Abstract
Ebola viruses and Marburg viruses, members of the filovirus family, cause severe hemorrhagic fever. The ability of these viruses to potently counteract host innate immune responses is thought to be an important component of viral pathogenesis. Several mechanisms of filoviral innate immune evasion have been defined and are reviewed here. These mechanisms inclue suppression of type I interferon (IFN) production; inhibition of IFN-signaling and mechanisms that either prevent cell stress responses or allow the virus to replication in the face of such responses. A greater understanding these innate immune evasion mechanisms may suggest novel therapeutic approaches for these deadly pathogens.
The filovirus family includes the Ebola viruses and the Marburg viruses (Sanchez et al., 2007). The family is divided into the ebolavirus genus which has five species, Zaire ebolavirus, Sudan ebolavirus (SUDV), Bundibugyo ebolavirus (BDBV), Tai Forrest ebolavirus (TAFV) and Reston ebolavirus (RESTV). The marburgvirus genus consists of a single species, Marburg marburgvirus (MARV), but is divided into two clades. These are zoonotic pathogens that likely used bats as reservoir hosts (Amman et al., 2012; Pourrut et al., 2009; Towner et al., 2009). Among the various species, Reston virus is unique in that it has not been associated with human illness. Of the pathogenic members, filoviruses have been associated with repeated outbreaks of viral hemorrhagic fever with high fatality rates (Feldmann and Geisbert, 2010). Before 2014, outbreaks in human populations had been recognized in equatorial regions of Africa or arose due to export of non-human primates from this region of the continent. However, in March of 2014 an Ebola virus outbreak was recognized in the West African country of Guinea (Baize et al., 2014). This outbreak spread to the neighboring countries of Sierra Leone and Liberia, becoming the largest filovirus outbreak on record, having caused, according to World Health Organization numbers, 22,092 cases of Ebola virus disease and 8,810 deaths as of January 21, 2015 (2015). Infected individuals also brought the virus to the United States, the United Kingdom and Europe, highlighting the global public health importance of these viruses.
Many aspects of the structure and molecular biology of filoviruses are well-described (Sanchez et al., 2007). They are filamentous, enveloped, negative-sense RNA viruses. The surface of the virus has a single virus-encoded glycoprotein (GP) that mediates virus attachment and entry. Underlying the viral membrane is a viral matrix comprised mainly of viral protein 40 (VP40). Within the particle is the uncapped, single-stranded RNA genome which is coated by the viral nucleoprotein (NP). Also associated with the encapsidated genomic RNA are the virus encoded proteins VP35, VP30, VP24 and the large protein (L). The filoviral genome is approximately 19 kilobases in length and has 7 distinct transcriptional units (Figure 1). Viral genome replication and transcription, resulting in the production of 5′-capped, 3′-polyadenylated mRNAs encoding viral proteins, are carried out in the cell cytoplasm by a virus encoded RNA-dependent RNA polymerase (RDRP) complex comprised of NP, VP35, VP30 and L, the enzymatic component of the RDRP complex.
Figure 1. Genome organization of filoviruses.

The names of genes, designated according to proteins encoded by each, are indicated. NP, nucleoprotein; VP35, viral protein 35; VP40, viral protein 40; GP/sGP, glycoprotein, soluble glycoprotein; VP30, viral protein 30; VP24, viral protein 24; L, Large protein (the viral polymerase). Note that Marburg virus encodes GP but not sGP. The spacing between genes is variable and is not drawn to scale.
The connection between filovirus disease and immune evasion mechanisms
The severe disease associated with filoviral infection is characterized by systemic virus replication, which results in very high titers in the blood (Feldmann and Geisbert, 2010). A presumed consequence of this robust virus replication is the appearance of damaging host responses. These include excessive cytokine production, release of tissue factors and other mediators that contribute to a severe disease featuring liver damage, vascular leakage and bleeding(Feldmann and Geisbert, 2010). The excessive replication reflects an ability of Ebola and Marburg viruses to very effectively counteract host antiviral defenses, particularly interferon (IFN) responses, which serve as critical innate immune responses toward virus infection(Basler and Amarasinghe, 2009; Bray and Geisbert, 2005). An overview of filoviral mechanisms of innate immune evasion, including several recent developments, is provided below.
