Abstract
Sigma-1 receptor (Sig-1R) has been proposed as a novel therapeutic target for drug and alcohol addiction. We have shown previously that Sig-1R agonists facilitate the reinforcing effects of ethanol and induce binge-like drinking, while Sig-1R antagonists block excessive drinking in both genetic and environmental models of alcoholism, without affecting intake in outbred non-dependent rats. Even though significant progress has been made in understanding the function of Sig-1Rs in alcohol reinforcement, its role in the early and late stage of alcohol addiction remains unclear.
Administration of the selective Sig-1R antagonist BD-1063 dramatically reduced the acquisition of alcohol drinking behavior as well as the preference for alcohol in genetically selected TSRI Sardinian alcohol preferring (Scr:sP) rats; the treatment had no effect on total fluid intake, food intake or body weight gain, proving selectivity of action. Furthermore, BD-1063 dose-dependently decreased alcohol-seeking behavior in rats trained under a second-order schedule of reinforcement, in which responding is maintained by contingent presentation of a conditioned reinforcer. Finally, an innate elevation in Sig-1R protein levels was found in the nucleus accumbens of alcohol-preferring Scr:sP rats, compared to outbred Wistar rats, alteration which was normalized by chronic, voluntary alcohol drinking.
Taken together these findings demonstrate that Sig-1R blockade reduces the propensity to both acquire alcohol drinking and to seek alcohol, and point to the nucleus accumbens as a potential key region for the effects observed. Our data suggest that Sig-1R antagonists may have therapeutic potential in multiple stages of alcohol addiction.
Keywords: Ethanol, Addiction, Intake, Rat, Reinforcement, Reward
1. Introduction
Sigma-1 receptor (Sig-1R) has been object of pharmacological studies for decades. Originally categorized as an opioid receptor [1] and then mistaken for the phencyclidine (PCP) binding site on NMDA receptors [2], Sig-1R was finally recognized as a non-opioid, non-PCP unique receptor [3, 4] which was then cloned in 1996 [5–7]. More recently, Sig-1R was identified as a ligand-operated molecular chaperone located post-synaptically in the endoplasmic reticulum and on the plasma membrane through a dynamic translocation [8]. Once activated the Sig-1R receptor can modulate glutamatergic, cholinergic, noradrenergic, and dopaminergic neurotransmissions [9–13], as well as the release of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and glial cell line derived neurotrophic factor (GDNF) [14–16].
Given its wide distribution in the limbic system and the brainstem, Sig-1R has been shown to play a key role in motivation, learning and memory [17–20]. Furthermore, Sig-1R is considered among the most promising targets in addiction. Several studies have shown that Sig-1R is involved in the motivational effect of psychostimulants such as cocaine and methamphetamine. Antagonists exert a plethora of actions, including the blockade of psychostimulant-induced behavioral sensitization, conditioned place preference, and cue-induced reinstatement of seeking behavior [21–25]. Interestingly, Sig-1R also mediates the motivational effect of ethanol: Sig-1R antagonists are able to block ethanol-induced hyper-locomotion and conditioned place preference [26], and our laboratory has shown that Sig-1R antagonists reduce excessive drinking in ethanol-dependent and alcohol-preferring rats, [27–29], while agonists induce binge-like drinking [27].
While the effects of Sig-1R antagonism on the reinforcing effects of alcohol have been investigated, the role of Sig-1Rs on the acquisition of alcohol drinking as well as alcohol-seeking behavior has yet to be elucidated.
The first aim of this study was to test the effects of the Sig-1R antagonist BD-1063 on the home-cage acquisition of alcohol-drinking behavior in TSRI Sardinians alcohol-preferring rats (Scr:sP). Our line descends from the original line which was selectively bred from a Wistar stock by Prof. G.L. Gessa (University of Cagliari, Italy). This line provides a model with significant face and predictive validity for alcoholism [30–32] as these rats voluntarily drink high quantities of ethanol, respond to pharmacological treatments which are proven to work in humans, and have a heritable component similar to human ethanol dependence [33–36].
The second aim of this study was to investigate the effect of BD-1063 rats on a later stage alcohol–seeking behavior using a second order schedule of reinforcement, in which seeking behavior is maintained by a response-contingent exposure to a drug-associated stimulus [37–41].
Finally, the third aim was to evaluate innate levels of Sig-1R in the nucleus accumbens (NAcc) and the central nucleus of the amygdala (CeA) of ethanol-naïve Scr:sP and Wistar rats, two brain areas highly involved in the rewarding properties of alcohol.
2. Material and Methods
2.1. Subjects
Subjects of this study were adult male Wistar rats (Charles River, Wilmington, MA) and rats derived from the Scripps Research Institute subline of Sardinian alcohol-preferring rats (Scr:sP, 29–30th generation, http://rgd.mcw.edu/rgdweb/report/strain/main.html?id=2302666) which were then maintained for 12 generations at Boston University without further selective breeding. Scr:sP rats were generated from intra-line breeding at The Scripps Research Institute from sP rats generously provided after 32 generations of selective breeding by Prof. G.L. Gessa (University of Cagliari, Italy). Scr:sP rats were housed in a humidity- and temperature-controlled vivarium on a 12-h light–dark cycle (lights off at 9:00 am), with water and regular rodent chow available ad libitum, except otherwise specified. Experiments were conducted during the rats’ dark cycle. Different sets of rats were used for each experiment. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Principles of Laboratory Animal Care, and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee.
2.2. Drugs
Ethanol solutions for the home-cage alcohol drinking acquisition procedure (10% v/v) and for the operant alcohol seeking procedure (10% w/v) were prepared using 95% ethyl-alcohol and tap water. BD-1063 × 2 HBr salt (1-[2-(3,4-Dichlorophenyl)ethyl]-4-methylpiperazine dihydrobromide]) was synthesized according to the previously reported procedure [42]. BD-1063 was solubilized in sterile, isotonic saline and administered subcutaneously (s.c. 1 ml/kg).
