Abstract
Variability in fear conditionability is common, and clarity regarding the neural regions responsible for individual differences in fear conditionability could uncover brain-based biomarkers of resilience or vulnerability to trauma-based psychopathologies (e.g., post-traumatic stress disorder). In recent years, neuroimaging work has yielded a detailed understanding of the neural mechanisms underlying fear conditioning common across participants, however only a minority of studies have investigated the brain basis of inter-individual variation in fear learning. Moreover, the majority of these studies have employed small sample sizes (mean n = 17; range n = 5–27) and all have failed to meet the minimum recommended sample size for functional magnetic resonance imaging (fMRI) studies of individual differences. Here, using fMRI, we analyzed blood-oxygenation level dependent (BOLD) response recorded simultaneously with skin conductance response (SCR) and ratings of unconditioned stimulus (US) expectancy in 49 participants undergoing Pavlovian fear conditioning. On average, participants became conditioned to the conditioned stimulus (CS+; higher US expectancy ratings and SCR for the CS+ compared to the unpaired conditioned stimulus, CS−); the CS+ also robustly increased activation in the bilateral insula. Amygdala activation was revealed from a regression analysis that incorporated individual differences in fear conditionability (i.e., a between-subjects regressor of mean CS+ > CS− SCR). By replicating results observed using much smaller sample sizes, the results confirm that variation in amygdala reactivity covaries with individual differences in fear conditionability. The link between behavior (SCR) and brain (amygdala reactivity) may be a putative endophenotype for the acquisition of fear memories.
Keywords: fear conditioning, amygdala, fMRI, skin conductance response, SCR, individual differences
1.0 Introduction
In the domain of fear conditioning, inter-individual differences are the rule rather than the exception; for a given conditioned stimulus, some individuals display robust fear responding, while others display little or no fear response. Evidence suggests that individual differences in fear responding are stable [1] and heritable [2], suggesting that they may reflect key neural differences. Importantly, such differences could be associated with resistance or vulnerability to anxiety disorders [3]. That is, individuals whose neural circuity predisposes them to remember fear more readily might be more likely to develop trauma-related psychopathology if exposed to a traumatic event [4]. In the past two decades, neuroimaging work has generated a detailed understanding of how fear responses are acquired in the human brain [5–7]. However, the majority of this work has focused on commonalities across participants, seeking to identify the neural regions involved in “typical” fear responding, while considering inter-individual variation in conditionability to be a source of statistical noise (e.g., necessitating the exclusion of “non-responders”).
In a typical Pavlovian fear conditioning paradigm, participants are presented with a neutral stimulus, such as a colored light (conditioned stimulus, CS+) that is paired repeatedly with an aversive stimulus, such as mild electric shock (unconditioned stimulus, US). After multiple pairings, the CS+ comes to elicit a fear response (conditioned response, CR), which can be observed in contrast to the response elicited by an unpaired neutral stimulus (CS−). Animal studies of fear conditioning have consistently implicated the amygdala in learning this association and in the production of conditioned fear responses [8,9]. In humans, neuroimaging studies have revealed activation of the amygdala, the insula and the anterior cingulate cortex (ACC) during fear conditioning [10,11], using measures such as blood-oxygenated level dependent response (BOLD), assessed via functional magnetic resonance imaging (fMRI).
Peripheral measures of fear learning such as skin conductance response (SCR), a measure of autonomic arousal, can be used to index fear conditioning success. SCR is represented in the brain by a number of regions overlapping with those involved in emotion [12,13]. While the amygdala does not appear to be essential for the production of SCRs [e.g., patients with bilateral amygdala damage produce normal SCRs to a number of visual and auditory stimuli; 14], trials that elicit larger conditioned SCRs are associated with increased amygdala reactivity to the CS, suggesting that the amygdala may be central to the expression of conditioned fear [15–17].
Prior neuroimaging work has elucidated commonalities in fear learning across individuals, and has begun to shed light on the neural correlates of trial-to-trial (i.e., within-subject) variability in conditioned SCR responding. However, examination of the neural generator(s) of individual differences (i.e., between-subject variability) in fear conditioning has been relatively limited. Those studies that have investigated the neural basis of individual differences in fear conditionability have generally been plagued by small sample sizes, ranging from 5-27 participants [17–22]. Small sample sizes are problematic in studies of individual differences and the fMRI literature in particular has been criticized on this point [23,24]. Among the problems associated with small sample sizes are that lack of power may lead to erroneous conclusions about which brain regions are and are not associated with individual differences and that effects which are observed may capitalize on chance, which may lead to overestimations of effect size [23].
In the largest study yet published on the neural basis of individual differences in fear conditionability (n = 27), Petrovic and colleagues [21] sought to investigate neural mechanisms underlying affective evaluations of social stimuli. To this end, participants viewed pictures of 4 different faces over the course of an experiment. Two of the faces (CS+) were paired with mild electric shock (US) on 50% of trials; the other two faces (CS−) were never paired with shock. While they failed to observe an overall increase in SCR for the CS+ versus the CS−, Petrovic and colleagues [21] observed greater conditioning related increases in SCR from the second half of the experiment compared to the first half of the experiment that were positively correlated with BOLD activation in the bilateral amygdala, using a region of interest (ROI) approach focused on the amygdala and the fusiform gyrus, a region involved in face processing.
