Abstract
Background
Parkinson’s disease (PD) is a neurodegenerative disorder that generally begins with asymmetric motor symptoms that persist over time. This suggests that the dysfunction in the nigrostriatal motor circuit may be lateralized. The present study examined whether the asymmetric motor presentation is associated with hemisphere-specific cognitive decline and lateralized gray matter volume loss.
Methods
Data from comprehensive cognitive tests that measured visuospatial and verbal functions and high-resolution T1-weighted magnetic resonance images of the brain were acquired in 23 PD subjects with left-side motor symptom onset (PDL), 23 PD subjects with right-side onset (PDR), and 23 matched Controls. GM volume differences were assessed using voxel-based morphometry (VBM). Cognitive results and VBM were compared among the three groups, and correlation analyses were performed between those cognitive domains and brain areas that showed significant differences.
Results
PDL subjects had lower performance on visuospatial memory tasks compared to PDR. Furthermore, PDL subjects experienced lateralized GM loss, which was localized predominantly in the right hemisphere contralateral to the side of motor symptom onset. Visuospatial memory performance in PDL was correlated with GM loss in the right middle frontal gyrus and precuneus.
Conclusion
These data suggest that the onset of asymmetric motor symptoms in PD may be associated with hemisphere-specific memory decline and lateralized GM loss, particularly in PDL. This study underscores the importance of classifying PD subgroups based on the side of motor symptom onset for clinical care and research to optimize cognitive outcomes.
Keywords: Parkinson's disease, motor asymmetry, cognition, Voxel-based morphometry, gray matter volume
1. Introduction
Parkinson’s disease (PD) presents often with lateralized motor symptoms that are associated with asymmetric dopamine uptake in striatum, especially the posterior putamen (1, 2). This asymmetry also can be observed in non-motor features such as lateralized fatigue, pain, and autonomic dysfunctions (3). Because the dopaminergic system of the striatum also is connected with the orbitofrontal, dorsolateral prefrontal, and other integrative cortices (4), hemisphere-specific cognitive and behavioral dysfunction has been suspected to be dependent on the side of motor symptom onset. Consistent with this hypothesis, previous studies have reported that PD subjects with left-side motor symptom onset (PDL) showed poorer performance on visuospatial tasks associated with the right cerebral hemisphere, whereas PD subjects with right-side onset (PDR) had poorer performance on verbal tasks associated with the left hemisphere (5, 6). Some studies, however, failed to show any cognitive performance difference between PDL and PDR (7, 8). The discrepancy may be due to patient selections and/or choices of individual tasks or domains tested (9). None of past studies investigated the structural substrate of the potential cognitive differences between PDL and PDR.
The current study was designed to provide a structure-function analysis of cognitive and anatomical changes in PD subjects with asymmetric motor onset. The purpose was to examine whether hemisphere-specific cognitive changes, if observed, are associated with a corresponding lateralized cortical degeneration. We tested the following hypotheses: (1) PD subjects will display different patterns of cognitive decline and cortical GM volume loss depending on the side of motor symptom onset. Specifically for PDL, there will be a significant reduction in visuospatial aspects of cognition and GM volume in the right cerebral hemisphere; for PDR, there will be a significant reduction in verbal aspects of cognition and GM volume in the left hemisphere (2) There will be positive correlations between cognitive performance and GM volume in those cognitive tests and brain areas that show significant differences.
2. Methods
2.1 Subjects
Ninety-seven PD subjects and 83 Controls were originally recruited for an ongoing longitudinal study approved by the Institutional Review Board/Human Subjects Protection Office (IRB/HSPO) of the Penn State Hershey Medical Center, and conducted in accordance with the principles of the Declaration of Helsinki. PD subjects were diagnosed by a movement disorder specialist (XH) according to published criteria (10) and were negative for other neurological disorders. Written informed consent was obtained from all participants according to IRB/HSPO guidelines. For this study, twenty-three pairs of PD subjects with different symptom onset sides were selected and matched in age, gender, education, disease duration, disease severity, and MMSE scores. Only right-handed PD subjects< 70 years of age with a Mini-Mental State Examination (MMSE) Score≥ 26 were selected for inclusion. Handedness was assessed by the Edinburgh Handedness Inventory (11).
Except for two subjects who had very mild symptoms and were drug naïve, PD subjects were treated with anti-parkinsonian medications. Three PDL subjects were treated with an acetylcholinesterase inhibitor (donepezil), and two PDL and one PDR subjects were on anti-cholinergic medication for incontinence. Twenty-three healthy Controls, matched in age, gender, education, and handedness, were randomly selected from the pool of Controls. To accomplish optimal matching, Controls who were close to the existing PD subject pool in terms of age, gender, and education were selected. Controls were free from any history of neurologic or psychiatric disorders.
