Abstract
Young adult childhood cancer survivors are at increased risk for frailty, a physiologic phenotype typically found among older adults. This phenotype is associated with new onset chronic health conditions and mortality among both older adults and among childhood cancer survivors. Mounting evidence suggests that poor fitness, muscular weakness and cognitive decline are common among adults treated for childhood malignancies, and that risk factors for these outcomes are not limited to those treated with cranial radiation. Although the pathobiology of this phenotype is not known, early cellular senescence, sterile inflammation and mitochondrial dysfunction in response to initial cancer or treatment related insults are hypothesized to play a role. Interventions to prevent or remediate frailty among childhood cancer survivors have not been tested. Pharmaceutical, nutriceutical and lifestyle interventions show some promise.
Keywords: Childhood cancer survivor, frailty, aging, fitness, weakness, senescence, inflammation, mitochondrial dysfunction
One of the greatest medical success stories over the past five decades is the improvement in survival among children newly diagnosed with a malignancy. Five-year survival rates now exceed 80 percent(%).1 Current estimates indicate that there are over 420,000 survivors of childhood cancer living in the United States.2 However, cure does not come without cost. Adults treated for childhood cancer are at increased risk for chronic health conditions,3-6 frequent hospitalization,7-9 and early mortality.10-13 The prevalence of chronic diseases among childhood cancer survivors increases with age; rates among survivors in their twenties are similar to rates among siblings in their fifties.3 Elevated rates of other outcomes typically associated with aging, like minimal cognitive dysfunction,14, 15 reduced muscle strength16-18 and poor exercise tolerance19, 20 are also reported among childhood cancer survivors and appear decades earlier than expected.21 This suggests that some survivors of childhood cancer have a physiological phenotype consistent with that found among older adults, frailty. This construct likely precedes or accompanies the development of clinically significant chronic disease that eventually results in reduced function, hospitalization and death.
Articles included in this review specific to childhood cancer survivors were identified in the PubMed and Web of Science database for clinical trials, observational studies, case series, and reviews, using the search strategy (“survivors” OR “survivor”) AND (“aging” OR “frailty”) AND (“childhood”) AND (“neoplasms” OR “cancer”) and were limited to those written in English. Included manuscripts were selected after reviewing the abstracts for relevance. The search was augmented with selected publications from reference lists of studies retrieved from databases.
Frailty
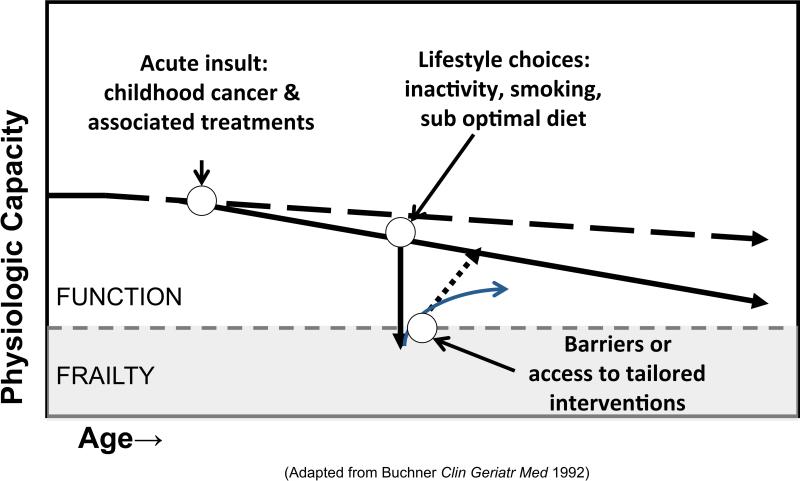
Frailty is a state of reduced physiologic reserve that increases susceptibility to chronic disease and disability.22 With typical aging, the innate or attained physiologic capacity of body systems declines over time. Initial changes in neuromuscular control, mechanical performance and energy metabolism are subtle and are not associated with noticeable loss of function during daily life. Unfortunately, sub-optimal lifestyle choices like inactivity, smoking and a high fat, high sugar content diet add to subtle age related changes, resulting in gradual loss of physiologic capacity. This trajectory of declining function is accelerated with exposure to acute insults (illness, injuries, major life events), particularly insults that damage organ systems and or result in periods of prolonged bed rest or inactivity. Frailty occurs when either there is not enough physiologic reserve to overcome organ system damage, or when access to interventions that promote recovery is limited (Figure 1).23, 24 In typical aging, declining physiologic reserve is clinically silent, detectable only with specific measures of functional, behavioral or biological performance or if acute illness or other stress unmasks decreased reserve.25
Figure 1.
