Abstract
Objectives
HIV-associated brain injury persists despite antiretroviral therapy (cART), but contributing factors remain poorly understood. We postulated that inflammation-associated biomarkers will be associated with cerebral injury on proton magnetic resonance spectroscopy (MRS) in chronically HIV-infected subjects.
Methods
Five biomarkers were measured in 197 HIV-infected subjects: soluble CD14, MCP-1, IP-10, MIP-1β, and fractalkine. Levels of N-acetyl aspartate (NAA), Choline (Cho), Myoinositol (MI), Glutamate+Glutamine (Glx), and Creatine (Cr) were acquired in the midfrontal cortex (MFC), frontal white matter (FWM), and basal ganglia (BG). Predictive models were built via linear regression and the best models were chosen using the Akaike Information Criterion.
Results
Increases in plasma or CSF MCP-1 were associated with lower NAA/Cr in the MFC and BG while metabolite changes in the FWM for NAA/Cr, GlxCr and Cho/Cr were explained almost exclusively by a single factor, sCD14. Plasma and CSF levels of this factor were also significantly associated with Glx/Cr in MFC and BG. Higher CSF FKN was associated with higher NAA/Cr in BG. Best predictors for higher Cho/Cr in BG and MFC were CSF sCD14 and CSF MIP-1β. Plasma and CSF IP-10 were only associated with Cho/Cr in MFC. Of the three models that simultaneously accounted for both plasma and CSF, there were more associations between CSF biomarkers and MRS metabolites.
Conclusions
Markers of inflammation and immune activation, in particular MCP-1 and sCD14, predominantly reflecting CNS sources, contribute to the persistence of brain injury in a metabolite and region dependent manner in chronically HIV-infected patients on stable cART.
Keywords: HIV, AIDS, HIV-associated neurocognitive disorder, cerebrospinal fluid
Introduction
HIV disease is associated with pathological changes in the brain resulting in neurocognitive, motor, and behavioral disturbances, including syndromes that were previously termed AIDS Dementia Complex (ADC), known now as HIV-associated neurocognitive disorder (HAND).1 While the incidence of severe dementia has decreased in the combination antiretroviral (cART) era, the prevalence of milder disease has remained stable or perhaps even increased.2–5 Several studies have shown that cognitive impairment and brain injury persist in chronically infected patients on stable cART, though the underlying mechanism is poorly understood.6,7 Proton magnetic resonance spectroscopy (MRS) provides a sensitive and noninvasive in vivo method to detect metabolite changes in the brain.8 Specific metabolites that have been identified include N-acetyl aspartate (NAA), a neuronal and axonal marker of integrity; choline (Cho), derived from a complex of transmembrane markers whose presence reflects membrane remodeling after injury; glutamate+glutamine, an excitatory neurotransmitter plus its precursor (together referred to as Glx), which are elevated in encephalopathic states and may reflect damage to neuronal glial cell environment; myo-inositol (MI), a carbohydrate synthesized primarily by glial cells, generally considered a marker of glial cell proliferation in response to neuronal injury; and creatine (Cr), a marker of energy production that is often used as a reference in ratios with other metabolites. Several studies in HIV-infected individuals and SIV-infected macaques have found decreased levels of NAA/Cr in the frontal white matter, basal ganglia and occasionally in the mesial frontal gray matter.7,9–11 High levels of Cho/Cr and MI/Cr have also been found in these regions, consistent with a pattern of neuronal injury and inflammation.
Multiple biomarkers found in cerebrospinal fluid have been associated with HAND. These include markers of monocyte/macrophage activation such as soluble CD14 (sCD14) and chemotactic cytokines such as monocyte chemotactic protein-1 (MCP-1), interferon gamma inducible protein-10 (IP-10), macrophage inflammatory protein-1β (MIP-1β), and fractalkine (FKN).12–17 One small study found that lower NAA/Cr is associated with higher MCP-1, suggesting a link between monocyte chemotaxis and neuronal injury during HIV infection.18 The HIV Neuroimaging Consortium (HIVNC), a 12-center collaborative group was formed to investigate the patterns and correlates of brain injury and cognitive impairment in over 300 subjects with chronic HIV infection on cART.7,19 Recent studies suggest than chronic immune activation plays an important role in systemic complications observed in HIV-infected patients on cART.20 The contribution of inflammatory factors to the persistence of brain injury in this setting, however, remains relatively unexplored. We therefore hypothesized that markers of immune activation would contribute to the persistence of brain injury in these patients in a metabolite and region dependent manner.
