all of the observed differences between the CO2 treatments.
Our results have profound implications for research on plant responses to elevated CO2. Some free-air CO2 enrichment experiments expose plants to elevated CO2 only during the day and let CO2 return to ambient levels at night. This is to avoid the added expense of applying concentrated CO2 at night and to avoid the difficulties of controlling atmospheric CO2 concentrations when photosynthesis does not provide a strong sink for the CO2. The large variation in plant responses to elevated CO2 among field experiments (Ainsworth and Long, 2005) may derive in part from differences in nighttime CO2 concentrations.
Few studies on plant responses to elevated CO2 have attempted to define the form of N that the plants are using. Our data demonstrate that N form and nighttime atmospheric CO2 concentration are critical factors in determining plant performance under the environmental conditions anticipated during the next few decades. Indeed, the future of food quality, in terms of protein and other nutrients (Myers et al., 2014), and the extent to which plants serve as sinks for human CO2 emissions (Bloom, 2010) will depend on the relationship between elevated CO2 and N form.
MATERIALS AND METHODS
Plant Material
We used Arabidopsis (Arabidopsis thaliana) ‘Columbia’ and wheat (Triticum aestivum) ‘Veery.’ For the gas exchange experiments, we also used two Arabidopsis ‘Columbia’ genotypes that exhibited different levels of NR activity (Rachmilevitch et al., 2004): a transgenic line harboring the chimeric gene Lhch1*3::Nia1*2 (the Arabidopsis nitrate reductase gene under the regulation of the light-harvesting chlorophyll a/b protein promoter) that had about twice the NR activity of the wild type (Heimer et al., 1995) and a genotype with mutations in both structural genes for NR, nia1 nia2, which had little detectable NR activity (Wilkinson and Crawford, 1993).
Growth Conditions
Arabidopsis seeds were germinated and grown in Magenta boxes for 12 d. For the first 3 d, the boxes were covered with foil, and then over the next 9 d, the plants were gradually acclimated to light. A pool of seedlings (60 per tub) were transplanted and kept for 3 d in 5-L opaque polyethylene tubs filled with an aerated nutrient solution, and the shoots were covered with transparent plastic trays. During this period (12 d + 3 d), all plants received the same nutrient solution: macronutrients (mm) at 1.25 CaSO4, 0.2 KNO3, 0.2 NH4Cl, 0.75 MgSO4, 0.25 KH2PO4, and 0.75 K2HPO4; micronutrients (µm) at 50 KCl, 25 H3BO3, 2 MnSO4H2O, 2 ZnSO4, 7 H2O, 0.5 CuSO4, 5 H2O, and 0.5 Na2MoO4; and Fe-NaDPTA (Sequestrene 330, Becker Underwood) at 0.2 g L–1. The most uniform seedlings were transplanted to 5-L tubs (10 plants per tub) and placed in controlled environmental growth chambers (Conviron PGR15), four tubs per chamber. The chambers had a 9-h-light period of 350 µmol m–2 s–1 of photosynthetic active radiation at plant height, 21°C, and 80% humidity, and a 15-h-dark period, 21°C, and 60% humidity. Nutrient solution was changed twice during the first week, three times during the second week, and every other day thereafter.
Wheat seeds were surface sterilized with 20% (v/v) NaOCl and then washed thoroughly with water. Healthy seeds were rolled up in a paper towel soaked with 10 mm CaSO4 for 4 d at 25°C in the dark, with the bottom one-fourth of the rolled towel sitting in a 10 mm CaSO4 solution. The most uniform wheat seedlings were transplanted to 20-L opaque polyethylene tubs (10 seedlings per tub) filled with a nutrient solution containing: macronutrients (mm) at 1.0 CaSO4, 0.2 KNO3, 0.2 NH4Cl, 1.0 MgSO4, 0.5 KH2PO4, and 0.5 K2HPO4; micronutrients (µm) at 50 KCl, 25 H3BO3, 2 MnSO4H2O, 2 ZnSO4, 7 H2O, and 0.5 H2MoO4; and Fe-NaDPTA (Sequestrene 330, Becker Underwood) at 0.2 g L–1. Plants grew in controlled environmental chambers (Conviron PGR15) with 15 h of 500 µmol m–2 s–1 of photosynthetic active radiation at plant height, 25°C, and 70% humidity, and with 9 h of dark, 16°C, and 60% humidity. Nutrient solution was changed every 3 d during the first week and every other day thereafter.
