Abstract
Background
Coital dilution, the reduction in the coital frequency per partner when an additional ongoing partner is added, may reduce the transmission potential of partnership concurrency for HIV and other sexually transmitted infections. Empirical estimates of dilution, especially dilution of sexual acts unprotected by condoms, are needed to inform prevention research.
Methods
Sexually active adults in Accra, Ghana were recruited in a multi-stage household probability sample. Degree (number of ongoing partners), total acts, and unprotected acts were measured retrospectively for each month in the past year through an event-history calendar. Random effects negative binomial models estimated the association between degree and coital frequency.
Results
Compared to person-months with a single partner (monogamy), 2.06 times as many total acts and 1.94 times as many unprotected acts occurred in months with 2 partners. In months with 3 partners, 2.90 times as many total acts and 2.39 times as many unprotected acts occurred compared to monogamous months. Total acts but not unprotected acts also declined with partnership duration.
Conclusions
No dilution was observed for total acts with up to three concurrent partners, but a small amount of dilution was observed for unprotected acts for months with multiple concurrencies. This suggests moderate selective condom use in months with multiple concurrencies. The implications of the observed dilution for future HIV transmission must be investigated with mathematical models.
Keywords: Coital dilution, coital frequency, concurrency, HIV/AIDS, Ghana, West Africa
INTRODUCTION
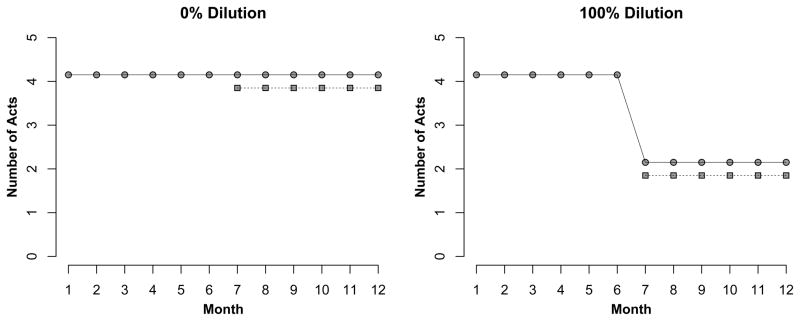
Coital dilution is the reduction in the number of sexual acts per partner when an additional, concurrent partner is added [1]. Concurrency is defined as having two or more sexual partnerships overlapping in time [2]. Figure 1 shows one extreme, in which total (100%) coital dilution would occur if a person who has a primary partner and a coital frequency of 4 acts per month acquires a secondary partner, but splits the 4 acts between the two partners (equally, or in any combination preserving the total across partners). At the other extreme, 0% dilution would occur if this person now has twice as many acts, so the number per partner remains constant. While this metric does not allow for increasing activity per partner, this is also certainly possible (e.g., a long-term partnership supplemented by a new partnership with sufficiently high coital frequency).
Figure 1.
Conceptual Examples of Coital Dilution. Under 0% dilution, a person has 4 acts per month with a main partner (circle), adds a secondary partner (square) also with 4 acts per month, and the per-partnership acts remains constant. Under 100% dilution, the act rate per partnership drops to 2 upon concurrency. Dilution does not require that acts be equally divided as they are here for illustration purposes.
Dilution may mitigate the effects of concurrency for the transmission of HIV and other sexually transmitted infections. Concurrency has been shown to increase disease incidence by allowing for the virus to transmit backwards from those partners established later to those earlier, and by decreasing the length of the generation interval [2]. But dilution decreases the per-partnership transmission rate, since this is a function of the number of sexual acts in each partnership per unit time. A common assumption in mathematical models is to preserve the number of acts per partnership under concurrency [3], but that implies the number of potential transmission events increases linearly with degree (the number of ongoing partners at any time). One model testing this assumption suggested that relatively modest levels of dilution (25% or higher) could erase the effects of concurrency [1], though the assumptions of this model also led to decreasing rates of activity over time.
The critical empirical question, therefore, is whether dilution occurs to the level required to offset the effects of concurrency. Despite scientific interest in concurrency, few empirical studies have addressed dilution. Modest dilution was found within the context of polygamous marriages, but the prevalence of these partnerships and their epidemic potential is minimal in many African populations [4]. Another study investigated concurrency in Kenya and Malawi using various analytic approaches, finding evidence for dilution in some cases but not others [5]. A review of three studies in Uganda, Thailand, and the United States found consistently lower rates of coital frequency with secondary compared to primary partners, with that difference the smallest in the country with the highest prevalence (Uganda) [6].