Methods
Design
This cross-sectional project included 197 HIV-infected subjects from 7 sites: UC-San Diego, UC-Los Angeles, Harbor-UCLA, Stanford University, University of Colorado, University of Pittsburgh, and University of Rochester. The study was conducted after approval by all local Institutional Review Boards (IRBs) and protections for subjects followed the Helsinki Declaration. Inclusion criteria included: Nadir CD4 count ≤ 200 cells/μl and stable cART regimen for at least 12 consecutive weeks prior to screening. Exclusion criteria included severe premorbid or comorbid psychiatric disorders, chronic seizures, stroke, head trauma resulting in loss of consciousness > 30 minutes, multiple sclerosis, non-HIV brain infection, brain neoplasms, active alcohol or drug abuse within 6 months of study; hemoglobin ≤ 9.0 g/dL; > 3 x upper limit of normal (ULN) of creatinine, AST, ALT, or alkaline phosphatase; or diabetes mellitus with a fasting glucose > 140. Subjects were enrolled for this study between the years of 2005 and 2008.
Magnetic Resonance Spectroscopy
The 1H-MRS protocol has been previously described.6,7 Briefly, levels of cerebral metabolites NAA, MI, Cho, Glx, and Cr were measured by single-voxel 1H spectra using a PRESS pulse sequence. Voxels 6 ml in volume were obtained in three regions: gray matter in mid-frontal cortex (MFC), right or left mid-frontal white matter (FWM), and right or left basal ganglia (BG). Field homogeneity and water suppression were adjusted using automated algorithms. Water-suppressed spectra were collected with echo time/repetition time (TE/TR) of 35/3000 ms, bandwidth 2500 Hz, 128 averages. MRS data were acquired using matched pulse sequence parameters on GE and Siemens MRI units. In addition to the water-suppressed MRS acquisition, single-scan fully relaxed unsuppressed water FIDs were acquired from each voxel at 7 different echo times (TE = 30, 45, 65, 100, 200, 500, and 1500 ms; TR = 15 s). These data were used to infer the voxel’s CSF content. To control for instrument bias, we collected phantom MRS data concurrently with the subject evaluation, using the same protocol described above.
The time domain spectral data were transferred to a central MRS processing site at the University of Hawaii. MR spectra were evaluated for quality based on visual inspection and the %SD (Cramer-Rao lower bounds) and FWHM outputs from the LC Model spectral analysis software.21 Cerebral metabolite concentrations were computed using LC Model with unsuppressed water FID at TE=35ms used for eddy-current correction. The automated processing method yields metabolite concentration estimates with coefficient of variation < 15.22 The variability in measurements of metabolite ratios over the group of human subjects was about 3-fold larger than the variability measured in the phantoms. The variability in the phantom metabolite ratio measurements between the seven sites did not exceed 7% of the overall mean, with most of the variation falling within 2–3%. Because the phantom agreement between sites (including between acquisitions) that used Siemens and GE scanners was within a few percent, we did not make scanner-specific corrections to the derived metabolite concentrations and ratios.
Assessment procedures
Neurocognitive status was assessed at each imaging time point using the AIDS Dementia Complex Scale as previously described23 (much of the study was performed prior to publication of the most recent HAND nosology).1 The ADC staging was performed by a trained clinician at each site and was based on the neurological examination, assessment of functional impairment, and neuropsychological performance.7 Neuropsychological impairment was defined as performance of at least 1.0 standard deviation below normative values on two or more neuropsychological tests or at least 2.0 standard deviations below normative values on one or more tests. Participants were classified at baseline using the ADC staging as follows: ADC stage 0- neurologically asymptomatic with no evidence of cognitive, functional or neuropsychological impairment; ADC stage 0.5- subclinical impairment, with evidence of neuropsychological impairment only, ADC stage 1- mild neurocognitive impairment, with evidence of definite cognitive and functional impairment on activities of daily living but without loss of independent functioning, ADC stage 2 -moderate impairment, requiring assistance on ADLs, or ADC stage 3- severe impairment. CSF was collected by lumbar puncture from 99 subjects and was stored at −80C.
Laboratory Procedures
Soluble CD14 was measured using a commercial enzyme-linked immunosorbent assay (ELISA) with a sensitivity of 125 pg/mL (R&D Systems, Minneapolis, MN). Other biomarkers (MCP-1, IP-10, MIP-1β, FKN) were measured by multiplex bead suspension array (Millipore, Billerica, MA) on a BioPlex 100 platform (Bio-Rad, Hercules, CA) with sensitivities of 3.2 pg/mL. All results were reviewed for quality assurance and assays were repeated when coefficients of variation exceeded 20% or if concentration distributions revealed possible batch effects. HIV RNA levels were quantified in plasma and CSF by RT-PCR using commercial assays with lower limits of quantitation of either 400 or 50 c/mL. CD4+ T-cells were measured by flow cytometry.
Statistical Analyses
Biomarker concentrations were log-transformed to reduce skewness. Predictive models for cerebral metabolites as functions of biomarker concentrations in plasma and CSF were built via linear regression models. The best models were then chosen using the Akaike Information Criterion (AIC), which balances the parsimony and the fit of the regression models.24 We prefer the AIC over other methods as a model selection criterion, since it balances the estimation error associated with including the additional covariates with the approximation error that associated with excluding the covariates. Analyses were performed in 3 groups: plasma biomarkers alone (N=186), CSF biomarkers alone (N=99), or both plasma and CSF markers (N=88). We deem the associations to be statistically significant if the test of the regression coefficients equal to zero results in a p-value less than 0.05.
