Abstract
The pathophysiology of antibody-mediated rejection (AMR) in solid organ transplants is multi-faceted and predominantly caused by antibodies directed against polymorphic donor human leukocyte antigens (HLA). Despite the clearly detrimental impact of HLA antibodies (HLA-Ab) on graft function and survival, the prevention, diagnosis and treatment of AMR remain a challenge. Histological manifestations of AMR reflect signatures of HLA-Ab-triggered injury, specifically endothelial changes, recipient leukocytic infiltrate, and complement deposition. We review the interconnected mechanisms of HLA-Ab-mediated injury that might synergize in a “perfect storm” of inflammation. Characterization of antibody features that are critical for effector functions may help identify HLA-Ab more likely to cause rejection. We also highlight recent advancements that may pave the way for new, more effective therapeutics.
Keywords: Organ Transplantation, Antibody-mediated Rejection, HLA Antibodies, Classical Complement Pathway
Rejection of solid organ transplants challenges long term allograft survival
Organ failure is an immense human and economic burden, which can be successfully reversed with transplantation, substantially improving quality of life and life expectancy. In the United States, more than 100,000 patients currently await transplant of major solid organs. Significant advances in histocompatibility and immunosuppression have dramatically improved short-term graft and patient survival rates. Recipient recognition of donor human leukocyte antigen (HLA; see Glossary) present in the allograft induces an allogeneic immune response, resulting in the production of donor specific HLA antibodies (DSA). These antibodies, through many different effector functions, are responsible for the damage, and ultimately graft rejection, which occurs in antibody mediated rejection (AMR). AMR has emerged as a leading cause of graft dysfunction and reduced outcomes, yet it is often unresponsive to current therapies [1]. Histological markers of AMR are often unreliable, and it is controversial whether intervention is required for patients with DSA but no graft dysfunction. Clinical evidence suggests that DSA alone in the absence of histological or molecular evidence of antibody-mediated injury is not detrimental to renal allograft survival [2, 3]. However, long-term follow-up studies of asymptomatic or subclinical AMR in cardiac [4, 5] and renal [6] transplantation have demonstrated increased risk for chronic rejection. Consequently, AMR remains a diagnostic and therapeutic challenge. Here, we highlight the recent developments in the understanding of how antibodies against HLA (HLA-Ab) function to cause graft injury, emphasizing the multiple effector mechanisms of HLA-Ab, specifically IgG, and how they relate to risk and manifestations of AMR.
The alloimmune response
Immunity to alloantigens is surprisingly robust, mediated by the major histocompatibility complex (MHC), and based on exposure to allogeneic tissues. The MHC locus covers nearly 4000kb on human chromosome 6, and is polygenic, containing 3 loci of HLA class I (HLA-A, -B, and -C), 6 to 9 functional HLA class II loci (α and β chains of HLA-DR, -DP, and -DQ), as well as many non-classical MHC, minor histocompatibility antigens and immune-related genes. Balancing selection has resulted in extreme polymorphism within HLA class I and class II genes. To date, over 10,000 nucleotide sequences encoding more than 6000 class I and 2000 class II unique proteins have been reported [7]. The high allelic diversity of MHC genes is advantageous for protection of populations against pathogens, but is highly unfavorable for cell and organ transplantation.
Immune sensitization to HLA occurs after exposure to allogeneic tissue, either through pregnancy, transfusion, or transplantation. Twenty percent of healthy individuals [8, 9] and up to 30% of transplant candidates have HLA-Ab. Another 8–25% of recipients develop de novo DSA after receiving a graft [10–12]. Half of pre-sensitized patients and one third of patients with de novo DSA will experience AMR within the first year after transplant [10, 12]. Antibody responses against donor HLA proteins are not well controlled by current immunosuppression regimens [1]. Therefore AMR can occur at any time and is a common occurrence more than one year post-transplant [13]. DSA and subsequent rejection episodes are strongly associated with risk of chronic rejection and late graft failure [13–15].
Histological manifestations and diagnostic criteria of AMR
AMR is best defined in renal [16], cardiac [17], and pancreas [18] transplantation, although the diagnostic histological criteria for AMR differ somewhat from organ to organ. Central features include endothelial cell (EC) swelling, microvascular inflammation (subendothelial mononuclear cell infiltration), and intravascular CD68+ macrophages, with or without complement C4d deposition, often in the presence of circulating DSA (Figure 1) [17, 19, 20]. While HLA-Ab are indeed detrimental to liver [21], lung [22], and small bowel [23] allograft survival, clear pathological definitions of AMR remain contentious [17], as the utility of C4d and other histological markers remains unclear in these tissues.
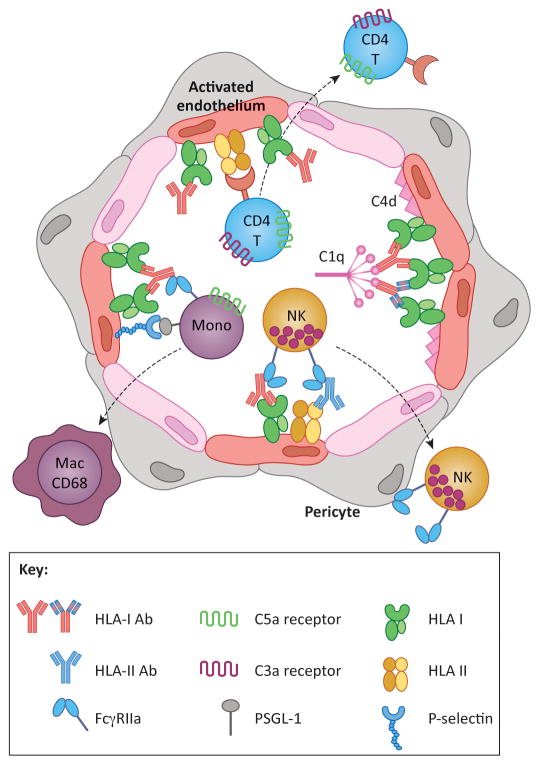
Figure 1. HLA antibodies cause graft injury by inducing phenotypic changes in the donor vasculature.
HLA crosslinking by antibodies of any subclass causes intracellular signaling leading to endothelial cell (EC) activation. Activated ECs express P-selectin, which promotes recruitment of leukocytes via interactions with PSGL-1. Recruited monocytes differentiate into CD68+ macrophages, which can be detected histologically in the capillaries and subendothelial space. Crosslinking of HLA molecules also enhances EC immunogenicity to recipient CD4 T cells, which proliferate and differentiate in response to alloantigen HLA class II. Complement activating antibodies trigger the classical pathway through binding of C1q, resulting in production of anaphylatoxins C3a and C5a, which have the potential to directly augment leukocyte recruitment and T cell alloresponses. Complement activation can be detected by immunohistochemical staining for C4d. Monocytes, neutrophils and NK cells also express FcγRs, which can interact with the heavy chain of HLA antibodies bound to donor ECs. FcγR functions augment leukocyte recruitment, and mediate phagocytosis and antibody-dependent cellular cytotoxicity. Taken together, the pleiotropic functions of HLA antibodies on the allograft ECs cause microvascular inflammation characteristic of antibody-mediated rejection. Antibodies in the figure with the same coloration of the Fc region are of the same subclass, whereas the varied colors within the F(ab′)2 denote unique antigenic specificities.
The donor vasculature present at the interface between donor tissue and the recipient immune system is the primary target of the alloimmune response. AMR is increasingly viewed as predominant endothelial injury and vascular inflammation [24, 25], and the principal involvement of the endothelium in AMR has been revealed by gene profiling studies of renal biopsies undergoing AMR [2, 3, 26].