One aspect of dilution requiring attention is condom use. Given the effectiveness of condoms for HIV/STI prevention [7], the most epidemiologically relevant dilution involves unprotected acts. Unprotected dilution occurs even if the total acts (protected and unprotected) increase with degree as long as the number of unprotected acts across partners remains constant. If 10 unprotected acts with a primary partner are supplemented with 10 protected acts with a secondary partner, no dilution has occurred with respect to total acts, but 100% dilution has occurred for unprotected acts. In South Africa, condom use did not vary by concurrency [8], but more precise quantification of the association between degree and unprotected acts is still needed. The impact of unprotected dilution on disease transmission is complex, since condom use may vary by partner type and partnership duration [9].
In this study, we investigated the association between partnership degree and both total and unprotected sexual acts, using time-varying exposure and outcome measures collected in retrospective panel data. As our target population for this study was sexually active adults in a dynamic urban slum area in Accra, Ghana [10], this is the first study of dilution in West Africa, which has experienced less HIV-1 burden compared to other African regions [11]. Our goal is to quantify dilution there in order to support future mathematical modeling of HIV transmission dynamics to investigate these regional disease disparities.
METHODS
Procedures
This analysis uses data from the Migration & HIV in Ghana (MHG) study, a cross-sectional study of sexually active adults in Agbogbloshie, Ghana in 2012. Agbogbloshie is an urban slum area in the capital city of Accra, selected for this study based on its hypothesized high-risk profile and lack of prior epidemiological research. The methods have been described in detail [12]. Briefly, MHG used a two-stage cluster randomized sampling scheme to obtain a probability sample of the population. Starting with an area census, we first randomly selected households with a probability proportional to household size, and then randomly selected one adult household member. Given differences in household size, a weighting scheme was employed to account for differential inclusion probabilities. Eligibility criteria to participate were current residence in the selected household, age 18 to 49 years, and lifetime history of consensual sexual intercourse. The Institutional Review Boards of the University of Washington and University of Ghana approved all procedures.
Measures
Trained field staff administered a standardized survey and drew serum via finger stick for a diagnostic HIV-1/2 test. The survey focused on demographics, migration and travel, and sexual behavior. For sexual behavior, summary data were collected on the number of lifetime sexual partners, past-year partners, and past-year partners with whom condoms were not always used. We used an event-history calendar to collect detailed partnership data for partners in the past year (up to three), with responses for each month during that period [13]. For each partner, data included the duration of the partnership and monthly information on the number of total and unprotected sexual acts. The degree for each person-month is defined as the number of overlapping partnerships in that month (up to three). To be categorized as a degree-2 month, for example, acts with the second partner must have occurred between those with the first partner. Ambiguous months, such as those in which one partnership ended and another started, were conservatively categorized as with a single partner (16 out of 4465 person-months).
For the HIV testing, dried blood spots were collected in the field on standard filter paper and maintained in refrigerators before delivery to the Department of Virology, Noguchi Memorial Institute for Medical Research, University of Ghana for processing. Serum was tested on the INNO-LIA™ HIV-1/2 test platform (Innogenetics, Belgium), shown to have good sensitivity and specificity for diagnosis and HIV type differentiation. All subjects who tested for HIV were asked to return in one week to receive their test results, which were provided by a trained nurse counselor. HIV-infected subjects were referred to medical care.
Statistical Analysis
Descriptive statistics were estimated for the full sample of sexually active adults in the past year. Because past-year sexual activity was not a study eligibility criterion, the analytic sample therefore excludes subjects inactive during the entire year. To account for the complex survey design, data were weighted by the inverse of the selection probability given the cluster sampling scheme, necessary because a discrepancy in household sizes from the initial census was observed [12]. Robust cluster-based standard errors were used for variance estimation [14]. Statistics for degree and monthly acts were calculated on person-months, while statistics that are temporally invariant are calculated on persons.