2.3. Home cage alcohol drinking acquisition
Scr:sP rats (n=18) were singly-housed and habituated to two water bottles for 3 days. Rats were then divided into two groups, matched for body weight and water intake over the last three days. Rats were treated daily with BD-1063 or vehicle 15 min before the dark cycle onset, using a between-subject design. Pre-weighed ethanol and water solutions and food, withdrawn during the 15 min between drug administration and the onset of the dark cycle, were provided at the beginning of the dark cycle. Ethanol, water, food intake and body weight gain were measured 24 h from the onset of the dark cycle.
2.4. Apparatus for operant second order schedule of reinforcement
The test chambers used for operant second order schedule of reinforcement (Med Associates, Inc., St. Albans, VT) were located in sound-attenuating, ventilated environmental cubicles. Syringe pumps (Med Associates, St. Albans, VT) dispensed ethanol in one of the two stainless steel drinking cups mounted 2 cm above the grid floor in the middle of one side panel. Two retractable levers were located 3.2 cm to either side of the drinking cups. Ethanol delivery and recording of operant responses were controlled by microcomputers.
2.5. Alcohol-seeking behavior procedure
The procedure was adopted and modified from [43]. Scr:sP rats were first allowed continuous (24 h/day) two-bottle choice access to ethanol (10% w/v) and water in their home cages for 1 week. Rats were then allowed one or two overnight (16 h) operant sessions to ethanol with chow available ad libitum. Subjects were trained to press a lever to acquire 0.05 ml of 10% w/v ethanol solution under a fixed ratio 1 (FR1) schedule of reinforcement. Responses on the inactive lever resulted in no consequences, but were recorded as an index of motor activity. Lever presses on the active lever resulted in ethanol solution delivery concomitantly with illumination of a conditioned stimulus (CS) light above the active lever for 20 s (time out, TO) followed by lever retraction. The session ended when subjects reached 30 rewards or after 2 h, whichever occurred first. Subjects were then introduced to the next step of the training, a fixed interval (FI) schedule of reinforcement. The FI schedule increased daily from FI1 min to FI2, FI4, FI8, and FI10 min before stabilizing at FI15 min. After the FI schedule, a press on the active lever resulted in ethanol delivery; the volume of ethanol delivered after the FI progressively increased from 0.05 to 0.12 ml according to the following schedule: FI2 0.05 ml, FI4 0.08 ml FI 8 0.10 ml and FI10 and FI15 0.12 ml. After three consecutive completed session of FI15, subjects were moved to a second-order schedule of reinforcement. The second order schedule was divided in two trials. During the FI15 min interval, every 10th active lever press resulted in a brief CS presentation for 1 s above the active lever (FI15(FR10:S)); after the FI15 min, ten active lever presses resulted in a delivery of 0.12 ml of ethanol solution and CS presentation for 20 s. The session ended when subjects completed two trials or after 40 min, whichever occurred first. Sessions were conducted daily during the dark cycle and, at the end of the session, subjects were returned to the animal facility. When stable responding over three consecutive days was achieved (<20%), rats were administered BD-1063 15 min prior to the alcohol seeking session in a latin square design (0, 3, 10 and 30 mg/kg), allowing at least 2–3 intervening treatment-free days.
2.6. Sigma-1 receptor western blotting
For the analysis of Sig-1R protein levels, Scr:sP rats (n=14) and Wistar rats (n=7) were habituated to two water bottles. Half of the Scr:sP rats (n=7) were then allowed to drink a 10%(v/v) ethanol solution and a water solution 24 h/day for 4 consecutive weeks. Rats were anesthetized using isoflurane and rapidly decapitated 0 to 2 h before dark cycle onset. Brains were sectioned coronally (2 mm slices) in a rat brain matrix and the brain regions of interested (NAcc and CeA) were punched using stainless steel needles (Fine Science Tools, Inc., Foster City, CA) guided by a brain atlas [44]. Samples were stored at −80°C until processed for western blotting. Tissue was homogenized with a microsonicator in sodium dodecyl sulfate lysis buffer without β-mercaptoethanol. After protein determination with a BCA assay kit (Thermo Scientific, Waltham, MA), 50–70 μg of protein were boiled for 3 min in the presence of β-mercaptoethanol, separated electrophoretically, and then transferred to a PVDF membrane (Bio-Rad, Hercules, CA). Membranes were blocked in nonfat dry milk and then incubated with the following either an anti-Sig-1R rabbit polyclonal antibody (1:1,000 recognizing C-terminal 143-165aa Sig-1R or an anti-β-tubulin mouse monoclonal antibody, Santa Cruz Biotechnology, Dallas, TX) [8]. After washing, membranes were incubated with secondary antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX). The blots were then developed with the SuperSignal West Femto detection system according to the manufacturer’s instructions (Thermo Scientific, Waltham, MA) Sig-1R expression levels were calculated as percentage relative to β-tubulin expression.
2.7. Statistical analysis
Data from the acquisition experiment were analyzed using a two-way mixed design ANOVA with Treatment as between-subject factor and Time as within-subject factor. Data from the alcohol-seeking experiment and Sigma-1 receptor protein expression were analyzed using one-way ANOVAs. Pairwise post-hoc comparisons were made using the Student Newman Keuls test; student-t test was used to compare two groups. Significance was set at p<0.05. The software/graphic packages used were Systat 11.0, InStat 3.0, Statistica 7.0, and SigmaPlot 11.0.