HLA antibodies and subclass biology
The fact that some patients with DSA do not experience AMR suggests that other factors influence susceptibility or risk of rejection in the presence of antibodies that bind the graft. The histological manifestations of AMR are reflective of the injurious functions of HLA-Ab binding to the vasculature, causing endothelial signaling and inflammation, activation of the classical complement cascade, and recruitment of effector cells. Immunoglobulin G (IgG) is the most common isotype of circulating Ig, and is divided into four subclasses with unique patterns of biological activity. IgG3 is the strongest activator of complement, followed closely by IgG1, and to a far lesser extent IgG2 [27]. IgG4 has no detectable complement activity, and is often linked with IgG2 as “noncomplement fixing.” However, it should be noted that under unique conditions, such as high antigen/epitope density or increased concentrations of complement and IgG [28, 29], all subclasses including IgG2 and IgG4 effectively activate complement. In addition, work with murine MHC antibodies has demonstrated synergism between high and lowly complement fixing IgG subclasses [30–32]. While not yet explored using human IgG and complement, this is pertinent given that most antibody responses are polyclonal and HLA is often recognized by an admixture of subclasses.
IgG subclass interaction with Fc receptors (FcγRs) is more complex (Table 1). In general, IgG3 and IgG1 have the highest affinity for most FcγRs, while IgG2 and IgG4 are bound by a more restricted repertoire of FcγRs. Unfortunately the disparity between murine and human immunoglobulin systems limits the translation of in vivo mechanistic studies of IgG subclass effector functions in murine models of AMR to human disease [33].
Table 1.
Summary of the biological properties of human FcγRs and IgG subclassesa.
| Name | FcγRI, CD64 | FcγRIIa, CD32a | FcγRIIb, CD32b | FcγRIIIa, CD16a | FcγRIIIb, CD16b | ||
|
| |||||||
| Expression | Mono, Mac, Activated PMN | Mono, Mac, PMN, DC, platelets | All immune cells except T and NK | APCs (mono, DC, B), NK cells | PMN, some mono | ||
|
| |||||||
| Activating or Inhibitory | Act | Act | Inh | Act | Act | ||
|
| |||||||
| Polymorphism | None Known | R131 | H131 | I232T | F158 | V158 | NA1/NA2 |
|
| |||||||
| Affinity for: | |||||||
| IgG1 | ++++ | + | ++ | ± | + | + | ± |
| IgG2 | − | + | + | − | − | − | − |
| IgG3 | ++++ | + | + | ± | ++ | +++ | + |
| IgG4 | +++ | + | + | ± | ± | ± | − |
|
| |||||||
| Murine Counterpart | FcγRI | FcγRIII | FcγRIIb | FcγRIV | |||
After transplant, IgG1 antibodies are directed against approximately 90% of HLA specificities, whereas those of IgG2/3/4 recognize roughly 40% or less of HLA specificities [34–36]. These results are indicative of a polyclonal response wherein each donor HLA antigen is recognized by multiple subclasses, most commonly including IgG1. It has been difficult to reconcile the apparently conflicting results regarding the association of DSA subclass and clinical outcome, despite reports of IgG1/3 dominating the alloantibody responses [37]. IgG3 DSA were associated with increased risk of allograft loss in liver [35] and renal transplantation [38]. In contrast, others have reported no correlation between DSA subclass and risk of AMR or graft loss, although one study found a trend toward lower AMR in patients with only IgG2/4 DSA [39].
HLA antibodies and complement activation
The historical paradigm of AMR was one of complement-mediated damage caused by classical pathway activation by Fc regions of DSA bound to the allograft [30]. In recent years, complement fixing DSA have become a controversial topic. C4d-negative AMR is becoming increasingly recognized, and the diagnostic schema for heart and renal AMR have been updated to reflect this entity [20]. Experimental mouse models of AMR suggest that acute rejection is dependent upon complement fixation [40]. In contrast, intimal thickening during antibody-induced chronic rejection occurred in complement-deficient murine recipients, suggesting there was no requirement for complement in this process [41]. Importantly, local production of complement by donor EC could not be ruled out [42]. These results from animal models are consistent with clinical observations that terminal complement inhibitors could not prevent chronic rejection [43, 44]. Furthermore, studies using methods to define DSA that are complement fixing, and determine whether complement fixation translates to graft damage, have had conflicting results [45–49].
Of the three complement pathways [50], the classical pathway is primarily responsible for DSA-mediated complement activation. Early activation results in the production of soluble mediators, such as anaphylatoxins C3a and C5a, which are potent chemoattractants for leukocytes, and alter the microvasculature by increasing vascular permeability and inducing expression of adhesion molecules. The later stages are characterized by membrane attack complex (MAC) formation, which causes osmotic lysis of the target. Given the general resistance of EC to complement-mediated lysis, due to high expression of complement regulatory proteins, the physiological relevance of lytic terminal MAC formation during rejection is unclear [30]. Indeed, early complement proteins, rather than terminal MAC formation, are likely to be the mediators of the majority of complement-associated damage to the graft (Figure 2A).
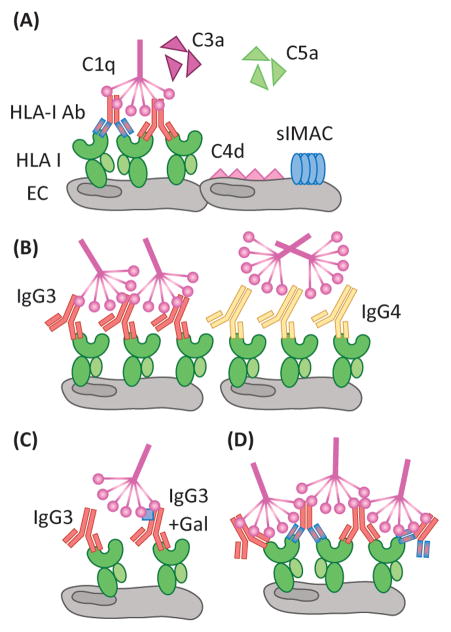
Figure 2. Complement activation by antibody/antigen determinants.
(A) Activation of the classical complement pathway by HLA-Ab is mediated by C1q recognition of the Fc region of IgG. Through a series of subsequent enzymatic cleavages, the complement pathway yields the soluble anaphylatoxins, C3a and C5a, which are potent chemoattractants and stimulators of immune responses. C4d is covalently linked to the cell surface, and is a defining marker of AMR in renal and cardiac transplants. Additionally, sublytic MAC (slMAC), the terminal complex bound to cells but unable to induce lysis, is proving to be an important mediator of endothelial cell (EC) activation. Differences in antibody clonality, as demonstrated by the antibodies of varying specificity (red or blue F(ab′)2 region), allow for increased ratios of IgG:HLA, allowing for more C1q binding. (B) Antibody subclass determines the propensity of C1q binding as IgG3, a prominent complement fixer, is recognized by C1q, whereas the structure of IgG4 makes it a poor C1q binding partner. (C) Differential patterning of the N297 glycan (blue square) of IgG also modulates the level of C1q interaction. Terminal galactose residues confer maximal C1q binding to antibodies. (D) The density of HLA antigen on the surface of the cell, as well as the number of epitopes, heavily dictates the level of complement activation. The proximity of antibody Fc regions is increased when multiple antibodies can bind the same molecule of HLA. Patients with high titer polyclonal DSA may be predisposed to exacerbated complement activation during times of heightened inflammation, such as infection, when HLA expression is increased on the surface of endothelium.
Factors which dictate complement activation
Many components modulate complement fixation by IgG. Of these, three are intrinsic to the antibody itself: IgG subclass, glycosylation, and affinity (Figure 2B and 2C). Multiple studies have defined the importance of antibody affinity in dictating the level of complement activation [51]. Repeated injury and consistent antigen exposure may increase affinity of DSA over time, resulting in HLA-Ab that are more inflammatory and induce robust complement induction.
Additionally, extrinsic factors, such as antigen density/epitopes and complement concentration, also regulate antibody induced complement activation [52]. Despite constitutive allograft endothelium expression of HLA class I and II [53], these levels are altered in response to inflammatory cues [54]. Many in vitro studies have shown that increased alloantibody bound to cells resulted in enhanced complement deposition, and this was augmented under inflammatory conditions [55, 56]. Moreover, binding of multiple antibodies with distinct epitopes to a single HLA molecule synergistically enhanced complement activation [36]. If antibody subclass and antigen density/epitopes coordinate to determine complement activation by DSA, polyclonal antibodies should elicit more complement activation than monoclonal antibodies. Indeed, sera with >80% PRA (panel reactive antibody) are strong inducers of complement activation [55, 56], supporting the notion that differing levels of HLA antigen/epitopes determine both the quantity and quality of DSA bound to the graft (Figure 2D).