Frailty and function.91 The dashed line above the shading represents the cut-point between function and frailty. The dashed line with arrow represents decline in physiologic reserve with typical aging. The solid line with arrow represents potential decline in physiologic reserve among childhood cancer survivors whose acute insult occurs early in life. The solid line with arrow pointing directly down represents the potential impact of lifestyle on the trajectory of physiologic capacity; the additional arrows pointing upward potential recovery from loss of function based on access to intervention.
Because frailty is distinguishable from, and often precedes symptomatic chronic disease and disability, detecting frailty in a vulnerable population, like childhood cancer survivors, is potentially useful. Identification of individuals at risk for future chronic disease and disability so they can be referred for early intervention is important.25 Additionally, changing the trajectory of physiologic decline is theoretically possible. Survivors who adopt a healthy lifestyle have the opportunity to prevent early manifestation of frailty. Frailty prevention requires a system that promotes cost effective screening, not only for serious diseases amenable to early detection and treatment, but also a system that incorporates screening and intervention for early deficits in neuromuscular control, muscle strength and energy metabolism.26
Models of Frailty
Investigators who study aging have used different approaches to characterize frailty. Fried and colleagues were one of the first groups to describe a specific “frailty” phenotype.24 Theyused five indicators of physiological reserve; individuals with two were considered “pre-frail” and those with three or more “frail.” The criteria were applied at baseline, four and seven year follow-ups in the Cardiovascular Health Study. This sample of 5,317 men and women 65 years of age or older were evaluated for: 1) Sarcopenia - unintentional weight loss of ten pounds or 5% of body mass or more in past year; 2) Decreased muscle strength - hand grip strength in the lowest 20th percentile for sex and body mass index; 3) Poor endurance - self-reported exhaustion (from two questions on the Center for Epidemiologic Studies Depression Scale); 4) Slowness - walking time per 15 feet in the lowest 20th percentile for gender and height; 5) Low activity - physical activity < 383 kilocalories per week for males, < 270 kilocalories per week for females. Overall, 7% were classified as “frail”, and 47% were classified as “pre-frail.” Incident frailty was 7% for years 0-3 and 7% for years 4-7. Adjusted hazard ratios for falls, disability, hospitalization and death at seven years ranged from 1.2-1.8 when persons with baseline frailty were compared to those with no frailty. This model was validated in the Women's Health and Aging Study,27, 28 and the Women's Health Initiative.29
Others have proposed different frailty phenotypes by applying fewer,30, 31 modifying,29 or adding measures to Fried criteria,32-34 by using clinician assessment to characterize fitness and health,35 or by counting known clinical comorbidities or deficits to define a frailty threshold.36, 37 There are basically two approaches. The first, like the Fried Index, employs physical measures with set cut-points, classifies persons with and without frailty at a baseline time point, and evaluates whether or not those who meet frailty criteria differ from those who do not meet frailty criteria on future adverse outcomes, like chronic disease, hospitalization and death. The second approach, characterized in the Canadian Study of Health and Aging,37 does not employ measures of physiologic reserve, but counts impairments and co-morbidities (up to 70 items) to create an index score representing increasing degrees of frailty. Each approach has strengths and weaknesses. The Fried Index is reproducible and coherent.38 However, its focus on physiologic measures has been criticized,39 as physiologic measures do not take into account cognition, mood or social items known to impact health.38 The model derived using data from the Canadian Study of Health and Aging has excellent mathematical properties, but is criticized because it is long and not easily applied in a general clinical setting. deSouto Barreto40 suggests that frailty should be operationalized locally so that definitions can take into account the unique characteristics of a given population.