For the primary analysis, we investigated coital dilution by regressing sexual acts on degree with two random-effects negative binomial models. The outcome in the first model was the number of total acts summed across all partners (up to 3) in each person-month. In the second model, the outcome was unprotected acts across partners. The main predictor in both models was degree in each person-month, parameterized as an indicator variable with the levels measured in the survey (1, 2, or 3 partners). Two additional variables were selected a priori as precision variables: sex of the subject and the duration of the longest-running partnership in that month. The duration variable was included to allow the predicted acts to vary for long-term partnerships. Concurrency in sub-Saharan Africa often occurs within the context of these partnerships [6], and we expected fewer acts in those established partnerships.
The random effects parameterization nested person-months within persons. Person-months with a degree of zero were dropped from the analysis as coital frequency must always be zero in these months. The negative binomial response distribution allowed for overdispersion in the outcomes. Exponentiated coefficients from these models were interpreted as incidence rate ratios for degree conditional on sex and duration. If 100% coital dilution holds, then these ratios for both degree comparisons (2 vs. 1 and 3 vs. 1) should be one. If 0% dilution holds, then the ratios should be a linear function of degree: degree-2 person-months should have twice as many acts as degree-1 months, and degree-3 should be three times as high. Finally, we estimated the predicted number of acts in two ways: first by degree and sex averaged over duration, and second by degree and duration averaged over sex. Prediction intervals incorporated the stochastic variation in the outcome data and the uncertainty in estimating the model coefficients [15].
RESULTS
A total of 484 subjects were recruited into the study, representing a 70% response rate. Of these, the 416 who reported past-year sexual activity were included in this analysis. Additionally, the 7% of person-months with a degree of zero among those active in the past year were dropped. Table 1 provides descriptive statistics for this subpopulation included in the analysis. Over half the population was female and below the age of 30 years. The average number of lifetime sexual partners was 4.58 (median = 3) and varied significantly by sex (although the difference in medians was smaller). Men also had higher past-year total partners and unprotected partners compared to women. Most of remaining person-time was monogamous, but there were clear differences in degree by sex: overall 12% of person-time was concurrent, with 21% of men’s time concurrent compared to 3% of women’s’ time. Across sex, the mean was 4.77 total acts per month and 4.34 unprotected acts, indicating the majority of acts were unprotected. Finally, the estimated HIV-1 prevalence in the study population was 4.4%, with infection twice as high among women compared to men. No HIV-2 infection was observed.
Table 1.
Demographic, Behavioral and Epidemiologic Characteristics of Sexually Active Adults in Agbogbloshie, Accra, Ghana, 2012 (n = 416)
| Total | Males | Females | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| %/mean | 95% CI | %/mean | 95% CI | %/mean | 95% CI | |
| Sex | ||||||
| Male | 42.5% | 36.6% – 48.5% | ||||
| Female | 57.5% | 51.5% – 63.4% | ||||
| Age | ||||||
| 18–29 | 55.7% | 49.6% – 61.9% | 50.5% | 41.7% – 59.3% | 59.6% | 51.8% – 67.4% |
| 30–39 | 29.1% | 23.7% – 34.4% | 33.0% | 24.8% – 41.2% | 26.2% | 19.6% – 32.7% |
| 40–49 | 15.2% | 10.3% – 20.0% | 16.5% | 9.8% – 23.2% | 14.2% | 8.5% – 20.0% |
| Sexual History | ||||||
| Total Partners, Lifetime | 4.58 | 3.67 – 5.49 | 6.77 | 4.79 – 8.75 | 2.99 | 2.71 – 3.26 |
| Total Partners, Past Year | 1.48 | 1.33 – 1.63 | 1.90 | 1.57 – 2.22 | 1.17 | 1.09 – 1.25 |
| Unprot. Partners, Past Year | 1.20 | 1.11 – 1.29 | 1.37 | 1.20 – 1.53 | 1.07 | 0.98 – 1.16 |
| Degree1 | ||||||
| 1 | 89.1% | 86.4% – 91.8% | 79.3% | 73.9% – 84.6% | 96.3% | 85.7% – 95.8% |
| 2 | 9.0% | 6.7% – 11.4% | 17.1% | 12.4% – 21.9% | 3.0% | 1.3% – 4.7% |
| 3 | 1.9% | 0.1% – 3.0% | 3.6% | 1.3% – 5.8% | 0.1% | 0.0% – 1.5% |
| Partnership Duration1,2 | ||||||
| Maximum Duration | 5.9 | 6.2 | 5.3 | 5.3 | 6.4 | 6.8 |
| Coital Frequency1 | ||||||
| Total | 4.77 | 4.27 – 5.28 | 5.52 | 4.59 – 6.45 | 4.22 | 3.69 – 4.75 |
| Unprotected | 4.34 | 3.89 – 4.80 | 4.75 | 3.96 – 5.54 | 4.05 | 3.52 – 4.57 |
| HIV-1 Infection | ||||||
| Infected | 4.4% | 1.8% – 7.0% | 2.7% | 0.0% – 5.4% | 5.6% | 1.6% – 9.7% |
Degree, maximum partnership duration, and coital frequency were calculated on person-months, whereas the remaining variables are fixed and calculated on persons.