Growth and N Balance Experiments
Arabidopsis and wheat plants were exposed for 20 and 10 d, respectively, to either 0.2 mm KNO3 or 0.2 mm NH4Cl as an N source and one of four CO2 treatments: ambient CO2 during the day and night (390/390 µmol mol–1), ambient CO2 during the day and elevated CO2 at night (390/720 µmol mol–1), elevated CO2 during the day and ambient CO2 at night (720/390 µmol mol–1), or elevated CO2 during the day and night (720/720 µmol mol–1). Two controlled environmental chambers were equipped with nondispersive infrared analyzers (Horiba APBA-250E) and control systems that added CO2 (filtered through a KMnO4 column to remove contaminating hydrocarbons such as ethylene) to maintain one at ambient (390 μmol mol−1) and the other at elevated (720 μmol mol−1) CO2 concentrations. The chambers were shifted to the alternative CO2 concentration in replicate experiments. The lids of the 5- and 20-L tubs were cut into halves; this allowed us to shift one-half of the plants from one chamber to the other chamber for the treatments that had different CO2 concentrations during the day and night. The shift occurred within 30 min of the chamber lights turning on or off. At this time, even plants that remained in the same chamber were briefly lifted out of the nutrient solution tub.
After 35 d for Arabidopsis and 14 and 30 d, respectively, for the growth and N balance of wheat, plants were harvested in the morning soon after the lights turned on when shoot carbohydrate levels were relatively low. The roots were separated from shoots and rinsed in a chilled solution containing 1 mm CaSO4. Then, both shoots and roots were placed in a forced-air drying oven for 3 d at 60°C. Shoots and roots were ground to a fine powder in a ball mill.
Total N and total N isotope ratios were determined by a PDZ Europa ANCA-GSL elemental analyzer interfaced to an isotope ratio mass spectrometer (Sercon Ltd.) at the University of California Davis Stable Isotope Facility. During analysis, samples were interspersed with several replicates of at least two different laboratory standards. These laboratory standards, selected to be compositionally similar to the samples being analyzed, were previously calibrated against National Institute of Standards and Technology standard reference materials (IAEA-N1, IAEA-N2, IAEA-N3, IAEA-CH7, and NBS-22). The final delta values were expressed relative to air. The NO3− concentration of the diluted extracts was determined spectrophotometrically (Doane and Horwath, 2003). Organic N was estimated from the difference between total N and unassimilated NO3− because NH4+ concentrations in these species are low and do not vary significantly with CO2 treatment (Bloom et al., 2002). We conducted two replicate experiments for each species.
Natural Abundance of Organic 15N
15N-organic N was estimated from the difference between total 15N and 15N-NO3−. The N isotopic composition of plant NO3− extracts was analyzed from N2O generated by denitrifying bacteria lacking N2O reductase (Sigman et al., 2001) at the UC Davis Stable Isotope Facility. In brief, Pseudomonas chlororaphis were grown in a tryptic soy broth amended with NO3− for 7 d. During this time, the O2 in the headspace of the medium bottles and the NO3− in the medium were consumed. Concentrated 2-mL aliquots of this culture were then divided into 20-mL headspace vials that were sealed and purged for 2 h with N2 gas to remove N2O and O2. Samples of the plant tissue extracts containing 0.1 µmol NO3−-N were injected through the septae of the vials. The conversion of NO3− to N2O was complete within less than 1 h. The N2O was flushed from the vials with helium, trapped cryogenically, and then released into the isotope ratio mass spectrometer. Standards of KNO3 (IAEA-N1, IAEA-N2, and IAEA-N3), having δ15N values that bracketed the values of our samples, were processed in the same manner as the plant tissue extracts and converted to N2O by the bacteria. A linear regression between measured versus known δ15N values of the standards was used to adjust the δ15N values of the samples. The adjustments were typically between 1‰ and 2‰ δ15N. The final delta values were expressed relative to air.
