Abstract
An epithelial-to-mesenchymal transition (EMT) is thought to be an important process in the acquisition of capabilities required for metastasis. Until recently, studies of EMT involved mostly in vitro assays and transplantation experiments of cancer cells that overexpressed known EMT drivers. While valuable, these studies do not allow us to conclude if an EMT sustained under “physiologic conditions” within the tumor microenvironment leads to the myriad changes in phenotype observed in vitro. Here we review our recently published work using a lineage labeled genetically engineered mouse model of pancreatic ductal adenocarcinoma to characterize cells that have sustained an EMT in vivo.
An epithelial-to-mesenchymal transition (EMT) is a complex reprogramming event during which epithelial cells lose many of the properties of such cells, including maintaining tight cell-to-cell contacts and apical-basal polarity, and gain abilities and characteristics commonly ascribed to mesenchymal cells, including invasive capacity, motility, and anoikis and apoptosis resistance1. Initially described in the context of embryonic development2, a wealth of published reports have implicated EMT as being involved in allowing for cancer cells to invade, disseminate and metastasize3. Indeed, pioneering work by the laboratories of Thiery, Weinberg, and Polyak, among many others, have established a potentially central role for EMT in the metastatic cascade4 and in the generation of cancer cells that are enriched for stem cell-like traits5. In this brief synopsis, we will review the state of evidence implicating EMT in metastasis and cancer and review recently published data that provides the first evidence that EMT under physiologic conditions may be involved in the acquisition of a disseminating phenotype that is enriched for tumor-initiating capability.
Theoretically, EMT provides a compelling mechanism by which epithelial cancer cells can acquire the abilities necessary to establish metastatic disease. The preponderance of data implicating EMT to cancer, however, involves genetically manipulated cells in vitro or transplantation models in vivo, neither of which fully recapitulate the salient aspects of tumor formation in humans. The vast majority of studies in the field feature human cancer cell lines in which selected drivers of EMT are either silenced or overexpressed, the implications of which are assessed through various in vitro assays or orthotopically in immunocompromised murine hosts. Indeed, through these systems, strong evidence has been presented that various EMT drivers, such as Twist1, Snail1, and Zeb1, among others, may be required for the acquisition and/or maintenance of an invasive and motile phenotype6–9. Furthermore, elegant studies by the Weinberg group and others has established then upon an EMT, induced by overexpression of Snail or Twist, mammary cells can acquire a phenotype reminiscent of stem cells, replete with the capacity to self-renew and initiate tumors in xenografts5 and may lead to a change in the chemosensitivity within these cells10–13.
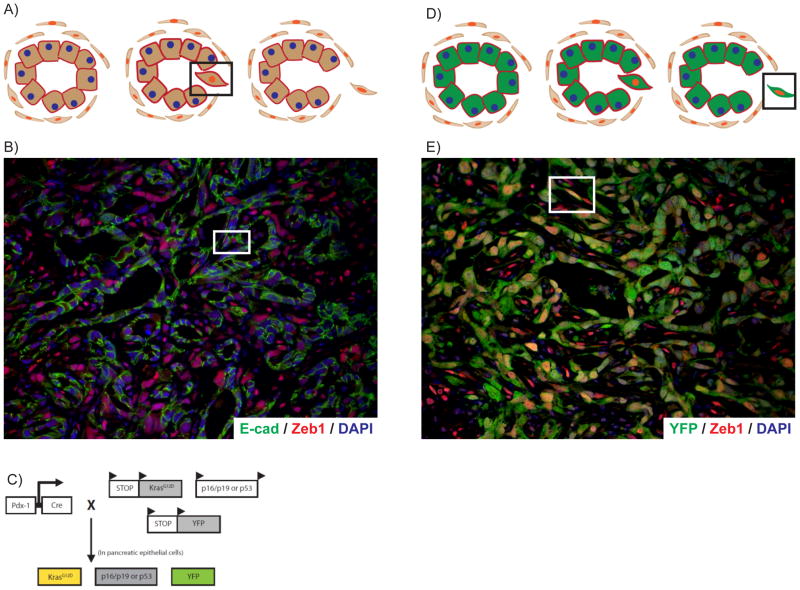
However, it has yet to be determined, whether cancer cells that undergo EMT under “physiologic” conditions, within the tumor microenvironment and in the absence of genetic manipulation, also acquire an invasive and stem cell-like phenotype. Addressing this question has been hindered by the intrinsic difficulty of identifying (and hence isolating) cancer cells from a tumor that have sustained an EMT in vivo in the first place. As illustrated in Fig. 1A, when epithelial cells undergo an EMT, de novo expression of a mesenchymal program is accompanied by the progressive downregulation of epithelial-specific markers and proteins. When an EMT is completed, cells lack epithelial markers and the typical polarized morphology used to identify such cells on histology, making it almost impossible to identify which cells within a tumor derive from an EMT. However, investigators used double-immunofluorescence analysis to identify tumor cells that express both epithelial and mesenchymal markers, representing cells in the process of undergoing an EMT (highlighted in square); indeed, using this strategy, pathologists have been able to identify approximately 9% of all tumor cells have evidence of an EMT (Fig. 1B). However, since most reliable mesenchymal markers are intracellular, the isolation of such cells using FACS sorting has heretofore not been successful.