Frailty among childhood cancer survivors
Pre-frailty and frailty were recently described, using the Fried criteria, among 1922 survivors of childhood cancer participating in the St. Jude Lifetime Cohort (SJLife) study.41 In this young adult cohort, with a mean age of 33.6 (standard deviation (SD) 8.1) years, pre-frailty and frailty were evident among 2.7 and 12.9% of males and 12.9 and 31.5% of females. These rates are similar to those reported in a meta-analysis of 44,894 persons ≥65 years of age ,42 where the prevalence of frailty was 9.9%. In SJLife, frailty increased with age, and was associated with new onset chronic health conditions (Relative risk (RR) 2.2, 95% Confidence interval (CI) 1.2-4.2) and mortality (Hazard ratio (HR) 2.6, 95% CI 1.2-6.2). Among male survivors, frailty was also associated with smoking and being underweight or obese. Although previous exposure to cranial radiation (CRT) increased risk for frailty, suggesting a role for hypothalamic pituitary axis dysfunction (growth hormone deficiency) in frail health, frailty was not limited to survivors exposed to CRT. Among survivors with no CRT exposure, no associations between specific chemotherapy agents or doses and frailty were identified, supporting the hypothesis that any exposure to cytotoxic agents may have a lasting impact on physiologic function across the lifespan.43
Findings from SJLife are supported by other published literature indicating that physical performance among young adult survivors of childhood cancer resembles that expected in older adults. For example, in a cohort of 78 adults (median age 22, range 18-58 years) treated for a brain tumor during childhood, both cardiopulmonary fitness and handgrip strength values were lower among survivors than among age-, sex-, race- and zip code-matched peers, but similar to values expected among persons in their sixties (Table 1).44 These impairments are evident in even younger cohorts. Wolfe et al45 reported lower than expected peak oxygen uptake during exercise (31.8 (SD 7.2) vs. 49.3 (SD 7.9) ml/kg/min) among twelve adolescent (mean age 14.41 (SD 1.86) years) survivors of posterior fossa tumors. Piscione et al46 documented strength deficits among 30% of another group (N=30, mean age 11.4 (SD 4.1)) of adolescent survivors of posterior fossa tumors.
Table 1.
Strength and fitness values among adult survivors of childhood brain tumors compared to age-, sex-, race-matched peers and to values among adults 60-70 years of age.
| Outcome | Brain tumor survivors Mean (SD) | Comparison group Mean (SD) | Healthy adults ages 60-69 years Mean (SD) |
|---|---|---|---|
| Hand grip strength, kg | |||
| Females | 24.7(9.2) | 31.5(5.8) | 23.5(5.2)92 |
| Males | 39.0(12.2) | 53.0(10.1) | 39.0(8.2)92 |
| Knee extension strength, N | |||
| Females | 246.6(95.5) | 339.1(92.4) | 270.3(81.6)93 |
| Males | 304.7(116.4) | 466.6(92.1) | 381.8(80.8)93 |
| Exercise tolerance, ml/kg/min | |||
| Females | 25.1(8.6) | 31.1(5.1) | 22.3(2.1)48 |
| Males | 24.6(9.5) | 33.2(3.4) | 30.0(2.7)48 |
Kg=kilograms; N=Newton; ml/kg/min=milliliters per kilogram per minute
Impairments in cardiopulmonary fitness and strength are not limited to brain tumor survivors. In a study of 415 adult (median age 35.6 (range 21.9-52.3 years) survivors of childhood acute lymphoblastic leukemia (ALL), 46.5% had reduced six minute walk distances and 30.1% had impaired quadriceps strength. In an even younger cohort of childhood ALL survivors, Tonorezos et al47 reported mean cardiopulmonary fitness values (N=115, median age 23.5, range 18-37 years) lower than peers (30.7±7.6 vs. 39.9±7.8 milliliters/kilogram/minute (ml/kg/min)), but similar to values predicted for persons aged 50 to 59 (29.7±2.7 ml/kg/min) years.48 In a cohort of 75 ALL survivors (30.2 (SD 7.1) years), mean quadriceps strength values were one SD less than expected when compared to population-norms.17 Again, these problems appear early in young survivors, among children still receiving cancer therapy, and among children with newly diagnosed ALL. van Brussel et al49 documented impaired cardiopulmonary fitness and quadriceps weakness among thirteen adolescent ALL survivors (mean age 15.5 years), Marchese et al50 found impaired mobility and quadriceps weakness among eight children with ALL after delayed intensification, and we reported impaired fitness and quadriceps weakness among 109 children with newly diagnosed ALL.51
Additional studies indicate that fitness and physical performance are impaired in survivors not exposed to central nervous system (CNS) therapies (i.e. CRT, intrathecal or high dose methotrexate). Hartman et al16 reported lower muscle strength and physical performance battery scores among 92 children (age range 5.1-12.9 years) after treatment for ALL, Wilms tumor, non-Hodgkin lymphoma and malignant mesenchymal tumors compared to 155 healthy controls. Similarly, in a study that evaluated fitness and strength among 183 survivors (mean age 13.5 (SD 2.5 years)) of leukemia, lymphoma, CNS tumors, and bone and soft tissue sarcoma and 147 siblings (mean age 13.4 (SD 2.4) years), survivors performed lower than siblings on measures of fitness and leg strength.21 Bone sarcoma survivors had the largest deficits in muscle strength. In this study, an analysis was done to evaluate the hypothesis that survivors and siblings who participated in similar levels of physical activity would perform similarly on physical function measures. Although there were strong associations between activity levels and physical performance for both survivors and siblings, for any given level of physical activity, survivors performed lower than siblings on every measure of physical function.21 This suggests the possibility that cancer or treatment-related injury to underlying structure(s) or physiologic process(es) is responsible for functional loss, even when lifestyle is optimized.