Partnership duration defined as the length of the longest-running partnership in any person-month
In the first model for total acts, there was no evidence of coital dilution. As shown in Table 2, the incidence rate ratio (IRR) for total acts comparing person-months with a degree of 2 to months with a degree of 1 was 2.07 (95% CI = 1.85 – 2.33). For a degree of 3, the IRR was 2.93 (95% CI = 2.35 – 3.69). The conditional rate for males did not significantly differ from females. There was a negative trend for maximum partnership duration: the number of total acts declined by 1% with each increasing year of partnership duration. In the second model for unprotected acts, there was some dilution, but only for months with 3 concurrent partners. The IRR for unprotected acts comparing person-months with a degree of 2 to months with a degree of 1 was 1.94 (95% CI = 1.72 – 2.20). For a degree of 3, the IRR was 2.41 (95% CI = 1.90 – 3.11). Both sex and partnership duration were not significantly associated with unprotected acts in this model.
Table 2.
Incidence Rate Ratios and 95% Confidence Intervals for Monthly Total Acts and Unprotected Acts
| Total Acts | Unprotected Acts | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| IRR | 95% CI | p | IRR | 95% CI | p | |
| Degree | ||||||
| 1 | 1.00 | 1.00 | ||||
| 2 | 2.07 | 1.85 – 2.33 | <0.001 | 1.94 | 1.72 – 2.20 | <0.001 |
| 3 | 2.93 | 2.35 – 3.69 | <0.001 | 2.41 | 1.90 – 3.11 | <0.001 |
| Male | 1.06 | 0.99 – 1.14 | 0.08 | 0.99 | 0.92 – 1.07 | 0.85 |
| Duration | 0.99 | 0.99 – 1.00 | <0.01 | 1.00 | 0.99 – 1.00 | 0.51 |
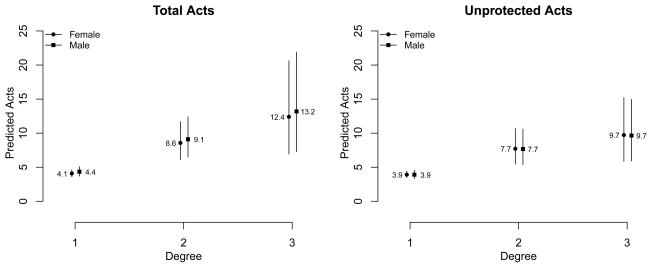
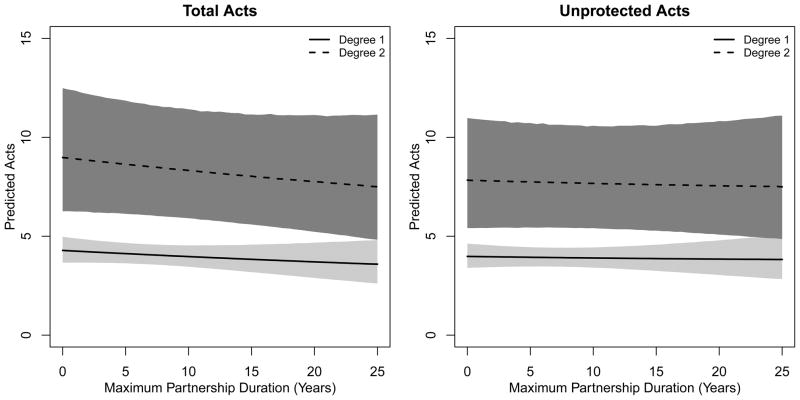
Figures 1 and 2 show the predicted number of acts by degree with 95% prediction intervals. In Figure 1, the predicted total and unprotected acts are plotted for males and females separately, averaging over partnership duration. The predicted number of acts increases with degree, but the slope over degree is more gradual for unprotected acts compared to total acts. Expressed as per-partnership rates, the females with 2 and 3 partnerships would have 4.3 and 4.1 acts per partnership compared to 4.1 among monogamous females; males with 2 and 3 partnerships would have 4.5 and 4.4 acts per partnership compared to 4.4 among monogamous males. The predictive uncertainty is higher with degree-3 months both because of the greater variance in outcomes and greater uncertainty in coefficient estimates. The predictions are non-overlapping when comparing degree-2 to degree-1 person-months for both total and unprotected acts, implying no evidence for complete dilution. Figure 2 plots the predicted total and unprotected acts by degree and partnership duration, limited to degree-1 and degree-2 person-months for clarity. In both panels, the predicted number of acts for degree-2 months is higher than degree-1 months across all durations. The predicted total acts declines with higher duration but is flat for unprotected acts, consistent with the two model coefficients for degrees 1 and 2.