Figure 1. Lineage labeling is a sensitive tool for the detection of EMT in tumors.
A) Illustration of the detection of EMT using conventional methods. In this scenario, transitional cells undergoing an EMT can only be detected using immunofluorescence by finding cells that express both epithelial and mesenchymal markers (highlighted by square). This strategy does not allow for robust detection of cells that have completed an EMT. B) Example of using conventional methods to identify bi-phenotypic transitional cells (see square). Green, E-cad (epithelial marker); Red, Zeb1 (mesenchymal marker); Blue, DAPI (nuclear stain). C) Schematic of the lineage labeled mouse model to study PanIN and PDAC in vivo. D) Illustration of how genetic lineage labeling allows for more sensitive detection of EMT in vivo. Since all pancreas epithelial derived cells are marked with YFP (green), cells undergoing and having completed and EMT can be identified as cells that are YFP+ and express a mesenchymal marker (red). E) Example of using the YFP lineage label to identify EMT in situ within a murine tumor. A cell exhibiting both morphologic features and marker expression of an EMT is highlighted in the square. Green, YFP (pancreas epithelial lineage label); Red, Zeb1 (mesenchymal marker); Blue, DAPI (nuclear stain).
We have recently developed a system to identify and study EMT under “physiologic conditions” within a genetically engineered mouse model of pancreatic ductal adenocarcinoma (PDAC), termed PKCY14. In this model, depicted in Fig. 1C, Cre-recombinase technology is used to achieve pancreas epithelial-specific expression of two of the most common genetic alterations in human PDAC: an oncogenic mutation in Kras (codon 12) and a single inactivated allele of the p53 tumor suppressor. In this well-established model, derived from seminal work by David Tuveson and colleagues15, 10-wk old mice contain only precancerous pancreatic intraepithelial neoplasias (PanINs) and no histologic evidence of cancer (heretofore called PanIN mice) and by about 16 weeks of age, pancreas tumors with metastases form (PDAC mice). Moreover, the tumors that arise in this model recapitulate most of the salient features of PDAC that are lacking within conventional xenograft or orthotopic transplantation models, including a dense inflammatory desmoplasia and relative hypovascularity within the tumor microenvironment16. To indelibly mark all pancreas epithelial-derived cells, a conditional yellow fluorescent protein (YFP) allele was bred into mice. This lineage label allows for the detection of all cancer cells derived from the epithelial compartment within the pancreas, including those that have completed an EMT. Thus, by finding YFP+ cells that also express a mesenchymal marker (Fig. 1D, highlighted by square), one may be able to more sensitively identify cells that have undergone an EMT compared to conventional methods. Indeed, using this approach, 42.2% of all YFP+ tumor cells within PKCY mice were found to have evidence of an EMT—a 4–5-fold increase in the rate of detection (Fig. 1E). Thus, lineage labeling is a sensitive tool to detect EMT in situ, within the confines of a tumor. Using this tool, we were also able to identify evidence of EMT within a portion of PanIN 2 and 3 lesions in mice aged 10 weeks, prior to the histologic appearance of cancer. This “early” EMT was confirmed on the transcriptional level (data not shown).
Genetic lineage labeling also creates an opportunity to specifically isolate populations of pancreas epithelial-derived cells within PKCY mice (Fig. 2A). Since all pancreas epithelial-derived cells endogenously express YFP, FACS sorting can be used to isolate these cells away from stromal cells within pancreas preps and circulating pancreas cells (CPCs) from blood specimens. Indeed, CPCs could be recovered from not only tumor-bearing mice, but also 10 week old PanIN mice. CPCs were found to be enriched for cells that expressed mesenchymal markers such as Zeb1 and Snail1 and were devoid of epithelial markers such as E-cadherin (Ecad) and cytokeratin 19 using a FACS-based assay. Thus, in a spontaneous genetic mouse model of PDAC, cells that have acquired the ability to enter the bloodstream were enriched for a mesenchymal immunophenotype, supporting the notion that an EMT under physiologic conditions was indeed associated with an invasive and disseminating phenotype. Moreover, 100-fold more CPCs expressed both CD24 and CD44, putative pancreatic cancer stem cell markers as identified by Simeone and colleagues17, compared to sister YFP+ tumor cells in the pancreas in PanIN and PDAC mice (Fig. 2B). This provided further evidence that a physiologic EMT may be associated with a stem cell-like phenotype.
Figure 2. Functional analysis of PDAC cells in PKCY mice reveals EMT+ cells may be enriched for stem cell-like traits.
