Abstract
Objective
To determine the effect of therapeutic plasma exchange (TPE) on hemodynamics, organ failure, and survival in children with multiple organ dysfunction syndrome (MODS) due to sepsis requiring extracorporeal life support (ECLS).
Design
A retrospective analysis.
Setting
A pediatric intensive care unit (PICU) in an academic children’s hospital.
Patients
14 consecutive children with sepsis and MODS who received TPE while on ECLS from 2005 to 2013.
Interventions
Median of 3 cycles of TPE with median of 1.0 times the estimated plasma volume per exchange.
Measurements and Main Results
Organ Failure Index (OFI) and Vasoactive-Inotropic Score (VIS) were measured before and after TPE use. PICU survival in our cohort was 71.4%. OFI decreased in patients following TPE [pre: 4.1 ± 0.7 vs. post: 2.9 ± 0.9 (mean ±SD); p = 0.0004]. Patients showed improved VIS following TPE [pre: 24.5 (13.0–69.8) vs. post: 5.0 (1.5–7.0), median (25th–75th); p = 0.0002]. Among all patients, the change in OFI was greater for early TPE use than late use [pre: −1.7 ±1.2 vs. post: −0.9 ±0.6; p = 0.14], similar to the change in VIS [pre: −67.5 (28.0–171.2) vs. post: −12.0 (7.2–18.5); p = 0.02]. Among survivors, the change in OFI was greater among early TPE use than late use [early: −2.3 ±1.0 vs. late: −0.8 ± 0.8; p = 0.03], as was the change in VIS [early: −42.0 (16.0–76.3) vs. late: −12.0 (5.3–29.0); p=0.17]. The mean duration of ECLS after TPE according to timing of TPE was not statistically different among all patients or among survivors.
Conclusions
The use of TPE in children on ECLS with sepsis-induced MODS is associated with organ failure recovery and improved hemodynamic status. Initiating TPE early in the hospital course was associated with greater improvement in organ dysfunction and decreased requirement for vasoactive and/or inotropic agents.
Keywords: sepsis, septic shock, multiple organ failure, extracorporeal membrane oxygenation, plasma exchange, renal replacement therapy
Introduction
The incidence of severe sepsis/septic shock has been increasing in the United States (1, 2). Treatment for severe sepsis/septic shock is time-sensitive and includes provision of aggressive fluid resuscitation, titration of vasoactive and/or inotropic agents, early administration of appropriate antimicrobials, and attaining infectious source control (3). Despite these interventions, severe sepsis can progress to septic shock, and ultimately to multiple organ dysfunction syndrome (MODS). Survival for patients who progress to MODS continues to be low depending on the number of organ systems involved (4–9). This low rate of survival has led to the aggressive use of extracorporeal therapies in patients with MODS, such as venoarterial (VA) extracorporeal life support (ECLS) for refractory shock (3). Another extracorporeal therapy that has shown promise in pediatric patients with MODS is therapeutic plasma exchange (TPE) (10–12).
TPE was first utilized as salvage therapy for severe sepsis due to meningococcemia in 1979 (13). Since then, multiple case series describing TPE use in sepsis have demonstrated improved survival compared to the expected outcome (11, 12, 14–20). The benefits of TPE have been postulated as “blood purification” by removal of cytotoxins and dysregulated cytokines and restoration of deficient or depleted humoral products such as immunoglobulins, pro- and anticoagulation proteins, growth factors, and enzymes necessary to regain homeostasis necessary for clinical recovery (17, 18, 21–25). The clinical entity of coexistent thrombocytopenia with MODS termed, “Thrombocytopenia-Associated Multiple Organ Failure (TAMOF),” in which patients develop a secondary thrombotic microangiopathy (TMA), represents a subgroup of patients with sepsis-induced MODS that are at exceptionally high risk for death (10, 12, 22, 26). Similar to thrombotic thrombocytopenic purpura (TTP), TAMOF is associated with decreased α disintegrin and metalloproteinase with thrombospondin motifs-13 (ADAMTS-13) leading to increased circulating ultra-large von Willebrand factor (vWF), platelet overconsumption, and organ failure secondary to vWF-rich microvascular thromboses. TAMOF can be treated with TPE by replenishing ADAMTS-13 activity and reversing organ dysfunction (10, 19).
Small, randomized controlled trials comparing plasma therapy use in sepsis to conventional management has demonstrated improved organ failure recovery and increased survival (10, 23, 27). Theoretically, patients with MODS who require ECLS would benefit from TPE by enhancing organ failure recovery while allowing adequate hemodynamic support and oxygen delivery provided by ECLS. However, the outcome of pediatric patients requiring TPE and ECLS for organ failure reversal and subsequent survival benefit remains largely unexplored even though addition of TPE could easily be placed in series with the ECLS while continuing to provide hemodynamic stability for the patient via the ECLS circuit. Anecdotal cases of simultaneous TPE and ECLS for MODS at our institution were felt to have better outcome than expected. We hypothesize that the use of TPE in children requiring ECLS for MODS due to sepsis is associated with improvement in organ failure reversal, less need for vasoactive and/or inotropic agents, and improved survival compared to historical controls. In this retrospective study, we report our single center accumulated experience with TPE use for children who require ECLS for sepsis-induced MODS.