Figure 2.
Predicted Number of Total Acts and Unprotected Acts by Monthly Degree and Sex among Sexually Active Adults in Agbogbloshie, Accra, Ghana.
DISCUSSION
In this study, we observed no coital dilution in total acts for up to 3 concurrent partners, nor for unprotected acts with up to 2 concurrent partners. Dilution was only observed for unprotected acts in the context of three concurrent partners (degree > 2). But there were few of these multiple concurrency person-months (1.9% of total months), and the confidence interval for this estimate does not exclude 0% dilution. If the point estimate is correct, this implies a moderate increase in condom use for these uncommon, high-risk time periods.
Dilution research to date has investigated populations in eastern and southern Africa, with mixed results. Gaydosh et al. find some dilution among residents persons on Likoma Island in Malawi in which behavioral reporting of partnership behavior within couples was linked, but conflicting evidence among young Kenyans in an analysis from another study [5]. Although different statistical approaches were used across their analyses, dilution was always defined as a binary outcome: no sexual acts with a non-primary partner in the same interval over which degree is considered. Morris et al. found evidence for dilution in studies from Thailand, Uganda, and the United States using measures based on average coital frequency in the last 6 or 12 months, but differences in study design did not permit quantitative comparisons across the countries [6]. Delva et al. define dilution in terms of a weekly per-partner coital frequency in South Africa, using similar statistical modeling methods as ours, and find little evidence of coital dilution [8]. Delva’s is also the only other study to distinguish between acts protected and unprotected by condoms (using a binary indicator for consistent vs. inconsistent/no use), and they found no difference in dilution by protection. In contrast, we found moderate unprotected dilution in months with multiple concurrencies (degree > 2).
The variability in results across these few studies on dilution to date may stem from several factors. One factor is differences in measurement methods, which makes comparisons difficult. While most of the studies have used some sort of event-history calendar to assess dilution, they have used quite different exposure and outcome measures. Dichotomizing dilution outcomes, in particular, leads to loss of information [16]. Therefore, we suggest that further empirical research on dilution adopt standardized measures for degree and acts as continuous variables, differentiating between total and unprotected acts.
Studies should also incorporate regression covariates useful for mathematical models of transmission dynamics. For example, we found that partnership duration is associated with coital frequency for long-term partnerships. By contrast, the effects of individual-level covariates, like age of the subject, are more complicated to assess because the attributes of both partners will influence their coital frequency, with the possibility of both main effects and interactions. It is therefore necessary to carefully interpret associations on coital frequency for individual-level variables like age (or sex). Our models feature the subject’s sex as an individual-level variable, but the purpose is to assess whether coital frequency is balanced for males and females, as it should be in a representative sample from a predominantly heterosexual population like ours (we did not limit survey questions to opposite-sex partners, but observed no same-sex partnerships). Along with standardized measurement, common statistical methods are needed. Statistical models like Poisson regression likely do not account for the overdispersed distribution of the coital frequency outcomes [8], so more flexible models like the negative binomial model should be considered.