Lastly, variations in complement can determine the degree of activation. Some complement proteins are located in the MHC locus (C2, C4), and are also polymorphic [57]. Genetic predisposition to specific polymorphisms may be useful for risk stratifying patients, and indeed polymorphisms in complement C4 [58] but not C3 [59] have been shown to influence renal allograft outcome. In addition, complement concentration is potentiated in response to local inflammation. Renal epithelium, macrophages, cardiocytes and vascular endothelium [42] are sources of extrahepatic complement production during episodes of rejection. As lowly lytic antibodies have enhanced activity when complement is elevated, and IgG4 activates complement when antigen density and complement levels are increased [28], patients with minimal complement-fixing DSA may have a higher degree of damage during rejection episodes, when complement and antigen are more abundant.
Measuring DSA induced complement activation
Complement activation by DSA is a highly dynamic process responsible for mediating damage to the allograft, therefore clinical assays which discern the complement fixing potential of DSA are in high demand. The lymphocytotoxicity crossmatch (CDC-XM) assay developed by McClelland and Terasaki [60] was established for highly sensitive detection of DSA to recipient HLA. Although this assay utilizes complement fixation as a readout, it is not fully reflective of potential physiological capacity of DSA to activate human complement, due to the use of rabbit serum as a source of complement. It should also be noted that human IgG2 is highly effective at activation of rabbit complement [61]; consequently DSA subclass and CDC-XM results may not always correlate.
Development of high-throughput single antigen bead-based assays has been an important tool for risk stratifying patients with complement fixing DSA [45, 62, 63]. Specifically, the C1q assay measures HLA-Ab that bind C1q, and although informative, this assay only recognizes binding, not physiological complement activation [62]. Recently, a new assay measuring DSA-induced complement deposition (C3d) reported C3d+ DSA were significant predictors of allograft loss [64]. Collectively, these in vitro diagnostics attempt to measure the pathogenicity of HLA antibodies with regard to their complement fixing potential. However, results differ regarding the predictive value of detecting complement fixing HLA-Ab in vitro with respect to clinical outcomes [39, 45, 49, 62, 65–67], and new diagnostic criteria for AMR include rejection without histological evidence of complement activation (C4d deposition) [17, 20, 68]
HLA antibodies and FcγRs
A nearly universal histological feature of AMR is the infiltration of CD68+ macrophages in the microvascular and perivascular spaces of heart and renal allografts [17, 19, 69, 70] and neutrophils in lung transplants [22], which is predictive of worse outcome [69]. In addition, gene expression profiling studies have uncovered a natural killer (NK) cell signature during AMR [2, 71, 72], results which were paralleled by experimental animal models of AMR implicating NK cells in chronic antibody-mediated rejection [73]. Monocytes, macrophages, neutrophils, and NK cells express receptors for the Fc region of antibodies (Table 1, [74]), and FcγRs mediate innate immune cell functions such as leukocyte recruitment, cytotoxicity, and phagocytosis which are highly relevant to the etiology of AMR (Figure 3).
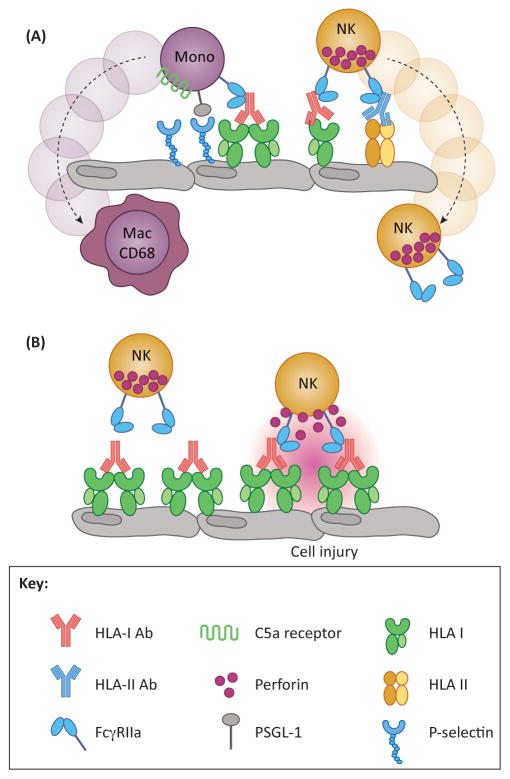
Figure 3. Fc-mediated functions which contribute to graft injury.
(A) Increased leukocytic infiltrate is a hallmark feature of AMR, and this occurrence is mediated by the Fc region of donor specific antibodies (DSA). Upon DSA binding to HLA, DSA-Fc are recognized by FcγR expressed on myeloid and NK cells. Additionally, monocytes are also able to interact with the endothelium through HLA-induced P-selectin to enhance tethering and extravasation. (B) An important feature of FcγR is their role in antibody-dependent cell-mediated cytotoxicity (ADCC). DSA bind to HLA on the surface of the endothelium, facilitating Fc interaction with FcγR expressed by myeloid and NK cells. This can lead to perforin-mediated lysis of target cells, in this case, endothelium, resulting in damage to the allograft.
FcγR families and alleles
FcγR families and alleles have distinct subclass specificities and divergent activities (Table 1) [33, 75]. Moreover, functional polymorphic variants of FcγRIIa (H131R), FcγRIIIa (F158V) and FcγRIIIb (NA1/NA2 alleles) are associated with differential phenotypes in response to antibody-based anti-tumor therapeutics, susceptibility to infection, and risk of autoimmune disease [76]. In the context of transplantation, the low affinity FcγRIIa-R131 allele was associated with increased risk of acute T cell-mediated rejection (TCMR) [77], but this is likely reflective of reduced responsiveness to antibody-based leukocyte depletion induction regimens rather than predisposition to rejection per se. However, the effect of transplant recipient FcγR polymorphism on risk of AMR has not yet been studied, and warrants investigation.
As with IgG subclasses, the human FcγR system is quite dissimilar from the murine system, complicating study of FcγRs in vivo and confounding translation of experimental results in murine models of AMR to the human setting. A recently described novel transgenic mouse carrying the full repertoire of human FcγRs [78] may enable future studies. Several important caveats, however, including cross-reactivity of human FcγRs with endogenous murine IgG and representation of only one FcγR genotype, may limit findings [33].
FcγR functions relevant to graft injury
FcγRs on monocytes, macrophages, and NK cells facilitate antibody-dependent cell-mediated cytotoxicity (ADCC). While HLA-Ab trigger NK cell degranulation and cytotoxicity against allogeneic target cells in vitro [79], and macrophages also perform ADCC, currently there is no direct evidence that these cells cause cytotoxicity in the graft. However, murine models of chronic AMR have revealed a novel role for NK cells in MHC antibody-induced transplant vasculopathy [73], through undefined FcγR-dependent mechanisms. An elegant study imaging the trafficking of recipient immune cells into murine cardiac allografts revealed elevated phagocytic activity during rejection, mediated by recipient macrophages [80]. HLA-Ab may provoke antibody-dependent cellular phagocytosis (ADCP) by macrophages and neutrophils, contributing to enhanced presentation of alloantigen to T cells, but the pathophysiological relevance of phagocytosis during rejection remains to be explored.
Finally, FcγRs are involved in capture of leukocytes by immune complexes and monomeric anti-endothelial cell antibodies, and enhanced trafficking of neutrophils to inflamed endothelium in autoimmune settings [81]. It is notable that there was a prerequisite for TNFα activation of endothelium, as deposition of antibody on resting cells did not cause efficient neutrophil adhesion. Moreover, concurrent expression of chemokines was required for efficient neutrophil adhesion to endothelial cells coated with monomeric IgG but not with immune complexes. It was recently demonstrated that monocyte recruitment to HLA-Ab activated endothelium was augmented by interaction of monocyte FcγRs with the Fc portion of HLA-Ab [82, 83]. This interaction was subclass-dependent, influenced by monocyte FcγRIIa allelic variants, and was abrogated by enzymatic modulation of antibody Fc regions using EndoS or IdeS [83]. In contrast to reports using murine anti-endothelial cell antibodies [81], efficient recruitment was observed by using HLA-Ab without preactivating endothelial cells with inflammatory cytokines, and it has been hypothesized that HLA-Ab are unique in their capacity to trigger direct endothelial activation and expression of selectins as well as stimulate FcγRs [82]. Interestingly, monocytes from donors who expressed the high affinity FcγRIIa-H131 allele exhibited significantly greater FcγR-dependent adhesion to EC activated with HLA-Ab of both IgG1 and IgG2 subclasses, compared with monocytes expressing only FcγRIIa-R131. These results suggest that transplant recipients carrying high affinity FcγR alleles may experience exacerbated leukocyte infiltration in response to HLA-Ab, predisposing them to AMR.