Materials and Methods
Patient selection
The study was approved by the Institutional Review Board (IRB) at University of Michigan Health System with a waiver of informed consent per IRB protocol IRB00001995. We retrospectively reviewed all cases of neonatal and pediatric patients from gestational age 37 weeks up to age 17 years who received TPE while on ECLS admitted to C.S. Mott Children’s Hospital from January 2005 through January 2013. The Division of Pediatric Nephrology and the Apheresis Procedure Unit had electronic databases of all children who underwent TPE. TPE was performed in patients who met evidence-based indications for TPE, such as TTP, and also performed in cases of MODS as a rescue therapy. In the latter case, the decision to perform TPE when patients developed MODS from sepsis was at the discretion of pediatric critical care, pediatric nephrology, and/or the apheresis team. All patients who simultaneously received TPE and ECLS were selected from the databases and their medical charts were reviewed. If the indication to initiate TPE was associated with a septic state, patients were included in this study.
Historical control group was defined as all cases of neonatal and pediatric patients from gestational age 37 weeks up to age 17 years who received ECLS for sepsis-induced MODS admitted to C.S. Mott Children’s Hospital 10 years prior to the use of TPE for sepsis from 1995 to 2004. Data of children placed on ECLS for sepsis-induced MODS was retrospectively collected from the University of Michigan ECLS Database that includes all children who are initiated on ECLS at our institution.
Membrane and centrifugation-based TPE
Patients received TPE via either membrane or centrifugation-based technologies. Membrane-based TPE was performed using the Prisma© or the PrismaFlex© (Gambro, Lakewood, CO, USA) continuous renal replacement therapy (CRRT) system utilizing a TPE2000 filter set. Centrifugation-based TPE was performed using the Cobe Spectra or Spectra Optia Apheresis Systems (Terumo BCT, Lakewood, CO, USA). The choice of membrane vs. centrifugal TPE was largely driven by whether or not the patient was concurrently on CRRT, with membrane TPE being primarily used for patients that were on CRRT. In both cases, TPE was dosed as follows: patients were prescribed exchange volumes of 1.0–1.5 times the estimated circulating plasma volume on consecutive days at the discretion of the treating physician, which included the estimated volumes of all extracorporeal circuits. The blood flow rate was 3–5 mL/kg/min (minimum 100 mL/min, maximum 200 mL/min). The replacement solution was a combination of 5% albumin and fresh frozen plasma (FFP). Proportion of FFP in the replacement fluid ranged from 0%–100% with the most common mixture of approximately 50% of each. Replacement fluid rate started at 25 mL/kg/h and was increased up to a maximum of 40 mL/kg/h (maximum flow rate 2000 mL/h). No net fluid was removed from the TPE circuit. Anticoagulation for the TPE circuit utilized regional citrate anticoagulation in addition to the heparinization provided by the ECLS circuit. To avoid hypocalcemia, patients received calcium chloride infusion on membrane TPE and calcium gluconate infusion on centrifugal TPE to maintain the patient’s serum ionized calcium between 1.1 and 1.4 mmol/L. Regular replacements of other electrolytes were not necessary during TPE.
Connection of the TPE and CRRT circuits to the ECLS circuit
Membrane-based TPE
Access and return lines were placed in parallel with the CRRT access via stopcocks, with the TPE first. The citrate infusion was placed before the TPE and CRRT accesses via stopcock. All stopcocks were in the open position such that if TPE flow was interrupted, the CRRT circuit would continue to receive flow. The blood flow rate was matched on the TPE and CRRT machines when running concurrently in order to prevent recirculation.
Centrifugation-based TPE
TPE circuit access was connected pre-pump/pre-bladder. In some cases, TPE return lines were connected post-oxygenator, while in other cases, TPE return lines were connected pre-pump/pre-bladder.