All PD subjects were assessed in a practically defined “off” state after withholding anti-parkinsonian medications overnight (~12 hours; 12) throughout the study except for MRI acquisition. Unified PD Rating Scale-III (UPDRS-III) scores were recorded. UPDRS Asymmetry Index was calculated using the formula: [(Right-side UPDRS – Left-side UPDRS) ÷ (Right-side UPDRS + Left-side UPDRS)]. The UPDRS-III items used for this index were range of motion, rigidity of each extremity, rapid opening/closing of each hand, finger tapping of each hand, rest tremor of each limb, and postural tremor of the right and left upper extremities. Levodopa equivalent daily dosage (LEDD) for PD subjects was calculated using previously published formulas (13). The Hamilton Depression Scale (HAM-D) was used to screen for depression and MMSE score and the Dementia Rating Scale (DRS-2) for dementia.
2.2 Cognitive Tests
Subjects were administered standardized neuropsychological batteries to assess cognitive functions including executive function, language, spatial perception, memory, and attention. The executive function, memory, and attention domains were further specified so that each functional domain could be assessed by either visuospatial or verbal materials, which led to the final eight subdomains: (1) visuospatial executive function, (2) verbal executive function, (3) language, (4) spatial perception, (5) visuospatial memory, (6) verbal memory, (7) visuospatial attention, and (8) verbal attention. Each subdomain was assessed by the following subtests:
Visuospatial (1) and verbal (2) executive function: The Design Fluency Test assessed visuospatial executive function, whereas the Verbal Fluency Test assessed verbal executive function.
Language (3): The Boston Naming Test provided standardized measures of semantic knowledge and word retrieval. The Color-word-interference (CWInt)-Word reading subtest assessed reading speed and accuracy.
Spatial perception (4): Visuospatial perception was evaluated with Benton’s Judgment of Line Orientation Test.
Visuospatial (5) and verbal (6) memory: To assess visuospatial memory, the Brief Visuospatial Memory Test was conducted with learning (3 repetitions of an immediate recall test) and delayed recall subtests, whereas the Hopkins Verbal Learning Test assessed verbal memory with verbal learning and delayed recall subtests.
Visuospatial (7) and verbal (8) attention: WAIS Spatial Span test evaluated visuospatial attention and WAIS Digit Span test assessed verbal attention.
2.3 Magnetic resonance imaging acquisition and voxel-based morphometry analysis
All images were acquired on a Siemens 3-Tesla TimTrio MRI with an 8-channel birdcage type coil. High-resolution T1-weighted (T1W) images (3D MPRAGE, TR=1540 ms, TE=2.3 ms, voxel spacing 1.0×1.0×1.0 mm, image resolution 256×256 mm2, 176 slices with no gap) were acquired.
A voxel-based morphometry (VBM) analysis was carried out using FMRIB Software Library (FSL) tools (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM). First, a study-specific template was created so that all images could be registered in the same stereotactic space (spatial normalization). To do this, brain-extracted structural images were segmented into gray matter, white matter (WM), and cerebrospinal fluid (CSF). GM images then were affine-registered to the GM ICBM-152 template and averaged to create an affine GM template. Next, GM images were re-registered to this affine GM template using a non-linear registration and averaged to create the study-specific non-linear GM template in the standard MNI space. Second, individual GM images were registered non-linearly to the study-specific template. After the normalization, the resulting GM images were modulated by multiplying with Jacobian determinants to correct for volume change induced by the nonlinear spatial normalization. Finally, the images were smoothed with an isotropic Gaussian kernel of 9 mm.
2.4 Statistical Analyses
Comparisons of demographic information and clinical characteristics (i.e., age, education, HAM-D, MMSE, DRS-2, and UPDRS-III) were completed using one-way analysis of variance (ANOVA). The sex ratio between groups was tested using Fisher’s Exact Test.
Cognitive test results were converted to standardized z-scores and domain-specific average z-scores were calculated from the subtests of each subdomain. One-way analysis of covariance (ANCOVA) with adjustment for depression was used to examine group differences in each cognitive subdomain and in individual cognitive subtests. ANCOVA analyses were corrected for multiple group comparisons using Tukey-Kramer correction but not for multiple test (eight subdomains) comparisons due to the exploratory nature of these analyses. To examine within-group differences between visuospatial and verbal performance in each subdomain, paired sample t-tests were conducted for each group separately.
For the imaging analysis, permutation-based non-parametric testing (5000 permutations) was used with adjustment for depression and total intracranial volume (TIV). First, group differences were considered significant at family-wise corrected (FWE) p<0.05 after initial cluster thresholding of t>2.3. For the group comparisons that failed to show significant differences at the FWE level of p<0.05, we relaxed the threshold to an uncorrected p< 0.0005 with more than 30 voxels (14) to examine patterns of GM atrophy.
To explore correlations between cognitive tests and cortical GM volume, Spearman’s partial correlation analyses were performed in PD subgroups in brain areas and cognitive subdomains that showed significant differences with adjustment for age. To do this, GM volume was extracted from the areas that showed group differences. Statistical significance for cognitive tests and correlation analyses was defined by α< 0.05.