IFN responses
Type I IFNs are critical components of the innate response to viral infection (Ivashkiv and Donlin, 2014). These are a family of proteins encoded by a single IFNβ gene and multiple IFNα genes. The interplay between the type I IFN response, called IFN–α/β hereafter, and filoviruses has been studied relatively intensively. When expressed, IFN–α/β are secreted from producing cells and can signal in an autocrine or paracrine manner by binding to a heterodimeric receptor, the IFN-α/β receptor, found on the cell surface. This triggers a JAK-STAT signaling cascade that upregulates hundreds of genes that cumulatively render cells resistant to virus infection and better able to block virus replication. IFN–α/β are encoded in humans and in mice by a single IFNβ gene and multiple IFNα genes. IFN–α/β gene expression is inducible following activation of several different pattern recognition receptor pathways, including the RIG-I-like receptor (RLR) pathways, select Toll-like receptor (TLR) pathways and the STING/cGAS pathway(Brubaker et al., 2015). Most likely, two RIG-I-like receptors, RIG-I and MDA5, have the most relevance to filoviruses. This reflects the facts that the RLRs reside in the cytoplasm of cells, where filoviruses replicate, and that they detect and signal in response to RNA products of virus replication. RIG-I senses RNA molecules with features such as 5′ triphosphates and dsRNA features and, while MDA5 appears to recognize longer dsRNAs. These are features that characterize or may characterize the products of filovirus replication and purified Ebola virus genomic RNA has in fact been demonstrated to activate RIG-I.
Filovirus VP35 proteins block IFN-α/β production
One major mechanism by which filoviruses evade innate antiviral defenses is by blocking the RLR pathways that would otherwise trigger IFN-α/β production. This mechanism is carried out by the VP35 proteins of both Ebola viruses and Marburg viruses. That Ebola virus VP35 can block IFN-α/β was first suggested by the observation that VP35 expression could complement the growth of a mutant influenza A virus that was unable to counteract the IFN-α/β response (Basler et al., 2000). VP35 expression also prevented activation of the IFN-β promoter following infection by Sendai virus, a potent IFN-α/β inducer, or following transfection of the IFN-inducing mimic of virus, polyI:C (Basler et al., 2000). VP35 was subsequently demonstrated to prevent phosphorylation of interferon regulatory factor 3 (IRF-3), a transcription factor critical for induction of the IFN-β promoter (Basler et al., 2003). VP35 was also shown to impair RIG-I signaling and this inhibition correlated with the capacity of VP35 to bind to dsRNA (Cardenas et al., 2006; Hartman, Towner, and Nichol, 2004)(Figure 2). In examining the mechanisms by which VP35 carries out these immune suppressive functions, several non-mutually exclusive models are supported by existing data. Because VP35 could impair activation of the IFN-β promoter in the presence of over-expressed IKKε or TBK1, the kinases that phosphorylate and activate IRF-3, the impact of VP35 on these kinases was assessed. VP35 was demonstrated to interact in co-immunoprecipitation studies with either IKKε or TBK1 via their more conserved kinase domains. The interaction of VP35 with the kinases was sufficient to block kinase interaction with, and phosphorylation of, either IRF-3 or IRF-7 and, in vitro, resulted in the phosphorylation of VP35 (Prins, Cardenas, and Basler, 2009). While the functional consequence of VP35 phosphorylation in unclear, the ability of VP35 to prevent kinase phosphorylation of IRF-3 or IRF-7 would be expected to disrupt induction of IFN-α/β gene expression. A second inhibitory activity of VP35 that would act downstream of IKKε and TBK1 was also described. This mechanism was first suggested by yeast two-hybrid assay results, where use of VP35 as bait identified Ubc9, the E2 enzyme for SUMOylation, and protein inhibitor of activated STAT (PIAS1, a SUMO E3 ligase) as interactors. Through this interaction, VP35 enhanced SUMOylation of IRF-7 and IRF-3, likely contributing to suppression of IFN-α/β gene transcription (Chang et al., 2009).