In the second-largest report on the neural basis of individual differences in fear conditionability published to-date, Schiller and Delgado [22] reanalyzed data from an earlier study [25]. In the original study, n = 17 participants viewed 2 faces, one of which (CS+) had been paired with a mild electric shock (US), and the other (CS−), which was never paired with shock. Using a whole-brain, between-subjects approach, Schiller and Delgado [22] found evidence of a positive correlation between CS+ SCR and activation in the striatum and the insula, suggesting that these brain regions, which have been implicated in the encoding of value signals, might underlie individual differences in fear conditionability.
The lack of congruence between results from these studies [e.g., lack of SCR-amygdala covariation in 22] makes it difficult to draw firm conclusions studies about the neural correlates of inter-individual variation in fear conditionability. For example, it is unclear whether Schiller and Delgado [22] failed to observe a correlation between the amygdala and SCR because of a lack of power, and whether Petovic and colleagues [21] might have observed correlations between SCR and BOLD activation in other brain regions (e.g., the insula, ventral striatum) had they not limited their analysis to the amygdala and the fusiform gyrus. Further, both studies used faces as the CS stimuli, which might vary in their perceived affective salience across individuals [e.g., 26] and might therefore confound effects of social stimuli processing and fear conditioning. Further, conditioned faces might potentiate activity in stimulus-specific regions (e.g., the fusiform gyrus) that may or may not be otherwise implicated in inter-individual variation in fear learning.
Therefore, the goal of the present study was to further investigate the brain mechanism underlying inter-individual variation in fear conditionability. Current recommendations are that fMRI studies of individual differences employ a minimum sample size of n = 40, in order to achieve an acceptable trade-off between statistical power and data collection costs [24]. To this end, we used a sample of n = 49 healthy volunteers and simultaneous SCR recording and fMRI BOLD during Pavlovian fear conditioning, in which a neutral object (a street lamp) was paired with a mild electric shock (US) on some trials (CS+) and not others (CS−). To assess contingency awareness during fear learning, participants were also asked to rate US expectancy on each trial (prior to US onset). Previous work has implicated the amygdala, the insula and the ACC in fear learning [10], and the amygdala, insula, cerebellum, medial prefrontal cortex, precentral gyrus and the superior temporal gyrus in the expression of conditioned fear responding [i.e., SCR production; 27]. Therefore, we hypothesized that individuals with greater fear conditionability (measured via SCR to the CS+ versus CS−) would show greater neural activation in these regions.
2.0 Materials and Method
2.1 Participants
Fifty-one healthy, right-handed participants participated in the study. One participant was excluded from analyses because of a technical difficulty that compromised recording of the SCR data; another participant was excluded because he felt claustrophobic during the scan and was unable to continue. Therefore, 49 participants (28 female; M age = 25.3 years, range = 21-40 years, SD = 4.8; Caucasian = 23, Asian = 12, African American/Black = 3, Native American or Native Hawaiian = 3, more than one race = 8) had both fMRI and skin conductance data and were included in the analyses. Participants were recruited from the community, consented and compensated $10/hour for their time. Participants were free from significant neurological, psychiatric or medical illness, as assessed by examination by a psychiatrist and the Structured Clinical Interview for DSM-IV [SCID-NP; 28]. Female volunteers were studied approximately one week prior to menses to minimize hormonal influences [i.e., when estrogen levels are consistently low; 29,30]. Participants also had negative urine toxicology and alcohol breathalyzer screens at the time of data collection. Study procedures were approved by the University of Illinois at Chicago Institutional Review Board.
2.2 Experimental Task
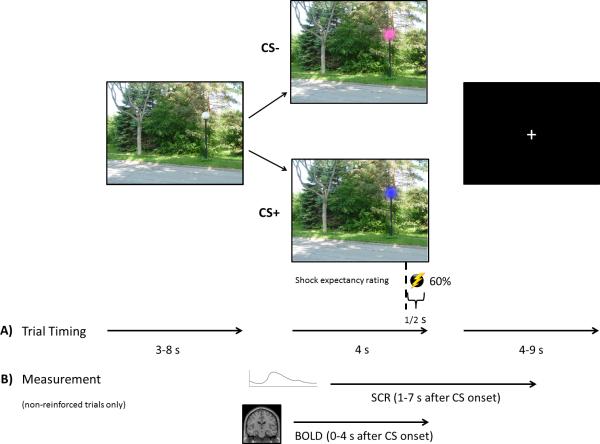
Participants performed a modified version of a fear conditioning task used previously [31,32]. Figure 1 depicts trial timing and the time points at which SCR and fMRI BOLD were measured. In brief, participants viewed different colored street lamps (e.g., blue, pink; conditioned stimuli, CSs) in a picture of an outdoor scene (a park or a school). The image of the outdoor scene was presented for 3-8s followed by 4s of CS presentation in the conditioning context, interleaved with a fixation cross inter-trial interval ranging from 4-9s. The paradigm consisted of 40 CS+s, which co-terminated with an aversive mild electric shock (US) at a partial reinforcement rate of 60% (24 trials) and 20 CS− trials, which were never paired with the US. The US consisted of highly annoying but not painful electrical stimulation to the left foot during the last 500 ms of CS+ presentation. Prior to the beginning of the experiment, participants selected their own shock level via a procedure in which a mild shock was slowly increased until participants indicated that it was “uncomfortable, but not painful”. The average shock intensity selected by participants was 4.41 mA (range 2.8-6.3; SD = .89). During the first 3.5 s of each CS presentation (i.e., prior to US onset), participants were asked to rate their expectancy that the US would occur on that trial (1= “Definitely Not”; 2 = “Unsure”; 3 = “Definitely Will”). Conditioned fear responses were indexed by changes in SCR. Trial types were intermixed and presented in pseudorandom order across two fMRI runs, each lasting approximately 8 minutes. Conditioning-to-light assignment and conditioning context were counterbalanced across participants.