3. Results
3.1 Demographics
Controls, PDL, and PDR groups did not differ in age, gender, education, DRS-2, or MMSE scores. PDL and PDR subjects were significantly different from Controls but not from each other in HAM-D and UPDRS–III scores (Controls vs. PD subgroups: ps< 0.008). PDL and PDR subgroups showed no significant differences in disease duration, disease stage, or LEDD (Table 1).
Table 1.
Demographics
| NC (n =23) |
PDL (n =23) |
PDR (n =23) |
P-value | |||
|---|---|---|---|---|---|---|
| NC vs. PDL |
NC vs. PDR |
PDL vs. PDR |
||||
| Age (yrs.) | 57.9 ± 6.7 | 60.7 ± 6.8 | 57.9 ± 6.5 | 0.35 | 1.00 | 0.35 |
| Gender (M:F) | 13:10 | 13:10 | 13:10 | 1.00 | 1.00 | 1.00 |
| Education (yrs.) | 15.6 ± 3.1 | 15.2 ± 2.5 | 15.2 ± 2.8 | 0.863 | 0.863 | 1.00 |
| MMSE | 29.4 ± 0.9 | 29.1 ±1.3 | 29.4 ± 1.0 | 0.603 | 1.00 | 0.603 |
| HAM-D Scale | 3.7 ± 2.1 | 7.2 ± 3.3 | 6.9 ± 4.0 | 0.002* | 0.004* | 0.946 |
| Dementia Rating Scale-2 | 12.8 ± 1.7 | 11.6 ± 2.2 | 12.3 ± 2.2 | 0.10 | 0.58 | 0.54 |
| Disease duration (yrs.) | n.a. | 3.7 ± 4.6 | 3.5 ± 3.5 | n.a. | n.a. | 0.91 |
| UPDRS - Total | 1.2 ± 2.6 | 20.8 ± 12.9 | 17.1 ± 9.5 | <0.0001* | <0.0001* | 0.27 |
| EHIq | 92.2 ± 13.1 | 92.2 ± 13.1 | 89.1 ± 11.2 | 1.00 | 0.69 | 0.69 |
| Asymmetry Index | n.a. | −0.49 ± 0.4 | 0.5 ± 0.4 | n.a. | n.a. | <0.0001* |
| H&Y stage | n.a. | 1.52 ± 0.67 | 1.39 ± 0.58 | n.a. | n.a. | 0.48 |
| (I/II/III) | n.a. | 13/8/2 | 15/7/1 | |||
| LEDD (mg/day) | n.a. | 366 ± 229 | 354 ± 282 | n.a. | n.a. | 0.88 |
p<0.05;
EHIq = Edinburgh Handedness Inventory quotient; LEDD = Levodopa Equivalent Daily Dosage; UPDRS = Unified Parkinson’s Disease Rating Scale III.
3.2 Cognitive tests
Compared to Controls, PDL and PDR subjects did not display any performance differences in the cognitive tests. Compared to PDR, PDL subjects had lower performance in visuospatial memory [t(22)= −2.74, p = 0.021, Tukey-Kramer corrected for multiple group comparisons]. There were no group differences in executive function, language, spatial perception, verbal memory, or attention between PD subgroups (Table 2).
Table 2.
Neuropsychological test scores and analyses results
| Test | NC (n = 23) |
PDL (n = 23) |
PDR (n = 23) |
P-value | ||
|---|---|---|---|---|---|---|
| NC vs. PDL |
NC vs. PDR |
PDL vs. PDR |
||||
| Executive function | ||||||
| Mean Visuospatial | 0.20 ± 0.66 | −0.13 ± 0.78 | 0.01 ± 0.82 | 0.091 | 0.286 | 0.770 |
| DesFlu Switch | 0.80 ± 0.83 | 0.26 ± 0.93 | 0.30 ± 1.03 | 0.053 | 0.077 | 0.975 |
| DesFlu Design Accuracy | −0.80 ± 1.15 | −0.87 ± 0.97 | −0.48 ± 1.03 | 0.686 | 0.919 | 0.384 |
| DesFlu Total Correct | 0.61 ± 1.01 | 0.23 ± 1.00 | 0.21 ± 0.93 | 0.200 | 0.176 | 0.999 |