Variability in dilution results across studies may also reflect actual differences in behavior across populations. For example, if the previous studies had measured dilution consistently, one could speculate that the dilution observed in populations with lower HIV prevalence (Malawi and Kenya) partially explains the disparity in disease burden between these areas and the higher-prevalence areas with no dilution (South Africa). But that hypothesis would be inconsistent with our results, since there was no dilution observed in our population, and it has the lowest disease burden. Again, however, while the goal of this body of research is to provide scientifically comparable results, the variation in measurement precludes such comparisons at this point.
All else equal, the protective effects of dilution on disease transmission will vary with the prevalence of concurrency, the rate of acts, the proportion of acts that are protected, and the effectiveness of condoms to prevent transmission. The probability of infection for a susceptible person given contact with an infected person will be a product of the two risks of transmission per contact, with and without condom use, given degree:
where τ is the probability of transmission per act, α is the number of acts, u connotes unprotected acts, p connotes protected acts, and d connotes degree. Therefore, dilution is defined as heterogeneity in α with respect to d. Because all of these parameters may vary across populations, along with other protective factors like male circumcision [17, 18], an ecological comparison of αd across studies is insufficient to quantify the epidemiological impact of dilution.
Mathematical modeling of HIV transmission dynamics is one framework that overcomes this limitation. Sawyers et al. modeled the effects of dilution on HIV prevalence, where dilution was expressed as the reduction in the transmission risk in “non-primary partnerships” [1]. Their simulated population could engage in primary and non-primary partnerships, having an effective maximum degree of 2, but dilution applied to non-primary partners only. In their model, the designation of primary/non-primary partner is fixed at the time the partnership begins, so a non-primary partner remains “non-primary” even if the primary partnership ends. It is worth noting that this assumption implicitly links concurrency to reduced total coital frequency in the population, a pattern that is not empirically supported, and would bias the observed effects of concurrency downward. Under these assumptions, they found that dilution above 25% resulted in disease extinction for concurrency levels up to a point prevalence of 14% (the maximum they tested). With our results translated to their metric, we effectively observed 0% dilution for total acts and 7% dilution for protected acts in this population where 10% of person-time was concurrent (with large variations in degree by sex). So even under their very conservative model assumptions, concurrency would still be likely to increase HIV transmission in Ghana. Our future mathematical modeling work will investigate the effects of dilution with more robust stochastic network models given the epidemiological parameters observed here.
Limitations
As noted, degree within each month corresponds to the number of active, overlapping partnerships within that month. We conservatively assumed that months in which one partnership ended and another started were not concurrent, but this may be a downward misclassification in degree. In a separate simulation study (not shown) we found that as long as any such misclassification is not correlated with coital frequency, the bias on IRR estimates would be conservative. A second limitation related to degree is degree truncation, due to the event history survey approach focusing on the last three partners in the prior year. This will also have a conservative impact, and requires modeling the relationship between degree and acts as a nonparametric function. However, the proportion of the population with a degree greater than 3 at any point in time is likely very small in both our target population. In the prior year, only 7% of men and 1% of women had 4 or more cumulative partners, and the degree would be much less than this. Another limitation is that the measurement of acts, total and unprotected, is subject to misreporting. This would only matter if misclassification was correlated with degree, leading to a conservative bias if those with higher degree were more likely to underreport their acts, and a positive bias otherwise. Finally, our modeling approach does not explicitly incorporate the temporal relationship between person-months: the time-effect was only controlled via the ever-increasing partnership duration for ongoing partnerships. More structured time-series approaches may better predict the relationship of interest but did not fit our data well.
Conclusions
Coital dilution has received little attention despite its potential to mitigate the effects of concurrency on HIV transmission dynamics. Our findings suggest that the impact of dilution in our population would be minimal. To verify this, we would need a well-specified mathematical model of HIV transmission dynamics that incorporates empirically observed differentials in degree, dilution given degree, condom use, and other biological and behavioral factors influencing transmission potential. Temporal exponential random graph models [19] is a promising statistical and mathematical framework for such analysis. These models will hopefully improve on past attempts to model dilution [1] to address important epidemiological questions on the impact of concurrency with dilution on population-level HIV transmission in sub-Saharan Africa.
Figure 3.