Cognitive correlates of frailty in childhood cancer survivors
In cohorts of aging adults, physical frailty and cognitive function are highly correlated.52, 53 This association may be present among survivors of childhood cancer, some of who have evidence of cognitive decline during young adulthood. Edelstein et al54 evaluated changes in neurocognitive function among 18 survivors of medulloblastoma (median age 21.9 (range 18-47 years), and reported both early impairment and declining working memory over time. Similarly, Armstrong et al,55 in a study of 265 ALL survivors (mean age 40.9 (SD 4.4) years), reported immediate and delayed memory impairments among 33.8% and 30.2% of those treated with 24 gray(Gy) CRT. Mean performance on a long-term narrative memory task was consistent with scores reported among persons in their eighth decade of life; neurocognitive impairments were associated with changes in brain structure and activation. Schuitema et al15 compared neuropsychological and imaging outcomes between 93 survivors of childhood leukemia or lymphoma 20-30 years after diagnosis (mean age 29.8 (SD 4.9) years) and 49 controls. Survivors treated with CRT had neuropsychological dysfunction and associated deficits in white matter integrity in frontal, parietal and temporal regions. These authors attributed the trajectory of declining white matter integrity among survivors exposed to CRT to accelerated aging. Although reports of age related cognitive deficits among survivors of childhood brain tumors or ALL appear to be related to CRT exposure, childhood cancer survivors with treatment exposures that impact the cardiopulmonary system may also be at risk. Krull et al14 documented impaired neurocognitive function and associated structural changes in both cardiopulmonary function and brain structure among 62 survivors of Hodgkin lymphoma (HL) (mean age 42.2 (SD 4.77)) exposed to high dose thoracic radiation or lower dose thoracic radiation and anthracycline.
Potential biological markers of frail health
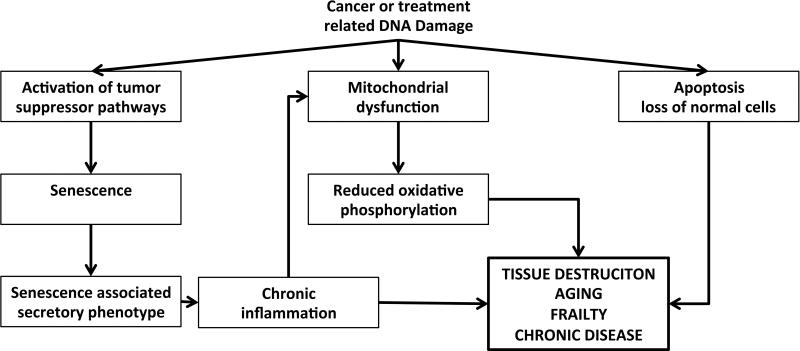
The pathobiology of frail health among survivors of childhood cancer is unknown. Imaging studies of the brain, skeletal muscle and heart documenting atrophy, sarcopenia and cardiomyopathy, suggest some degree of initial cancer- or treatment- related damage to normal, non-malignant cells, like neurons, skeletal muscle cells and cardiac myocytes. We hypothesize that this is compounded by DNA damage that induces cellular senescence, growth arrest that occurs when cells with regenerative potential are exposed to oncogenic insults.56 In fact, recent data indicate that even post-mitotic cells can develop a senescent-like phenotype in response to DNA damage.57 An overabundance of senescent cells is associated with aging and is also documented in progeroid syndromes.58 A similar accumulation of senescent cells is also possible in children with cancer, whose disease is hallmarked by the presence of rapidly dividing cells, and whose treatment exposures can confer new onset DNA damage59 and mutations,60 telomere shortening,61 protein aggregation,62 and increased production of reactive oxygen species.63, 64 Although telomere shortening is reported in tumor tissue among children with Wilms tumor,65 and in buccal cells among survivors of childhood cancer who develop second malignant neoplasms,61 there have been few reports in the literature evaluating other biological markers of cellular senescence among childhood cancer survivors. In a small pilot study (N=10), Marcoux et al,66 reported that among children previously treated with cranial radiation for acute lymphoblastic leukemia (ALL), expression of p16INK4a, a marker of cellular senescence, was 5.8 times higher in skin biopsies from the scalp than in skin biopsies from the buttocks.