Fig. 1.
Example of task timing. A) Stimulus presentation durations. B) Time windows during which SCR and fMRI BOLD activation were measured.
2.3 Subjective Ratings Measurement and Data Analysis
Subjective ratings of US expectancy were compared using a paired t-test contrasting CS+ and CS− trials. Only ratings for CS+ trials that were unpaired with the US were analyzed, in line with analysis of psychophysiological and fMRI data. Statistical analysis of subjective ratings and psychophysiological data was performed using IBM SPSS Statistics for Windows, Version 22 (IBM Corp., Armonk, NY).
2.4 Psychophysiological Measurement and Data Analysis
Skin conductance level was measured simultaneously with BOLD response using two disposable carbon fiber electrodes attached between the first and second phalanges of the second and third digits of the left hand (EL509, BIOPAC Systems Inc., Goleta, CA). An MRI-compatible BIOPAC Systems skin conductance module (EDA100C-MRI) was used to sample skin conductance at 1000 Hz and amplify this data. Data was stored for offline analysis using AcqKnowledge 4.2 software (BIOPAC Systems, Inc.). Waveforms were low-pass filtered using a Blackman window with a cutoff of 40Hz and mean-valued smoothed over 100 adjacent data points.
To assess the level of conditioned fear responding separate from unconditioned responses to the US, we included only non-reinforced trials of the CS+ in the analyses. SCR for each CS was calculated by subtracting the mean skin conductance level during the first second of CS presentation from the highest skin conductance level occurring in the 1-7 seconds following CS onset [33, see also 34,35 on SCR onset latency]. Our use of non-reinforced CS+ trials permitted examination of SCR after CS offset. Raw SCRs were square root transformed to normalize distributions [32,36]. SCRs to the CS+ versus CS− were compared using a paired t-test.
2.5 Image Acquisition
Functional MRI based on BOLD contrast was performed on a 3.0 Tesla GE MR 750 scanner (General Electric Healthcare; Waukesha, WI) using an 8-channel phased-array radio frequency head coil. A gradient-echo echo planar imaging (EPI) sequence was used (2s TR; 22.2ms TE; 90° flip angle; 64 × 64 matrix; 22cm FOV; 44 axial slices; 3.4 × 3.4 × 3.0 mm voxels; 488 volumes across 2 runs). The first 4 volumes from each run were discarded to allow the magnetization to reach equilibrium. Data from all participants met our inclusion criteria for image quality with minimal motion correction (all movements ≤ 2 mm in any direction across functional runs).
2.6 Functional MRI Data Analysis
Statistical Parametric Mapping (SPM 8) software (Wellcome Trust Centre for Neuroimaging, London, www.fil.ion.ucl.ac.uk/spm) was used to perform conventional preprocessing steps. In brief, slice-time correction was performed to account for temporal differences between slice collection order, images were spatially realigned to the first image of the first run, functional images were normalized to a Montreal Neurological Institute (MNI) template using the EPI template, resampled to 2 mm3 voxels and smoothed with an 8 mm isotropic Gaussian kernel.
The time series data were subjected to a general linear model, convolved with the canonical hemodynamic response function (HRF) and filtered with a 128s high-pass filter. We modeled non-reinforced CS+ and CS− trials separately and estimated effects for each voxel for each participant. Individual motion parameters were entered in the model as covariates of no interest. Following processing at the first-level, CS+ > CS− contrasts were taken to the second level for random effects analysis.
At the second level, we performed two sets of analyses. First, to examine overall neural correlates of conditioning, main effects of condition were assessed by comparing brain activity elicited by CS+ versus CS− trials using a one-sample t-test. For this analysis, activations were deemed significant at a p value of < .05, corrected for multiple comparisons across the whole brain using the false discovery rate (FDR) method.