Predicted Number of Total Acts and Unprotected Acts by Monthly Degree and Maximum Partnership Duration among Sexually Active Adults in Agbogbloshie, Accra, Ghana. Degree was capped at two for visual clarity. Maximum partnership duration is the duration in years of the subject’s longest-running partnership in any month.
Acknowledgments
Sources of Support
This work was supported in part by the NICHD (R00 HD057533) and the UW Center for AIDS Research SPRC (P30 AI027757). Computing support was provided by a NICHD research infrastructure grant (5R24HD042828) to the UW Center for Studies in Demography & Ecology.
The following provided invaluable assistance with this study: Kamil Fuseini, Fidelia Dake and the staff at the Regional Institute for Population Studies at the University of Ghana, the Ghana AIDS Commission, our field staff (Vincent Kantah, Patrick Nyarko, Charlotte Ofori, Cecilia Segbedji, Maame Yaa Konamah Siaw, Bilaal Tackie, Habakkuk Tarezina, Solomon Tetteh), and the laboratory staff at NMIMR (Prince Parbie and Joyce Appiah-Kubi). We also thank Martina Morris, Steven Goodreau, and other members of the UW Network Modeling Group for comments on this study.
References
- 1.Sawers L, Isaac AG, Stillwaggon E. HIV and concurrent sexual partnerships: modelling the role of coital dilution. Journal of the International AIDS Society. 2011;14:44. doi: 10.1186/1758-2652-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. Aids. 1997;11(5):641–8. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Goodreau SM, Cassels S, Kasprzyk D, Montano DE, Greek A, Morris M. Concurrent partnerships, acute infection and HIV epidemic dynamics among young adults in Zimbabwe. AIDS and behavior. 2012;16(2):312–22. doi: 10.1007/s10461-010-9858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reniers G, Tfaily R. Polygyny, partnership concurrency, and HIV transmission in Sub-Saharan Africa. Demography. 2012;49(3):1075–101. doi: 10.1007/s13524-012-0114-z. [DOI] [PubMed] [Google Scholar]
- 5.Gaydosh L, Reniers G, Helleringer S. Partnership Concurrency and Coital Frequency. AIDS and behavior. 2013;17(7):2376–86. doi: 10.1007/s10461-013-0525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris M, Epstein H, Wawer M. Timing is everything: international variations in historical sexual partnership concurrency and HIV prevalence. PLoS one. 2010;5(11):e14092. doi: 10.1371/journal.pone.0014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. The Cochrane database of systematic reviews. 2002;(1):CD003255. doi: 10.1002/14651858.CD003255. [DOI] [PubMed] [Google Scholar]
- 8.Delva W, Meng F, Beauclair R, et al. Coital frequency and condom use in monogamous and concurrent sexual relationships in Cape Town, South Africa. Journal of the International AIDS Society. 2013;16:18034. doi: 10.7448/IAS.16.1.18034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson AM, Mercer CH, Erens B, et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet. 2001;358(9296):1835–42. doi: 10.1016/S0140-6736(01)06883-0. [DOI] [PubMed] [Google Scholar]
- 10.Oberhauser AM, Yeboah MA. Heavy burdens: Gendered livelihood strategies of porters in Accra, Ghana. Singapore Journal of Tropical Geography. 2011;32(1):22–37. [Google Scholar]
- 11.HIV/AIDS JUNPo. Global report: UNAIDS report on the global AIDS epidemic 2012. UNAIDS; 2012. [Google Scholar]
- 12.Cassels S, Jenness SM, Biney A, Ampofo W, Dodoo F. Migration, Sexual Networks, and HIV in Ghana: Study Design and Preliminary Results. Under review. [Google Scholar]
- 13.Luke N, Clark S, Zulu EM. The relationship history calendar: improving the scope and quality of data on youth sexual behavior. Demography. 2011;48(3):1151–76. doi: 10.1007/s13524-011-0051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumley T. Complex surveys: A guide to analysis using R. John Wiley & Sons; 2011. [Google Scholar]
- 15.Imai K, King G, Lau O. Zelig: Everyone’s statistical software. R package version. 2009;3(5) [Google Scholar]
- 16.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Wolters Kluwer Health; 2008. [Google Scholar]
- 17.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 18.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 19.Krivitsky PN, Handcock MS. A separable model for dynamic networks. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2014;76(1):29–46. doi: 10.1111/rssb.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]