3. RESULTS
3.1. BD-1063 blocks the ethanol home cage acquisition in Scr:sP rats
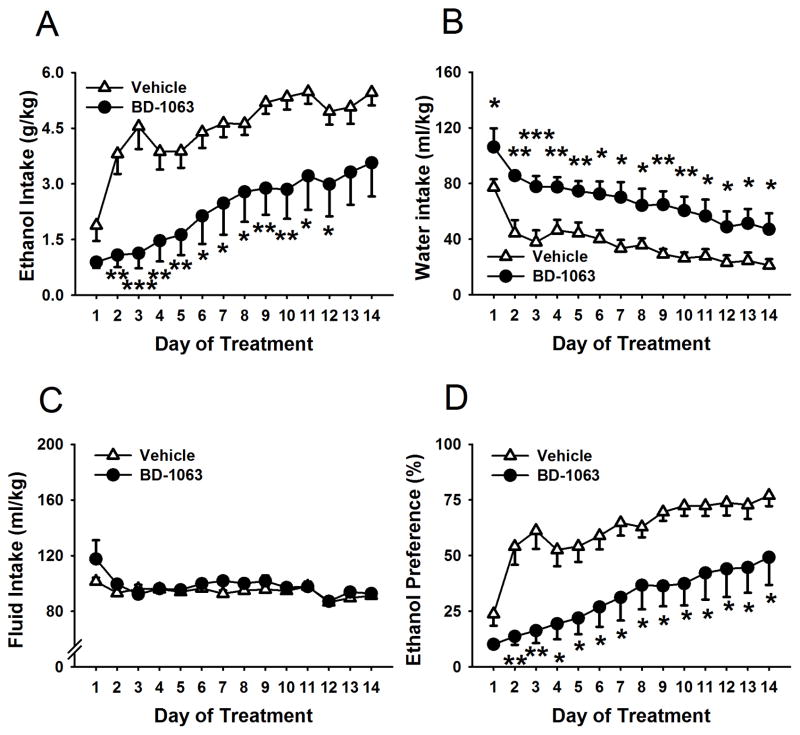
As shown in Fig. 1, panel A, chronic administration of BD-1063 decreased 24-h ethanol intake in Scr:sP rats (Treatment: F(1,14)=10.41, p< 0.01), with a more pronounced effect towards the first part of the 14-day treatment period (Treatment X day: F(13,182)=1.75, p= 0.05). Both the vehicle and the BD-1063 groups increased their alcohol intake over the course of the 14 days; however, rats treated with vehicle increased their intake from 1.88±0.42 g/kg on day 1 to 5.47±0.35 g/kg on day 14, while the intake of BD-1063-treated rats increased from 0.89±0.16 g/kg on day 1 to only 3.57±0.91 g/kg on day 14.
Figure 1.
Effect of chronic administration (14 days) of the Sig-1R antagonist BD-1063 (0, 30 mg/kg, s.c.) on daily ethanol intake (g/kg) (panel A), water intake (ml/kg) (panel B), ethanol preference (%) (panel C) and total fluid intake (ml/kg) (panel D) in Scr:sP rats (n=18). Data represent Mean ± SEM; * p < 0.05, ** p < 0.01; *** p < 0.001 vs. vehicle-treated group.
Water intake was also significantly affected by the treatment (Treatment: F(1,14)=11.34, p< 0.01; Treatment X day: F(13,182)=0.63, n.s.), with BD-1063-treated rats drinking more water than vehicle-treated rats during the entire treatment period, as shown in Fig. 1, panel B. This resulted in a significant reduction in ethanol preference on all 14 days of treatment (Treatment: F(1,14)=11.77, p<0.01; Treatment X Day: F(13,182)=1.55, n.s. Fig. 1, panel C) and no change in total fluid intake (Treatment: F(1,14)=2.03, n.s.; Treatment X Day: F(13,182)=1.36, n.s.; Fig. 1, panel D).
The treatment with BD-1063 did not affect food intake (Treatment: F(1,14)=4.07, n.s.; Treatment X Day: F(13,182)=1.13, n.s., Figure 2, panel A), total caloric intake (comprised of the calories coming from both food and ethanol; Treatment: F(1,14)=0.79, n.s; Treatment X Day: F(13,182)=1.13 n.s.; Figure 2, panel B). In addition, cumulative body weight gain was not affected by the treatment (Treatment: F(1,14)=1.76, n.s.; Treatment X Day: F(13,182)=1.21, n.s.; Fig. 2, panel C).
Figure 2.
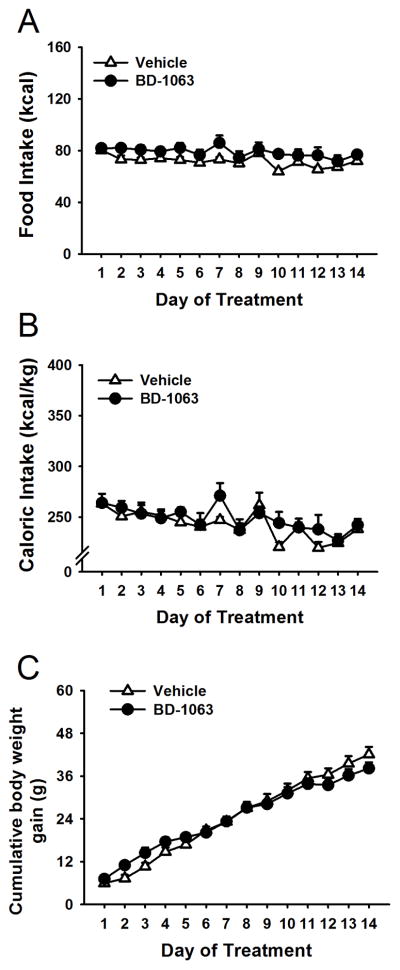
Effect of chronic administration (14 days) of the Sig-1R antagonist BD-1063 (0, 30 mg/kg, s.c.) on food intake (panel A), total caloric intake (panel B) and cumulative body weight gain (panel C) in Scr:sP rats exposed 24h/day to the ethanol-water choice (n=18). Data represent Mean ± SEM.