Second, we examined regional brain activity that covaried with individual differences in CS+ > CS− SCR measurements taken during scanning. At the between-subjects level, each participant's mean CS+ > CS− SCR across all nonreinforced trials (capturing individual differences in conditioned responding) provided the regressor-of-interest. This regressor was entered into the design matrix examining whole-brain CS+ > CS− brain activity, yielding a statistical map of regional brain activity that occurred during fear learning and covaried with individual differences in conditioned responding (CS+ > CS− SCR). For this covariation analysis, in which we used CS+ > CS− SCR to predict CS+ > CS− BOLD, we tested the a priori hypotheses that SCR strength would be related to brain activity in regions previously implicated in fear acquisition [10] or conditioned SCR production [27]. Specifically, bilateral amygdala, bilateral insula, ACC, cerebellum, medial prefrontal cortex, precentral gyrus and superior temporal gyrus were chosen based on prior findings. An ROI mask encompassing coordinates corresponding to these regions and reported by Sehlmeyer and colleagues [10] and Knight and colleagues [27] was created using anatomical landmarks taken from the Automated Anatomical Labelling (AAL) atlas [37,38]1. Clusters of activation were initially identified using an uncorrected voxel threshold of p < .001, and then subjected to correction for multiple comparisons within the ROI as determined via simulation using the ClusterSim utility (10,000 iterations; http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html). Given smoothness estimates of the data within the mask (total size = 331 cm3), a family-wise error correction at α < 0.05 was realized using a voxel threshold of p < 0.001 with minimum cluster size of 40 voxels (320 mm3). For completeness, to obviate bias, and to generate hypotheses for future studies, we also examined whole-brain activation that occurred outside the a priori ROI mask, and surpassed a threshold of p < .05, FDR corrected, however no additional clusters of activation surpassed this threshold. To clarify the direction of significant results from each analysis, we extracted BOLD signal responses (parameter estimates, β-weights in arbitrary units [a.u.] of activation), averaged across all voxels within a 10 mm radius sphere surrounding peak activations.
3.0 Results
3.1 US Expectancy Ratings
US expectancy ratings were missing for one participant; therefore, analysis of ratings was performed on 48 participants. Participants rated the US as more likely to occur during the CS+ (M = 2.45, SD = .44) versus the CS− trials [M = 1.21, SD = .30; t(47)=13.12, p < .001].
3.2 SCR
Figure 2 (A) depicts the average SCR elicited by CS− and CS+ trials. A paired t-test revealed that CS+ trials elicited significantly higher SCR (M = .13, SD = .16) than CS− trials [M = .04, SD = .10; t(48)=4.80, p < .001].
Fig. 2.
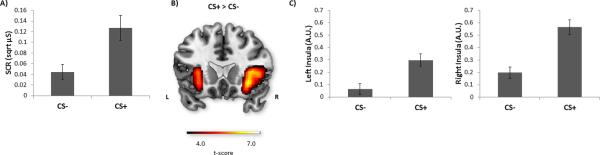
Peripheral and central responses during fear acquisition. A) Mean SCR elicited by the unpaired conditioned stimulus (CS−) and conditioned stimulus paired with shock (CS+). B) BOLD response during conditioning elicited by the CS+ contrasted with that elicited by the CS−, shown in the left insula and right insula. Activation maps are illustrated at a threshold of p < .001 and masked. C) Mean BOLD response (β weights) from the left insula [−28, 24, −4] and right insula [34, 24, 4] showing greater activation during CS+ versus CS− presentation. Error bars indicate standard error of the mean.
3.3 fMRI BOLD Response
3.3.1 Task effects
First, we examined the CS+ (> CS−) condition to determine whether CS+ trials effectively elicited increased brain activity associated with fear acquisition. Results showed that CS+ relative to CS− trials elicited greater activation in the left insula [peak MNI coordinate, −28, 24, −4; 3328 mm3; Z = 5.29, p < .001, corrected; Figure 2 (B)] and in the right insula [peak MNI coordinate, 34, 24, 4; 8000 mm3; Z = 6.02, p < .001, corrected; Figure 2 (B)]. Follow-up inspection of ROI-extracted BOLD signal (β weights) from the left and right insula confirmed the direction of increased insula activation during CS+ trials [left insula: CS+, M = .30, SD = .34, CS−, M = .06, SD = .31; right insula: CS+, M = .56, SD = .40, CS−, M = .20, SD = .32; Figure 2 (C)]. Greater activation for CS+ versus CS− trials was also observed in the supplementary motor area [peak MNI coordinate, 6, 24, 48; 2320 mm3; Z = 4.18, p = .004, corrected], the brainstem [peak MNI coordinate, 2, −24, −30; 1088 mm3; Z = 3.95, p = .009, corrected], the caudate [peak MNI coordinate, 14, 8, −8; 320 mm3; Z = 3.72, p = .02, corrected] and the cerebellum [peak MNI coordinate, −6, −52, −40; 192 mm3; Z = 3.69, p = .02, corrected]. In a post-hoc analysis, we examined CS+ > CS− amygdala activation at the group level with a more relaxed statistical threshold: small-volume correction (p < .05), performed separately for each of left and right amygdala with anatomically defined (AAL) masks [37,38]. Even with this relaxed statistical threshold, greater amygdala activation was not observed for CS+ (> CS−) trials. Because prior work has suggested rapid adaptation of CS+ responses in the amygdala [e.g., 39], we also examined BOLD responses separately for the first and second halves of acquisition. Conditioned amygdala reactivity was not observed across participants in either the first or second half of acquisition. Additionally, in a post-hoc analysis, we examined amygdala activation specifically for individuals showing evidence of heightened fear conditionability. Therefore, we performed a median split on CS+ > CS− SCRs and examined amygdala responding separately for each group [small-volume corrected, p < .05 using separate left and right AAL anatomically defined masks; 37,38]. Still, we failed to observe greater left or right amygdala activation for the CS+ versus the CS− condition.