Cellular senescence can be associated with a sterile pro-inflammatory state, or senescence associated secretory phenotype (SASP), resulting in elevated levels of inflammatory markers, including Interleukin-6, tumor necrosis factor alpha and immune cell cytokines.56 This chronic inflammation is thought to contribute to the development and progression of chronic conditions like insulin resistance, diabetes, hypertension, and atherosclerosis.67 Among childhood cancer survivors, published data is consistent with this hypothesis. Baker et al reported increased risk for insulin resistance among 319 adolescents (mean age 14.5 (range 9-18) years) previously treated with CRT and cisplatin or glucocorticoids when compared to siblings. Nottage et al68 reported increased risk for metabolic syndrome among adult survivors of childhood ALL (n = 784, median age 31·7 years (18.9-59.1)) treated with CRT when compared to population based controls. Holmquist et al69 reported an increased risk for diabetes in a population-based registry of childhood cancer survivors (N=32,903). Survivors of Wilms tumor, leukemia, CNS tumors, germ cell tumors, malignant bone tumors and HL were at greatest risk.69 Coronary artery disease, detected with computed tomography angiography, was prevalent among 39% of 31 survivors of HL (median age 40 (range 26-55 years)) exposed to chest radiation during childhood.70 Finally, Sulicka et al reported a chronic pro-inflammatory state among 27 survivors of childhood ALL (age range 18-27 years) when compared to normal controls.71 ALL survivors exhibited elevated levels of pentraxin 3, soluble vascular cell adhesion molecule-1, osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand and counts of intermediate monocytes.71
Although a pro-inflammatory state likely influences function across every organ system; organs that are directly exposed to toxins are most vulnerable. This is evidenced by the increased risk for heart disease among children exposed to chest radiation and/or chemotherapy72 and by the increased risk for neuroendocrine73 or neurocognitive74 decline among children exposed to CRT. This is probably also true at the cellular level. Mitochondria produce energy (adenosine tri-phosphatase (ATP)) and are a major source of reactive oxygen species (ROS), which when produced in excess, can be highly damaging to cells. Likely because of the proximity of Mitochondrial (mtDNA) to sites of ROS generation, mtDNA is prone to accumulating mutations with age,75, 76 and after exposure to chemotherapy and radiation.77 The contributions of these MtDNA mutations to the pathogenesis of age-related disorders are suggested by the phenotype of mtDNA mutator mice (polgD257A/D257A), genetically engineered to accumulate somatic mtDNA mutations at an accelerated rate. These mice show reduced levels of oxidative phosphorylation with age and develop a variety of age-related disorders, including osteoporosis, neurodegeneration, cardiomyopathy, diabetes and muscle wasting.78, 79 In addition, inherited mitochondrial disorders are often characterized by muscle atrophy and weakness, reduced exercise capacity and fatigue,80 all components of the frailty phenotype described among aging adults42 and among childhood cancer survivors.41 Thus, we hypothesize that mitochondrial dysfunction resulting from the therapy related increase in somatic mtDNA mutations and/or the suboptimal microenvironment conferred by the SASP56 contributes to the development of the frailty phenotype in aging childhood cancer survivors.
Preventing and treating frail health
Interventions specifically designed to prevent or remediate frailty among childhood cancer survivors have not been tested, but likely will include pharmaceutical or nutriceutical agents and lifestyle modifications.81 Therapies designed to interfere with damage caused by radical oxygen species,82 mimic the known protective effects of caloric restriction,82 or clear dysfunctional senescent cells83 show promise in some human and animal models, although additional work is needed to bring these agents to clinical trials in childhood cancer survivors. Behavioral interventions designed to optimize a healthy lifestyle, including caloric restriction and exercise, are possibilities with demonstrated efficacy in aging human populations,84, 85 and are probably the most immediately accessible possibilities for current childhood cancer survivors with frailty or at risk for frailty. Two recent studies indicate that lifestyle is as important as treatment-related risk factors for metabolic86 and cardiac health87 in childhood cancer survivors; another study indicates that exercise in this population, even in the presence of known cardiac dysfunction is safe, and likely effective.88 However, data have shown that childhood cancer survivors may not respond to lifestyle changes in the same way that others respond,21 interventions may need tailoring to account for the unique needs of this population.