Model Building Strategy
A stepwise variable selection algorithm using AIC as the selection criterion was used to choose the biomarkers that were associated with each of the four relative metabolite concentrations (NAA/Cr, Cho/Cr, MI/Cr and Glx/Cr) in three brain regions: frontal white matter (FWM), basal ganglia (BG) and mid-frontal cortex (MFC). For each relative metabolite concentration, the stepwise model selection procedure was repeated on 500 bootstrapped datasets, and only those biomarkers selected in at least 70% of the 500 bootstrapped model fits were used in the final model. To study the association of the metabolite concentrations and the biomarker levels in the presence of demographic, clinical and viral covariates (age, gender, race, education level, plasma HIV RNA, CD4+ count, CD4+ nadir, duration of HIV infection, and presence of ADC stage 1 or higher), we used the same model selection strategy as described above starting with the best metabolite-biomarker model chosen above.
Results
Cohort characteristics (Table 1)
Table 1. Demographic and disease characteristics of the cohort.
Values are either number (%) or median (IQR)
| Characteristic | Total N=197 unless otherwise stated |
|---|---|
| Gender (Men) | 173 (88%) |
|
| |
| Years of age | 46 (41,52) |
|
| |
| Race (White) | 135 (69%) |
|
| |
| Education ≤12 years | 82 (42%) |
|
| |
| Cognition | n=195 |
| Significant neurocognitive impairment (ADC Stage 1–3) | 44 (22%) |
| Subclinical neurocognitive impairment (ADC stage 0.5) | 55 (27.9%) |
| No neurocognitive impairment (ADC stage 0) | 96 (48.7%) |
|
| |
| Duration of HIV (Years) | 12 (8,17) |
|
| |
| Current CD4+ T-cells (/μL) | 288 (179,461) |
|
| |
| Nadir CD4+ T-cells (/μL) | 34 (12,81) |
|
| |
| Plasma HIV RNA ≤ 400 c/mL (% of n=186) | 72% |
|
| |
| CSF HIV RNA ≤ 75 c/mL (% of n=86) | 69% |
|
| |
| Current ART (%) | 100% |
|
| |
| ART Regimens Containing: | n=183 |
|
| |
| Protease Inhibitors (excluding ritonavir) | |
|
| |
| Lopinavir | 69 (37.7%) |
|
| |
| Atazanavir | 36 (19.7%) |
|
| |
| Fosamprenavir | 14 (7.7%) |
|
| |
| Saquinavir | 10 (5.5%) |
|
| |
| Nelfinavir | 7 (3.8%) |
|
| |
| Darunavir | 4 (2.2%) |
|
| |
| Amprenavir | 3 (1.6%) |
|
| |
| Indinavir | 2 (1.1%) |
|
| |
| Tipranavir | 1 (0.5%) |
|
| |
| Non-nucleoside Reverse Transcriptase Inhibitors | |
|
| |
| Efavirenz | 49 (26.8%) |
|
| |
| Nevirapine | 16 (5.6%) |
|
| |
| Tenofovir | 117 (64%) |
|
| |
| Lamivudine | 100 (54.6%) |
|
| |
| Abacavir | 69 (37.7%) |
|
| |
| Emtricitabine | 64 (35%) |
|
| |
| Zidovudine | 53 (29%) |
|
| |
| Stavudine | 15 (8.2%) |
|
| |
| Didanosine | 11 (6%) |
The entire cohort of 197 subjects who had at least one cerebral metabolite and the panel of soluble biomarkers in at least one body fluid measured had a median age of 46 years (IQR: 41,52) with a median duration of HIV disease of 12 years (IQR: 8,17). Median nadir CD4+ count was 34 cells/μL (IQR: 12,81) while median current CD4+ count was 288 cells/μL (IQR: 179,461). Of 197 subjects with ADC assessment, 96 (48.7%) were stage “0”, 55 (27.9%) were stage “0.5”, 33 (16.8%) were stage “1”, 11 (5.6%) were stage “2–4”, and 2 were missing. Therefore, nearly 78% of subjects were cognitively normal or had only subclinical cognitive impairment. Tables 2 and 3 summarize associations between biomarkers (plasma available for n=186, CSF available for n=99, and plasma plus CSF available for n=88) and MRS organized by brain region.
Table 2. Statistically significant associations between soluble biomarkers and MRS metabolites by brain region.