3.3.2 Correlation with SCR
Within our a priori regions, correlational analysis, in which participants’ CS+ > CS− hemodynamic activity was regressed onto participants’ CS+ > CS− SCR revealed a positive correlation between activation in the right amygdala [peak MNI coordinate, 22, −2, −16; 576 mm3; Z = 3.79, p < .05, corrected] and SCRs to the CS+ versus the CS− [Figure 3 (A) and (B)]. Also falling within the a priori mask, an additional positive correlation was observed between CS+ > CS− hemodynamic response and participants’ CS+ > CS− SCR in the supplementary motor area [peak MNI coordinate, −4, −8, 62; 2976 mm3; Z = 3.97, p < .05, corrected]. No other regional brain activity covaried with SCR using the ClusterSim statistical threshold. In a post-hoc analysis, we examined the correlation between CS+ > CS− SCR and BOLD response in the left amygdala using a more relaxed statistical threshold: small-volume correction (p < .05) with a left amygdala anatomical AAL mask [37,38]. Using this more relaxed statistical threshold, we observed a significant positive correlation between CS+ > CS− SCR and fMRI BOLD [peak MNI coordinate, −24, 2, −20; 1368 mm3; Z = 3.10, p < .05, corrected] in the left amygdala.
Fig. 3.
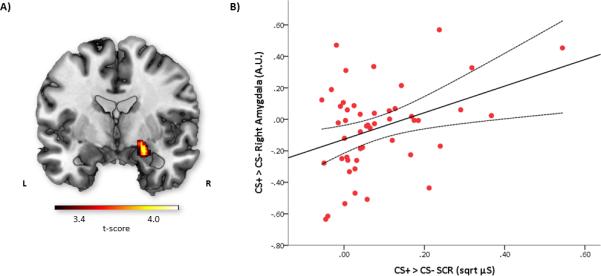
A) Correlational map showing the location of the significant correlation between the CS+ > CS− amygdala activation [22, −2, −16] and SCR during fear conditioning. Threshold is set at p < .001 and masked. B) Scatterplot plot depicting the correlation between the amygdala and SCR to the CS+ > CS−. Dashed lines indicate 95% confidence intervals.
To determine whether the correlation between CS+ > CS− amygdala reactivity and SCR was driven more by the CS+ or the CS−, we performed Pearson's correlations between activity in the right amygdala (β-weights for the CS+ > CS− contrast) and SCR for each of the CS+ and CS−. Results showed a significant correlation with CS+ SCR [r(47) = .30, p = .04] but not with CS− SCR [r(47) = .04, p = .77]; moreover, the difference between these correlations was statistically significant [two-tailed p = .02; 40,41]. To determine whether insula reactivity would predict SCR, we correlated CS+ > CS− BOLD responses extracted from the left and right insula with CS+ > CS− SCR. Correlations did not reach significance for the left insula [r(47) = .18, p = .23] or the right insula [r(47) = .23, p = .11]. Self-reported shock expectancy (CS+ > CS−) also failed to correlate with CS+ > CS− SCR [r(46) = .08, p = .61].
4.0 Discussion
Conditioned fear describes a series of coordinated behavioral effects that result when a neutral stimulus (which at first has no behavioral effects) is repeatedly paired with an aversive outcome [42]. Individuals vary greatly in their propensity to acquire conditioned fear responses, however the brain mechanism underlying this variability is unknown. Here, using a large sample size and simultaneous SCR-fMRI recording, we identified the amygdala as a neural correlate of between-subjects differences in fear learning. The more an individual showed fear acquisition at the behavioral level (as measured by SCR), the greater their conditioned amygdala reactivity. The results are in line with those of Petrovic and colleagues [21] and with other studies that have examined the neural basis of individual differences in fear conditionability, albeit using much smaller sample sizes [17–20].
By using a whole-brain approach and a sample size that meets recommendations for fMRI studies of individual differences [24] the current study confirms the role of the amygdala in the generation of individual differences in fear conditionability [cf 22]. Unlike some prior studies that defined fear conditionability as the magnitude of change in conditioned responding from the first to the second half of the experiment [i.e., (CS+ > CS−second) – (CS+ > CS−first)], here we observed that activity in the amygdala correlated with SCR potentiation to the CS+ versus the CS− measured across the experiment. Therefore, the current results suggest that the amygdala is involved in individual differences underlying the overall strength of fear conditioning (i.e., as opposed to the speed at which participants acquired conditioned responses). In addition, the covariation we observed here between peripheral fear responding and amygdala reactivity can be attributed to variation in CS+ responding rather than CS− responding [cf 43], as evidenced by correlations performed separately for SCR elicited by CS+ and CS−.
Work using a variety of experimental approaches has suggested that the amygdala plays a crucial role in fear acquisition and expression. Electrical stimulation of the amygdala elicits behaviors that emulate fear, whereas lesions of the amygdala can block innate or conditioned fear [e.g., 44,45]. Injection of anxiolytic compounds directly into the amygdala has been shown to reduce anxiety [e.g., 46] and amygdala plasticity may mediate the acquisition and extinction of conditioned fear [42]. Moreover, prior work has shown that the amygdala is coupled to conditioned SCR at the trial-level for individual subjects [15]. What the present study adds to this body of prior work is confirmation that individual differences in fear conditionability correspond to variability in amygdala reactivity, known to explain within- subject variability in fear responding.