15N-NO3− Labeling Experiments
Measurements of NO3− uptake and assimilation were made on 35- to 36-d-old Arabidopsis plants and 14-d-old wheat plants. These plants were grown on the nutrient solution described earlier but with both NO3− and NH4+ as N sources. The night before 15N-NO3− labeling, 12 plants were transferred from the controlled environment chamber to a multiplant measurement system in the laboratory (Kosola and Bloom, 1994). The root of each plant was sealed by a rubber stopper around the stem into cuvettes supplied with a continuous flow of nutrient solution. The nutrient solution contained 0.2 mm KNO3, 1 mm CaSO4, 5 µM KH2PO4, and 5 µM K2HPO4.
The plants were kept in the laboratory for two night periods interrupted by one light period. In the first night period (8 h), the plants were allowed to recover from any transplant shock. During the following light period, the shoots were exposed to either an ambient (390 µmol mol–1) or elevated (720 µmol mol–1) CO2 concentration. This light period was 9 h for Arabidopsis and 12 h for wheat; light intensity was 350 and 500 µmol m–2 s–1 for Arabidopsis and wheat, respectively; and the temperature was set at 25°C and 22°C for Arabidopsis and wheat, respectively. The next night period and after 1 h of acclimation to darkness, the nutrient solution containing natural abundance levels of 15N-NO3− was switched to one containing 25 atom % 15N-NO3−. During this second night period, NO3− uptake and assimilation were assayed using the tracer 15N in plants exposed to either an ambient (390 µmol mol–1) or elevated (720 µmol mol–1) CO2 concentration. This night period was 12 and 8 h for Arabidopsis and wheat, respectively, and the temperature was controlled at 20°C.
Before beginning the labeling period, we harvested five plants, and after the labeling period, we harvested seven plants. The roots were rinsed in a chilled solution with 1 mm CaSO4. Shoots and roots were dried at 60°C for 3 d and ground to a fine powder in a ball mill. Total N and NO3− tissue concentration and its ratio, 15N/14N, were analyzed as described earlier. Plant absorption of 15N-NO3− was calculated from the difference in total 15N between the plants harvested after the labeling period and those harvested before. Plant assimilation of 15N-NO3− was calculated from the difference in 15N-organic N between the plants harvested after the labeling period and those harvested before.
Gas Exchange Experiments
We monitored net CO2 evolution and net O2 consumption simultaneously from the whole canopy and calculated the RQ (ratio of net CO2 evolved to net O2 consumed). The differences in the RQ between NH4+-fed and NO3−-fed plants (ΔRQ) reflect NO3− assimilation in the nighttime because electrons generated from the catabolism of carbohydrates to CO2 are transferred to NO3− or nitrite rather than O2 (Bloom et al., 1992). Thus, ΔRQ (change in RQ with a shift in N source) has provided real-time, nondestructive estimates of NO3− assimilation for nearly a century (Warburg and Negelein, 1920; Van Niel et al., 1953). Measurements of net CO2 assimilation and net O2 evolution were made on 35- to 36-d-old Arabidopsis and 14-d-old wheat plants.