CRRT
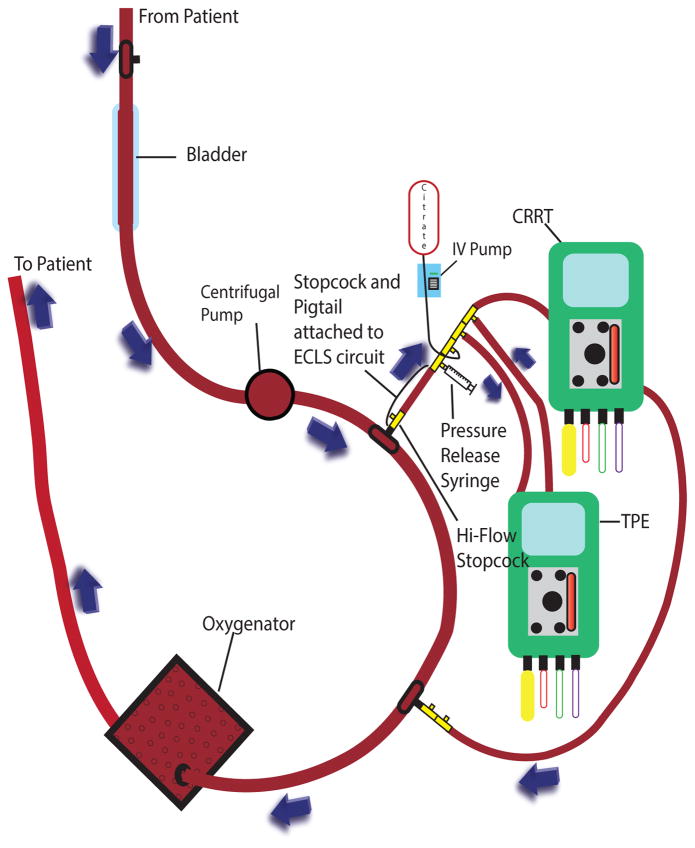
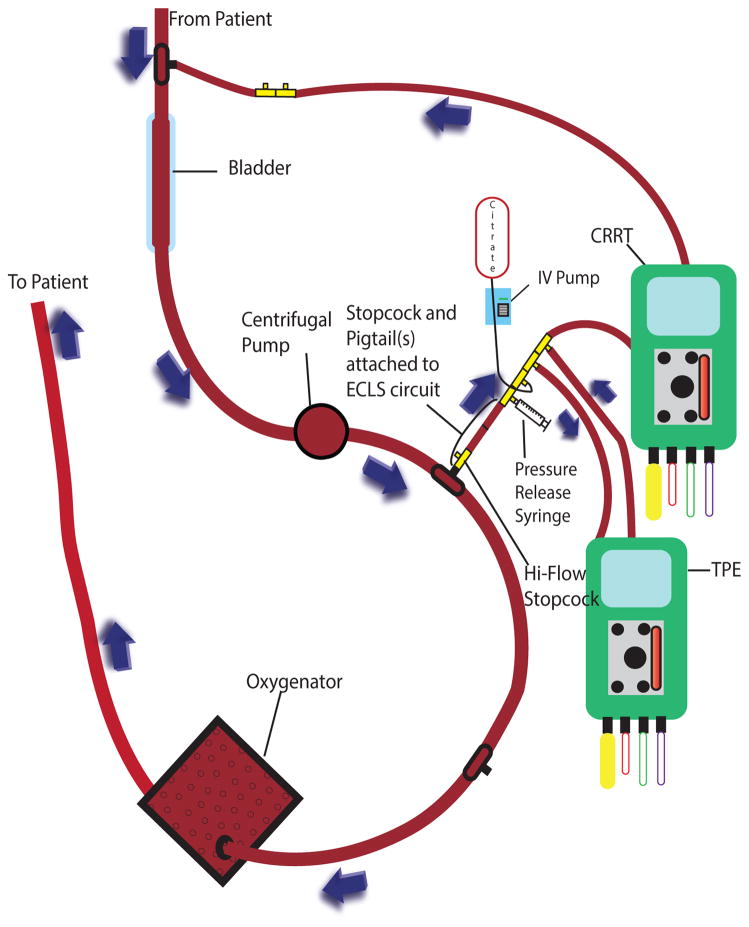
Access and return were in parallel with the ECLS circuit. In patients with ECLS pressures <300 mmHg (low pressure circuit), the CRRT access and return were both placed post-pump and pre-oxygenator (Figure 1A). Due to a maximum return pressure on the PrismaFlex© of 350 mmHg, for patients with ECLS circuit pressures >300 mmHg (high pressure circuit), the CRRT return was relocated to a lower pressure area of the circuit pre-pump and pre-bladder (Figure 1B). Although there is potential for recirculation with the CRRT return attaching upstream from the CRRT access, the high flow rates of even the smallest ECLS circuit makes this a negligible concern. When ECLS circuit pressures were >200 mmHg at the point of CRRT/TPE access, resistance was created using 6 inch increments of pigtail extension tubing from the ECLS circuit feeding the TPE access, as the maximum access pressure on the Prisma© or the PrismaFlex© is 250 mmHg.
Figure 1.
Figure 1A: Continuous Renal Replacement Therapy, Therapeutic Plasma Exchange, and Extracorporeal Life Support Low Pressure Circuit
CRRT = continuous renal replacement therapy; TPE = therapeutic plasma exchange; IV = intravenous; ECLS = extracorporeal life support
Figure 1B: Continuous Renal Replacement Therapy, Therapeutic Plasma Exchange, and Extracorporeal Life Support High Pressure Circuit
CRRT = continuous renal replacement therapy; TPE = therapeutic plasma exchange; IV = intravenous; ECLS = extracorporeal life support
Patient Analysis, Study Variables, and Definitions
The primary outcome was survival upon discharge from the pediatric intensive care unit (PICU). Secondary outcomes included changes in Organ Failure Index (OFI) (28) and Vasoactive-Inotropic Score (VIS) (29). OFI and VIS were calculated by selecting the worst vital signs and laboratory values and the highest infusion rate of vasoactive and/or inotropic agents 24 hours prior to the first TPE and 12 hours after completing the last TPE. The 24 hour mark was chosen prior to TPE since OFI was developed and calculated by using the “worst value of the day.” Data up to 12 hours after the last TPE was analyzed to capture the immediate effect of TPE. Organ failure criteria of OFI was modified as most patients were on mechanical ventilation and on ECLS before and/or after TPE regimen. The modifications were as follows:
PaO2:FIO2 ratio was calculated for all patients prior to going on ECLS, which was greater than 24 hours prior to the first TPE in 7 of 14 patients. After completing TPE, ECLS and mechanical ventilator dependent patients were assumed to have on-going respiratory failure and received 1 point.