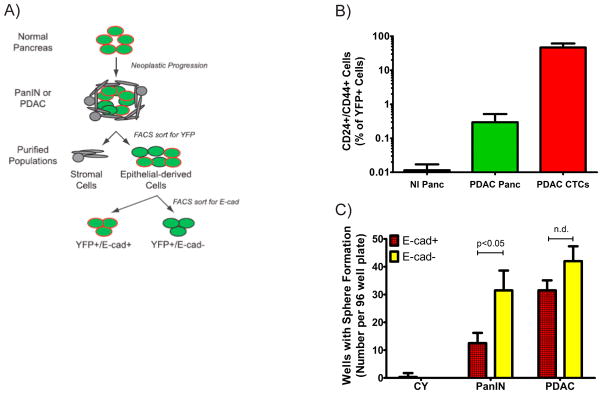
A) Schema depicting isolation of various populations of pancreas epithelial-derived cells from PKCY mice. B) Ratio of CD24+, CD44+ cells within sorted YFP+ cells in Pdx-Cre; YFP (nl) mice, and tumor-bearing PKCY mice from the pancreas (Panc) and the circulation (CTCs). C) Determination of pancreatosphere formation from single sorted YFP+ cells as denoted after seven days of culture. n=3–5 for each cohort.
Our genetic lineage labeling approach also allows for functional characterization of cells that have sustained an EMT. By staining for the surface epithelial marker, E-cadherin (E-cad), one can isolate YFP+ cells that are enriched for an EMT (i.e., YFP+, E-cad−; depicted in Fig. 2A) for functional analyses. Using this approach, we performed pancreatosphere assays, a surrogate test for self-renewal properties akin to the mammosphere assay18. In these experiments, we sorted individual YFP+ cells from PanIN and PDAC mice into individual wells of an ultra-low attachment plate. We further subdivided cells based on E-cad expression. In both PanIN and PDAC mice, YFP+, E-cad− (EMT+) cells were enriched for sphere-forming capability compared to YFP+, E-cad+ control cells from the same pancreas, though a statistical difference was only within PanIN mice (Fig. 2C).
To determine if an EMT is associated with enhanced tumor initiating capabilities, we orthotopically transplanted 100,000 EMT+ and EMT− cells from PanIN and PDAC into individual NOD/SCID mice. Surprisingly, but predicted by the pancreatosphere experiments, there was no difference in tumor incidence 3 weeks after transplantation (5/5 animals in each group formed tumors). Upon histologic analysis, tumors from EMT+ and EMT− transplanted animals were comprised of both E-cad+ and E-cad− cells. This result suggested that EMT is plastic within PDAC mice; and, because of this plasticity, interpretation of these experiments are difficult. That is, since PDAC cells are likely cycling between epithelial and mesenchymal states, it is difficult to parse if an EMT is indeed associated with enhanced tumor-initiating properties. However, starkly different results were found when the experiment was repeated with cells from PanIN mice. 0/5 mice transplanted with E-cad+ cells gave rise to tumors, but 3/5 mice with Ecad− (EMT+) cells formed tumors in 8 weeks. YFP+ cells were found within E-cad+ recipient mice, but all such cells were E-cad+ and formed what appeared to be normal-appearing acinar structures. This suggests that E-cad+ PanIN cells have limited propensity to undergo an EMT, thus providing an adequate negative control for these experiments. On the other hand, the tumors that arose from YFP+, E-cad− (EMT+) cells reflected a histology seen in PDAC transplanted mice—tumors were comprised of a mix of E-cad+ and E-cad− cells. Thus, based on these data, we concluded that an EMT under physiologic conditions was indeed associated with enhanced tumor-initiating capability at the PanIN stage.
Our studies provides first evidence that a “physiologic” EMT, that is one that arises from a spontaneous tumor in vivo, not from genetic manipulation ex vivo, is associated with an invasive and disseminating phenotype that is also enriched for enhanced tumor initiating potential. We suspect that genetic lineage labeling will provide an important for future studies of EMT in cancer in the future, as such experiments provide a profound advantage over highly controlled and simplified in vitro studies.
Acknowledgments
Supported by NIH/NIDDK ((K08DK088945) and pilot grant funding and Core facilities from P30DK050306 (ADR)), National Pancreas Foundation, and the American Gastroenterological Association Fellow.
References
- 1.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Lim J, Thiery JP. Epithelial-mesenchymal transitions: Insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 3.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29:1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing e-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 8.Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, et al. The transcriptional repressor zeb1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 9.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, et al. Deltaef1 is a transcriptional repressor of e-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 10.Singh A, Settleman J. Emt, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witta SE, Gemmill RM, Hirsch FR, Coldren CD, Hedman K, Ravdel L, et al. Restoring e-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- 12.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayan AE, Griffiths TR, Pal R, Browne GJ, Ruddick A, Yagci T, et al. Sip1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci U S A. 2009;106:14884–14889. doi: 10.1073/pnas.0902042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. Emt and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53r172h and krasg12d cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 18.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]