Two days before gas exchange measurements, a plant was switched from a nutrient solution containing 0.2 mm NH4NO3 to one containing 0.2 mm NH4Cl to deplete NO3− from the plant tissue. To monitor gas fluxes, the stem of an intact plant was wrapped with Teflon plumber’s tape, and a thin layer of silicon vacuum grease was applied to the outside. A slit rubber stopper with an appropriately sized hole was fit around the taped stem. The stopper sealed the root system of this intact plant into a root cuvette made of acrylic plastic and stainless steel for both Arabidopsis and wheat and its shoot system into a shoot cuvette made of glass and Teflon-coated aluminum for Arabidopsis and into a gold-plated cuvette with a glass top for wheat (Bloom et al., 1989). Roots remained in the dark at 18°C and were supplied with a continuous flow of an aerated nutrient solution containing 1 mm CaSO4, 0.5 µM K2HPO4, and either 200 µM KNO3 or 200 µM NH4Cl. The pH of the solution was 6.0. The leaves in the shoot cuvette retained their natural orientation to the light source (1,000-W metal-halide lamp; Wide-Lite). The light levels during the light cycles were 350 and 500 µmol m–2 s–1 for Arabidopsis and wheat, respectively. The light-dark cycle was the same as in the controlled environmental chambers. Two 0.07-mm copper-constantan thermocouples were placed on the abaxial side of two leaves to monitor leaf temperatures.
The plant was kept in the lab for 2 d, the first day with NH4+ as the sole N source and the second day with NO3− as the sole N source. During the first light period, the plant was allowed to recover from any transplant shock (Bloom and Sukrapanna, 1990). During the subsequent dark period, the gas exchange of the NH4+-fed plant was monitored. At the start of the second light period, the nutrient solution was switched to one containing NO3− as the sole N source. Finally, during the second dark period, the gas exchange of the NO3−-fed plant was monitored. Gas exchange measurements began 1 h into the dark period. Plants were subjected to each CO2 concentration, 390 and 720 µmol mol–1 CO2, for 30 min before taking a measurement, shifting back and forth three times between the two concentrations. The rate of respiration at a given CO2 concentration did not change significantly during the 2 h between the measurements (P > 0.9). After the 2-d measurement period, we determined the leaf area and calculated specific respiration rates on a leaf area basis (either CO2 evolution or O2 consumption in µmol m−2 s−1; Bloom et al., 1980). In addition, shoot and root dry weights were determined.
An open gas exchange system (Bloom et al., 1989) monitored net CO2 assimilation, net O2 evolution, and transpiration using a commercial nondispersive infrared CO2 analyzer (Horiba model VIA-500R), a custom-designed O2 analyzer, and relative humidity sensors (Vaisala), respectively. The custom O2 analyzer contained two cells of calcia-stabilized zirconium oxide ceramic similar to those found in an Applied Electrochemistry model N-37 M. When heated to 752 ± 0.01°C in an electric furnace, these cells become selectively permeable to O2, and at the ambient O2 concentration (20.97% O2), generate a Nernst potential of 106 nV per µmol mol–1 difference in O2 concentration. The oxygen analyzer resolves 2 µmol mol–1 O2 partial pressure difference on a background of 209,490 µmol mol–1. Mass flow controllers (Tylan) mixed 2% CO2 in air from a compressed gas cylinder and CO2-free air from a 100-L storage tank to obtain the 390 and 720 µmol mol–1 CO2 concentrations. The flow rate through the shoot chamber was 10 cm3 s–1. A pressure transducer (Validyne) monitored the pressure drop across a capillary to measure the gas flow through the shoot chamber. The leaf vapor pressure deficit was maintained at approximately 10 mbar. To check for leaks, we periodically confirmed that the net flux rates of CO2 and O2 in an empty cuvette were zero through a range of known cuvette CO2 concentrations.
Carbohydrate Analysis
Shoots of plants used in the dark 15N-labeling experiments were also analyzed for carbohydrates. Samples were extracted by hot deionized water, and the extract was analyzed for free sugars (Glc, Fru, and Suc). The samples for total Glc were enzymatically hydrolyzed at 55°C with amyloglucosidase for 12 h and analyzed for free Glc. The analyses were conducted by HPLC with mass selective detection (Johansen et al., 1996) using a Phenomenex Luna NH2 HPLC column (250 × 4.6 mm) at a flow rate of 2.75 mL min–1 acetonitrile:water (78:22). The method has a detection limit of 0.2% and is reproducible within 10% (relative). Total nonstructural carbohydrate was calculated from the sum of total Glc, free Fru, and free Suc, whereas starch was calculated from total Glc minus free Glc multiplied by 0.9 (Smith, 1969).