Patients on CRRT before and/or after TPE received 1 point for renal failure. All patients were initiated on CRRT due to acute renal failure and either met the oliguria or the creatinine criteria prior to initiation of CRRT.
Patients on ECLS were anticoagulated with heparin. Data for activated partial thromboplastin time was omitted and prothrombin time and thrombocytopenia were used to determine hematologic failure.
We did not include neurologic failure as part of calculating the OFI since all patients were sedated. Therefore, the maximum points a patient could have accumulated was 5 instead of 6.
Because previously published articles have commented on the likely benefit of starting TPE early in the hospital course, subgroup analyses compared patients receiving TPE within 30 hours (early group) vs. after 30 hours (late group) from the time of PICU admission to determine the differences in OFI and VIS changes and time spent on ECLS after TPE (10, 11). Analyses were performed separately among all patients and in survivors only. Survival rate was calculated by dividing the number of patients who were discharged from the PICU by the total number of patients in this cohort.
Statistical Analysis
All statistical analyses were performed using IBM SPSS version 21 for Windows (SPSS, Chicago, IL). Data for OFI, hours to TPE, and total hours spent on ECLS after TPE were determined to be normally distributed while the VIS data was non-normally distributed using the 1-sample Kolmogorov Smirnov test. All normally distributed data were represented as mean ± standard deviation and comparative analyses were done with Student’s T-test. All VIS data were represented as median (25–75 percentiles) and comparative analyses were done using Mann-Whitney rank sum test. In the subgroup analyses comparing the change in OFI and VIS in early vs. late group, the OFI and VIS prior to and after TPE were compared in early vs. late group using Student’s T-test and Mann-Whitney rank sum test, respectively. None of the comparative data had a significant difference, suggesting that the statistically significant differences in data for change in OFI and VIS in early vs. late group were truly due to the degree of change in OFI and VIS in early vs. late group. Differences were considered statistically significant at p values < 0.05. All reported p values were 2-sided.
Results
Fourteen patients aged 4 months to 16 years of age (9 males and 5 females) weighing 7.5kg to 109.0kg met study criteria. Subject identification and their clinical characteristics are represented in Table 1. Half of our cohort was infected with Staphylococcus aureus. Twelve of the 14 patients were initiated on VA ECLS. Except for Patient #2 who was on VA ECLS due to weight limitation of venovenous (VV) ECLS, all patients on VA ECLS had cardiovascular failure and hypoxic respiratory failure. Patient #8 was the only patient on VV ECLS who had hypoxic respiratory failure but eventually developed cardiovascular failure a few days after ECLS initiation. Patient #12 had hypoxic respiratory failure and was initially planned for VV ECLS. However, he became profoundly hypotensive, required cardiopulmonary resuscitation (CPR) prior to ECLS, and was converted to venoarteriovenous ECLS. A total of five patients, including Patient #12, required CPR prior to ECLS initiation. The first 9 patients received ECLS using a roller pump system while the last 5 patients were supported using a centrifugal pump system per institutional changes. Twelve of the 14 patients (85.7%) were on simultaneous CRRT and ECLS during the TPE regimen.
Table 1.
Patient Demographics.
| ID | Age | Sex | Weight (kg) | Co-morbidity | Etiologies of MODS | CRRT | ECLS | Admission to TPE (hours) | Cycles of TPE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 years | F | 69.0 | Hyperthyroidism, Depression | Thyrotoxicosis Enterobacter cloacae Pneumonia |
Y | VA | 128 | 2 |
| 2 | 29 months | F | 12.0 | None | MRSE Bacteremia | Y | VA | 52 | 4 |
| 3 | 15 years | M | 65.0 | None | MRSA Pneumonia MRSA Urinary Tract Infection MRSA Bacteremia |
Y | VA | 72 | 3 |
| 4 | 14 years | F | 50.0 | None | MRSA Pneumonia | N | VA | 98 | 3 |
| 5 | 9 years | F | 28.0 | Pre-B Cell ALL | Streptococcus viridans Bacteremia | Y | VA | 30 | 5 |
| 6 | 4 months | M | 7.5 | AAA Renovascular Hypertension | MSSA Pneumonia | Y | VA | 192 | 6 |
| 7 | 10 months | M | 8.5 | Eczema | Klebsiella pneumoniae Bacteremia Acinetobacter Species Bacteremia Stenotrophomonas Superinfection of Chronic Eczematous Dermatitis Candida albicans Bacteremia |
Y | VA | 19 | 5 |
| 8 | 12 years | M | 63.0 | Autism Asthma |
Blastomyces dermatitidis Pneumonia |
Y | VV | 148 | 3 |
| 9 | 6 years | M | 21.5 | None | Enterovirus (Coxsakivirus, Echovirus) Myocarditis Viral Pneumonia |
Y | VA | 48 | 3 |
| 10 | 7 years | F | 20.9 | Burkitt’s Leukemia | Pseudomonas aeruginosa Pneumonia Pseudomonas aeruginosa Peritonitis Pseudomonas aeruginosa Bacteremia |
Y | VA | 40 | 5 |
| 11 | 15 years | M | 42.1 | None | MRSA Pneumonia MRSA Bacteremia |
N | VA | 6 | 3 |
| 12 | 16 years | M | 109.0 | Asperger Syndrome | Influenza A Pneumonia MSSA Bacteremia |
Y | VAV | 28 | 3 |
| 13 | 15 years | M | 50.0 | None | Influenza A Pneumonia MRSA Pneumonia/Bacteremia |
Y | VA | 7 | 3 |
| 14 | 12 years | M | 72.6 | Autism | Influenza A Pneumonia MSSA Pneumonia/Bacteremia |
Y | VA | 9 | 3 |
ID = identification; F = female; M = male; ALL = acute lymphoblastic leukemia; AAA = abdominal aortic aneurysm; MODS = multiple organ dysfunction syndrome; MRSE = methicillin resistant Staphylococcus epidermidis; MRSA = methicillin resistant Staphylococcus aureus; MSSA = methicillin sensitive Staphylococcus aureus; CRRT = continuous renal replacement therapy; Y = yes; N = no; ECLS = extracorporeal life support; VA = venoarterial; VV = venovenous; VAV = venoarteriovenous; TPE = therapeutic plasma exchange
All patients met TAMOF criteria, defined as OFI greater than or equal to 2 and platelet count less than 100,000/mm3, within 24 hours prior to receiving TPE. Twelve patients received membrane-based TPE while 2 patients received centrifugation-based TPE. Patients underwent 2–6 cycles (median 3) of TPE with 0.2–1.5 times the estimated plasma volume (median 1.0 times) per exchange. Majority of replacement fluid consisted of FFP and 5% albumin with the most common mixture of 50% FFP and 50% albumin.