3.2. BD-1063 reduces alcohol-seeking behavior in Scr:sP rats
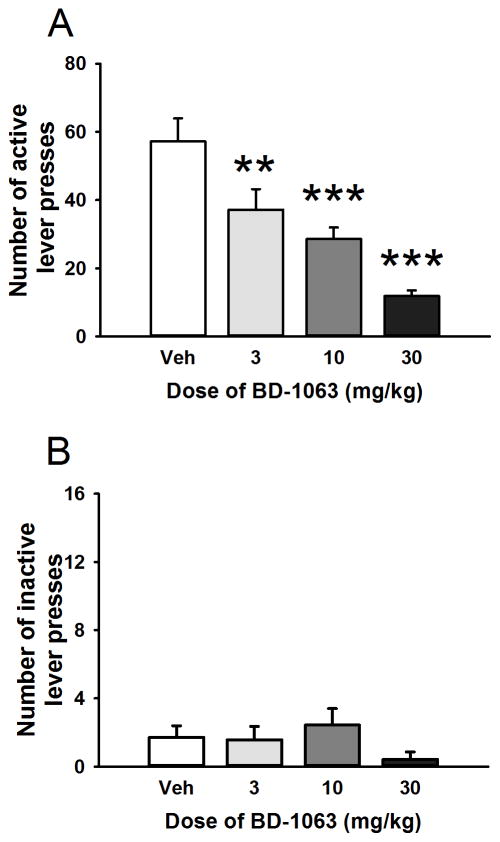
A separate set of animals (n=7) was used to assess the effects of the Sig-1R antagonist on alcohol-seeking behavior. As shown in Fig. 3, panel A, BD-1063 (0, 3, 10, 30 mg/kg s.c.) decreased alcohol-seeking behavior in Scr:sP rats (F(3,27)= 24.28, p< 0.001) in a second-order schedule of reinforcement, in which an alcohol-associated incentive stimulus maintains alcohol-seeking behavior. All three doses of BD-1063 significantly decreased the BD-1063 number of lever presses during the first (pre-ingestive) interval (79.2% reduction with the highest dose, compared to vehicle treatment). The treatment had no effect on inactive lever presses (F(3,27)=1.61, n.s.; Fig. 3, panel B).
Figure 3.
Effect of acute administration of the Sig-1R antagonist BD-1063 (0, 3, 10, 30 mg/kg) on an operant second-order schedule of reinforcement to assess alcohol-seeking behavior in Scr:sP rats (n=7). The number of active lever presses following BD-1063 administration is shown in panel A, the number of lever presses on the inactive lever in panel B. Data represent Mean ± SEM; ** p < 0.01, *** p < 0.001 vs. vehicle-treated group.
3.3. Protein expression of Sig-1 receptor is elevated in NAcc, but not the CeA, of ethanol-naive Scr:sP rats
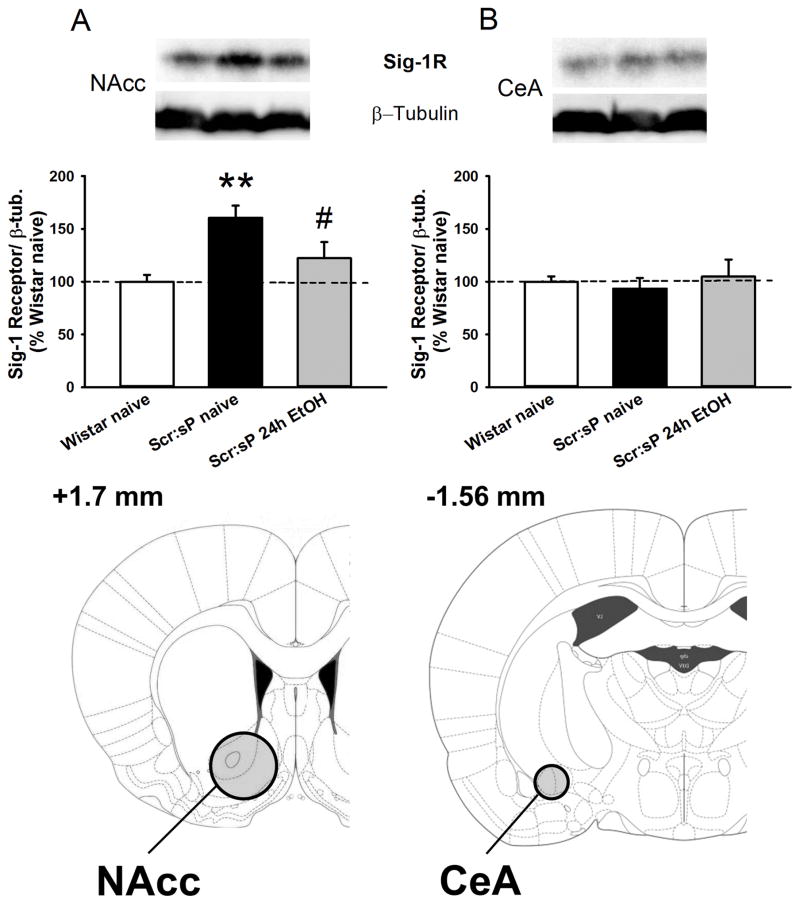
Sig-1R protein levels were measured in ethanol naïve outbred Wistar, ethanol naïve Scr:sP, and Scr:sP rats under continuous access to ethanol (24h/day for 4 weeks). One-way ANOVA showed a significant difference in protein levels among groups in the NAcc (F(2,18)= 6.91, p< 0.01). Post-hoc analysis revealed an increase of Sig-1R protein levels in ethanol-naïve Scr:sP rats compared to ethanol-naïve Wistar rats; in addition, Scr:sP rats which underwent continuous voluntary intake of ethanol for 4 consecutive weeks showed control-levels of Sig-1R protein expression (Fig. 4 panel A), as this group significantly differed from the ethanol-naïve Scr:sP but not from the ethanol-naïve Wistar. Sig-1R protein levels did not differ among groups in the CeA (F(2,18)= 0.25, n.s.; Fig. 4 panel B).
Figure 4.
Sig-1 receptor protein expression normalized to β-tubulin in the nucleus accumbens (NAcc) (panel A) and the central nucleus of the amygdala (CeA) (panel B) (n=14). Panel C contains drawings of rat brain coronal slices, where circles show brain regions that were punched out for the western blot. Values are expressed as % of the control Wistar group. Data represent Mean ± SEM; ** p < 0.01 vs. Wistar group, # p <0.05 vs. Scr:sP naïve group.