Conclusion
Childhood cancer survivors are at risk for frail health, a proportion demonstrating an aging-like phenotype characterized by muscle wasting and weakness, slow walking speed, reduced energy expenditure, fatigue and in some cases cognitive decline. Although the information in this review is based on cohorts that include some survivors treated with modalities eliminated from, or modified in, today's therapeutic protocols (e.g. cranial radiation for central nervous system prophylaxis among children with low or standard risk ALL, high dose thoracic radiation for children with Hodgkin lymphoma), it continues to be relevant. The frailty phenotype described was not limited to those who received radiation therapy.41 Additionally, many chemotherapy agents used in older treatment protocols continue to provide the backbone for today's therapeutic interventions.89, 90 Research is needed to determine if the prevalence of frailty remains significant among survivors with less common diagnoses (sarcoma, other solid tumors) treated on contemporary protocols. In addition, to determine the pathobiology of this phenomenon, aging research focused on cellular senescence, sterile inflammation and mitochondrial dysfunction has potential to provide insight for future interventions. Currently accessible interventions like medical management of chronic disease or behavioral management of diet/exercise should include relevant endpoints like quality of life, functional decline and long term survival.
Précis.
This manuscript provides a review of the evidence documenting physiologic frailty among childhood cancer survivors and describes potential biological mechanisms for this phenotype.
Figure 2.
Pathobiology of frail health
Acknowledgments
This work was supported by a Cancer Center Support Grant from the National Cancer Institute (CA 21765) and by ALSAC.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2011. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. [Google Scholar]
- 2.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–27. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–15. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 5.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landier W, Armenian SH, Lee J, et al. Yield of screening for long-term complications using the children's oncology group long-term follow-up guidelines. J Clin Oncol. 2012;30:4401–8. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchhoff AC, Fluchel MN, Wright J, et al. Risk of hospitalization for survivors of childhood and adolescent cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:1280–9. doi: 10.1158/1055-9965.EPI-13-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurt BA, Nolan VG, Ness KK, et al. Hospitalization rates among survivors of childhood cancer in the Childhood Cancer Survivor Study cohort. Pediatr Blood Cancer. 2012;59:126–32. doi: 10.1002/pbc.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winther JF, Boice JD, Jr., Christensen J, et al. Hospitalizations among children of survivors of childhood and adolescent cancer: a population-based cohort study. Int J Cancer. 2010;127:2879–87. doi: 10.1002/ijc.25286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–38. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewster DH, Clark D, Hopkins L, et al. Subsequent mortality experience in five-year survivors of childhood, adolescent and young adult cancer in Scotland: a population based, retrospective cohort study. Eur J Cancer. 2013;49:3274–83. doi: 10.1016/j.ejca.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Wilson CL, Cohn RJ, Johnston KA, Ashton LJ. Late mortality and second cancers in an Australian cohort of childhood cancer survivors. Med J Aust. 2010;193:258–61. doi: 10.5694/j.1326-5377.2010.tb03902.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Goddard K, Spinelli JJ, Gotay C, McBride ML. Risk of Late Mortality and Second Malignant Neoplasms among 5-Year Survivors of Young Adult Cancer: A Report of the Childhood, Adolescent, and Young Adult Cancer Survivors Research Program. J Cancer Epidemiol. 2012 doi: 10.1155/2012/103032. Epub Sep 12.:103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol. 2012;30:3618–24. doi: 10.1200/JCO.2012.42.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuitema I, Deprez S, Van Hecke W, et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol. 2013;31:3378–88. doi: 10.1200/JCO.2012.46.7050. [DOI] [PubMed] [Google Scholar]
- 16.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr Blood Cancer. 2008;50:833–7. doi: 10.1002/pbc.21325. [DOI] [PubMed] [Google Scholar]
- 17.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:975–81. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]
- 18.Talvensaari KK, Jamsen A, Vanharanta H, Lanning M. Decreased isokinetic trunk muscle strength and performance in long-term survivors of childhood malignancies: correlation with hormonal defects. Arch Phys Med Rehabil. 1995;76:983–8. doi: 10.1016/s0003-9993(95)81033-1. [DOI] [PubMed] [Google Scholar]
- 19.De Caro E, Fioredda F, Calevo MG, et al. Exercise capacity in apparently healthy survivors of cancer. Arch Dis Child. 2006;91:47–51. doi: 10.1136/adc.2004.071241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenney ME, Faragher EB, Jones PH, Woodcock A. Lung function and exercise capacity in survivors of childhood leukaemia. Med Pediatr Oncol. 1995;24:222–30. doi: 10.1002/mpo.2950240403. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31:2799–805. doi: 10.1200/JCO.2012.47.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchner DM, Wagner EH. Preventing frail health. Clinics in geriatric medicine. 1992;8:1–17. [PubMed] [Google Scholar]