Regression coefficients with the associated p-values are shown as well as the variation explained by the models resulting from the variable selection via AIC. Only the biomarkers that were present in the final models are reported below. Blank spaces indicate non-significant associations. Underlined associations became statistically non-significant when baseline demographic and disease characteristics were included in the models.
| Model 1: Plasma (N=186) | Model 2: CSF (N=99) | Model 3: CSF & Plasma (N=88) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Metabolite | Biomarker* | Fluid | β | p | Adj. R2 | β | P | Adj. R2 | β | P | Adj. R2 |
| FWM | NAA/Cr | sCD14 | CSF | −0.12 | 0.001 | 0.10 | −0.16 | 0.001 | 0.11 | |||
| MIP-1β | CSF | −0.06 | 0.243 | |||||||||
| Cho/Cr | sCD14 | Plasma | 0.02 | 0.008 | 0.07 | |||||||
| Glx/Cr | sCD14 | CSF | −0.13 | 0.042 | 0.04 | |||||||
| BG | NAA/Cr | MCP-1 | CSF | −0.17 | 0.009 | 0.10 | −0.24 | 0.001 | 0.24 | |||
| FKN | CSF | 0.06 | 0.015 | |||||||||
| MCP-1 | Plasma | −0.04 | 0.017 | 0.07 | −0.10 | <0.001 | ||||||
| MIP-1β | Plasma | 0.03 | 0.015 | |||||||||
| MIP-1β | CSF | −0.11 | 0.057 | |||||||||
| Cho/Cr | sCD14 | CSF | 0.017 | 0.037 | 0.05 | |||||||
| MIP-1β | Plasma | 0.006 | 0.073 | |||||||||
| Glx/Cr | sCD14 | Plasma | −0.06 | 0.048 | 0.02 | |||||||
| MIP-1β | CSF | 0.19 | 0.038 | 0.06 | ||||||||
| MFC | NAA/Cr | MCP-1 | CSF | −0.07 | 0.039 | 0.04 | −0.08 | 0.034 | 0.04 | |||
| MCP-1 | Plasma | −0.02 | 0.035 | 0.02 | ||||||||
| Cho/Cr | MIP-1β | CSF | 0.03 | 0.004 | 0.08 | 0.02 | 0.039 | 0.04 | ||||
| sCD14 | CSF | 0.02 | 0.015 | 0.01 | 0.104 | |||||||
| IP-10 | CSF | −0.01 | 0.047 | |||||||||
| IP-10 | Plasma | 0.01 | 0.021 | 0.03 | ||||||||
| Glx/Cr | sCD14 | CSF | −0.16 | 0.020 | 0.10 | |||||||
| sCD14 | Plasma | 0.06 | 0.010 | 0.04 | ||||||||
| FKN | Plasma | 0.06 | 0.006 | |||||||||
Value log transformed
Table 3. Summary of statistically significant associations between biomarkers and MRS metabolites.
Positive associations are denoted by up arrow (↑) while negative associations are denoted by down arrow (↓). (1 = plasma models, 2 = CSF models, 3 = combined CSF and plasma models).
Underlined associations became statistically non-significant when baseline demographic and disease characteristics were included in the models.
| Frontal White Matter | Basal Ganglia | Mid Frontal Cortex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Biomarker | Fluid | NAA/Cr | Cho/Cr | Glx/Cr | NAA/Cr | Cho/Cr | Glx/Cr | NAA/Cr | Cho/Cr | Glx/Cr |
| FKN | CSF | ↑(2) | ||||||||
| Plasma | ↑(3) | |||||||||
|
| ||||||||||
| IP-10 | CSF | ↓(2) | ||||||||
| Plasma | ↑(1) | |||||||||
|
| ||||||||||
| MCP-1 | CSF | ↓(2,3) | ↓(2,3) | |||||||
| Plasma | ↓(1,3) | ↓(1) | ||||||||
|
| ||||||||||
| MIP-1β | CSF | ↓(3) | ↓(3) | ↑(2) | ↑(2,3) | |||||
| Plasma | ↑(1) | ↑(3) | ||||||||
|
| ||||||||||
| sCD14 | CSF | ↓(2,3) | ↓(2) | ↑(3) | ↑(2,3) | ↓(3) | ||||
| Plasma | ↑(3) | ↓(1) | ↑(1) | |||||||
Frontal White Matter Associations
In the analysis of all plasma biomarker data (n=186), no statistically significant associations were found between the panel of biomarkers and cerebral metabolites. In the analysis of all CSF biomarker data (n=99), higher sCD14 levels were associated with lower NAA/Cr values (p = 0.001) and with lower Glx/Cr values (p=0.04) (Figure 1). In the subgroup that had matched plasma and CSF biomarkers measured (n=88), higher CSF sCD14 levels were again associated with lower NAA/Cr values (p = 0.001) and higher plasma sCD14 levels were associated with higher Cho/Cr values (p = 0.01).
Figure 1.