HLA antibodies and glycosylation
Patterns of antibody glycosylation strongly influence affinity of FcγRs [84]. The bulk of evidence comes from the fields of tumor immunology and recombinant therapeutic antibodies, through glycoengineering of antibodies to alter ADCC and CDC properties. In addition, several studies have correlated the degree of IgG-Fc glycosylation with the severity of antibody-mediated disease [85]. A common theme appears: antibodies with agalactosylated Fc-glycans are more pro-inflammatory than those containing glycans with terminal galactosylation or sialic acid. As properties of glycosylation moieties modulate the inflammatory nature of IgG, the Fc-glycan may participate in determining the degree of pathogenicity of DSA in regards to AMR.
The conserved yet highly heterogenous N297 glycan present on Fc of all IgG [27, 86] contains a biantennary core heptasaccharide that is further modified by addition of fucose (over 90% of IgG), galactose, and sialic acid to further diversify the IgG glycoform pool. Various changes to this structure can completely alter the function of IgG in regards to both FcγR and complement dependent activities (thoroughly reviewed elsewhere [27, 87]). In brief, removal of fucose increases ADCC whereas removal of galactose residues reduces ADCC mediated by FcγRIIIa and complement-dependent cytotoxicity (CDC). Interestingly, sialic acid has been identified as the mediator of anti-inflammatory properties of intravenous immunoglobulin (IVIg) [84], a common modality used in treating AMR. Whereas all sialic acid linkages contribute to decreased ADCC, the alpha-2,6 version is responsible for the anti-inflammatory effects of sialylated IgG, through direct binding of SIGN-R1/DC-SIGN, causing upregulation of inhibitory FcγR. Although there is minimal literature regarding differential glycosylation patterns of DSA, one would be remiss to disregard the potential role of DSA glycan heterogeneity during the course of AMR.
Regulation of IgG glycosylation
Given that both complement activation and FcγR engagement are key effector functions of HLA antibodies in causing allograft injury, the Fc region of antibody is a potential therapeutic target. The gram-positive bacterium Streptococcus pyogenes expresses a battery of immunomodulatory enzymes that aid in its pathogenicity, two of which have shown promise in preclinical autoimmune models through specific actions on IgG [88]. The peptidase IdeS cleaves off the Fc fragment of human IgG, generating an F(ab′)2 fragment, while the endoglycosidase EndoS hydrolyzes the N297-linked Fc glycan. Both ameliorate inflammation, complement activation and FcγR-dependent leukocyte recruitment in several experimental models, and treatment of HLA-Ab with either EndoS or IdeS dramatically reduced recruitment of monocytes to EC [83]. Clinical trials are currently underway testing the efficacy of IdeS in sensitized kidney transplant recipients (NCT02224820).
Glycan analysis of antibodies produced during inflammation in response to pathogens or autoimmune disease have shown an increased proportion of agalactosylated IgG. Moreover, IgG from active immune responses have altered glycan profiles which differ from normal serum IgG [89, 90]. The mechanism by which antibodies are glycosylated during immune responses is not well understood, although distinct glycan profiles from individual patients suggest differential glycosylation by unique B cell subsets [91, 92]. This indicates that the ability to regulate levels of glycosylation relies on B cell intrinsic factors, and would be subject to the immune milieu. In this regard, B cells presented with T-dependent antigens under proinflammatory conditions produced antibody which lacked galactose (proinflammatory) [89, 93], whereas antibodies produced in response to T-dependent antigens but under tolerogenic settings were heavily sialylated (anti-inflammatory) [89]. Finally, in the context of T-independent antigens, no matter the inflammatory surroundings, IgG were sialylated and immunosuppressive [93].
This comprehensive understanding of Fc-glycan contribution to immune function of IgG, and circumstances modulating the production of these glycosylated antibodies allows for conjecture regarding the pathogenic potential of DSA. One could surmise acute rejection episodes increase levels of agalactosylated DSA, which would incur damage to the graft through both complement and FcγR pathways, whereas DSA present in accommodated grafts may be heavily sialylated and somewhat tolerogenic. Future work detailing glycan profiles of DSA would determine if antibody glycosylation status correlates with severity of AMR. Additionally, new methodology described to simultaneously measure both the subclass and glycosylation of antigen-specific IgG [94] may be adapted to transplantation.
HLA antibodies and endothelial activation and regulation of immunogenicity
There has been resurgence in the appreciation of EC as important regulators of the immune response. EC can undergo acute (Type I) and chronic (Type II) activation, leading to expression of chemokines and adhesion molecules and recruitment of leukocytes to sites of inflammation [95]. Past work showed that crosslinking of HLA by antibodies triggers intracellular signaling through focal adhesion kinase (FAK), Akt, mammalian target of rapamycin (mTOR), S6 kinase (S6K), S6 ribosomal protein (S6RP) and extracellular regulated kinase (ERK1/2) in endothelial and smooth muscle cells leading to dynamic cytoskeletal reorganization, proliferation, migration and survival [96]. Multiple groups recently confirmed the activation of these signaling pathways in biopsies from cardiac allografts undergoing AMR [97, 98]. Importantly, the agonistic signaling capacity is an observed property of all HLA-Ab requiring the bivalent F(ab’)2 region of IgG, and does not appear to depend upon subclass, complement or FcγRs. Alternatively, complement activation, antigen expression, epitope density and antibody affinity will all significantly impact binding to and crosslinking of HLA on EC, in turn affecting intracellular signaling.
Recent studies demonstrated HLA class I signaling triggers Type I EC activation, resulting in a rapid increase of cell surface P-selectin and adhesion of neutrophils and monocytes to endothelium [99, 100]. Exocytosed von Willebrand Factor (vWF) and P-selectin also facilitated capture and activation of platelets, which aggregate in the microvasculature and support tethering of monocytes [101]. Platelets express FcγRIIa [102]; therefore additional mechanisms of FcγR-dependent platelet adhesion cannot be excluded. HLA crosslinking also activated transcription factors CREB and non-canonical NF-κB, resulting in increased protein expression of late phase adhesion molecules, cytokines, chemokines [56, 103], consistent with Type II EC activation.
An expanding paradigm of vascular endothelium in directly stimulating adaptive immune responses has garnered attention [104, 105]. HLA class II-expressing ECs trigger allogeneic CD4 T cell proliferation and promote generation of Th17 and Treg subsets [106, 107]. Interestingly, rapamycin treatment of ECs resulted in selective expansion of Tregs via PD-L1 and PD-L2 [107], pointing to a role for mTOR in regulation of endothelial alloimmunogenicity through modulation of costimulatory molecule expression. mTOR inhibitors sirolimus and everolimus also prevent HLA I antibody-induced endothelial migration and proliferation [108], suggesting that rapalogues may be beneficial in preventing multiple manifestations of graft injury by HLA-Ab. A recent study showed HLA-Ab increased expression of proinflammatory cytokines and activation of noncanonical NFκB [56], indicating that HLA-Ab modulate endothelial immunogenicity and antigen presentation to T cells.