The overall survival rate in this cohort was 71.4% (10 of 14). Patient #1 died 20 days after ECLS decannulation secondary to acute worsening of cardiac and respiratory failure, and patient’s goals of care was redirected to comfort care only. The remaining 3 patients died while on ECLS: Patient #6 failed multiple attempts to trial off of ECLS and Patients #13 and #14 developed irreversible CNS injury from intracerebral hemorrhage, and medical care was eventually withdrawn in all 3 patients.
In our historical control group, there were a total of 20 children who required ECLS for sepsis-induced MODS that did not receive TPE. Eleven of the 20 patients (55.0%) were on simultaneous CRRT. A total of 10 of 20 patients (50.0%) survived to PICU discharge. Of the patients who received ECLS and CRRT, 3 of 11 patients (27.3%) survived compared to 8 of 12 patients (66.7%) surviving in our cohort of patients on ECLS and CRRT who subsequently received TPE.
For the secondary outcome, the mean OFI decreased following TPE [pre: 4.1 ± 0.7 vs. post: 2.9 ± 0.9; p = 0.0004], and the median VIS decreased nearly 5 fold following TPE [pre: 24.5 (13.0–69.8) vs. post: 5.0 (1.5–7.0); p = 0.0002]. Every patient on vasoactive and/or inotropic agents had a decrease in VIS, and Patients #5 and #12 were able to discontinue all agents. Of all of the organ systems, hematologic failure was most commonly reversed with TPE (11 of 12; 91.7%). Liver failure was the second most commonly reversed organ failure (3 of 7; 42.9%). In addition, Patient #10 was able to discontinue ECLS after respiratory failure reversal and Patient #5 was able to discontinue CRRT use within 12 hours of completion of TPE. No known major complications (30), including cardiopulmonary arrest, bleeding/hematoma, pneumothorax, or hemothorax occurred in 51 total TPE sessions. Five sessions (5 of 51; 9.8%) in 4 patients were stopped early due to transient hypotension and new onset rash.
Based on a prior study using TPE in TAMOF (10), patients were assigned to an early group (TPE started < 30 hours from admission) or a late group (TPE started > 30 hours from admission) to investigate if the timing of initiation of TPE influenced outcomes (Table 2). The time points were based on previously published studies suggesting earlier initiation of TPE led to improved outcome (10, 11). Two of 6 patients in the early group and 2 of 8 patients in the late group died. Although there was no significant difference in OFI and VIS between the early and the late groups prior to TPE, there was a trend towards greater change in OFI in the early group compared to the late group [pre: −1.7 ±1.2 vs. post: −0.9 ±0.6; p = 0.14], while the change in VIS in the early group was 5 fold greater than the late group [pre: −67.5 (28.0–171.2) vs. post: −12.0 [7.2–18.5]; p = 0.02]. Among survivors, the change in OFI was almost 3 fold greater among early vs. late initiation of TPE [early: −2.3 ±1.0 vs. late: −0.8 ± 0.8; p = 0.03], while only a trend was apparent for improvement in VIS among early vs. late initiation [early: −42.0 (16.0–76.3) vs. late: −12.0 (5.3–29.0); p=0.17]. The mean duration of ECLS after TPE was not statistically different among either the early or the late group.
Table 2.