4. DISCUSSION
This series of studies demonstrates that pretreatment with BD-1063, a selective antagonist of Sig1-R [42], significantly reduces the acquisition of alcohol-drinking behavior in alcohol-preferring Scr:sP rats. The Sig-1R antagonist also increases the intake of the concurrently available water, resulting in a marked reduction of alcohol preference, while not reducing food intake or body weight. Importantly, treatment with BD-1063 is also able to reduce alcohol-seeking behavior in Scr:sP rats. Finally, we show that ethanol naïve Scr:sP rats display increased levels of Sig-1R expression in the NAcc, compared to ethanol naïve control Wistar rats, and that chronic voluntary alcohol consumption normalize the altered expression to control Wistar rats levels.
Alcohol-preferring rats are a well-established model for alcoholism with a strong face, predictive and construct validity [32, 45–47]. In the first part of our study, alcohol-naïve Scr:sP rats were treated daily with the Sig-1R antagonist BD-1063 for 2 weeks, concomitantly with the initiation of alcohol access. BD-1063 significantly reduced the acquisition of alcohol drinking (~1.5 g/kg reduction of intake compared to vehicle-treated rats on the last day of injection), suggesting a decrease as well as a delay of the expression of the genetic predisposition toward high alcohol intake. BD-1063 increased water intake and did not reduce food intake, demonstrating that the reduction in alcohol intake was not due to motor impairment, inability to drink, induction of nausea or another aversive state. The increase in water intake in BD-1063-treated rats can be interpreted as a compensatory behavior for the diminished ethanol intake, as the total fluid intake remained unchanged compared to vehicle-treated rats. Similar increases in water intake have been previously shown for several other drugs which are effective in reducing concurrent alcohol intake [48–51]. The results are also consistent with previous reports of lack of motor impairing effects of this dose of BD-1063 [52].
The second main finding of this study was that the Sig-1R antagonist BD-1063 decreased the alcohol-seeking behavior, suggesting a role for Sig-1R in incentive motivational mechanisms controlling ethanol-seeking and intake. This is a novel and highly relevant observation, as it proves that SigR antagonists can not only reduce alcohol consumption, but also alcohol-seeking behavior. We demonstrated this by means of a second-order schedule of reinforcement, a procedure widely used with psychostimulants and opiates [43, 53], which is characterized by the maintenance of responding not only by the self-administered drug, but also by the contingent presentation of drug-paired stimuli that serve as conditioned reinforcers of instrumental behavior [38]. BD-1063 was able to decrease the active lever presses during the first interval as a function of the dose administered; the first interval is the “drug-free” one, and is therefore not affected by the pharmacological effects of recently administered alcohol. Importantly, the effect was selective for alcohol-seeking, as responses on the inactive lever were unaffected by the treatment. Our results therefore suggest a major role for Sig-1R in either the incentive motivational mechanisms governing alcohol-seeking and intake or in the impact of alcohol-associated conditioned reinforcer (environmental cues) to maintain responding [38, 43].
Previously we and others have shown that Sig-1R antagonism reduces the consummatory and motivational behavior to work for alcohol in both operant and home-cage situations. Acute treatment with BD-1063 is indeed able to reduce ethanol self-administration in a fixed ratio-1 schedule of reinforcement as well as the motivation to work to obtain ethanol in a progressive ratio schedule of reinforcement in Scr:sP rats [29]. In addition, the selective Sig-1R antagonist NE-100 was shown to be able to suppress home-cage ethanol drinking in Scr:sP rats under continuous access conditions [28]. The present study adds further evidence supporting a key role for Sig-1Rs in alcohol drinking, and expands the previous findings by demonstrating that Sig-1R is also involved in the initial acceptance of an ethanol solution, i.e. the early acquisition phase of drinking, as well as in alcohol-seeking behavior. Blocking Sig-1Rs may therefore reduce the elevated probability of drinking that accompanies certain genetic backgrounds, thus allowing individuals with a genetic predisposition toward alcohol abuse to resist the impulse to start drinking heavily as well as to seek alcohol later in the condition.
Previous studies have shown that Sig-1R antagonists work to decrease alcohol intake selectively in alcohol-preferring rats, while the low to moderate drinking levels of outbred rats is not affected by the treatment [29]. In the present study we therefore aimed at identifying potential innate differences in the expression levels of the Sig-1R protein in Scr:sP rats in reward-related brain areas. Indeed, ethanol-naïve Scr:sP rats showed innately enhanced levels of Sig-1R protein in the NAcc, compared to ethanol-naïve Wistar rats. On the other hand, no differences were observed among groups in expression of Sig-1R protein in the CeA. The NAcc and the CeA both are intimately related to reward-related processes and play a major role in the reinforcing effects of drugs, including alcohol [54–56]. In particular, the NAcc integrates limbic information related to memory, drive, and motivation with the generation of goal-directed behaviors [57–59]. The NAcc also is implicated in excessive alcohol drinking [60, 61]. The NAcc shows moderately concentrated staining of Sig-1R [62–64]. The alteration in the NAcc expression of Sig-1R displayed by Scr:sP rats may therefore explain the previously reported higher sensitivity of these alcohol-preferring rats to pharmacological blockade with Sig-1R antagonists [29]. In addition, considering that chronic treatment with a SigR agonist has been shown to induce binge-like drinking in rats [27], we argue that an overexpression of Sig-1R in the NAcc may therefore mediate the susceptibility to excessive drinking, both innate (as in alcohol-preferring rats) and induced by chronic alcohol exposure (as in alcohol-dependent animals). The present study did not discriminate between the Core and the Shell of the NAcc, as the brain punches in which the Sig-1R levels were measured included both sub-regions; future studies will determine whether one of the two contributes more than the other to the effect observed. Our laboratory has previously demonstrated innate differences in the mRNA expression of Sig-1R in alcohol-preferring rats compared to outbred rats [29]; the present study, therefore, extends those findings to the Sig-1R protein, adding important information about the genetic basis of high alcohol drinking.