Inflammatory loops and interplay between antibody functions
Concurrent processes of EC activation, classical complement activation, and FcγR-dependent immune cell functions are likely to independently and cumulatively promote graft inflammation during AMR. Crosstalk between FcγR and complement adds another level of complexity to IgG modulation of the immune response [109]. Abrogation of either Fc/FcγR or C5a/C5aR signaling abolished inflammation induced by immune complexes (IC); and it is known that both are necessary for robust immune responses. C5a acts directly on macrophages, simultaneously upregulating activating FcγR and downregulating inhibitory FcγR [110, 111]. Additionally, IC binding to macrophages through FcγRIII induced C5a synthesis [112]. Furthermore, binding of C5a to Kupffer cells triggered increased expression of activating FcγR, which bound IC, thereby stimulating C5a production and creating a proinflammatory loop [113]. This cycle could potentially translate to exacerbated AMR-associated pathophysiology. Local activation of complement in the graft by DSA can activate macrophages, and increase FcγR expression, which may bind sequestered DSA-IC, thereby augmenting local C5a production (Figure 4A). In addition to direct effects of complement on macrophages, anaphylatoxins and MAC complex enhance EC activation. Endothelial NFκB signaling and inflammatory gene expression induced by DSA binding was augmented in the presence of sublytic MAC, and increased T cell stimulation [56]. These findings demonstrate an additional mechanism of synergy between complement and HLA-Ab on endothelial activation (Figure 4B). As C5a is a potent mediator of leukocyte recruitment, as well as a novel modulator of T cell alloimmunity [114], this DSA-induced inflammatory loop could exacerbate damage during episodes of AMR.
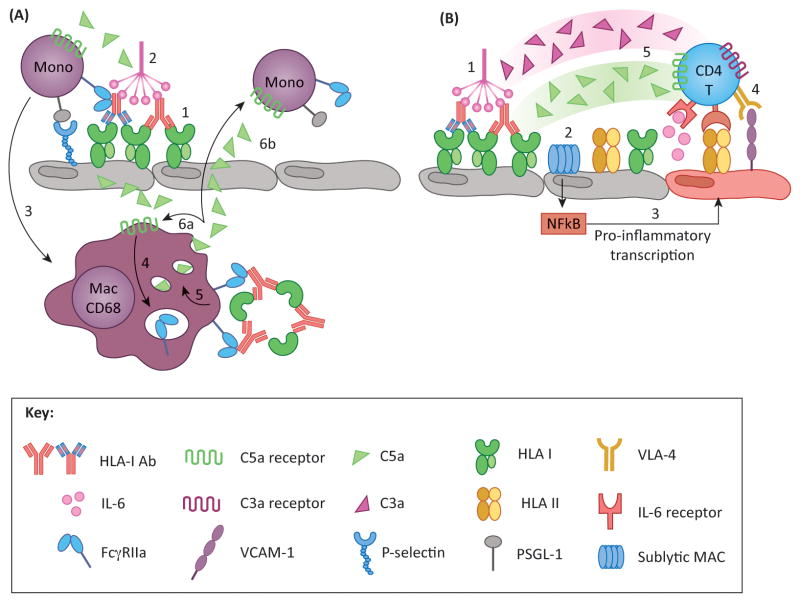
Figure 4. Proposed models of DSA-induced inflammatory loops.
(A) Macrophages perpetuate activation and recruitment via complement and FcγR pathways. DSA crosslinking of HLA on endothelium results in P-selectin mobilization to the cell surface, and provides a binding platform for C1q (1). Classical complement activation produces C5a (2), which has two functions: (i) C5a recruits monocytes to activated EC, which tether to P-selectin via PSGL-1, promoting graft infiltration and differentiation into macrophages (3); and (ii) C5a may act on intragraft CD68+ macrophages and induce FcγR expression (4). These cells can recognize immune complexes (IC) via FcγR, which can upregulate C5a production (5). Newly synthesized C5a may signal in either an autocrine (6a) or paracrine (6b) fashion, mediating further activation of intragraft macrophages and recruitment and activation of monocytes from the periphery, respectively. (B) Recent studies have identified a novel role for endothelial cells and complement in antigen presentation and stimulation of allogeneic T cells. Under inflammatory conditions (such as IFNγ activation) endothelial cells express HLA class II as well as ICAM-1, VCAM-1 and IL-6, molecules that are critical for promoting allo CD4 T cell proliferation (4) and differentiation into Th17 and Treg subsets. Preliminary work has shown that HLA antibodies modulate endothelial alloimmunogenicity through activation of the classical complement pathway (1) resulting in deposition of sublytic MAC (2). MAC triggers non-canonical NFκB signaling leading to inflammatory gene expression (3) and stimulation of allogeneic CD4 T cells (4). T cells also express receptors for complement split products C3a and C5a, which provide costimulatory signals and augment T cell proliferation. Therefore, it is likely that the presence of these anaphylatoxins at the endothelial-T cell interface might enhance T cell alloimmunity (5).
Concluding remarks and future perspectives
In summary, graft injury results from the pleiotropic function of antibodies (Figure 5), both through canonical Fc-mediated effector functions as well as novel agonistic actions on HLA molecules. The collective action of antibodies on donor vascular cells, including complement activation, FcγR-dependent macrophage and NK cell functions, and EC activation, likely synergize to cause damage to the allograft. Features such as antibody subclass, Fc glycosylation and FcγR polymorphisms may be key determinants of HLA-Ab pathogenicity and recipient risk of AMR. Therefore, characterization of both patient DSA and immune repertoire provides a foundation for individualized medicine, as well as possible guidelines for risk stratification of transplant patients. Highly tailored and specific immunotherapies could be used in the transplant field to modulate patient immune responses according to the details of the patient immune repertoire. Further experimental dissection of alloimmunity variables (Box 1) will guide future practice in allocation/antigen avoidance, management in sensitized patients, and development of new drugs to prevent and treat AMR.
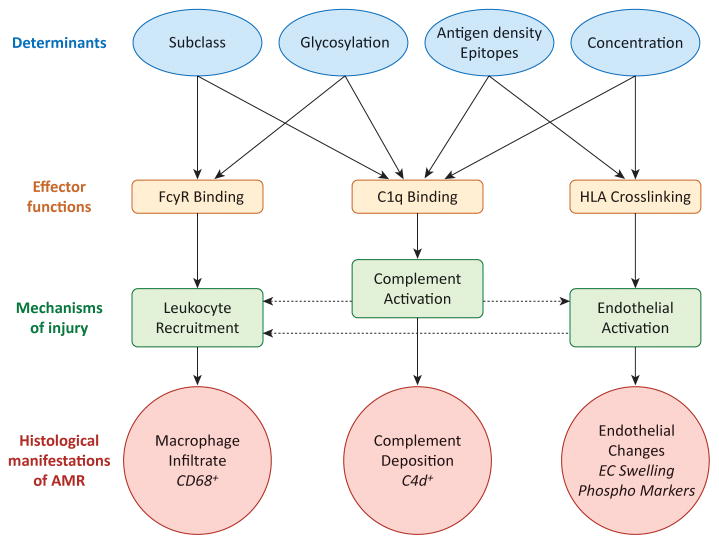
Figure 5. Mechanisms of DSA in graft pathogenesis.
Features of antibody-antigen and antibody-effector system interactions that influence pathogenic functions and mechanisms of injury are shown. Variable factors regulating antibody-antigen interactions (blue ovals) directly influence the capacity of an antibody to trigger effector functions (green boxes), and mechanisms causing graft injury (purple boxes), which ultimately manifest in the graft as common histological features (red bursts). Linear effects are indicated by solid arrows.
The functional endpoints of antibody-mediated injury are interrelated (with potential inflammatory loops indicated by dashed arrows), and likely synergize to cause maximal inflammation during AMR. For example, direct endothelial cell activation by HLA antibodies triggers adhesion of leukocytes, which can be enhanced when those leukocytes bind antibody through FcγRs. Activation of complement at the endothelial cell surface may cause production of anaphylatoxins C3a and C5a, which can act on leukocytes as chemoattractants, or enhance endothelial activation.
Box 1. Outstanding Questions.
-
Which mechanisms of HLA-Ab are critical for rejection and graft injury, and how do these mechanisms vary depending on antibody characteristics?
What are the effector functions of NK cells during AMR? Do ADCC and ADCP play a mechanistic role in AMR?
-
Can we reliably define the HLA-Ab repertoire, including specificity, glycosylation, complement fixing capacity and subclass distribution, of transplant patients?
In particular, do current in vitro assays of complement detection reliably predict whether HLA-Ab will cause complement-mediated injury?