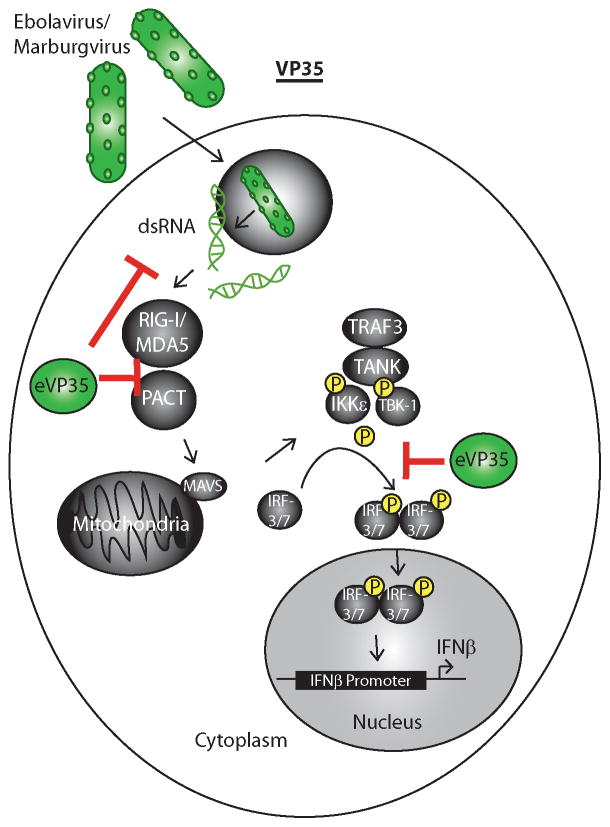
Figure 2. Filovirus VP35 proteins block RIG-I signaling at more than one step.
Filoviruses enter the host cell via micropinocytosis and escape the endosome (depicted as a circle containing a virus). The viral genome escapes into the cytoplasm where replication reactions occur. Products of viral RNA synthesis, which may include RNAs with dsRNA features (depicted) and RNA with 5′-triphosphates are recognized by RIG-I or MDA5. This, aided by host protein PACT, activates RIG-I or MDA5 signaling and stimulates a signaling pathway that leads to activation of kinases IKKε and TBK1. These phosphorylate interferon regulator factors 3 or 7 (IRF3/7) which then dimerize, move to the nucleus and contribute to IFN-α/β gene expression. VP35 can bind to dsRNA, can block PACT activation of RIG-I and can prevent IKKε and TBK1 phosphorylation of IRF-3 and IRF-7.
As noted above, VP35 is a dsRNA binding protein and point mutations that disrupt VP35 inhibition of virus or dsRNA-induced IFN-α/β responses have been described (Cardenas et al., 2006; Leung et al., 2010a). These mutations do not significantly impact VP35 function as part of the Ebola virus RDRP complex, indicating that they do not promote the misfolding of the protein (Leung et al., 2010a; Prins et al., 2010). In addition, when IKKε or TBK1 are over-expressed, the over-expression is sufficient to trigger IFN-β gene transcription. Expression of VP35 is sufficient to inhibit this activation and VP35 mutants unable to bind to dsRNA were as effective as wild-type VP35 in this assay. These same mutants are severely impaired in blocking IFN–α/β induction by Sendai virus or transfected dsRNA. Therefore, there is a dsRNA binding-dependent mechanism that makes the major contribution to suppression of RIG-I-dependent IFN-α/β responses.