| Mean Verbal | −0.08 ± 0.71 | −0.02 ± 0.96 | −0.14 ± 0.85 | 0.985 | 0.816 | 0.884 |
| VerbFlu Letter | −0.26 ± 0.77 | −0.13 ± 1.25 | −0.18 ± 1.19 | 0.872 | 0.999 | 0.988 |
| VerbFlu Category | 0.10 ± 1.04 | 0.10 ± 1.02 | −0.10 ± 0.87 | 0.903 | 0.554 | 0.792 |
| Language | ||||||
| Z-scores | 0.48 ± 0.51 | 0.29 ± 0.75 | 0.41 ± 0.65 | 0.998 | 0.880 | 0.825 |
| CWInt Word | 0.32 ± 0.67 | 0.32 ± 0.83 | 0.26 ± 1.16 | 0.876 | 0.965 | 0.962 |
| BNT | 0.64 ± 0.82 | 0.26 ± 0.97 | 0.56 ± 0.84 | 0.458 | 0.984 | 0.501 |
| Spatial cognition | ||||||
| JoLO | 0.23 ± 0.94 | −0.40 ± 1.63 | −0.49 ± 1.53 | 0.128 | 0.088 | 0.986 |
| Learning/Memory | ||||||
| Mean Visuospatial | −0.44 ± 1.42 | −0.93 ± 1.17 | 0.08 ± 1.21 | 0.266 | 0.617 | 0.022* |
| BVMT Total Learning | −0.54 ± 1.41 | −1.02 ± 1.16 | −0.05 ± 1.18 | 0.275 | 0.650 | 0.027* |
| BVMT Delayed Recall | −0.33 ± 1.54 | −0.84 ± 1.24 | 0.21 ± 1.27 | 0.296 | 0.618 | 0.026* |
| Mean Verbal | −0.46 ± 0.88 | −0.61 ± 0.65 | −0.60 ± 1.31 | 0.411 | 0.457 | 0.994 |
| HVLT Total Learning | −0.38 ± 0.87 | −0.71 ± 0.72 | −0.72 ± 1.33 | 0.194 | 0.199 | 0.999 |
| HVLT Delayed Recall | −0.54 ± 1.05 | −0.52 ± 0.87 | −0.49 ± 1.35 | 0.780 | 0.850 | 0.987 |
| Attention | ||||||
| Visuospatial | ||||||
| Spatial Span | 0.19 ± 0.80 | 0.26 ± 1.11 | 0.35 ± 0.97 | 0.996 | 0.974 | 0.941 |
| Mean Verbal | 0.19 ± 0.59 | 0.07 ± 0.59 | 0.25 ± 0.65 | 0.419 | 0.955 | 0.531 |
| Digit Span | 0.20 ± 0.90 | 0.06 ± 0.70 | 0.29 ± 0.79 | 0.624 | 1.000 | 0.570 |
| Letter Number Sequencing | 0.17 ± 0.50 | 0.09 ± 0.87 | 0.22 ± 0.70 | 0.492 | 0.871 | 0.758 |
p < 0.05;
DesFlu = Design Fluency; VerbFlu = Verbal Fluency; BNT = Boston Naming Test; JoLO = Judgment of Line Orientation; BVMT-R = Brief Visuospatial Memory Test-Revised; HVLT-R = Hopkins Verbal Learning Test-Revised.]
Within PDR, there was poorer performance on verbal memory compared to visuospatial memory tasks [t(22)= −2.98, p= 0.007]. For the other tasks, there were no performance differences between visuospatial and verbal tasks in PDR subjects. Within Controls and PDL, there were no differences between visuospatial and verbal tests.
3.3 VBM analysis
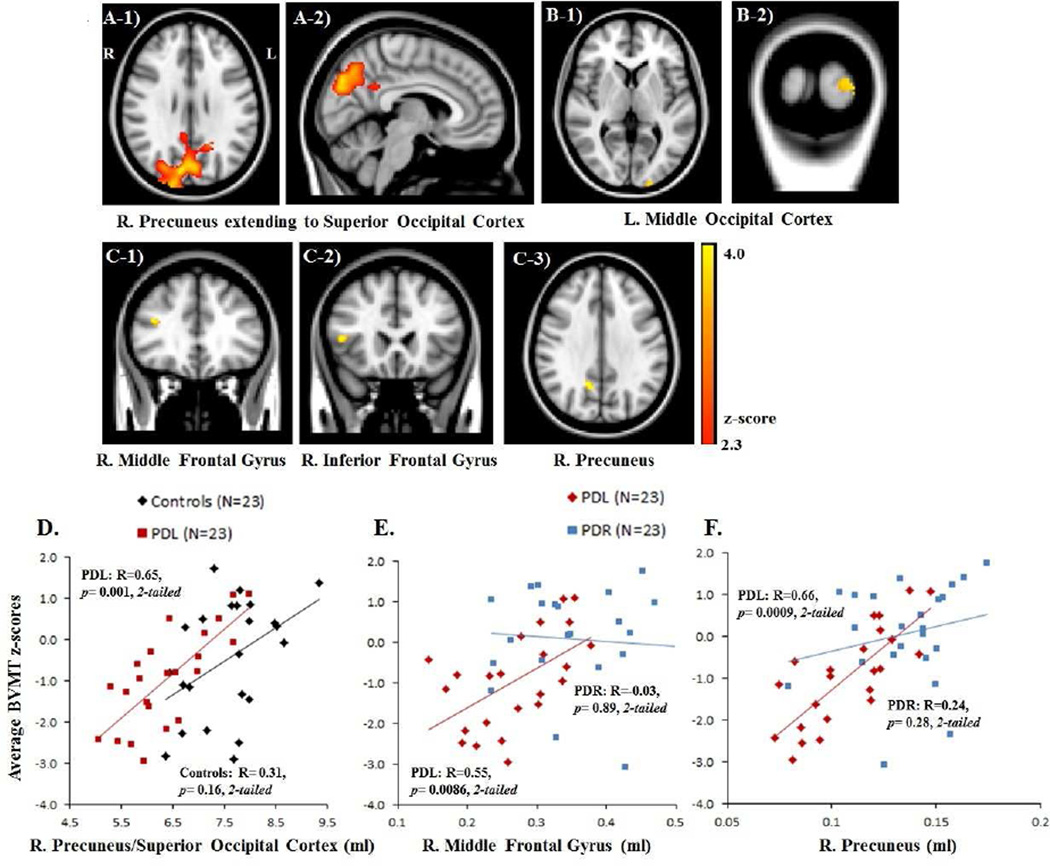
Compared to Controls, PDL subjects showed significant volume loss predominantly in the right precuneus (BA 7), which extended to the right superior occipital cortex (BA 18–19) and also encompassed part of the left precuneus [2, −76, 36; Z-score= 4.51; Figure 1A]. There was no significant group difference between Controls and PDR subjects or between PD subgroups at FWE corrected p< 0.05.
Figure 1.