-
Are some patients predisposed to experience rejection in the presence of antibodies?
Does the glycan profile of the DSA or recipient FcγR genotype influence transplant outcome?
Should patients be treated when they have donor specific antibodies, yet no evidence of graft dysfunction?
What is the significance of C4d-negative AMR? Does it represent complement-independent graft injury by non-complement fixing antibodies, or is it capturing AMR after complement is no longer active?
Highlights.
Antibody-mediated rejection is a major challenge to solid organ transplantation.
Complement, endothelial and FcγR mechanisms synergize to exacerbate inflammation.
A variety of antibody characteristics influence Fc-dependent effector functions.
Acknowledgments
This work was supported by the Ruth L Kirschstein National Research Service Award T32HL69766 (to KAT), by the National Institute of Allergy and Infectious Diseases Grant RO1 AI 042819 (to EFR). The authors would like to thank A. Roux and M. Rossetti for critical review of this manuscript.
Glossary
- Acute rejection
commonly refers to rejection that arises rapidly and causes graft function within days to weeks, often occurring in the early post-transplant period (less than one year); may be mediated primarily by T cells (called T cell-mediated rejection, TCMR, or acute cellular rejection, ACR) or primarily by antibodies (called humoral or antibody-mediated rejection, AMR); can often be reversed with aggressive treatment
- Allograft
transplanted cells or solid organ from a genetically disparate member of the same species
- Alloimmunity
adaptive immune responses against non-self cells or tissue from members of the same species as a result of polymorphisms in proteins that are then recognized as foreign antigens
- Chronic rejection
also called transplant allograft vasculopathy (TAV), transplant arteriopathy or arteriosclerosis (TA) in cardiac allograft, transplant glomerulopathy (TG) in renal allograft, bronchiolitis obliterans syndrome (BOS) in lung allograft, and vanishing bile duct syndrome in liver allograft; progressive and irreversible fibrosis and occlusion of the donor vasculature; distinct from native atherosclerosis in that it is concentric rather than focal and affects only the vessels of the allograft; thought to result from repair mechanisms in response to successive insults or indolent, ongoing injury from antibodies and/or CD4 T cells; manifests as an expanded subendothelial layer, consisting of endothelial cells and smooth muscle cells which have migrated and proliferated in the neointima, as well as CD4 T cells and macrophages
- Classical complement pathway
a system of proteases which consecutively cleave downstream components to generate catalytically active or inflammatory and cytolytic products; the classical pathway is activated by immunoglobulin (Ig), and initiated by binding of C1 complex to the Fc region of IgM or IgG
- Donor specific HLA antibodies (DSA)
antibodies directed against polymorphic HLA molecules expressed by donor tissue
- Fc receptors
receptors for the crystallizable fragment (Fc) of immunoglobulin, expressed by myeloid and some lymphoid cells; link the innate immune system with adaptive immunity; binding to complexed or immobilized antibody triggers intracellular signaling leading to activation and inflammatory effector functions
- Human leukocyte antigen (HLA)
genes encoded by the major histocompatibility complex; these proteins function in antigen presentation of peptides to T cells and are the most polymorphic loci in the human genome
- Transplant rejection
alloimmune response of the recipient against transplanted donor cells, tissues or organs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta G, et al. Late antibody-mediated rejection in renal allografts: outcome after conventional and novel therapies. Transplantation. 2014;97:1240–1246. doi: 10.1097/01.TP.0000442503.85766.91. [DOI] [PubMed] [Google Scholar]
- 2.Sellares J, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:971–983. doi: 10.1111/ajt.12150. [DOI] [PubMed] [Google Scholar]
- 3.Sis B, et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2312–2323. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 4.Wu GW, et al. Asymptomatic antibody-mediated rejection after heart transplantation predicts poor outcomes. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28:417–422. doi: 10.1016/j.healun.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kfoury AG, et al. A longitudinal study of the course of asymptomatic antibody-mediated rejection in heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31:46–51. doi: 10.1016/j.healun.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Loupy A, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2561–2570. doi: 10.1111/j.1600-6143.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 7.Robinson J, et al. The IMGT/HLA database. Nucleic Acids Res. 2011;39:D1171–1176. doi: 10.1093/nar/gkq998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Clippel D, et al. Screening for HLA antibodies in plateletpheresis donors with a history of transfusion or pregnancy. Transfusion. 2014 doi: 10.1111/trf.12727. [DOI] [PubMed] [Google Scholar]
- 9.Kakaiya RM, et al. Prevalence of HLA antibodies in remotely transfused or alloexposed volunteer blood donors. Transfusion. 2010;50:1328–1334. doi: 10.1111/j.1537-2995.2009.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everly MJ, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95:410–417. doi: 10.1097/TP.0b013e31827d62e3. [DOI] [PubMed] [Google Scholar]
- 11.Kaneku H, et al. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:1541–1548. doi: 10.1111/ajt.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiebe C, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 13.Halloran PF, et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. Journal of the American Society of Nephrology : JASN. 2014 doi: 10.1681/ASN.2014060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank R, et al. Circulating donor-specific anti-human leukocyte antigen antibodies and complement c4d deposition are associated with the development of cardiac allograft vasculopathy. Am J Clin Pathol. 2014;142:809–815. doi: 10.1309/ajcptlbeu5bq8shn. [DOI] [PubMed] [Google Scholar]
- 15.Torres IB, et al. Comparing transplant glomerulopathy in the absence of C4d deposition and donor-specific antibodies to chronic antibody-mediated rejection. Clinical transplantation. 2014;28:1148–1154. doi: 10.1111/ctr.12433. [DOI] [PubMed] [Google Scholar]
- 16.Haas M, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 17.Berry GJ, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. The Journal of Heart and Lung Transplantation. 2013;32:1147–1162. doi: 10.1016/j.healun.2013.08.011. http://dx.doi.org/10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Drachenberg CB, et al. Guidelines for the diagnosis of antibody-mediated rejection in pancreas allografts-updated Banff grading schema. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1792–1802. doi: 10.1111/j.1600-6143.2011.03670.x. [DOI] [PubMed] [Google Scholar]
- 19.Fishbein GA, Fishbein MC. Morphologic and immunohistochemical findings in antibody-mediated rejection of the cardiac allograft. Hum Immunol. 2012;73:1213–1217. doi: 10.1016/j.humimm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Haas M. Pathology of C4d-negative antibody-mediated rejection in renal allografts. Current opinion in organ transplantation. 2013;18:319–326. doi: 10.1097/MOT.0b013e32835d4daf. [DOI] [PubMed] [Google Scholar]
- 21.O’Leary JG, et al. Acute liver allograft antibody-mediated rejection: an inter-institutional study of significant histopathological features. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2014;20:1244–1255. doi: 10.1002/lt.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeNicola MM, et al. Pathologic findings in lung allografts with anti-HLA antibodies. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:326–332. doi: 10.1016/j.healun.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farmer DG, et al. Pretransplant predictors of survival after intestinal transplantation: analysis of a single-center experience of more than 100 transplants. Transplantation. 2010;90:1574–1580. doi: 10.1097/TP.0b013e31820000a1. [DOI] [PubMed] [Google Scholar]
- 24.Lefaucheur C, et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381:313–319. doi: 10.1016/s0140-6736(12)61265-3. [DOI] [PubMed] [Google Scholar]
- 25.Tavora F, et al. Endothelitis in cardiac allograft biopsy specimens: possible relationship to antibody-mediated rejection. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:435–444. doi: 10.1016/j.healun.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Halloran PF, et al. Microarray diagnosis of antibody-mediated rejection in kidney transplant biopsies: an international prospective study (INTERCOM) American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:2865–2874. doi: 10.1111/ajt.12465. [DOI] [PubMed] [Google Scholar]