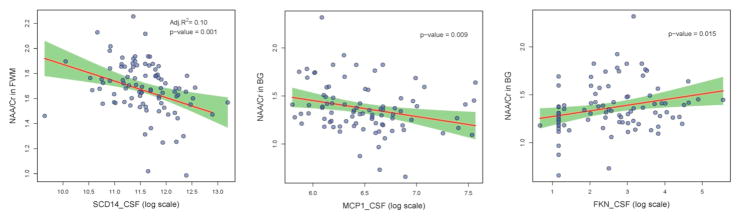
In all figures, the red line shows the linear regression fit of the association between the soluble biomarker levels in CSF and cerebral metabolite values, the green shaded area indicates the 95% pointwise confidence interval, and the blue dots are the raw data. Figures 1A (left): Higher sCD14 levels in CSF correlate with lower NAA/Cr ratios is FWM. Figure 1B (center): Higher MCP-1 levels in CSF correlate with lower NAA/Cr ratios is BG. Figure 1C (right): Lower FKN levels in CSF correlate with lower NAA/Cr ratios in BG.
Basal Ganglia Associations
In the analysis of all plasma biomarker data, higher MCP-1 levels (p=0.02) and lower MIP-1β levels (p=0.02) were significantly associated with lower NAA/Cr while higher sCD14 levels were associated with lower Glx/Cr (p = 0.05). In the analysis of all CSF biomarker data, higher MCP-1 (p = 0.01) and lower FKN (p = 0.02) levels were associated with lower NAA/Cr values (figure 2). Higher MIP-1β levels were associated with higher Glx/Cr values (p = 0.04). In the subgroup that had matched plasma and CSF biomarkers measured, higher CSF MCP-1 (p = 0.001) and plasma MCP-1 (p < 0.001) as well as higher CSF MIP-1β levels (p = 0.06) were associated with lower NAA/Cr. Higher CSF sCD14 levels were associated with higher Cho/Cr (p = 0.04).
Mid Frontal Cortex Associations
In the analysis of all plasma biomarker data, higher MCP-1 levels were associated with lower NAA/Cr values (p=0.04) while higher sCD14 levels were related to increases in Glx/Cr (p=0.01). Higher IP-10 levels were associated with higher Cho/Cr (p=0.02). In the analysis of all CSF biomarker data, higher MCP-1 levels (p = 0.04) was associated with lower NAA/Cr. Higher MIP-1β levels (p = 0.004), higher sCD14 levels (p = 0.02), and lower IP-10 levels were associated with higher Cho/Cr values (p = 0.047). In the subgroup that had matched plasma and CSF biomarkers measured, higher MCP-1 levels in CSF (p = 0.03) were again associated with lower NAA/Cr values. Higher sCD14 levels in CSF (p = 0.02) and lower FKN levels in plasma (p=0.01) were associated with lower Glx/Cr values. Again, higher MIP-1β levels (p = 0.04) in CSF were associated with higher values Cho/Cr values.
Analyses accounting for demographic and disease covariates
All the associations remained statistically significant in the multivariable analysis with exceptions only in the basal ganglia (BG) region. In analyses of all plasma biomarker data, the p values for the associations of NAA/Cr with MCP-1 levels (p=0.08) and Glx/Cr with sCD14 levels (p=0.10) rose above 0.05. In analyses of all CSF biomarker data, the p value for the association between FKN and NAA/Cr (p=0.11) rose above 0.05. In the subgroup that had matched plasma and CSF biomarkers measured, the p values for the associations of CSF MIP-1β with NAA/Cr (p=0.82) or Cho/Cr (p=0.07) rose above 0.05.
Discussion
HAND and HIV- associated brain injury remain common complications in chronically infected patients despite cART. 25 Several recent studies including those from the HIVNC have shown persistence and progression of neuronal injury and atrophy as measured by MRS and MRI.6,7,19,26,27 Risk factors such as the nadir CD4+ T-cell count, HCV co-infection, substance abuse, and cardiovascular disease may increase the risk for neurocognitive impairment and brain atrophy.28 While the underlying mechanisms remain incompletely understood, one possible convergent mechanism is immune activation,29,30 which in turn may lead to neuronal injury. The results from the current study point to certain inflammatory markers, in particular MCP-1 and sCD14, as important factors that contribute significantly to distinct patterns of brain injury in these patients.
A strength of the study is the use of robust multivariable analyses using AIC, which allowed for a more accurate characterization of individual biomarkers, given the likelihood of collinearity between those that may reflect similar immune processes. The only similar analysis that we found was for two completely different biomarkers (neopterin and β2-microglobulin) measured in only 38 adults.31 The multivariable analyses in our study revealed that multiple biomarkers were simultaneously associated with distinct regional patterns, e.g. MCP-1 with NAA/Cr in the BG/MFC and sCD14 with metabolites in the FWM, suggesting that the pathogenesis of HIV-associated brain injury may be a multifactorial process due to the combined effects of several immune or inflammatory events.