To investigate whether voluntary alcohol drinking could alter Sig-1R expression, we also measured Sig-1R protein levels in the NAcc and CeA of Scr:sP rats continuously exposed to voluntary alcohol drinking. We found that a 4-week consumption of relatively high amounts of ethanol (~5.5 g/kg/day) by Scr:sP rats was associated with reduced (normalized) Sig-1R protein levels in the NAcc, a region-specific effect (no effect in the CeA). Our observation is in line with several reports showing that ethanol drinking –especially chronic– can differentially affect ethanol-naive alcohol-preferring rats, eliminating or introducing neurochemical behavioral differences [65–68]. The now decreased Sig-1R levels, which may appear counter-intuitive at a first glance, may instead be in part responsible for the reduced motivation to drink alcohol which follows recent alcohol consumption; future studies can assess whether an abstinence period, which increases the motivation to drink and can drive relapse [69–72], can restore Sig-1R levels. This hypothesis is consistent with the proven ability of a Sig-1R antagonist to reduce alcohol-deprivation effect in Scr:sP rats after a 7-day abstinence [28]. In this study we recorded the alcohol intake of each individual cage, but not each individual rat; it will be interesting in future studies to assess whether the mean daily alcohol intake over the exposure period shows a correlation with NAcc Sig-1R protein levels, i.e. whether Scr:sP rats which drink more alcohol show also lower levels.
Previous studies in limbic areas have shown the interaction between Sig-1R and dopamine system [73, 74]. Sig-1R is indeed localized in brain areas in which dopamine innervation is highly distributed [62, 75, 76]. Sig-1R has been indeed demonstrated to affect the whole process of dopamine production, from synthesis to release, and uptake [77–79], with the activation of Sig-1R generally increased the firing rate of dopaminergic pathways [80, 81]. Therefore, the blockade of Sig-1R may attenuate dopamine neurotransmission in reward-related areas, which in turn may suppress the acquisition of ethanol drinking behavior as well as the seeking behavior. The dopamine mesolimbic system has been shown to be essential for acquisition of alcohol drinking behavior, as lesions with 6-hydroxy dopamine in the NAcc for example attenuate the acquisition of ethanol consumption in Indiana preferring rats [82]. Dopamine release in the NAcc has also been shown to be increased by non-contingent presentations of drug-associated conditioned stimuli [83]. Nevertheless, the potential involvement of other neurotransmitter systems (e.g. glutamatergic) in the observed effects of the Sig-1R antagonist cannot be ruled out, especially considering the involvement of glutamatergic transmission in the NAcc in drug-seeking behavior [84] and the interaction reported between the Sig-1R and the glutamatergic system, mainly NMDA [85–87].
The data here presented give novel, important insights regarding the potential genetic basis of excessive alcohol consumption and seeking, suggesting that innate differences in the Sig-1R system are associated with higher alcohol preference in Scr:sP rats and that the modulation of this system regulates acquisition and seeking of alcohol. The findings also indicate a modulation of Sig-1R levels following chronic alcohol consumption, suggesting that the NAcc may be a key brain region for the Sig-1R modulation of alcohol-drinking behavior observed after systemic administration of ligands.
Altogether these results suggest that SigR system plays a major role in the susceptibility to alcohol addiction and strengthen the notion that Sig-1Rs are a very important therapeutic target for alcohol abuse and dependence.
Acknowledgments
The authors thank Alyssa C. DiLeo and Stephen A. St. Cyr for technical assistance. We also thank The Scripps Research Institute and the University of Cagliari, CNR Neuroscience Institute for providing the Scr:sP and the original sP rats, respectively. This publication was made possible by grant numbers AA016731, MH093650, MH091945, and DA030425 from the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Mental Health and the National Institute on Drug Abuse, by the Peter McManus Trust, the Peter Paul Career Development Professorship (P.C.), and the Boston University Undergraduate Research Opportunity Program.. A portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–32. [PubMed] [Google Scholar]
- 2.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, et al. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–6. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 3.Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–21. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 4.Vaupel DB. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. European journal of pharmacology. 1983;92:269–74. doi: 10.1016/0014-2999(83)90297-2. [DOI] [PubMed] [Google Scholar]
- 5.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, et al. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8072–7. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a sigma receptor from rat brain. J Neurochem. 1998;70:922–31. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- 7.Mei J, Pasternak GW. Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochem Pharmacol. 2001;62:349–55. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Ault DT, Radeff JM, Werling LL. Modulation of [3H]Dopamine release from rat nucleus accumbens by neuropeptide Y may involve a sigma1-like receptor. J Pharmacol Exp Ther. 1998;284:553–60. [PubMed] [Google Scholar]
- 10.Gronier B, Debonnel G. Involvement of sigma receptors in the modulation of the glutamatergic/NMDA neurotransmission in the dopaminergic systems. European journal of pharmacology. 1999;368:183–96. doi: 10.1016/s0014-2999(99)00025-4. [DOI] [PubMed] [Google Scholar]
- 11.Skuza G, Kolasiewicz W. Repeated treatment with SA4503, a selective sigma1 receptor agonist, up-regulates alpha-adrenergic system. a behavioral study. Pol J Pharmacol. 2001;53:547–50. [PubMed] [Google Scholar]