A) VBM results contrasting PDL < Controls at FWE corrected p< 0.05 indicate GM volume loss in PDL subjects in the right precuneus extending to the right superior occipital cortex and part of the left precuneus [A-1): axial view and A-2): sagittal view]; (B) VBM results contrasting PDR < Controls at uncorrected p< 0.0005 indicate GM volume loss in PDR patients in the left middle occipital cortex [B-1): axial view and B-2): coronal view]; C) VBM results contrasting PDL < PDR subjects at uncorrected p< 0.0005 indicate GM volume loss in PDL subjects in (C) Right middle/inferior frontal gyri [C-1) – C-2): coronal views], and the right precuneus [C-3): axial view]. D) Scatter plots of gray matter volume with the average Brief Visuospatial Memory Test (BVMT) z-scores for Controls and PDL subjects (D) and for both PD subgroups (E–F): PDL GM volume (ml) in the right hemisphere cluster (precuenus/superior occipital cortex) correlated with the average BVMT scores (D). PDL GM volume (ml) in the middle frontal gyri (E), and the right precuneus (F) correlated with the average BVMT scores.
After relaxing the threshold to an uncorrected p< 0.0005, PDR subjects showed significant volume reduction compared to Controls in the left middle occipital cortex [BA 18; −20, 102, 4; Z-score= 3.83; Figure 1B]. Comparison of PD subgroups demonstrated that PDL subjects had GM volume loss compared to PDR subjects in the right middle [BA 46; 38, 32, 22; Z-score= 4.05]/inferior [BA 45; 48, 22, 8; Z-score= 4.13] frontal gyri and the right precuneus [BA 7; 14, −58, 34; Z-score= 4.75; Figure 1C], whereas PDR subjects still showed no discernible volume reduction compared to PDL subjects (Table 3).
Table 3.
Local peaks of clusters indicating significant gray matter loss in PD subgroups compared to Controls and PDL compared to PDR subjects.
| Anatomical Region | MNI coordinates a | Zmax b | Voxel Size c | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| PDL vs Controls1 | |||||
|
R. Precuneus (BA 7)/ R. Superior Occipital Cortex (BA 18–19) |
2 | −76 | 36 | 4.51 | 3047 |
| PDR vs Controls2 | |||||
| L. middle Occipital Cortex (BA 18) | −20 | 102 | 4 | 3.83 | 40 |
| PDL vs PDR3 | |||||
| R. middle Frontal gyruse (BA 10,11) | 38 | 32 | 22 | 4.05 | 40 |
| R. inferior Frontal gyrus (BA 10, 32) | 48 | 22 | 8 | 4.13 | 36 |
| R. Precuneus (BA 7) | 14 | −58 | 34 | 4.75 | 47 |
- FWE corrected p< 0.05;
- uncorrected p < 0.0005;
- coordinates of local maxima are given in MNI (Montreal Neurological Institute) standard space;
- Z-scores of the local maxima;
- Cluster extent is given in voxel size (1 voxel = 2×2×2 mm3);
BA= Brodmann Area; R.- right; L.- left.