The positive correlation between conditioned SCR responding was evident at a more stringent statistical threshold for the right compared to the left amygdala, in line with hemispheric effects observed in prior work [20,17, but see 19,18]. This lateralization of effects might be taken to support the notion of right hemispheric control of fear conditioning, which could be driven by anatomical and neurochemical differences between the left and right amygdala [e.g., 47]. Alternatively, involvement of the right amygdala in fear conditioning might be more likely when the aversive stimulus is visual, such as in the present study, as compared to when the aversive nature of the stimulus is learned verbally [48]. Nevertheless, as in prior work [e.g., 20], SCR in the present study was always recorded from the left hand and shock was always delivered to the left foot, which limits evaluation of hemispheric effects.
Interestingly, we did not observe task-related (CS+ > CS−) moderation of amygdala activity in the current study, in line with several prior studies [e.g., 19 of the 44 studies in 10, also see 49]. Significant between-subject variability in fear conditionability may underlie the absence of a mean effect, however it is also worth noting that we failed to observe CS+ > CS− amygdala activation when limiting our analysis to individuals with greater than average fear conditionability (as measured via SCR). We also failed to observe amygdala activation when examining BOLD response only during the first half of acquisition, which would seem to rule-out amygdala habituation as a potential explanation [similar results were observed by 27]. There is evidence to suggest that to thoroughly assess the temporal course of amygdala activation during fear conditioning, it may be necessary to employ a higher temporal resolution by focusing image acquisition on the amygdala and to use longer CS presentations to assess habituation within trials [i.e., during early versus later portions of CS presentation; 50]. Therefore, future work may wish to employ some of these procedures in order to more thoroughly understand the role of the amygdala in fear learning.
In contrast to the amygdala results, robust discrimination between conditioned stimuli was observed in the bilateral insula across participants [10,11]. Prior work suggests that both the insula and the amygdala may be involved in arousal, but that the insula may be uniquely involved in conscious awareness of CS+ contingency and anticipation of upcoming US presentation [e.g., 13]. Along these lines, Phelps and colleagues [48] found that activation in the insula was especially robust during an instructed fear paradigm, reflecting its role in threat awareness. Therefore, robust insula activation in the present study may reflect relatively invariant conscious threat awareness across participants.
Could greater amygdala reactivity during fear acquisition be related to enhanced fear memories? Using emotional films, Cahill and colleagues [51,52] found that right amygdala reactivity during emotional encoding predicted enhanced memory for emotional (but not neutral) items at retrieval [see also 53]. Therefore, it is possible that greater amygdala reactivity during fear learning might signal enhanced encoding of fear memories and might predict enhanced recall of these memories at a later time. However, it is also possible that amygdala reactivity during fear conditioning might be less related to stimulus encoding/processing and more to the production of conditioned fear responses. In other words, inter-individual variation in amygdala response during fear conditioning might track inter-individual variation in fear expression rather than stimulus input/CS processing [27]. Given the close relationship in the temporal pattern of CS presentation and CR production during fear acquisition, these responses are difficult to dissociate, however it will be important for future work to do so.
Limitations of the current study include our inability to determine whether individual differences present during fear conditioning translate into variation in the strength of fear memories over time or whether they affect extinction/extinction recall. Another limitation is our inability to link individual differences in brain and behavior to individual differences in anxiety. Future work may be directed towards evaluating the functional significance of the results presented here by assessing fear memory at subsequent testing sessions and/or assessing relationships with anxiety. In addition, future work could use a different experimental design to evaluate habituation effects in the amygdala and to help dissociate neural correlates of individual differences in fear expression versus fear learning.
5.0 Conclusions
In sum, our results suggest that the insula responds preferentially to threatening compared to non-threatening conditioned stimuli, but that it is insensitive to individual differences in the strength of these conditioned associations [but see 48]. The amygdala, however, did activate differentially depending on the extent to which participants showed evidence of fear responding, even though it did not activate to CS+ versus CS− trials across participants. Therefore, amygdala activity might track the brain mechanism associated with individual differences in fear conditioning, and might serve as an endophenotype for susceptibility to anxiety or affective disorders in the context of stressful or traumatic life experiences [54,55]. The current study is the largest study yet conducted on the brain basis of individual differences in fear conditionability measured using SCR, and replicates results observed in studies that employed much smaller samples [17–21]. Further research on the neural mechanisms underlying individual differences in fear learning may provide insight into the transition from adaptive to pathological fear acquisition [56,57] and could help identify individuals who are at risk of developing trauma-related psychopathology following an aversive experience [58–60].
Highlights.