Impact of Timing of Initiation of Therapeutic Plasma Exchange on Organ Failure Index, Vasoactive-Inotropic Score, and Extracorporeal Life Support Requirement.
| Early Group (n=6) | Late Group (n=8) | p value | ||
|---|---|---|---|---|
| Hours to TPE from Admission | All | 16.5 ± 10.7 | 97.3 ± 54.6 | 0.004 |
| Change in OFI | All | −1.7 ± 1.2 | − 0.9 ± 0.6 | 0.14 |
| Survivors Only | − 2.3 ± 1.0 | − 0.8 ± 0.8 | 0.03 | |
| Change in VIS | All | − 67.5 (28.0–171.2) | − 12.0 (7.2–18.5) | 0.02 |
| Survivors Only | − 42.0 (16.0–76.3) | − 12.0 (5.3–29.0) | 0.17 | |
| Hours Spent on ECLS After TPE | All | 172.5 ± 169.6 | 248.0 ± 197.9 | 0.47 |
| Survivors Only | 133.3 ± 72.8 | 214.0 ± 208.2 | 0.48 |
TPE = therapeutic plasma exchange; OFI = Organ Failure Index; VIS = Vasoactive-Inotropic Score; ECLS = extracorporeal life support
Early group = patients receiving TPE within 30 hours from the time of PICU admission
Late group = patients receiving TPE after 30 hours from the time of PICU admission
Discussion
In our pediatric cohort with sepsis-induced MODS requiring ECLS who received TPE as an adjunctive therapy, we detected a nearly 30% improvement in mean OFI and 80% reduction in median VIS at 12 hours after completing the TPE regimen compared to the 24 hours prior to the initiation of TPE. Our cohort had a mean OFI of 4.1, resulting in an expected survival rate of less than 40% as reported in previous studies (4–9). Despite this fact, survival rate in our cohort was over 70%. We also compared the survival rate of this cohort to the children who required ECLS for sepsis-induced MODS in the 10 years prior to the use of TPE for sepsis in our institution, and TPE use was associated with a trend towards improved survival. These results were achieved with no major complications, and simultaneous TPE and ECLS was safely performed, as also concluded by Dyer et al (31).
The first randomized controlled trial (RCT) of plasma therapy use in sepsis demonstrated that the use of plasma filtration compared to conventional sepsis management led to significant attenuation of initial acute-phase septic response, a trend towards reduction of risk of death, and fewer organ failures in children and adults (23). In a larger, adult RCT comparing TPE use to conventional severe sepsis/septic shock management, Busund et al. concluded that TPE use led to an absolute risk reduction in mortality at 28 days by 20.5% compared to conventional sepsis management (27). The only pediatric RCT by Nguyen et al. reported no mortality in patients diagnosed with TAMOF randomized to receive TPE while 80% of patients randomized to standard sepsis therapy without TPE died (10). Other case series, retrospective/prospective studies, and meta-analysis have also shown improved clinical outcomes with plasma therapy use in MODS (11, 12, 14–20, 32–35). Our results are in agreement with published studies of the potential benefit of plasma therapy in sepsis.
Interestingly, patients who received TPE within 30 hours from the time of admission had 3 fold greater degree of OFI improvement in survivors compared to late receivers while only a trend existed when change in OFI was analyzed in all patients. It is possible that the therapeutic benefit of TPE is missed, especially in the most severely ill patients, if instituted late in the patient’s hospital course. This theory further highlights the importance of detecting severe sepsis/septic shock early and to consider the use of TPE. Degree of VIS reduction was 5 fold greater with patients receiving TPE earlier as opposed to later in the hospital course. The likely theory is that the most severely ill patients began with higher VIS prior to TPE and had the highest likelihood to lower their VIS, which was accentuated when looking at early vs. late receivers of TPE. Lastly, our study showed that the total time dependent on ECLS after completing TPE was not statistically different whether receiving TPE early vs. late in the hospital course. Although there was a trend towards requiring less ECLS time when TPE was initiated early, this association is limited by the small number of patients in our cohort and the heterogeneous nature of the diseases that required ECLS.
TPE is thought to be beneficial for sepsis-induced MODS for multiple reasons including attenuation of exaggerated inflammatory response by removal of cytokines, removal of cytotoxins such as Panton-Valentine leukocidin released by community-associated methicillin resistant Staphylococcus aureus, removal of harmful antibodies such as inhibitors of ADAMTS-13, and replenishing the patients with proteins and enzymes needed to achieve balance between procoagulant and anticoagulant homeostasis (17, 18, 21–25). As expected, patients in this cohort most commonly had hematologic organ recovery as providing patients with FFP directly improves its function. However, we also saw cardiovascular, pulmonary, renal, and hepatic dysfunction recovery in our cohort.
Nguyen et al. postulated that TAMOF is a clinical indicator of secondary TMA which could benefit greatly from TPE (26). Improvement in the degree of thrombocytopenia with TPE can be a sign of organ failure reversal, and some have recommended resolution of thrombocytopenia as an end point for plasma therapy in MODS (10, 11, 26). Twelve of our 14 patients did develop thrombocytopenia prior to going on ECLS and initiating TPE. All 14 of our patients had thrombocytopenia at the time of TPE initiation, but it is unclear whether thrombocytopenia developed in the 2 patients due to secondary TMA vs. platelet adhering to ECLS circuits, which is a known phenomenon (36). The mean platelet count within 24 hours prior to TPE initiation was 70,000/mm3 and increased to a mean count of 73,000/mm3 within 12 hours from the completion of TPE. The minimal difference in the degree of thrombocytopenia could be explained by the continued consumption of platelets from ECLS circuits, especially since all patients except one remained on ECLS after completion of TPE. Interestingly, the 2 patients without thrombocytopenia prior to ECLS were the only patients among survivors in this cohort who did not achieve a lower OFI with TPE, though their VIS did decrease after TPE regimen.