3.4 Correlation analyses
Spearman’s partial rank correlation analyses adjusted for age revealed that the visuospatial memory scores in PDL subjects were positively correlated with the posterior right hemisphere cluster [precuneus/superior occipital cortex; R=0.65, p= 0.001, 2-tailed, N=23; Figure 1D], in which PDL showed significant volume reduction compared to Controls. The GM volume in the left middle occipital cortex, in which PDR subjects showed volume reduction compared to Controls, failed to show any correlation with visuospatial and verbal memory tasks in both PDR and Controls.
PDL subjects also showed positive correlations between visuospatial memory and the volume in the middle frontal gyrus [R=0.55 p= 0.0086, 2-tailed, N=23] and the right precuneus [R=0.66, p= 0.0009, 2-tailed, N=23; Figure 1E–F], in which PDL showed significant volume reduction compared to PDR subjects.
4. Discussion
The present study demonstrated that the onset side of PD motor symptoms is associated with lateralized cognitive decline and GM loss. Results showed that 1) PDL subjects performed worse on memory tasks that are known to be mediated by the right cerebral hemisphere (i.e., contralateral to the motor symptom onset side); 2) PDL subjects experienced lateralized GM loss that was localized predominantly in the right hemisphere; and 3) there were positive associations between lateralized memory decline and GM loss in PDL. To our knowledge, this is the first study demonstrating lateralized structure-function pathophysiology in PD subjects with asymmetric motor onset.
Hemisphere-specific memory decline depending on motor symptom onset side
The poorer performance on visuospatial memory tasks in PDL compared to PDR subjects supports previous findings reporting PD subjects having hemisphere-specific decline depending on the motor symptom onset side (6, 15). Consistent with this, verbal memory performance also was significantly worse than visuospatial memory within PDR subjects. PDR subjects, however, showed no performance deficit compared to PDL subjects. This may be due to the finding that verbal memory in the PDL subjects was as poor as the visuospatial memory. The right-handed PDL subjects are conceivably challenged in both hemispheres: predominant PD pathology in the right hemisphere and potentially increased metabolic stress due to right handedness in the left hemisphere (16).Thus, PDL subjects may be vulnerable to visuospatial but also verbal memory decline. Consistent with this hypothesis, prior studies reported that right-handed PDL subjects can be impaired in multiple cognitive areas that extend beyond that expected with right hemisphere pathology in comparison with right-handed PDR subjects (17).
Some previous literature reported that lateralized cognitive changes seem to mostly impact the memory domain (6, 9). Our findings are consistent with this notion, though it is unclear what makes the memory domain unique in terms of this lateralized cognitive dysfunction. It is possible that some cognitive tests used in the current study were not sensitive enough to detect the insidious, gradual lateralized pattern of cognitive decline in PD (9). Including more tests tapping different aspects of each subdomain with parameters that can increase information processing load and/or speed of processing may increase the chance to detect consistently existing differences. Interestingly though, our study demonstrated specific GM volume reductions in the right prefrontal-medial parietal cortices in PDL compared to PDR subjects, which is known to be involved in memory retrieval processes (18). These results suggest that cortical degeneration (atrophy) may underlie the lateralized cognitive dysfunction observed in our PDL subjects. It is possible that it is too early in the disease process to observe changes in other cognitive domains and/or cortical areas. Consistent with this hypothesis, we found no GM volume reduction, however, in the right lateral posterior parietal cortex, an area often associated with spatial cognition (19). In addition, the absence of any lateralized cognitive alterations has been reported for newly diagnosed, untreated PD patients (8). Since our PD subjects were at a relatively early stage (H&Y stage ≤ 2), lateralized cognitive decline in other domains may not have been apparent but may emerge with advancing disease stage (20). It also is possible that some cognitive functions are complex and include higher-level mental activities (e.g., executive function may refer to mental abilities including cognitive control, planning, and problem solving). To successfully perform these complex cognitive functions, it may be necessary to utilize both hemispheres rather than predominantly relying on one (9). Hence, subtle deficits may be masked or compensated in various ways. Consistent with this hypothesis, previous studies found no differences between PD subjects with different motor asymmetric onsets for executive function tasks (5, 9).
Lateralized gray matter loss depending on motor symptom onset side
Compared to Controls, PDL demonstrated volume reductions localized predominantly in the right hemisphere, contralateral to the symptom onset side. The same was true for PDR but at a lower statistical threshold. In addition, PDL subjects displayed GM reduction in the right hemisphere compared to PDR subjects. These results suggest that asymmetric motor onset may be associated not only with functional (1) but also structural changes by leading to greater neuronal loss in the hemisphere contralateral to the more affected limb (21, 22).
The lateralized cortical degeneration observed in the current study may reflect progressive dysfunction of cortico-striatal and/or striato-thalamo-cortical pathways, which may progress in an asymmetric fashion (1, 21). It also is possible that cortical GM pathology occurs asymmetrically independent of disrupted cortico-striatal circuitries because the presence of Lewy bodies and neurites, pathological hallmarks in PD, may extend beyond the basal ganglia to cortical regions as the disease progresses (23).