We recorded fMRI BOLD response and skin conductance response (SCR) to CS+ and CS−
The insula activated in response to CS+ versus CS− trials across participants
Amygdala reactivity to CS+ versus CS− was not observed across participants
Individual differences in CS+ > CS− SCR covaried with activity in right amygdala
Results suggest brain mechanism for individual differences in fear conditionability
Acknowledgments
This material is based upon work supported by a National Institutes of Health (NIH) grant R21MH093917 (KLP); AM is supported by NIH grant T32MH067631-09. The authors would like to thank Donald McNair for his assistance in data collection and Edward F. Pace-Schott for his assistance with data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The following bilateral, AAL anatomically defined regions were included in the ROI mask used to examine correlations between CS+ > CS− SCR and CS+ > CS− fMRI BOLD response: postcentral gyrus; superior frontal gyrus, dorsolateral; superior frontal gyrus, medial; supplementary motor area; paracentral lobule; superior temporal gyrus; anterior cingulate and paracingulate gyri; insula; amygdala; cerebellum, lobule 4-5.
The authors of this study have no conflicts of interest to declare.
References
- 1.Zeidan MA, Lebron-Milad K, Thompson-Hollands J, Im JJY, Dougherty DD, Holt DJ, et al. Test-retest reliability during fear acquisition and fear extinction in humans. CNS Neurosci Ther. 2012;18:313–7. doi: 10.1111/j.1755-5949.2011.00238.x. doi:10.1111/j.1755-5949.2011.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M. A twin study of the genetics of fear conditioning. Arch Gen Psychiatry. 2003;60:702–8. doi: 10.1001/archpsyc.60.7.702. doi:10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- 3.Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B, et al. Fear load: The psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int J Psychophysiol. doi: 10.1016/j.ijpsycho.2014.11.005. in press. doi:10.1016/j.ijpsycho.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–8. [PubMed] [Google Scholar]
- 5.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. doi:10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–28. doi: 10.1038/nrn3492. doi:10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maren S. Neurobiology of Pavlovian Fear Conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. doi:10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 8.Davis M. The role of the amygdala in emotional learning. In: Bradley RJ, Harris RA, editors. Int. Rev. Neurobiol. Vol. 36. Academic Press; San Diego, CA: 1994. pp. 225–66. [DOI] [PubMed] [Google Scholar]
- 9.LeDoux JE. Emotion: Clues from the Brain. Annu Rev Psychol. 1995;46:209–35. doi: 10.1146/annurev.ps.46.020195.001233. doi:10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 10.Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human Fear Conditioning and Extinction in Neuroimaging: A Systematic Review. PLoS ONE. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. doi:10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. doi:10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: A functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchley HD, Mathias CJ, Dolan RJ. Fear Conditioning in Humans: The Influence of Awareness and Autonomic Arousal on Functional Neuroanatomy. Neuron. 2002;33:653–63. doi: 10.1016/s0896-6273(02)00588-3. doi:10.1016/S0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 14.Tranel D, Damasio H. Intact electrodermal skin conductance responses after bilateral amygdala damage. Neuropsychologia. 1989;27:381–90. doi: 10.1016/0028-3932(89)90046-8. doi:10.1016/0028-3932(89)90046-8. [DOI] [PubMed] [Google Scholar]
- 15.Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behav Neurosci. 2006;120:1187–95. doi: 10.1037/0735-7044.120.5.1187. doi:10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- 16.Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behav Neurosci. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- 17.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human Amygdala Activation during Conditioned Fear Acquisition and Extinction: a Mixed-Trial fMRI Study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. doi:10.1016/S0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 18.Carter RM, O’Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage. 2006;29:1007–12. doi: 10.1016/j.neuroimage.2005.09.011. doi:10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Dunsmoor JE, Prince SE, Murty VP, Kragel PA, LaBar KS. Neurobehavioral mechanisms of human fear generalization. NeuroImage. 2011;55:1878–88. doi: 10.1016/j.neuroimage.2011.01.041. doi:10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furmark T, Fischer H, Wik G, Larsson M, Fredrikson M. The amygdala and individual differences in human fear conditioning. Neuroreport. 1997;8:3957–60. doi: 10.1097/00001756-199712220-00021. [DOI] [PubMed] [Google Scholar]
- 21.Petrovic P, Kalisch R, Pessiglione M, Singer T, Dolan RJ. Learning affective values for faces is expressed in amygdala and fusiform gyrus. Soc Cogn Affect Neurosci. 2008;3:109–18. doi: 10.1093/scan/nsn002. doi:10.1093/scan/nsn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14:268–76. doi: 10.1016/j.tics.2010.04.002. doi:10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarkoni T. Big correlations in little studies: Inflated fMRI correlations reflect low statistical power—Commentary on Vul et al.(2009). Perspect Psychol Sci. 2009;4:294–8. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 24.Yarkoni T, Braver TS. Cognitive Neuroscience Approaches to Individual Differences in Working Memory and Executive Control: Conceptual and Methodological Issues. In: Gruszka A, Matthews G, Szymura B, editors. Handb. Individ. Differ. Cogn. Springer; New York: 2010. pp. 87–107. [Google Scholar]