- 27.Vidarsson G, et al. IgG subclasses and allotypes: from structure to effector functions. Frontiers in immunology. 2014;5 doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9–19. doi: 10.1046/j.0019-2805.2001.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucisano Valim YM, Lachmann PJ. The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes: a systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clinical and experimental immunology. 1991;84:1–8. doi: 10.1111/j.1365-2249.1991.tb08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata K, Baldwin WM., 3rd Mechanisms of complement activation, C4d deposition, and their contribution to the pathogenesis of antibody-mediated rejection. Transplant Rev (Orlando) 2009;23:139–150. doi: 10.1016/j.trre.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murata K, et al. Synergistic deposition of C4d by complement-activating and non-activating antibodies in cardiac transplants. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7:2605–2614. doi: 10.1111/j.1600-6143.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 32.Rahimi S, et al. Non-complement- and complement-activating antibodies synergize to cause rejection of cardiac allografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:326–334. doi: 10.1111/j.1600-6143.2004.00334.x. [DOI] [PubMed] [Google Scholar]
- 33.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 34.Cicciarelli JC, et al. Immunoglobulin G subclass analysis of HLA donor specific antibodies in heart and renal transplant recipients. Clinical transplants. 2013:413–422. [PubMed] [Google Scholar]
- 35.Kaneku H, et al. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18:984–992. doi: 10.1002/lt.23451. [DOI] [PubMed] [Google Scholar]
- 36.Kushihata F, et al. Human leukocyte antigen antibodies and human complement activation: role of IgG subclass, specificity, and cytotoxic potential. Transplantation. 2004;78:995–1001. doi: 10.1097/01.TP.0000136966.63957.E2. [DOI] [PubMed] [Google Scholar]
- 37.Schaub S, et al. Determinants of C1q binding in the single antigen bead assay. Transplantation. 2014;98:387–393. doi: 10.1097/TP.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 38.Everly MJ, et al. Impact of IgM and IgG3 anti-HLA alloantibodies in primary renal allograft recipients. Transplantation. 2014;97:494–501. doi: 10.1097/01.TP.0000441362.11232.48. [DOI] [PubMed] [Google Scholar]
- 39.Honger G, et al. C4d-fixing capability of low-level donor-specific HLA antibodies is not predictive for early antibody-mediated rejection. Transplantation. 2010;89:1471–1475. doi: 10.1097/TP.0b013e3181dc13e7. [DOI] [PubMed] [Google Scholar]
- 40.Wasowska BA, et al. New concepts of complement in allorecognition and graft rejection. Cell Immunol. 2007;248:18–30. doi: 10.1016/j.cellimm.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirohashi T, et al. Complement independent antibody-mediated endarteritis and transplant arteriopathy in mice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:510–517. doi: 10.1111/j.1600-6143.2009.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamer R, et al. Human leukocyte antigen-specific antibodies and gamma-interferon stimulate human microvascular and glomerular endothelial cells to produce complement factor C4. Transplantation. 2012;93:867–873. doi: 10.1097/TP.0b013e31824b3762. [DOI] [PubMed] [Google Scholar]
- 43.Orandi BJ, et al. Eculizumab and Splenectomy as Salvage Therapy for Severe Antibody-Mediated Rejection After HLA-Incompatible Kidney Transplantation. Transplantation. 2014;98:857–863. doi: 10.1097/TP.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 44.Stegall MD, et al. The role of complement in antibody-mediated rejection in kidney transplantation. Nature reviews Nephrology. 2012;8:670–678. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]
- 45.Chin C, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:158–163. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 46.Piazza A, et al. Post-transplant development of C1q-positive HLA antibodies and kidney graft survival. Clinical transplants. 2013:367–375. [PubMed] [Google Scholar]
- 47.Sutherland SM, et al. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatric transplantation. 2012;16:12–17. doi: 10.1111/j.1399-3046.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence C, et al. Preformed complement-activating low-level donor-specific antibody predicts early antibody-mediated rejection in renal allografts. Transplantation. 2013;95:341–346. doi: 10.1097/TP.0b013e3182743cfa. [DOI] [PubMed] [Google Scholar]
- 49.Hönger G, et al. Pretransplant IgG subclasses of donor-specific human leukocyte antigen antibodies and development of antibody-mediated rejection. Transplantation. 2011;92:41–47. doi: 10.1097/TP.0b013e31821cdf0d. [DOI] [PubMed] [Google Scholar]
- 50.Ehrnthaller C, et al. New insights of an old defense system: structure, function, and clinical relevance of the complement system. Mol Med. 2011;17:317–329. doi: 10.2119/molmed.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diebolder CA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nature reviews Drug discovery. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 53.Muczynski KA, et al. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: characterization, isolation, and regulation of MHC class II expression. Journal of the American Society of Nephrology : JASN. 2003;14:1336–1348. doi: 10.1097/01.asn.0000061778.08085.9f. [DOI] [PubMed] [Google Scholar]
- 54.Mannam VK, et al. The fate of renal allografts hinges on responses of the microvascular endothelium. Experimental and molecular pathology. 2013;94:398–411. doi: 10.1016/j.yexmp.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 55.AlMahri A, et al. Detection of complement-fixing and non-fixing antibodies specific for endothelial precursor cells and lymphocytes using flow cytometry. Tissue Antigens. 2012;80:404–415. doi: 10.1111/j.1399-0039.2012.01954.x. [DOI] [PubMed] [Google Scholar]
- 56.Jane-wit D, et al. Alloantibody and Complement Promote T Cell–Mediated Cardiac Allograft Vasculopathy Through Noncanonical Nuclear Factor-κB Signaling in Endothelial Cells. Circulation. 2013;128:2504–2516. doi: 10.1161/circulationaha.113.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkinson JP, et al. H-2 S region determined polymorphic variants of the C4, Slp, C2, and B complement proteins: a compilation. Immunogenetics. 1982;16:617–623. doi: 10.1007/BF00372032. [DOI] [PubMed] [Google Scholar]
- 58.Bay JT, et al. Low C4 gene copy numbers are associated with superior graft survival in patients transplanted with a deceased donor kidney. Kidney international. 2013;84:562–569. doi: 10.1038/ki.2013.195. [DOI] [PubMed] [Google Scholar]
- 59.Varagunam M, et al. C3 Polymorphisms and Allograft Outcome in Renal Transplantation. New England Journal of Medicine. 2009;360:874–880. doi: 10.1056/NEJMoa0801861. [DOI] [PubMed] [Google Scholar]
- 60.TERASAKI PI, MCCLELLAND JD. MICRODROPLET ASSAY OF HUMAN SERUM CYTOTOXINS. Nature. 1964;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 61.Dangl JL, et al. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. The EMBO journal. 1988;7:1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen G, et al. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Human Immunology. 2011;72:849–858. doi: 10.1016/j.humimm.2011.07.001. http://dx.doi.org/10.1016/j.humimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Loupy A, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. The New England journal of medicine. 2013;369:1215–1226. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 64.Sicard A, et al. Detection of C3d-Binding Donor-Specific Anti-HLA Antibodies at Diagnosis of Humoral Rejection Predicts Renal Graft Loss. Journal of the American Society of Nephrology : JASN. 2014 doi: 10.1681/ASN.2013101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crespo M, et al. Clinical relevance of pretransplant anti-HLA donor-specific antibodies: Does C1q-fixation matter? Transplant Immunology. 2013;29:28–33. doi: 10.1016/j.trim.2013.07.002. http://dx.doi.org/10.1016/j.trim.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Freitas MC, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95:1113–1119. doi: 10.1097/TP.0b013e3182888db6. [DOI] [PubMed] [Google Scholar]
- 67.Lachmann N, et al. Systematic comparison of four cell- and Luminex-based methods for assessment of complement-activating HLA antibodies. Transplantation. 2013;95:694–700. doi: 10.1097/TP.0b013e31827b3dc3. [DOI] [PubMed] [Google Scholar]
- 68.Haas M. An updated Banff schema for diagnosis of antibody-mediated rejection in renal allografts. Current opinion in organ transplantation. 2014;19:315–322. doi: 10.1097/mot.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 69.Xu L, et al. Increased macrophage density of cardiac allograft biopsies is associated with antibody-mediated rejection and alloantibodies to HLA antigens. Clinical transplantation. 2014;28:554–560. doi: 10.1111/ctr.12348. [DOI] [PubMed] [Google Scholar]
- 70.Papadimitriou JC, et al. Antibody-mediated allograft rejection: morphologic spectrum and serologic correlations in surveillance and for cause biopsies. Transplantation. 2013;95:128–136. doi: 10.1097/TP.0b013e3182777f28. [DOI] [PubMed] [Google Scholar]