- 23.Conti AA, Conti A. Frailty and resilience from physics to medicine. Medical hypotheses. 2010;74:1090. doi: 10.1016/j.mehy.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 25.De Alfieri W, Costanzo S, Borgogni T. Biological resilience of older adults versus frailty. Medical hypotheses. 2011;76:304–5. doi: 10.1016/j.mehy.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 26.Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing research reviews. 2012;11:390–8. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 28.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–71. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 29.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–30. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 30.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–8. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–9. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 32.Forti P, Rietti E, Pisacane N, Olivelli V, Maltoni B, Ravaglia G. A comparison of frailty indexes for prediction of adverse health outcomes in an elderly cohort. Arch Gerontol Geriatr. 2012;54:16–20. doi: 10.1016/j.archger.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Freiheit EA, Hogan DB, Eliasziw M, et al. Development of a frailty index for patients with coronary artery disease. J Am Geriatr Soc. 2010;58:1526–31. doi: 10.1111/j.1532-5415.2010.02961.x. [DOI] [PubMed] [Google Scholar]
- 34.Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–9. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–33. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 37.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–7. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 38.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–7. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15:1–29. [PubMed] [Google Scholar]
- 40.de Souto Barreto P. One operational definition by population: the need for local evaluations of frailty. J Physiol Anthropol. 2011;30:259–62. doi: 10.2114/jpa2.30.259. [DOI] [PubMed] [Google Scholar]
- 41.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496–503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 43.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67:212–5. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 44.Ness KK, Morris EB, Nolan VG, et al. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116:3034–44. doi: 10.1002/cncr.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolfe KR, Hunter GR, Madan-Swain A, Reddy AT, Banos J, Kana RK. Cardiorespiratory fitness in survivors of pediatric posterior fossa tumor. J Pediatr Hematol Oncol. 2012;34:e222–7. doi: 10.1097/MPH.0b013e3182661996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piscione PJ, Bouffet E, Mabbott DJ, Shams I, Kulkarni AV. Physical functioning in pediatric survivors of childhood posterior fossa brain tumors. Neuro Oncol. 2014;16:147–55. doi: 10.1093/neuonc/not138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tonorezos ES, Snell PG, Moskowitz CS, et al. Reduced cardiorespiratory fitness in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:1358–64. doi: 10.1002/pbc.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med. 1990;61:3–11. [PubMed] [Google Scholar]
- 49.van Brussel M, Takken T, van der Net J, et al. Physical function and fitness in long-term survivors of childhood leukaemia. Pediatr Rehabil. 2006;9:267–74. doi: 10.1080/13638490500523150. [DOI] [PubMed] [Google Scholar]
- 50.Gocha Marchese V, Chiarello LA, Lange BJ. Strength and functional mobility in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 2003;40:230–2. doi: 10.1002/mpo.10266. [DOI] [PubMed] [Google Scholar]
- 51.Ness KK, Kaste SC, Zhu L, et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk Lymphoma. 2014 doi: 10.3109/10428194.2014.944519. Epub Aug 20:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology. 2013;80:2055–61. doi: 10.1212/WNL.0b013e318294b462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JS, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc. 2014;15:281–6. doi: 10.1016/j.jamda.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Edelstein K, Spiegler BJ, Fung S, et al. Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neuro Oncol. 2011;13:536–45. doi: 10.1093/neuonc/nor015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong GT, Reddick WE, Petersen RC, et al. Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J Natl Cancer Inst. 2013;105:899–907. doi: 10.1093/jnci/djt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–72. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jurk D, Wang C, Miwa S, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11:996–1004. doi: 10.1111/j.1474-9726.2012.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh S, Zhou Z. Genetics of aging, progeria and lamin disorders. Curr Opin Genet Dev. 2014;26C:41–46. doi: 10.1016/j.gde.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Saffhill R, Margison GP, O'Connor PJ. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985;823:111–45. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- 60.Alexandrov LB, Stratton MR. Mutational signatures: the patterns of somatic mutations hidden in cancer genomes. Curr Opin Genet Dev. 2014;24:52–60. doi: 10.1016/j.gde.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gramatges MM, Liu Q, Yasui Y, et al. Telomere content and risk of second malignant neoplasm in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Clin Cancer Res. 2014;20:904–11. doi: 10.1158/1078-0432.CCR-13-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaime-Perez JC, Gomez-Almaguer D. Platelet aggregation is stimulated by L-asparginase in children with acute lymphoblastic leukemia and normal individuals. Haematologica. 2002;87:891–2. [PubMed] [Google Scholar]