Lateralized structural-function relationship
Previous studies reported that the right parieto-prefrontal and parieto-medial temporal regions are involved in visuospatial processing and memory functions (19, 24) whereas the left inferior frontal- superior/medial temporal regions are involved in language processing and verbal memory functions (25, 26). Consistent with past literature, the present study demonstrated that visuospatial memory performance in PDL subjects had positive correlations with GM volume in the right middle frontal-precuneus areas. These results suggest a lateralized structure-function relationship in PD patients with asymmetric motor onset.
The middle frontal gyrus (BA 46) is well known to be crucial for various higher-order mental processes such as executive function, top-down attention, and memory retrieval by forming fronto-parietal and fronto-striatal connections (27). The precuneus (BA 7) has been characterized as a parietal association area involved in various integrative functions [e.g., visuospatial imagery, episodic (personal experience-based) memory retrieval, and self-awareness (28, 29)] by having widespread connections with the frontal and temporal lobes (29, 30), as well as subcortical basal ganglia areas (caudate and putamen; 29). Furthermore, some studies report the predominant involvement of the right precuneus in memory retrieval processes by utilizing visuospatial imagery (18, 29, 30). Thus, the current finding of lateralized association of visuospatial memory performance with the right middle frontal-precuneus volume in PDL subjects suggests that poorer visuospatial memory may be due to altered memory retrieval process.
It is worth noting that lateralized GM loss in PD subgroups was found even in the absence of clear performance deficits compared to Controls. This result raises the hypothesis that lateralized GM loss may be a more sensitive marker of underlying PD pathology than neurobehavioral tests that may benefit from various compensatory processes via cognitive strategies and involvement of other brain regions. Nevertheless, the positive association of lateralized cortical atrophy (right precuneus/superior occipital cortex) with visuospatial memory performance in PDL subjects in comparison with Controls suggests that the lateralized volume reduction observed in PD subgroups may indicate risk for lateralized cognitive decline.
Conclusion
The results support the hypotheses that among PD subjects with asymmetric motor symptom onset those with PDL sustain lateralized cognitive decline in the visuospatial memory domain, together with GM loss in the right cerebral hemisphere, implicating dysfunction of the right cerebral hemisphere. To our knowledge, this is the first study to report lateralized cognitive function-structural changes in PD subjects with asymmetric motor symptom onset. The findings underscore the importance of classifying PD based on motor symptom onset side in future studies, as well as in clinical assessment and treatment strategies to optimize behavioral outcomes.
Highlights.
PDL subjects performed worse on visuospatial memory tasks that are known to be mediated by the right cerebral hemisphere (i.e., contralateral to the motor symptom onset side).
PDL subjects experienced lateralized GM loss that was localized predominantly in the right cerebral hemisphere.
There were positive associations between lateralized memory decline and GM loss in PDL.
Acknowledgments
Study funding: This work was supported by NS060722, U0140726 and the HMC GCRC (NIH M01RR10732) and GCRC Construction Grants (C06RR016499).
Statistical analysis: Drs. S. Sen (Neurology), E.Y. Lee (Neurology), and L. Kong (Public Health Sciences).
List of abbreviations used in the paper
- ANOVA
analysis of variance
- ANCOVA
Analysis of covariance
- BA
Brodmann area
- CSF
Cerebral spinal fluid
- CWInt
Color-word-interference
- DRS-2
Dementia Rating Scale- 2nd Edition
- GM
Gray matter
- FWE
family-wise error
- HAM-D
Hamilton Depression Scale
- LEDD
Levodopa equivalent daily dosage
- MMSE
Mini-mental state examination
- MRI
Magnetic resonance imaging
- PD
Parkinson’s disease
- PDL
Parkinson’s patients with left-side onset
- PDR
Parkinson’s patients with right-side onset
- T1W
T1-weighted
- TIV
Total intracranial volume
- UPDRS
Unified PD Rating Scale III
- VBM
Voxel-based morphometry
- WM
White matter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have reported no conflicts of interest.
Specific Contributions of Authors
Eun-Young Lee: Data analysis and interpretation; statistical analysis; primary drafting
Suman Sen: Conception and design of the study; data acquisition, analysis and interpretation; statistical analysis; primary drafting and critical revision of the manuscript.
Paul J. Eslinger: Conception and design of the study; data acquisition, analysis, and interpretation; drafting and critical revision of the manuscript.
Daymond Wagner: Data acquisition and interpretation; critical revision of the manuscript.
Lan Kong: Statistical analysis of the data; critical revision of the manuscript.
Mechelle M. Lewis: Data acquisition; critical revision of the manuscript; administrative support for the study.
Guangwei Du: Data acquisition; critical revision of the manuscript.
Xuemei Huang: Conception and design of the study; data acquisition and interpretation; drafting and critical revision of the manuscript; obtaining funds for the study; administration and supervision of the project.