Statistics
An ANOVA was conducted (PROC MIXED in SAS 9.3, SAS Institute). All of the data met the assumptions of normality and homogeneity of variance as evaluated via the Shapiro-Wilks and Levene’s tests, respectively. We determined the effects of N form and CO2 treatment and their interaction on the different parameters evaluated. Tukey’s post hoc test was conducted on the differences between means.
Acknowledgments
We thank Brian Hua and the staff of the Stable Isotope Facility at the University of California (Davis) for help with analyzing samples and Francisco del Amor, Matthew Gilbert, William Horwath, James Richards, Francisco García-Sánchez, Lucas Silva, Astrid Volder, and Maciej Zwieniecki for providing many suggestions about the article.
Glossary
- N
nitrogen
- NO3−
nitrate
- NH4+
ammonium
- RQ
respiratory quotient
- NR
nitrate reductase
Footnotes
This work was supported by the National Science Foundation (grant nos. IOS–13-58675 and IOS–08-18435), the U.S. Department of Agriculture National Research Initiative Competitive Research Grant (grant no. 2008–0214546), and the Agencia Regional de Ciencia y Tecnología de la Región de Murcia, Spain (fellowship to J.S.R.A.).
Articles can be viewed without a subscription.
References
- Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165: 351–371 [DOI] [PubMed] [Google Scholar]
- Amthor JS. (1991) Respiration in a future, higher-CO2 world. Plant Cell Environ 14: 13–20 [Google Scholar]
- Amthor JS. (1995) Terrestrial higher-plant response to increasing atmospheric [CO2] in relation to the global carbon cycle. Global Change Biol 1: 243–274 [Google Scholar]
- Baker JT, Allen LH, Boote KJ, Pickering NB (2000) Direct effects of atmospheric carbon dioxide concentration on whole canopy dark respiration of rice. Global Change Biol 6: 275–286 [Google Scholar]
- Bloom AJ. (2010) Global Climate Change: Convergence of Disciplines. Sinauer Assoc., Sunderland, MA [Google Scholar]
- Bloom AJ. (2015) Photorespiration and nitrate assimilation: a major intersection between plant carbon and nitrogen. Photosynth Res 123: 117–128 [DOI] [PubMed] [Google Scholar]
- Bloom AJ, Asensio JS, Randall L, Rachmilevitch S, Cousins AB, Carlisle EA (2012) CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 93: 355–367 [DOI] [PubMed] [Google Scholar]
- Bloom AJ, Burger M, Kimball BA, Pinter PJ (2014) Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nature Clim Change 4: 477–480 [Google Scholar]
- Bloom AJ, Burger M, Rubio Asensio JS, Cousins AB (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328: 899–903 [DOI] [PubMed] [Google Scholar]
- Bloom AJ, Caldwell RM, Finazzo J, Warner RL, Weissbart J (1989) Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol 91: 352–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Mooney HA, Björkman O, Berry J (1980) Materials and methods for carbon dioxide and water exchange analysis. Plant Cell Environ 3: 371–376 [Google Scholar]
- Bloom AJ, Smart DR, Nguyen DT, Searles PS (2002) Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc Natl Acad Sci USA 99: 1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Sukrapanna SS (1990) Effects of exposure to ammonium and transplant shock upon the induction of nitrate absorption. Plant Physiol 94: 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Sukrapanna SS, Warner RL (1992) Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol 99: 1294–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn D, Mikkelsen TN, Atkin OK (2002) Does the direct effect of atmospheric CO2 concentration on leaf respiration vary with temperature? Responses in two species of Plantago that differ in relative growth rate. Physiol Plant 114: 57–64 [PubMed] [Google Scholar]
- Bunce JA. (2001) Effects of prolonged darkness on the sensitivity of leaf respiration to carbon dioxide concentration in C3 and C4 species. Ann Bot (Lond) 87: 463–468 [Google Scholar]