- 12.Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–80. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- 13.Weatherspoon JK, Gonzalez-Alvear GM, Frank AR, Werling LL. Regulation of [3H] dopamine release from mesolimbic and mesocortical areas of guinea pig brain by sigma receptors. Schizophr Res. 1996;21:51–62. doi: 10.1016/0920-9964(96)00030-8. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog Neurobiol. 2013;100:15–29. doi: 10.1016/j.pneurobio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto M, Hayashi T, Urfer R, Mita S, Su TP. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse. 2012;66:630–9. doi: 10.1002/syn.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penas C, Pascual-Font A, Mancuso R, Fores J, Casas C, Navarro X. Sigma receptor agonist 2-(4-morpholinethyl)1 phenylcyclohexanecarboxylate (Pre084) increases GDNF and BiP expression and promotes neuroprotection after root avulsion injury. J Neurotrauma. 2011;28:831–40. doi: 10.1089/neu.2010.1674. [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu M, Hoshino T. Involvement of kappa-opioid receptors and sigma receptors in memory function demonstrated using an antisense strategy. Brain Res. 2004;1030:247–55. doi: 10.1016/j.brainres.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Hiramatsu M, Hoshino T. Improvement of memory impairment by (+)- and (−)-pentazocine via sigma, but not kappa opioid receptors. Brain Res. 2005;1057:72–80. doi: 10.1016/j.brainres.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Meunier J, Demeilliers B, Celerier A, Maurice T. Compensatory effect by sigma1 (sigma1) receptor stimulation during alcohol withdrawal in mice performing an object recognition task. Behav Brain Res. 2006;166:166–76. doi: 10.1016/j.bbr.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology. 2007;32:514–21. doi: 10.1038/sj.npp.1301047. [DOI] [PubMed] [Google Scholar]
- 21.Hiranita T, Soto PL, Tanda G, Katz JL. Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther. 2010;332:515–24. doi: 10.1124/jpet.109.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–73. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. European journal of pharmacology. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 24.Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–45. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Maurice T, Casalino M, Lacroix M, Romieu P. Involvement of the sigma 1 receptor in the motivational effects of ethanol in mice. Pharmacol Biochem Behav. 2003;74:869–76. doi: 10.1016/s0091-3057(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 27.Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, et al. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36:1207–18. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabino V, Cottone P, Zhao Y, Steardo L, Koob GF, Zorrilla EP. Selective reduction of alcohol drinking in Sardinian alcohol-preferring rats by a sigma-1 receptor antagonist. Psychopharmacology (Berl) 2009;205:327–35. doi: 10.1007/s00213-009-1548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr, Steardo L, et al. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology. 2009;34:1482–93. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabino V, Kwak J, Rice KC, Cottone P. Pharmacological characterization of the 20% alcohol intermittent access model in Sardinian alcohol-preferring rats: a model of binge-like drinking. Alcoholism, clinical and experimental research. 2013;37:635–43. doi: 10.1111/acer.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, et al. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–86. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- 32.Colombo G. ESBRA-Nordmann 1996 Award Lecture: ethanol drinking behaviour in Sardinian alcohol-preferring rats. Alcohol Alcohol. 1997;32:443–53. doi: 10.1093/oxfordjournals.alcalc.a008279. [DOI] [PubMed] [Google Scholar]
- 33.Bohman M, Sigvardsson S, Cloninger CR. Maternal inheritance of alcohol abuse. Cross-fostering analysis of adopted women. Arch Gen Psychiatry. 1981;38:965–9. doi: 10.1001/archpsyc.1981.01780340017001. [DOI] [PubMed] [Google Scholar]
- 34.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–8. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 35.Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- 36.Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm Adoption Study of alcoholism. Confirmatory cross-fostering analysis. Arch Gen Psychiatry. 1996;53:681–7. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- 37.Giuliano C, Robbins TW, Wille DR, Bullmore ET, Everitt BJ. Attenuation of cocaine and heroin seeking by mu-opioid receptor antagonism. Psychopharmacology (Berl) 2013;227:137–47. doi: 10.1007/s00213-012-2949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- 39.Di Ciano P, Everitt BJ. Neuropsychopharmacology of drug seeking: Insights from studies with second-order schedules of drug reinforcement. European journal of pharmacology. 2005;526:186–98. doi: 10.1016/j.ejphar.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg SR, Kelleher RT, Morse WH. Second-order schedules of drug injection. Fed Proc. 1975;34:1771–6. [PubMed] [Google Scholar]
- 41.Kelleher RT, Goldberg SR. Fixed-interval responding under second-order schedules of food presentation or cocaine injection. J Exp Anal Behav. 1977;28:221–31. doi: 10.1901/jeab.1977.28-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Costa BR, He XS, Linders JT, Dominguez C, Gu ZQ, Williams W, et al. Synthesis and evaluation of conformationally restricted N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamines at sigma receptors. 2. Piperazines, bicyclic amines, bridged bicyclic amines, and miscellaneous compounds. J Med Chem. 1993;36:2311–20. doi: 10.1021/jm00068a007. [DOI] [PubMed] [Google Scholar]
- 43.Giuliano C, Robbins TW, Nathan PJ, Bullmore ET, Everitt BJ. Inhibition of opioid transmission at the mu-opioid receptor prevents both food seeking and binge-like eating. Neuropsychopharmacology. 2012;37:2643–52. doi: 10.1038/npp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Amsterdam; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- 45.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction biology. 2006;11:270–88. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 46.Ciccocioppo R. Genetically selected alcohol preferring rats to model human alcoholism. Current topics in behavioral neurosciences. 2013;13:251–69. doi: 10.1007/7854_2012_199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, et al. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addiction biology. 2006;11:339–55. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. European journal of pharmacology. 1998;358:189–96. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- 49.Rezvani AH, Overstreet DH, Levin ED, Rosenthal DI, Kordik CP, Reitz AB, et al. Effects of atypical anxiolytic N-phenyl-2-[1-[3-(2-pyridinylethynyl)benzoyl]-4-piperidine]acetamide (JNJ-5234801) on alcohol intake in alcohol-preferring P rats. Alcoholism, clinical and experimental research. 2007;31:57–63. doi: 10.1111/j.1530-0277.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- 50.Suchankova P, Steensland P, Fredriksson I, Engel JA, Jerlhag E. Ghrelin receptor (GHS-R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long-term voluntary alcohol consumption. PloS one. 2013;8:e71284. doi: 10.1371/journal.pone.0071284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brammer MK, Gilmore DL, Matsumoto RR. Interactions between 3,4-methylenedioxymethamphetamine and sigma1 receptors. Eur J Pharmacol. 2006;553:141–5. doi: 10.1016/j.ejphar.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nature neuroscience. 2004;7:389–97. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 54.Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–65. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- 55.Herring BE, Mayfield RD, Camp MC, Alcantara AA. Ethanol-induced Fos immunoreactivity in the extended amygdala and hypothalamus of the rat brain: focus on cholinergic interneurons of the nucleus accumbens. Alcoholism, clinical and experimental research. 2004;28:588–97. doi: 10.1097/01.alc.0000122765.58324.6d. [DOI] [PubMed] [Google Scholar]