Although this retrospective study is the largest to date looking at the outcomes of TPE in patients requiring ECLS for sepsis, there are limitations to this study. The most obvious is its retrospective and non-randomized nature in which patient selection bias cannot be excluded. We used a historical control group from previously published studies by gathering data on survival rates in patients with similar number of organ failure to determine the approximate survival rate of our cohort (4–9). We also presented the survival rate of children with sepsis-induced MODS on ECLS and/or CRRT in our institution prior to the use of TPE for comparison. Due to the absence of an immediate control group, our conclusions are limited to associations of TPE with the outcome measures. Since we were unable to assess neurological dysfunction due to sedation medications used while on ECLS, the cohort may have had a higher mean OFI.
The single institution and limited numbers of patients, only 14 patients in 8 years, also limits the conclusions that can be drawn from our data, especially when comparing the survival rates of the cohort to our historical control group. The lack of standardized TPE protocols including initiation of therapy, number of cycles, plasma replacement volume, the type of replacement fluid, and the end point for TPE treatment resulted in individualized treatments. The TPE regimens were determined at the discretion of pediatric critical care, pediatric nephrology, and/or the apheresis team limiting our ability to determine the decision processes leading to various prescriptions. Most patients received TPE until clinical improvement and stability were achieved, which was suggested by decrease in OFI and/or VIS. Even with these limitations, our data represents an association with positive clinical outcomes with TPE use in even the most critically ill pediatric population requiring ECLS.
Conclusions
TPE in children with MODS due to sepsis requiring ECLS appears to be associated with significant organ failure recovery and decreased requirement for vasoactive and/or inotropic agents, which may have the potential to improve survival rate. Initiating TPE earlier compared to later in the hospital course was associated with greater improvement in organ dysfunction and less need for vasoactive and/or inotropic agents. These data support the need for larger, prospective studies identifying the indication, clinical benefits, and safety of TPE use in pediatric patients with sepsis-induced MODS requiring ECLS.
Acknowledgments
TTC (K08 HD062142) and NBB (K08 DK093785) are supported by NIH Career Development Awards.
We want to thank Moni Weber, a clinical research program manager, in the Pediatric Critical Care Department at C.S. Mott Children’s Hospital and staff in the Medical Center Information Technology - Clinical Research Services for helping identify the patients for this study. We would also like to thank the University of Michigan Center for Statistical Consultation and Research for performing an in-depth review of our statistical analyses.
Footnotes
Reprints will not be ordered.
References
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, et al. Trends in the epidemiology of pediatric severe sepsis*. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14(7):686–693. doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 2.Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487–494. doi: 10.1542/peds.2006-2353. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical care medicine. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 4.Johnston JA, Yi MS, Britto MT, et al. Importance of organ dysfunction in determining hospital outcomes in children. The Journal of pediatrics. 2004;144(5):595–601. doi: 10.1016/j.jpeds.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Lacroix J, Cotting J, et al. Pediatric Acute Lung I. Severity of illness and organ dysfunction scoring in children. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2005;6(3 Suppl):S126–134. doi: 10.1097/01.PCC.0000161287.61028.D4. [DOI] [PubMed] [Google Scholar]
- 6.Leclerc F, Leteurtre S, Duhamel A, et al. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. American journal of respiratory and critical care medicine. 2005;171(4):348–353. doi: 10.1164/rccm.200405-630OC. [DOI] [PubMed] [Google Scholar]
- 7.Tantalean JA, Leon RJ, Santos AA, et al. Multiple organ dysfunction syndrome in children. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2003;4(2):181–185. doi: 10.1097/01.PCC.0000059421.13161.88. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson JD, Pollack MM, Glass NL, et al. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. The Journal of pediatrics. 1987;111(3):324–328. doi: 10.1016/s0022-3476(87)80448-1. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson JD, Pollack MM, Ruttimann UE, et al. Outcome of pediatric patients with multiple organ system failure. Critical care medicine. 1986;14(4):271–274. doi: 10.1097/00003246-198604000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Critical care medicine. 2008;36(10):2878–2887. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu L, Kiss JE, Dargo G, et al. Outcomes of previously healthy pediatric patients with fulminant sepsis-induced multisystem organ failure receiving therapeutic plasma exchange. Journal of clinical apheresis. 2011;26(4):208–213. doi: 10.1002/jca.20296. [DOI] [PubMed] [Google Scholar]
- 12.Sevketoglu E, Yildizdas D, Horoz OO, et al. Use of Therapeutic Plasma Exchange in Children With Thrombocytopenia-Associated Multiple Organ Failure in the Turkish Thrombocytopenia-Associated Multiple Organ Failure Network. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2014 doi: 10.1097/PCC.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharfman WB, Tillotson JR, Taft EG, et al. Plasmapheresis for meningococcemia with disseminated intravascular coagulation. The New England journal of medicine. 1979;300(22):1277–1278. [PubMed] [Google Scholar]