Our study extends prior findings of this group in a different cohort where significant associations were found between CSF MCP-1 and decreases in NAA/Cr.32 MCP-1 is a potently chemotactic protein expressed on brain macrophages of adults with HIV encephalitis.33 Interestingly, CSF levels of this biomarker were elevated in adults with HIV-associated dementia even in the pre-CART era,14 while polymorphisms in the genes encoding MCP-1 and its receptor, CCR2, have been associated with increased risk of this severe form of HAND.30,34 Of note, the associations between MCP-1 and NAA/Cr reflected mostly CNS activity, where in the basal ganglia it accounted for 24 percent of the variance (Table 2), a finding that has not been previously reported. Given the importance of this region in HAND,11 the in vivo observations in this study further support the pathogenic importance of this chemokine in neuronal injury and HAND among chronically HIV- infected patients.
More than half of the statistically significant models included sCD14, a marker of activated monocytes that has been linked to HIV disease progression and HAND.13,17,35 Of note, sCD14 accounted for nearly all the associations with cerebral metabolites in FWM, including the decrease in NAA/Cr, suggesting this region may be particularly susceptible to the deleterious effects of monocyte activation and migration. As with the basal ganglia, evidence suggests that white matter plays a key role in cognitive function in HIV-infected patients and recent studies have shown significant disturbances in functional connectivity in this region in HIV-infected patients.36 MIP-1β entered over one third of statistically significant models. Elevated CSF levels of MIP-1β have been found in adults with HAND and decrease during treatment with cART.16,37 However, in contrast to MCP-1 and sCD14, the associations between MIP-1β and cerebral metabolites did not exhibit a strong regional preference but did favor the importance of CSF over blood (5 of 7 models that included MIP-1β included the CSF level).
Other soluble biomarkers were included in relatively few models. IP-10 (CXCL10) is interesting because its involvement may indicate a pathogenic role for activated T cells in the CNS,15,38 particularly in the MFC. IP-10 was associated with evidence of inflammation in this region (Cho/Cr) but not neuronal injury (NAA/Cr). The current analysis did not confirm our prior findings linking IP-10 to NAA/Cr, though the subject groups were somewhat different between the two studies, particularly in neurocognitive function (only 22% had ADC score ≥1 compared with 77% with ADC score ≥1 in the prior study).32 FKN (CX3CL1) is produced by neurons and has neuroprotective properties that were evident in the BG.12,39 Here, FKN was the only biomarker that was associated with better NAA/Cr levels. FKN is involved in crosstalk between neurons and glia and we acknowledge that it has been found to be neuroprotective in some settings but contributing to neuronal damage in others, possibly through mediation of chemotaxis.40 Our finding supports that its predominant effect in our sample was neuroprotective.
The findings for the plasma biomarkers in our study are provocative since the direction of the associations with MRS metabolites were dissimilar in some cases from those for CSF biomarkers (sCD14 with Glx in MFC, IP-10 with Cho/Cr in MFC). These discordances may reflect the pleiotropic nature of biomarkers (e.g., MIP-1β can be produced by monocytes, B-lymphocytes, T-lymphocytes) or extraneural processes that can adversely affect the nervous system. For instance, some of these same biomarkers are also associated with vascular disease,41,42 which may be an important determinant of neurocognitive functioning among adults taking suppressive cART.43
There were several limitations to this study. Reflecting when the neurocognitive data were generated, the neurocognitive assessments in our study were not based on the most recent criteria for HIV-associated neurocognitive disorder (HAND),1 which was published subsequent to the inception of our study. Our study population had a history of advanced immunosuppression (median nadir CD4+ T-cell count < 50 cells/μL) and remained relatively immunocompromised (median CD4+ T-cell count < 300 cells/μL) at the time of assessment. Thus, the findings may not reflect HIV-infected adults who have started cART at higher CD4+ T-cell counts and have not progressed to AIDS. Additionally, our study had a relatively low percentage of black and female subjects compared to the HIV-infected population in the US and thus the findings may not generalize to these demographic groups. At the time that the study was designed, the CNS effects of hepatitis C virus (HCV) were not fully appreciated. As a result, HCV serostatus was available on very few subjects, which precluded HCV-focused analyses.
We acknowledge that many other biomarkers, including neopterin, neurofilament-light, beta-2-microglobulin and interferon-alpha have been found to reflect HIV neuropathogenesis.44–46 Patients with HIV-associated dementia have particularly high concentrations of CSF neopterin and severity of ADC has been shown to correlate with CSF beta-2-microglobulin levels.45,46 However, the primary aim of our study was to focus on chemokines, and therefore we did not include an analysis of all biomarkers. We did consider the potential effect of all biomarkers on ADC stage and performed an exploratory analysis, finding that only plasma IP-10 (p=0.01) and CSF sCD14 (p=0.02) were significantly associated with ADC stage. Conclusions based on this ADC-biomarker analysis are limited due to the low number of subjects with significant neurocognitive impairment in this study. We also acknowledge that the extent to which variance was explained in this study was relatively modest. This limitation is consistent with prior analyses,32 which also did not explain substantial variance in MRS metabolites, and is mitigated by the fact that only a small number of our observed associations were substantially weakened by inclusion of demographic and disease covariates. Additional research, particularly in the form of longitudinal studies, is needed to better understand the pathogenesis and evolution of HIV-associated brain injury despite cART.