- 71.Hidalgo LG, et al. Interpreting NK cell transcripts versus T cell transcripts in renal transplant biopsies. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:1180–1191. doi: 10.1111/j.1600-6143.2011.03970.x. [DOI] [PubMed] [Google Scholar]
- 72.Hidalgo LG, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:1812–1822. doi: 10.1111/j.1600-6143.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 73.Hirohashi T, et al. A Novel Pathway of Chronic Allograft Rejection Mediated by NK Cells and Alloantibody. American Journal of Transplantation. 2012;12:313–321. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herter S, et al. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. Journal of immunology. 2014;192:2252–2260. doi: 10.4049/jimmunol.1301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shashidharamurthy R, et al. Dynamics of the interaction of human IgG subtype immune complexes with cells expressing R and H allelic forms of a low-affinity Fc gamma receptor CD32A. Journal of immunology. 2009;183:8216–8224. doi: 10.4049/jimmunol.0902550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mellor JD, et al. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. Journal of hematology & oncology. 2013;6:1. doi: 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan FF, et al. Association of Fc gamma receptor IIA polymorphisms with acute renal-allograft rejection. Transplantation. 2004;78:766–769. doi: 10.1097/01.tp.0000132560.77496.cb. [DOI] [PubMed] [Google Scholar]
- 78.Smith P, et al. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6181–6186. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shin BH, et al. Regulation of anti-HLA antibody-dependent natural killer cell activation by immunosuppressive agents. Transplantation. 2014;97:294–300. doi: 10.1097/01.TP.0000438636.52085.50. [DOI] [PubMed] [Google Scholar]
- 80.Christen T, et al. Molecular imaging of innate immune cell function in transplant rejection. Circulation. 2009;119:1925–1932. doi: 10.1161/circulationaha.108.796888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Florey OJ, et al. Antiendothelial cell antibodies mediate enhanced leukocyte adhesion to cytokine-activated endothelial cells through a novel mechanism requiring cooperation between Fc{gamma}RIIa and CXCR1/2. Blood. 2007;109:3881–3889. doi: 10.1182/blood-2006-08-044669. [DOI] [PubMed] [Google Scholar]
- 82.Valenzuela NM, et al. HLA class I antibodies trigger increased adherence of monocytes to endothelial cells by eliciting an increase in endothelial P-selectin and, depending on subclass, by engaging FcgammaRs. Journal of immunology. 2013;190:6635–6650. doi: 10.4049/jimmunol.1201434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valenzuela NM, et al. Monocyte Recruitment by HLA IgG-activated Endothelium: The Relationship Between IgG Subclass and FcgRIIa Polymorphisms. American Journal of Transplantation. 2015 doi: 10.1111/ajt.13174. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anthony RM, Nimmerjahn F. The role of differential IgG glycosylation in the interaction of antibodies with FcγRs in vivo. Current opinion in organ transplantation. 2011;16:7–14. doi: 10.1097/MOT.0b013e328342538f. [DOI] [PubMed] [Google Scholar]
- 85.Goulabchand R, et al. Impact of autoantibody glycosylation in autoimmune diseases. Autoimmunity Reviews. 2014;13:742–750. doi: 10.1016/j.autrev.2014.02.005. http://dx.doi.org/10.1016/j.autrev.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Jefferis R, et al. Recognition sites on human IgG for Fc gamma receptors: the role of glycosylation. Immunol Lett. 1995;44:111–117. doi: 10.1016/0165-2478(94)00201-2. [DOI] [PubMed] [Google Scholar]
- 87.Lux A, Nimmerjahn F. Impact of differential glycosylation on IgG activity. Adv Exp Med Biol. 2011;780:113–124. doi: 10.1007/978-1-4419-5632-3_10. [DOI] [PubMed] [Google Scholar]
- 88.Yang R, et al. Successful treatment of experimental glomerulonephritis with IdeS and EndoS, IgG-degrading streptococcal enzymes. Nephrol Dial Transplant. 2010;25:2479–2486. doi: 10.1093/ndt/gfq115. [DOI] [PubMed] [Google Scholar]
- 89.Oefner CM, et al. Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. J Allergy Clin Immunol. 2012;129:1647–1655. e1613. doi: 10.1016/j.jaci.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 90.Scherer HU, et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62:1620–1629. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 91.Omtvedt LA, et al. Glycan analysis of monoclonal antibodies secreted in deposition disorders indicates that subsets of plasma cells differentially process IgG glycans. Arthritis Rheum. 2006;54:3433–3440. doi: 10.1002/art.22171. [DOI] [PubMed] [Google Scholar]
- 92.Wuhrer M, et al. Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J Proteome Res. 2009;8:450–456. doi: 10.1021/pr800651j. [DOI] [PubMed] [Google Scholar]
- 93.Hess C, et al. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. The Journal of clinical investigation. 2013;123:3788–3796. doi: 10.1172/jci65938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lundström SL, et al. IgG antibodies to cyclic citrullinated peptides exhibit profiles specific in terms of IgG subclasses, fc-glycans and a fab-Peptide sequence. PLoS One. 2014;9:e113924. doi: 10.1371/journal.pone.0113924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 96.Valenzuela NM, et al. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Current opinion in organ transplantation. 2014;19:33–40. doi: 10.1097/mot.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li F, et al. Antibody ligation of human leukocyte antigen class I molecules stimulates migration and proliferation of smooth muscle cells in a focal adhesion kinase-dependent manner. Hum Immunol. 2011;72:1150–1159. doi: 10.1016/j.humimm.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tible M, et al. Pathologic classification of antibody-mediated rejection correlates with donor-specific antibodies and endothelial cell activation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:769–776. doi: 10.1016/j.healun.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Valenzuela NM, et al. Blockade of p-selectin is sufficient to reduce MHC I antibody-elicited monocyte recruitment in vitro and in vivo. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:299–311. doi: 10.1111/ajt.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamakuchi M, et al. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1301–1306. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuo HH, et al. Platelets in Early Antibody-Mediated Rejection of Renal Transplants. Journal of the American Society of Nephrology : JASN. 2014 doi: 10.1681/ASN.2013121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.López JA. The platelet Fc receptor: a new role for an old actor. Blood. 2013;121:1674–1675. doi: 10.1182/blood-2013-01-475970. [DOI] [PubMed] [Google Scholar]
- 103.Naemi FM, et al. Anti-donor HLA class I antibodies: pathways to endothelial cell activation and cell-mediated allograft rejection. Transplantation. 2013;96:258–266. doi: 10.1097/TP.0b013e3182985504. [DOI] [PubMed] [Google Scholar]
- 104.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cravedi P, et al. Complement regulation of T-cell alloimmunity. Semin Nephrol. 2013;33:565–574. doi: 10.1016/j.semnephrol.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taflin C, et al. Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2891–2896. doi: 10.1073/pnas.1011811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang C, et al. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. The Journal of clinical investigation. 2013;123:1677–1693. doi: 10.1172/JCI66204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin YP, et al. Everolimus Inhibits Anti-HLA I Antibody-Mediated Endothelial Cell Signaling, Migration and Proliferation More Potently Than Sirolimus. American Journal of Transplantation. 2014;14:806–819. doi: 10.1111/ajt.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karsten CM, Kohl J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology. 2012;217:1067–1079. doi: 10.1016/j.imbio.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 110.Shushakova N, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest. 2002;110:1823–1830. doi: 10.1172/JCI16577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Konrad S, et al. Phosphoinositide 3-kinases gamma and delta, linkers of coordinate C5a receptor-Fcgamma receptor activation and immune complex-induced inflammation. J Biol Chem. 2008;283:33296–33303. doi: 10.1074/jbc.M804617200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skokowa J, et al. Macrophages induce the inflammatory response in the pulmonary Arthus reaction through G alpha i2 activation that controls C5aR and Fc receptor cooperation. J Immunol. 2005;174:3041–3050. doi: 10.4049/jimmunol.174.5.3041. [DOI] [PubMed] [Google Scholar]
- 113.Kumar V, et al. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116:512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cravedi P, et al. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:2530–2539. doi: 10.1111/ajt.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]