References
- 1.Eidelberg D, Moeller J, Dhawan V, Sidtis J, Ginos J, Strother S, et al. The metabolic anatomy of Parkinson's disease: complementary [18F] fluorodeoxyglucose and [18F] fluorodopa positron emission tomographic studies. Movement Disorders. 1990;5(3):203–213. doi: 10.1002/mds.870050304. [DOI] [PubMed] [Google Scholar]
- 2.Morrish P, Sawle G, Brooks D. Clinical and [18F] dopa PET findings in early Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry. 1995;59(6):597–600. doi: 10.1136/jnnp.59.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D. Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology. 2004;62(12):2171–2175. doi: 10.1212/01.wnl.0000130455.38550.9d. [DOI] [PubMed] [Google Scholar]
- 4.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research Reviews. 2000;31(2):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 5.Mohr E, Mann U, Miletich R, Sampson M, Goldberg T, Grimes J, et al. Neuropsychological and glucose metabolic profiles in asymmetric Parkinson's disease. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 1992;19(2):163–169. [PubMed] [Google Scholar]
- 6.Amick M, Grace J, Chou K. Body side of motor symptom onset in Parkinson's disease is associated with memory performance. Journal of the International Neuropsychological Society. 2006;12(05):736–740. doi: 10.1017/S1355617706060875. [DOI] [PubMed] [Google Scholar]
- 7.Foster ER, Black KJ, Antenor-Dorsey JAV, Perlmutter JS, Hershey T. Motor asymmetry and substantia nigra volume are related to spatial delayed response performance in Parkinson disease. Brain and cognition. 2008;67(1):1–10. doi: 10.1016/j.bandc.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erro R, Santangelo G, Picillo M, Vitale C, Amboni M, Longo K, et al. Side of onset does not influence cognition in newly diagnosed untreated Parkinson's disease patients. Parkinsonism & related disorders. 2013;19(2):256–259. doi: 10.1016/j.parkreldis.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Verreyt N, Nys GM, Santens P, Vingerhoets G. Cognitive differences between patients with left-sided and right-sided Parkinson’s disease. A review. Neuropsychology review. 2011;21(4):405–424. doi: 10.1007/s11065-011-9182-x. [DOI] [PubMed] [Google Scholar]
- 10.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery & Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 12.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations (CAPIT) Movement Disorders. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Movement Disorders. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 14.Pan P, Song W, Shang H. Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson’s disease. European Journal of Neurology. 2012;19(2):199–206. doi: 10.1111/j.1468-1331.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- 15.Katzen HL, Levin BE, Weiner W. Side and type of motor symptom influence cognition in Parkinson's disease. Movement Disorders. 2006;21(11):1947–1953. doi: 10.1002/mds.21105. [DOI] [PubMed] [Google Scholar]
- 16.van der Hoorn A, Burger H, Leenders KL, de Jong BM. Handedness correlates with the dominant Parkinson side: A systematic review and meta-analysis. Movement Disorders. 2012;27(2):206–210. doi: 10.1002/mds.24007. [DOI] [PubMed] [Google Scholar]
- 17.Tomer R, Levin BE, Weiner WJ. Side of onset of motor symptoms influences cognition in Parkinson's disease. Annals of neurology. 1993;34(4):579–584. doi: 10.1002/ana.410340412. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher P, Frith C, Baker S, Shallice T, Frackowiak R, Dolan R. The mind's eye—precuneus activation in memory-related imagery. Neuroimage. 1995;2(3):195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- 19.Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nature Reviews Neuroscience. 2011;12(4):217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poletti M, Frosini D, Pagni C, Baldacci F, Giuntini M, Mazzucchi S, et al. The relationship between motor symptom lateralization and cognitive performance in newly diagnosed drug-naïve patients with Parkinson's disease. Journal of clinical and experimental neuropsychology. 2013;35(2):124–131. doi: 10.1080/13803395.2012.751966. [DOI] [PubMed] [Google Scholar]
- 21.Kempster P, Gibb W, Stern G, Lees A. Asymmetry of substantia nigra neuronal loss in Parkinson's disease and its relevance to the mechanism of levodopa related motor fluctuations. Journal of Neurology, Neurosurgery & Psychiatry. 1989;52(1):72–76. doi: 10.1136/jnnp.52.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis MM, Smith AB, Styner M, Gu H, Poole R, Zhu H, et al. Asymmetrical lateral ventricular enlargement in Parkinson’s disease. European Journal of Neurology. 2009;16(4):475–481. doi: 10.1111/j.1468-1331.2008.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braak H, Tredici KD, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 24.Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learning & Memory. 2007;14(10):645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell H, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32(1):388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Friederici AD. Pathways to language: fiber tracts in the human brain. Trends in cognitive sciences. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48(1):82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends in cognitive sciences. 2003;7(1):38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 29.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 30.Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39(6):545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]