Footnotes
This work was presented in part (Poster 444) at the 18th Conference on Retroviruses and Opportunistic Infections (CROI) in Boston, Massachusetts, February 27- March 2, 2011
Disclosures/Conflict of interest:
Dr. Anderson previously received research support from Gilead Sciences. He is currently funded by NIHK23MH095679. No conflicts of interest
Dr. Harezlak is supported by NIH1R01NS080655-01A1 and NIH1R01DK089070. No conflicts of interest
Dr. Bharti is funded by NIH K23MH085512. No conflicts of interest
Dr. Mi reports no disclosures and no conflicts of interest
Dr. Taylor reports no disclosures and no conflicts of interest.
Dr. Daar has served as a consultant/advisor for Abbvie, Bristol Myers Squibb, Gilead, Janssen, Merck, Teva and Viiv as well as received research grant support from Bristol Myers Squibb, Gilead and ViiV. No conflicts of interest
Dr. Schifitto reports no disclosures and no conflicts of interest
Dr. Zhong reports no disclosures and no conflicts of interest
Dr. Alger reports no disclosures and no conflicts of interest
Dr. Brown reports no disclosures and no conflicts of interest
Dr. Singer has received support from NIH: 1U24MH100929 and 1U01MH083500 and also serves as a site investigator for Pfizer. No conflicts of interest
Dr. Campbell previously served as a consultant for Gilead Sciences. He receives funding from the following NIH grants AI069432, RR025780, HL098996, AI068614 TW008881, CA172050 and HL121816. No conflicts of interest.
Dr. McMahon reports no disclosures and no conflicts of interest
Dr. Buchthal reports no disclosures and no conflicts of interest
Dr. Cohen was funded by the following NIH grants: IH R01MH074368, 403 P01AA019072, K99AA020235, P30AI042853. He also served as a reviewer on NIH study sections of IRGs that reviewed research proposals for NIA and NIDA on topics related to HIV effects on the brain. No conflicts of interest.
Dr. Yiannoutsos reports no disclosures and no conflicts of interest
Dr. Letendre is currently funded by NIH grants R01MH092225 and K24MH097673. No conflicts of interest.
Dr. Navia reports NIH funding R01NS36524. No conflicts of interest.
References
- 1.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct 30;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Topics in HIV medicine: a publication of the International AIDS Society, USA. 2008 Jun-Jul;16(2):94–98. [PubMed] [Google Scholar]
- 3.Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ National HIVSC. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. Aids. 2003 Jul 4;17(10):1539–1545. doi: 10.1097/00002030-200307040-00015. [DOI] [PubMed] [Google Scholar]
- 4.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. Aids. 2007 Sep 12;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 5.Tozzi V, Balestra P, Serraino D, et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS research and human retroviruses. 2005 Aug;21(8):706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- 6.Cohen RA, Harezlak J, Gongvatana A, et al. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. Journal of neurovirology. 2010 Nov;16(6):435–444. doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. Aids. 2011 Mar 13;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen Y, Lenkinski RE. Recent advances in magnetic resonance neurospectroscopy. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2007 Jul;4(3):330–345. doi: 10.1016/j.nurt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacktor N, Skolasky RL, Ernst T, et al. A multicenter study of two magnetic resonance spectroscopy techniques in individuals with HIV dementia. Journal of magnetic resonance imaging: JMRI. 2005 Apr;21(4):325–333. doi: 10.1002/jmri.20272. [DOI] [PubMed] [Google Scholar]
- 10.Tracey I, Lane J, Chang I, Navia B, Lackner A, Gonzalez RG. 1H magnetic resonance spectroscopy reveals neuronal injury in a simian immunodeficiency virus macaque model. Journal of acquired immune deficiency syndromes and human retrovirology: official publication of the International Retrovirology Association. 1997 May 1;15(1):21–27. doi: 10.1097/00042560-199705010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Yiannoutsos CT, Ernst T, Chang L, et al. Regional patterns of brain metabolites in AIDS dementia complex. NeuroImage. 2004 Nov;23(3):928–935. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Cotter R, Williams C, Ryan L, et al. Fractalkine (CX3CL1) and brain inflammation: Implications for HIV-1-associated dementia. Journal of neurovirology. 2002 Dec;8(6):585–598. doi: 10.1080/13550280290100950. [DOI] [PubMed] [Google Scholar]
- 13.Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of acquired immune deficiency syndromes. 2012 Jul 1;60(3):234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Annals of neurology. 1998 Nov;44(5):831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 15.Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. Journal of neuroimmunology. 1999 Jan 1;93(1–2):172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 16.Letendre SL, Lanier ER, McCutchan JA. Cerebrospinal fluid beta chemokine concentrations in neurocognitively impaired individuals infected with human immunodeficiency virus type 1. The Journal of infectious diseases. 1999 Aug;180(2):310–319. doi: 10.1086/314866. [DOI] [PubMed] [Google Scholar]