- Bunce JA. (2003) Responses of seedling growth to daytime or continuous elevation of carbon dioxide. Int J Plant Sci 164: 377–382 [Google Scholar]
- Bunce JA. (2004) A comparison of the effects of carbon dioxide concentration and temperature on respiration, translocation and nitrate reduction in darkened soybean leaves. Ann Bot (Lond) 93: 665–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle E, Yarnes C, Toney MD, Bloom AJ (2014) Nitrate reductase (15)N discrimination in Arabidopsis thaliana, Zea mays, Aspergillus niger, Pichea angusta, and Escherichia coli. Front Plant Sci 5: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Booker FL, Tu C, Burkey KO, Zhou L, Shew HD, Rufty TW, Hu S (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337: 1084–1087 [DOI] [PubMed] [Google Scholar]
- Cotrufo MF, Ineson P, Scott A (1998) Elevated CO2 reduces the nitrogen concentration of plant tissues. Global Change Biol 4: 43–54 [Google Scholar]
- Cousins AB, Bloom AJ (2004) Oxygen consumption during leaf nitrate assimilation in a C3 and C4 plant: the role of mitochondrial respiration. Plant Cell Environ 27: 1537–1545 [Google Scholar]
- Doane TA, Horwáth WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36: 2713–2722 [Google Scholar]
- Drake BG, Azcon-Bieto J, Berry J, Bunce J, Dijkstra P, Farrar J, Gifford RM, Gonzalez-Meler MA, Koch G, Lambers H. , et al (1999) Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant Cell Environ 22: 649–657 [Google Scholar]
- Easlon HM, Carlisle E, McKay JK, Bloom AJ (2015) Does low stomatal conductance or photosynthetic capacity enhance growth at elevated CO2 in Arabidopsis thaliana? Plant Physiol 167: 793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Bloom AJ (2005) Mineral Nutrition of Plants: Principles and Perspectives, Ed 2 Sinauer Associates, Sunderland, MA [Google Scholar]
- Farrar JF, Williams ML (1991) The effects of increased atmospheric carbon dioxide and temperature on carbon partitioning, source-sink relations and respiration. Plant Cell Environ 14: 819–830 [Google Scholar]
- Foyer CH, Bloom AJ, Queval G, Noctor G (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu Rev Plant Biol 60: 455–484 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Meler MA, Ribas-Carbo M, Siedow JN, Drake BG (1996) Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol 112: 1349–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzàlez-Meler MA, Siedow JN (1999) Direct inhibition of mitochondrial respiratory enzymes by elevated CO(2): does it matter at the tissue or whole-plant level? Tree Physiol 19: 253–259 [DOI] [PubMed] [Google Scholar]
- Griffin KL, Sims DA, Seemann JR (1999) Altered night-time CO2 concentration affects the growth, physiology and biochemistry of soybean. Plant Cell Environ 22: 91–99 [Google Scholar]
- Hamilton JG, Thomas RB, Delucia EH (2001) Direct and indirect effects of elevated CO2 on leaf respiration in a forest ecosystem. Plant Cell Environ 24: 975–982 [Google Scholar]
- Heimer YM, Brusslan JA, Kenigsbuch D, Tobin EM (1995) A chimeric Lhcb:Nia gene: an inducible counter selection system for mutants in the phytochrome signal transduction pathway. Plant Mol Biol 27: 129–136 [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge U. Press, Cambridge. [Google Scholar]
- Jahnke S, Krewitt M (2002) Atmospheric CO2 concentration may directly affect leaf respiration measurement in tobacco, but not respiration itself. Plant Cell Environ 25: 641–651 [Google Scholar]
- Johansen HN, Glitso V, Knudsen KEB (1996) Influence of extraction solvent and temperature on the quantitative determination of oligosaccharides from plant materials by high-performance liquid chromatography. J Agric Food Chem 44: 1470–1474 [Google Scholar]