- 63.Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs. 1997;54(Suppl 4):1–7. doi: 10.2165/00003495-199700544-00003. [DOI] [PubMed] [Google Scholar]
- 64.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 65.Stewenius Y, Jin Y, Ora I, et al. Defective chromosome segregation and telomere dysfunction in aggressive Wilms' tumors. Clin Cancer Res. 2007;13:6593–602. doi: 10.1158/1078-0432.CCR-07-1081. [DOI] [PubMed] [Google Scholar]
- 66.Marcoux S, Le ON, Langlois-Pelletier C, et al. Expression of the senescence marker p16INK4a in skin biopsies of acute lymphoblastic leukemia survivors: a pilot study. Radiat Oncol. 2013;8:252. doi: 10.1186/1748-717X-8-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study). Am J Cardiol. 2003;92:522–8. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 68.Nottage KA, Ness KK, Li C, Srivastava D, Robison LL, Hudson MM. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia - From the St. Jude Lifetime Cohort. Br J Haematol. 2014;165:364–74. doi: 10.1111/bjh.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmqvist AS, Olsen JH, Andersen KK, et al. Adult life after childhood cancer in Scandinavia: diabetes mellitus following treatment for cancer in childhood. Eur J Cancer. 2014;50:1169–75. doi: 10.1016/j.ejca.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 70.Mulrooney DA, Nunnery SE, Armstrong GT, et al. Coronary artery disease detected by coronary computed tomography angiography in adult survivors of childhood Hodgkin lymphoma. Cancer. 2014 doi: 10.1002/cncr.28925. Epub Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sulicka J, Surdacki A, Mikolajczyk T, et al. Elevated markers of inflammation and endothelial activation and increased counts of intermediate monocytes in adult survivors of childhood acute lymphoblastic leukemia. Immunobiology. 2013;218:810–6. doi: 10.1016/j.imbio.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merchant TE, Rose SR, Bosley C, Wu S, Xiong X, Lustig RH. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol. 2011;29:4776–80. doi: 10.1200/JCO.2011.37.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harila MJ, Winqvist S, Lanning M, Bloigu R, Harila-Saari AH. Progressive neurocognitive impairment in young adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53:156–61. doi: 10.1002/pbc.21992. [DOI] [PubMed] [Google Scholar]
- 75.Batlevi Y, La Spada AR. Mitochondrial autophagy in neural function, neurodegenerative disease, neuron cell death, and aging. Neurobiol Dis. 2011;43:46–51. doi: 10.1016/j.nbd.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaziev AI, Abdullaev S, Podlutsky A. Mitochondrial function and mitochondrial DNA maintenance with advancing age. Biogerontology. 2014 doi: 10.1007/s10522-014-9515-2. Epub Jul 12. [DOI] [PubMed] [Google Scholar]
- 77.Wardell TM, Ferguson E, Chinnery PF, et al. Changes in the human mitochondrial genome after treatment of malignant disease. Mutat Res. 2003;525:19–27. doi: 10.1016/s0027-5107(02)00313-5. [DOI] [PubMed] [Google Scholar]
- 78.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 79.Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 80.Wallace DC. Mitochondria as chi. Genetics. 2008;179:727–35. doi: 10.1534/genetics.104.91769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–43. doi: 10.1016/j.pcad.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 82.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–24. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–6. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mercken EM, Crosby SD, Lamming DW, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–51. doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fragala MS, Dam TT, Barber V, et al. Strength and Function Response to Clinical Interventions of Older Women Categorized by Weakness and Low Lean Mass Using Classifications From the Foundation for the National Institute of Health Sarcopenia Project. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu110. Epub Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith WA, Li C, Nottage KA, et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. Cancer. 2014;120:2742–50. doi: 10.1002/cncr.28670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones LW, Ness KK, Liu Q, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study (CCSS). J Clin Oncol. 2014 doi: 10.1200/JCO.2014.56.7511. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith WA, Ness KK, Joshi V, Hudson MM, Robison LL, Green DM. Exercise training in childhood cancer survivors with subclinical cardiomyopathy who were treated with anthracyclines. Pediatr Blood Cancer. 2013 doi: 10.1002/pbc.24850. Epub March 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58:334–43. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60:1083–94. doi: 10.1002/pbc.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 92.Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand Grip Strength: age and gender stratified normative data in a population-based study. BMC Res Notes. 2011;4:127. doi: 10.1186/1756-0500-4-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78:26–32. doi: 10.1016/s0003-9993(97)90005-8. [DOI] [PubMed] [Google Scholar]