Two dsRNA-dependent mechanisms of VP35 function have also been proposed: 1) the sequestration of RLR-activating RNAs and 2) the interaction of VP35 with cellular protein PACT, which interacts with RIG-I and facilitates its activation by dsRNA (Kimberlin et al.; Leung et al., 2010a; Luthra et al., 2013; Prins et al., 2010). VP35 suppresses gene silencing by small interfering RNAs (Fabozzi et al., 2011; Haasnoot et al., 2007; Zhu et al.). The biological significance of this observation is unclear, but it led investigators to examine VP35 interactions with the RNA silencing machinery(Fabozzi et al., 2011); this identified an interaction between VP35 and PACT. PACT has multiple functions, including modulation of Dicer activity and protein activation of the IFN-induced antiviral kinase, PKR (Lee et al., 2013a; Patel and Sen, 1998). However, PACT can also interact with RIG-I and promote its activation (Kok et al., 2011). Therefore, studies were performed to evaluate the consequences of VP35-PACT interaction for IFN-α/β induction via RIG-I (Luthra et al., 2013). The data demonstrated that VP35 interacts with PACT in such a manner that PACT is unable to interact with RIG-I. This impairs PACT activation of RIG-I and induction of IFN-α/β gene expression by either Sendai virus or transfected dsRNAs. Cells lacking PACT were impaired for induction of IFN-β promoter activation and therefore, introduction of VP35 into these cells had little impact. However, adding back PACT by transfection restored the IFN-α/β response and under these circumstances VP35 suppressed the response. Point mutations in VP35 that disrupted dsRNA binding abrogated VP35-PACT interaction and VP35 became unable to disrupt PACT-RIG-I interaction. This resulted in a loss of VP35 inhibition of PACT-facilitated activation of RIG-I. Given that PACT is also a dsRNA-binding protein, a dsRNA bridge between VP35 and PACT seemed plausible. However, in vitro binding studies using purified components in the absence or presence of RNAses suggest a direct protein-protein interaction. This suggests that the same VP35 amino acid residues that mediate interaction with dsRNA also contribute to direct interaction with PACT(Luthra et al., 2013). As discussed below, some of the basic residues in VP35 that directly contact the dsRNA phosphodiester backbone in VP35-dsRNA X-ray crystal structures also make VP35-VP35 contacts in the structure (Leung et al., 2010b). Therefore, it is plausible that these same residues can also participate in protein-protein interactions with a host factor.
The relevance of PACT for EBOV infection was demonstrated with PACT-over-expression and PACT knockdown studies (Luthra et al., 2013). PACT over-expression enhanced IFN-α/β responses when cells were infected with either wild-type or mutant VP35 Ebola viruses, presumably because excess PACT can overcome the inhibitory effects of even wild-type VP35. Knockdown of PACT could reverse the IFN-α/β response in cells infected with a VP35 mutant virus. These observations provide compelling evidence that interaction with PACT contributes to the immune suppression caused by VP35. However, these results do not rule out other mechanisms of inhibition, including the sequestration of RLR-activating dsRNAs (Bale et al., 2012; Bale et al., 2013; Leung et al., 2010b).
The interaction of PACT with VP35 has a second functional consequence. As noted above, VP35 plays a critical role in filoviral genome replication and mRNA synthesis, because it serves as a co-factor for the enzymatic subunit of the viral RNA-dependent RNA polymerase, the large protein (L). Through interaction with L and the viral nucleoprotein (NP), VP35 brings L to the NP-encapsidated template RNA. The filoviral RNA polymerase complex can be reconstituted by expression of its protein components and its function can be assayed by co-expression of a model viral genomic RNA encoding a reporter gene (Muhlberger et al., 1998; Muhlberger et al., 1999). When PACT is co-expressed with this system, it impairs viral RNA synthesis (Luthra et al., 2013). This impairment requires interaction with VP35, because the RNA synthesis system is affected by PACT when wild-type VP35 is used, but not when the PACT binding-defective mutants of VP35 are used in the system. Co-immunoprecipitation experiments indicated that PACT could impair VP35 interaction with L, providing a likely explanation for the inhibition. Together, these observations identify a unique impact of a host innate immune signaling protein on the function of a negative-sense RNA virus polymerase. It is intriguing to hypothesize that the filovirus RDRP complex may respond to the innate immune status of the host cell, as this would allow the virus to modulate production of RLR activating RNAs. However, it is unclear whether PACT levels or PACT availability change in response to virus infection.
In addition to suppressing IFN-α/β production, VP35 inhibits the activation of the IFN-induced, dsRNA-activated kinase PKR, a well-characterized IFN-induced, dsRNA-activated kinase with antiviral activity (Feng et al., 2007; Schumann, Gantke, and Muhlberger, 2009). Although activated by dsRNA or by PACT, some VP35 point mutants defective for dsRNA binding still inhibit PKR. Therefore, the as yet to be defined mechanism of inhibition appears to be dsRNA-binding independent. It is suggestive that PACT is a PKR activator, but a direct connection between VP35-PACT interaction and VP35-PKR inhibition has not been demonstrated. How VP35 inhibition of PKR influences EBOV replication is also, as yet, undefined; however, there is some evidence that this function can impact virus growth in vitro. Phosphorylation of PKR and phosphorylation of the PKR substrate eIF-2α are not detectable in infected 293 cells, suggesting effective suppression of PKR by the virus. Further, reactivation of lytic Ebola virus replication could be restored in persistently infected cells by modulating the status of eIF-2α phosphorylation (Schumann et al., 2009; Strong et al., 2008).