- Kosola KR, Bloom AJ (1994) Methylammonium as a transport analog for ammonium in tomato (Lycopersicon esculentum L.). Plant Physiol 105: 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J, Hetzger I, Hänsch R, Mendel RR, Walch-Liu P, Engels C, Rennenberg H (2002) Elevated pCO(2 )favours nitrate reduction in the roots of wild-type tobacco (Nicotiana tabacum cv. Gat.) and significantly alters N-metabolism in transformants lacking functional nitrate reductase in the roots. J Exp Bot 53: 2351–2367 [DOI] [PubMed] [Google Scholar]
- Kruse J, Hetzger I, Mai C, Polle A, Rennenberg H (2003) Elevated pCO2 affects N-metabolism of young poplar plants (Populus tremula × P. alba) differently at deficient and sufficient N-supply. New Phytol 157: 65–81 [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60: 2859–2876 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang G, Sun B, Zhang S, Zhang Y, Liao Y, Zhou Y, Xia X, Shi K, Yu J (2013) Stimulated leaf dark respiration in tomato in an elevated carbon dioxide atmosphere. Sci Rep 3: 3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55: 591–628 [DOI] [PubMed] [Google Scholar]
- Markelz RJC, Lai LX, Vosseler LN, Leakey ADB (2014) Transcriptional reprogramming and stimulation of leaf respiration by elevated CO2 concentration is diminished, but not eliminated, under limiting nitrogen supply. Plant Cell Environ 37: 886–898 [DOI] [PubMed] [Google Scholar]
- Myers SS, Zanobetti A, Kloog I, Huybers P, Leakey ADB, Bloom AJ, Carlisle E, Dietterich LH, Fitzgerald G, Hasegawa T, et al. (2014) Increasing CO2 threatens human nutrition. Nature 510: 139–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Fernie AR, Stitt M (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant 3: 973–996 [DOI] [PubMed] [Google Scholar]
- Pleijel H, Högy P (2015) CO2 dose-response functions for wheat grain, protein and mineral yield based on FACE and open-top chamber experiments. Environ Pollut 198: 70–77 [DOI] [PubMed] [Google Scholar]
- Pleijel H, Uddling J (2012) Yield vs. quality trade-offs for wheat in response to carbon dioxide and ozone. Global Change Biol 18: 596–605 [DOI] [PubMed] [Google Scholar]
- Rachmilevitch S, Cousins AB, Bloom AJ (2004) Nitrate assimilation in plant shoots depends on photorespiration. Proc Natl Acad Sci USA 101: 11506–11510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman DM, Casciotti KL, Andreani M, Barford C, Galanter M, Böhlke JK (2001) A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem 73: 4145–4153 [DOI] [PubMed] [Google Scholar]
- Smart DR, Bloom AJ (1993) Relationships between the kinetics of NH4+ and NO3− absorption and growth in the cultivated tomato (Lycopersicon esculentum Mill, cv T-5). Plant Cell Environ 16: 259–267 [Google Scholar]
- Smith D. (1969) Removing and analyzing total nonstructural carbohydrates from plant tissue. Wisconsin Agricultural Experimental Station Research Reports 41: 1–11 [Google Scholar]
- Stiles W. (1994) Principles of Plant Physiology. Discovery House Publishers, Grand Rapids, MI [Google Scholar]
- Van Niel CB, Allen MB, Wright BE (1953) On the photochemical reduction of nitrate by algae. Biochim Biophys Acta 12: 67–74 [DOI] [PubMed] [Google Scholar]
- Wang X, Curtis P (2002) A meta-analytical test of elevated CO2 effects on plant respiration. Plant Ecol 161: 251–261 [Google Scholar]
- Wang X, Lewis JD, Tissue DT, Seemann JR, Griffin KL (2001) Effects of elevated atmospheric CO2 concentration on leaf dark respiration of Xanthium strumarium in light and in darkness. Proc Natl Acad Sci USA 98: 2479–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O, Negelein E (1920) Über die Reduktion der Salpetersäure in grünen Zellen. Biochem Z 110: 66–115 [Google Scholar]
- Wilkinson JQ, Crawford NM (1993) Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 and NIA2. Mol Gen Genet 239: 289–297 [DOI] [PubMed] [Google Scholar]