- 25.Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From Fear to Safety and Back: Reversal of Fear in the Human Brain. J Neurosci. 2008;28:11517–25. doi: 10.1523/JNEUROSCI.2265-08.2008. doi:10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE. Amygdala Response to Happy Faces as a Function of Extraversion. Science. 2002;296:2191–2191. doi: 10.1126/science.1068749. doi:10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- 27.Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. NeuroImage. 2005;26:1193–200. doi: 10.1016/j.neuroimage.2005.03.020. doi:10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version – Non-patient Edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 29.Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats. Brain Res. 2001;888:356–65. doi: 10.1016/s0006-8993(00)03116-4. doi:10.1016/S0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- 30.Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. doi:10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. doi:10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–64. doi: 10.1111/j.1469-8986.2005.00302.x. doi:10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 33.Pace-Schott EF, Verga PW, Bennett TS, Spencer RMC. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. J Psychiatr Res. 2012;46:1036–44. doi: 10.1016/j.jpsychires.2012.04.015. doi:10.1016/j.jpsychires.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handb. Psychophysiol. 3rd ed. Cambridge University Press; New York: 2007. pp. 159–81. [Google Scholar]
- 35.Pineles SL, Orr MR, Orr SP. An alternative scoring method for skin conductance responding in a differential fear conditioning paradigm with a long-duration conditioned stimulus. Psychophysiology. 2009;46:984–95. doi: 10.1111/j.1469-8986.2009.00852.x. doi:10.1111/j.1469-8986.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitman RK, Orr SP. Test of the conditioning model of neurosis: Differential aversive conditioning of angry and neutral facial expressions in anxiety disorder patients. J Abnorm Psychol. 1986;95:208–13. doi: 10.1037//0021-843x.95.3.208. doi: http://dx.doi.org/10.1037/0021-843X.95.3.208. [DOI] [PubMed] [Google Scholar]
- 37.Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, et al. MARINA: An easy to use tool for the creation for MAsks for Region of INterest Analyses. Neuroimage. 2003:19. [Google Scholar]
- 38.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. doi:10.1006/nimg.2001.0978 [doi] S1053811901909784 [pii]. [DOI] [PubMed] [Google Scholar]
- 39.Quirk GJ, Armony JL, LeDoux JE. Fear Conditioning Enhances Different Temporal Components of Tone-Evoked Spike Trains in Auditory Cortex and Lateral Amygdala. Neuron. 1997;19:613–24. doi: 10.1016/s0896-6273(00)80375-x. doi:10.1016/S0896-6273(00)80375-X. [DOI] [PubMed] [Google Scholar]
- 40.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–51. doi:10.1037/0033-2909.87.2.245. [Google Scholar]
- 41.Lee IA, Preacher KJ. Calculation for the test of the difference between two dependent correlations with one variable in common. 2013 [Google Scholar]
- 42.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 43.Van Well S, Visser RM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: Evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cogn Affect Behav Neurosci. 2012;12:499–512. doi: 10.3758/s13415-012-0089-7. doi:10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanchard CD, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–90. doi: 10.1037/h0033521. doi:10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 45.Gold PE, Hankins L, Edwards RM, Chester J, McGaugh JL. Memory interference and facilitation with posttrial amygdala stimulation: effect on memory varies with footshock level. Brain Res. 1975;86:509–13. doi: 10.1016/0006-8993(75)90905-1. [DOI] [PubMed] [Google Scholar]
- 46.Costall B, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB. Neuroanatomical sites of action of 5-HT3 receptor agonist and antagonists for alteration of aversive behaviour in the mouse. Br J Pharmacol. 1989;96:325–32. doi: 10.1111/j.1476-5381.1989.tb11821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker KB, Kim JJ. Amygdalar Lateralization in Fear Conditioning: Evidence for Greater Involvement of the Right Amygdala. Behav Neurosci. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. doi: http://dx.doi.org/10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- 48.Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–41. doi: 10.1038/86110. doi:10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 49.Mechias M-L, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. NeuroImage. 2010;49:1760–8. doi: 10.1016/j.neuroimage.2009.09.040. doi:10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 50.Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learn Mem. 2007;14:485–90. doi: 10.1101/lm.632007. doi:10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, et al. Sex-Related Difference in Amygdala Activity during Emotionally Influenced Memory Storage. Neurobiol Learn Mem. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. doi:10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- 52.Canli T, Desmond JE, Zhao Z, Gabrieli JDE. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci. 2002;99:10789–94. doi: 10.1073/pnas.162356599. doi:10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–93. doi: 10.1038/6404. doi:10.1038/6404. [DOI] [PubMed] [Google Scholar]
- 54.Bergman O, Ahs F, Furmark T, Appel L, Linnman C, Faria V, et al. Association between amygdala reactivity and a dopamine transporter gene polymorphism. Transl Psychiatry. 2014;4:e420. doi: 10.1038/tp.2014.50. doi:10.1038/tp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 56.Davis M, Whalen P j. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 57.Hariri AR, Whalen PJ. The amygdala: inside and out. F1000 Biol Rep. 2011;3:2. doi: 10.3410/B3-2. doi:10.3410/B3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bomyea J, Risbrough V, Lang AJ. A consideration of select pre-trauma factors as key vulnerabilities in PTSD. Clin Psychol Rev. 2012;32:630–41. doi: 10.1016/j.cpr.2012.06.008. doi:10.1016/j.cpr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jovanovic T, Ressler KJ. How the Neurocircuitry and Genetics of Fear Inhibition May Inform Our Understanding of PTSD. Am J Psychiatry. 2010;167:648–62. doi: 10.1176/appi.ajp.2009.09071074. doi:10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–24. doi: 10.1038/nn1944. doi:10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]