- 14.Bridges BC, Hardison D, Pietsch J. A case series of the successful use of ECMO, continuous renal replacement therapy, and plasma exchange for thrombocytopenia-associated multiple organ failure. Journal of pediatric surgery. 2013;48(5):1114–1117. doi: 10.1016/j.jpedsurg.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 15.De Simone N, Racsa L, Bevan S, et al. Therapeutic plasma exchange in the management of sepsis and multiple organ dysfunction syndrome: A report of three cases. Journal of clinical apheresis. 2013 doi: 10.1002/jca.21296. [DOI] [PubMed] [Google Scholar]
- 16.Hadem J, Hafer C, Schneider AS, et al. Therapeutic plasma exchange as rescue therapy in severe sepsis and septic shock: retrospective observational single-centre study of 23 patients. BMC anesthesiology. 2014;14(1):24. doi: 10.1186/1471-2253-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt J, Mann S, Mohr VD, et al. Plasmapheresis combined with continuous venovenous hemofiltration in surgical patients with sepsis. Intensive care medicine. 2000;26(5):532–537. doi: 10.1007/s001340051200. [DOI] [PubMed] [Google Scholar]
- 18.Stegmayr BG, Banga R, Berggren L, et al. Plasma exchange as rescue therapy in multiple organ failure including acute renal failure. Critical care medicine. 2003;31(6):1730–1736. doi: 10.1097/01.CCM.0000064742.00981.14. [DOI] [PubMed] [Google Scholar]
- 19.Darmon M, Azoulay E, Thiery G, et al. Time course of organ dysfunction in thrombotic microangiopathy patients receiving either plasma perfusion or plasma exchange. Critical care medicine. 2006;34(8):2127–2133. doi: 10.1097/01.CCM.0000227659.14644.3E. [DOI] [PubMed] [Google Scholar]
- 20.Gardlund B, Sjolin J, Nilsson A, et al. Plasmapheresis in the treatment of primary septic shock in humans. Scandinavian journal of infectious diseases. 1993;25(6):757–761. doi: 10.3109/00365549309008575. [DOI] [PubMed] [Google Scholar]
- 21.Carcillo JA, Kellum JA. Is there a role for plasmapheresis/plasma exchange therapy in septic shock, MODS, and thrombocytopenia-associated multiple organ failure? We still do not know--but perhaps we are closer Intensive care medicine. 2002;28(10):1373–1375. doi: 10.1007/s00134-002-1428-x. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TC, Carcillo JA. Understanding the role of von Willebrand factor and its cleaving protease ADAM TS13 in the pathophysiology of critical illness. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2007;8(2):187–189. doi: 10.1097/01.CCM.0000257468.75474.D4. [DOI] [PubMed] [Google Scholar]
- 23.Reeves JH, Butt WW, Shann F, et al. Continuous plasmafiltration in sepsis syndrome. Plasmafiltration in Sepsis Study Group Critical care medicine. 1999;27(10):2096–2104. doi: 10.1097/00003246-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Stegmayr BG. Plasmapheresis in severe sepsis or septic shock. Blood purification. 1996;14(1):94–101. doi: 10.1159/000170250. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz J, Winters JL, Padmanabhan A, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. Journal of clinical apheresis. 2013;28(3):145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen TC, Carcillo JA. Bench-to-bedside review: thrombocytopenia-associated multiple organ failure--a newly appreciated syndrome in the critically ill. Critical care. 2006;10(6):235. doi: 10.1186/cc5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busund R, Koukline V, Utrobin U, et al. Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive care medicine. 2002;28(10):1434–1439. doi: 10.1007/s00134-002-1410-7. [DOI] [PubMed] [Google Scholar]
- 28.Doughty L, Carcillo JA, Kaplan S, et al. The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113(6):1625–1631. doi: 10.1378/chest.113.6.1625. [DOI] [PubMed] [Google Scholar]
- 29.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 30.Winters JL. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2012;2012:7–12. doi: 10.1182/asheducation-2012.1.7. [DOI] [PubMed] [Google Scholar]
- 31.Dyer M, Neal MD, Rollins-Raval MA, et al. Simultaneous extracorporeal membrane oxygenation and therapeutic plasma exchange procedures are tolerable in both pediatric and adult patients. Transfusion. 2013 doi: 10.1111/trf.12418. [DOI] [PubMed] [Google Scholar]
- 32.Berlot G, Agbedjro A, Tomasini A, et al. Effects of the Volume of Processed Plasma on the Outcome, Arterial Pressure and Blood Procalcitonin Levels in Patients with Severe Sepsis and Septic Shock Treated with Coupled Plasma Filtration and Adsorption. Blood purification. 2014;37(2):146–151. doi: 10.1159/000360268. [DOI] [PubMed] [Google Scholar]
- 33.Zhou F, Peng Z, Murugan R, et al. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Critical care medicine. 2013;41(9):2209–2220. doi: 10.1097/CCM.0b013e31828cf412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Formica M, Olivieri C, Livigni S, et al. Hemodynamic response to coupled plasmafiltration-adsorption in human septic shock. Intensive care medicine. 2003;29(5):703–708. doi: 10.1007/s00134-003-1724-0. [DOI] [PubMed] [Google Scholar]
- 35.Ronco C, Brendolan A, Lonnemann G, et al. A pilot study of coupled plasma filtration with adsorption in septic shock. Critical care medicine. 2002;30(6):1250–1255. doi: 10.1097/00003246-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Annich GM, Meinhardt JP, Mowery KA, et al. Reduced platelet activation and thrombosis in extracorporeal circuits coated with nitric oxide release polymers. Critical care medicine. 2000;28(4):915–920. doi: 10.1097/00003246-200004000-00001. [DOI] [PubMed] [Google Scholar]