- 17.Marcotte TD, Deutsch R, Michael BD, et al. A Concise Panel of Biomarkers Identifies Neurocognitive Functioning Changes in HIV-Infected Individuals. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2013 Oct 8; doi: 10.1007/s11481-013-9504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antiviral therapy. 2004 Jun;9(3):431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- 19.Gongvatana A, Harezlak J, Buchthal S, et al. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. Journal of neurovirology. 2013 Jun;19(3):209–218. doi: 10.1007/s13365-013-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013 Oct 17;39(4):633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in biomedicine. 2001 Jun;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 22.Lee PL, Yiannoutsos CT, Ernst T, et al. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. Journal of magnetic resonance imaging: JMRI. 2003 Jun;17(6):625–633. doi: 10.1002/jmri.10295. [DOI] [PubMed] [Google Scholar]
- 23.Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- 24.Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. Journal of pharmacokinetics and biopharmaceutics. 1994 Oct;22(5):431–445. doi: 10.1007/BF02353864. [DOI] [PubMed] [Google Scholar]
- 25.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010 Dec 7;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hua X, Boyle CP, Harezlak J, et al. Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatment. NeuroImage Clinical. 2013;3:132–142. doi: 10.1016/j.nicl.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu T, Zhong J, Hu R, et al. Patterns of white matter injury in HIV infection after partial immune reconstitution: a DTI tract-based spatial statistics study. Journal of neurovirology. 2013 Feb;19(1):10–23. doi: 10.1007/s13365-012-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen RA, Harezlak J, Schifitto G, et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. Journal of neurovirology. 2010 Feb;16(1):25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen RA, de la Monte S, Gongvatana A, et al. Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. Journal of neuroimmunology. 2011 Apr;233(1–2):204–210. doi: 10.1016/j.jneuroim.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature reviews. Immunology. 2005 Jan;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 31.Cysique LA, Moffat K, Moore DM, et al. HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a (1)H MRS study. PloS one. 2013;8(4):e61738. doi: 10.1371/journal.pone.0061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Letendre SL, Zheng JC, Kaul M, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. Journal of neurovirology. 2011 Feb;17(1):63–69. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. Aids. 1998 Jun 18;12(9):1021–1026. [PubMed] [Google Scholar]
- 34.Singh KK, Ellis RJ, Marquie-Beck J, et al. CCR2 polymorphisms affect neuropsychological impairment in HIV-1-infected adults. Journal of neuroimmunology. 2004 Dec;157(1–2):185–192. doi: 10.1016/j.jneuroim.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Nockher WA, Bergmann L, Scherberich JE. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clinical and experimental immunology. 1994 Dec;98(3):369–374. doi: 10.1111/j.1365-2249.1994.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013 Mar 26;80(13):1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monteiro de Almeida S, Letendre S, Zimmerman J, Lazzaretto D, McCutchan A, Ellis R. Dynamics of monocyte chemoattractant protein type one (MCP-1) and HIV viral load in human cerebrospinal fluid and plasma. Journal of neuroimmunology. 2005 Dec;169(1–2):144–152. doi: 10.1016/j.jneuroim.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Sui Y, Potula R, Dhillon N, et al. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. The American journal of pathology. 2004 May;164(5):1557–1566. doi: 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience. 2006 Jul;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 40.Sheridan GK, Murphy KJ. Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open biology. 2013 Dec;3(12):130181. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones KL, Maguire JJ, Davenport AP. Chemokine receptor CCR5: from AIDS to atherosclerosis. British journal of pharmacology. 2011 Apr;162(7):1453–1469. doi: 10.1111/j.1476-5381.2010.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiner AP, Lange EM, Jenny NS, et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arteriosclerosis, thrombosis, and vascular biology. 2013 Jan;33(1):158–164. doi: 10.1161/ATVBAHA.112.300421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009 Oct 20;73(16):1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson AMLD, Lennox JL, Patel N, Fritz-French C, Sethi N, Rosen J, Koneru R, Tyor WR. CSF Interferon Alpha Levels Inversely Correlate with Processing and Motor Speed in HIV-infected Subjects with Cognitive Complaints with and without HAND. 20th Conference on Retroviruses and Opportunistic Infections. 2013. Poster Abstract 437. 20th Conference on Retroviruses and Opportunistic Infections; 2013. [Google Scholar]
- 45.Brew BJ, Bhalla RB, Paul M, et al. Cerebrospinal fluid beta 2-microglobulin in patients with AIDS dementia complex: an expanded series including response to zidovudine treatment. Aids. 1992 May;6(5):461–465. [PubMed] [Google Scholar]
- 46.Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS research and therapy. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