- 56.Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 57.Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14:763–8. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–11. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 59.Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–61. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 60.Koob GF. Alcoholism: allostasis and beyond. Alcoholism, clinical and experimental research. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 61.Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–47. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 62.Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, et al. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–70. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 63.Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 1997;76:467–77. doi: 10.1016/s0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- 64.Phan VL, Urani A, Sandillon F, Privat A, Maurice T. Preserved sigma1 (sigma1) receptor expression and behavioral efficacy in the aged C57BL/6 mouse. Neurobiol Aging. 2003;24:865–81. doi: 10.1016/s0197-4580(02)00231-2. [DOI] [PubMed] [Google Scholar]
- 65.Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–4. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 66.Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and -nonpreferring rats: a quantitative microdialysis study. Alcoholism, clinical and experimental research. 2001;25:198–205. [PubMed] [Google Scholar]
- 67.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–8. [PubMed] [Google Scholar]
- 68.Zhou Y, Colombo G, Gessa GL, Kreek MJ. Effects of voluntary alcohol drinking on corticotropin-releasing factor and preprodynorphin mRNA levels in the central amygdala of Sardinian alcohol-preferring rats. Neurosci Lett. 2013;554:110–4. doi: 10.1016/j.neulet.2013.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koob GF. Animal models of craving for ethanol. Addiction. 2000;95 (Suppl 2):S73–81. doi: 10.1080/09652140050111663. [DOI] [PubMed] [Google Scholar]
- 70.Li TK. Clinical perspectives for the study of craving and relapse in animal models. Addiction. 2000;95 (Suppl 2):S55–60. doi: 10.1080/09652140050111645. [DOI] [PubMed] [Google Scholar]
- 71.Boening JA, Lesch OM, Spanagel R, Wolffgramm J, Narita M, Sinclair D, et al. Pharmacological relapse prevention in alcohol dependence: from animal models to clinical trials. Alcoholism, clinical and experimental research. 2001;25:127S–31S. doi: 10.1097/00000374-200105051-00022. [DOI] [PubMed] [Google Scholar]
- 72.McBride WJ, Le AD, Noronha A. Central nervous system mechanisms in alcohol relapse. Alcoholism, clinical and experimental research. 2002;26:280–6. [PubMed] [Google Scholar]
- 73.Goldstein SR, Matsumoto RR, Thompson TL, Patrick RL, Bowen WD, Walker JM. Motor effects of two sigma ligands mediated by nigrostriatal dopamine neurons. Synapse. 1989;4:254–8. doi: 10.1002/syn.890040311. [DOI] [PubMed] [Google Scholar]
- 74.Ceci A, Smith M, French ED. Activation of the A10 mesolimbic system by the sigma-receptor agonist (+)SKF 10,047 can be blocked by rimcazole, a novel putative antipsychotic. European journal of pharmacology. 1988;154:53–7. doi: 10.1016/0014-2999(88)90362-7. [DOI] [PubMed] [Google Scholar]
- 75.Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–70. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weissman AD, Su TP, Hedreen JC, London ED. Sigma receptors in post-mortem human brains. The Journal of pharmacology and experimental therapeutics. 1988;247:29–33. [PubMed] [Google Scholar]
- 77.Bastianetto S, Rouquier L, Perrault G, Sanger DJ. DTG-induced circling behaviour in rats may involve the interaction between sigma sites and nigro-striatal dopaminergic pathways. Neuropharmacology. 1995;34:281–7. doi: 10.1016/0028-3908(94)00156-m. [DOI] [PubMed] [Google Scholar]
- 78.Iyengar S, Dilworth VM, Mick SJ, Contreras PC, Monahan JB, Rao TS, et al. Sigma receptors modulate both A9 and A10 dopaminergic neurons in the rat brain: functional interaction with NMDA receptors. Brain research. 1990;524:322–6. doi: 10.1016/0006-8993(90)90709-k. [DOI] [PubMed] [Google Scholar]
- 79.Weiser SD, Patrick SL, Mascarella SW, Downing-Park J, Bai X, Carroll FI, et al. Stimulation of rat striatal tyrosine hydroxylase activity following intranigral administration of sigma receptor ligands. European journal of pharmacology. 1995;275:1–7. doi: 10.1016/0014-2999(94)00718-m. [DOI] [PubMed] [Google Scholar]
- 80.Minabe Y, Matsuno K, Ashby CR., Jr Acute and chronic administration of the selective sigma1 receptor agonist SA4503 significantly alters the activity of midbrain dopamine neurons in rats: An in vivo electrophysiological study. Synapse. 1999;33:129–40. doi: 10.1002/(SICI)1098-2396(199908)33:2<129::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 81.Sanchez-Arroyos R, Guitart X. Electrophysiological effects of E-5842, a sigma1 receptor ligand and potential atypical antipsychotic, on A9 and A10 dopamine neurons. European journal of pharmacology. 1999;378:31–7. doi: 10.1016/s0014-2999(99)00440-9. [DOI] [PubMed] [Google Scholar]
- 82.Ikemoto S, McBride WJ, Murphy JM, Lumeng L, Li TK. 6-OHDA-lesions of the nucleus accumbens disrupt the acquisition but not the maintenance of ethanol consumption in the alcohol-preferring P line of rats. Alcoholism, clinical and experimental research. 1997;21:1042–6. [PubMed] [Google Scholar]
- 83.Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–95. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–60. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 85.Kim HW, Roh DH, Yoon SY, Seo HS, Kwon YB, Han HJ, et al. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008;154:1125–34. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoon SY, Roh DH, Seo HS, Kang SY, Moon JY, Song S, et al. An increase in spinal dehydroepiandrosterone sulfate (DHEAS) enhances NMDA-induced pain via phosphorylation of the NR1 subunit in mice: involvement of the sigma-1 receptor. Neuropharmacology. 2010;59:460–7. doi: 10.1016/j.neuropharm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 87.Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, et al. Role of N-methyl-D-aspartate receptors in antidepressant-like effects of sigma 1 receptor agonist 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine dihydrochloride (SA-4503) in olfactory bulbectomized rats. J Pharmacol Exp Ther. 2007;322:1305–14. doi: 10.1124/jpet.107.124685. [DOI] [PubMed] [Google Scholar]