VP35 promotes suppression of dendritic cell function
Another consequence of VP35 innate immune suppression is disruption of dendritic cell (DC) maturation. DCs serve as a critical link between innate and adaptive immunity; upon encountering pathogens. DCs are important targets of Ebola and Marburg viruses in vivo (Geisbert et al., 2003); both viruses also productively replicate in DCs and potently suppress maturation and function (Bosio et al., 2003; Lubaki et al., 2013; Mahanty et al., 2003). Upon infection by Ebola virus, DCs undergo aberrant maturation where production of IFN-α/β and inflammatory cytokines and upregulation of costimulatory markers is impaired; the infected DCs also fail to activate naïve T cells. However, infection of cells with recombinant Ebola viruses expressing mutant VP35s results in DC activation, suggesting a critical role for VP35 in DC suppression (Lubaki et al., 2013). Further demonstrating this role for VP35 in DCs, VP35 expression recapitulates many of the suppressive effects seen with EBOV infection (Bosio et al., 2003; Jin et al., 2009; Yen et al.). The inhibitory role of VP35 is related to its ability to antagonize the function of RLRs, IKKε and TBK1 (Yen et al., 2014). This is evidenced by the ability of VP35 to impair several aspects of DC maturation and function induced by RLR agonists. Functions affected include IFN-α/β and cytokine production, upregulation of costimulatory markers, and T cell activation. When Toll-like receptors were activated, VP35 could impair IFN-α/β production induced by TLR4 agonist LPS, however VP35 did not impair cytokine production induced by LPS or TLR2 agonist zymosan (Leung et al., 2011; Yen et al.). This suggests that VP35 does not globally impair TLR signaling, a conclusion reached by other studies as well (Leung et al., 2011). Rather, the specific impairment of TLR-induced IFN-α/β response may reflect the inhibition of IKKε and TBK1. Although Ebola virus infection does trigger specific T cell responses in vivo, the suppression of RLR signaling and resulting block in DC maturation may slow T cell responses and contribute to the failure of adaptive immune responses. That TLR signaling can at least partially bypass the effects of VP35 suggests that it may be possible to stimulate maturation of filovirus-infected DCs.
Structural Basis for VP35 function
Because the dsRNA binding activity of VP35 correlates with its IFN-antagonist function, the basis by which VP35 binds to dsRNA is of significant interest. Biophysical and structural approaches have therefore been applied to this problem. Ebola virus VP35 possesses a carboxy-terminal domain, comprised of residues 220–340, that is sufficient to bind dsRNA (Bale et al., 2013; Leung et al., 2010a). This same domain is sufficient for suppression of IFN production, although inclusion of the amino-terminal half of VP35, which provides an oligomerization activity, enhances IFN inhibition (Leung et al., 2010a; Reid et al., 2005). Because of its role in IFN-antagonist function, the carboxy-terminal domain has been called the “interferon inhibitory domain” or IID. The X-ray crystal structure of the IID has been solved for Zaire Ebola virus, Reston virus and Marburg virus. These are very structurally similar, despite limited sequence homology (42.9% percent amino acid identity between Ebola and Marburg virus IIDs). The IID has an alpha-helical subdomain, a beta-sheet subdomain and two basic patches. The “central basic patch” (CBP) contributes to VP35 suppression of RLR signaling, whereas the “first basic patch” is important for VP35 function in the RDRP complex (Leung et al., 2010b).
Despite their similarities, some differences between the Ebola and Marburg VP35 IIDs have been described. For example, the minimum length of dsRNA bound by Ebola virus IID (8bp) is shorter than that for Marburg virus IID (12bp), and Ebola virus IID binds dsRNAs with higher affinity than does Marburg virus IID (Bale et al., 2012; Ramanan et al., 2012). There are also differences in how each IID recognizes RNA. Ebola virus IID recognizes the dsRNA phosphodiester backbone of the RNA but also engages the blunt ends of the dsRNA. In contrast, Marburg virus IID lacks the “endcapping” function of Ebola virus; this could conceivably affect the ability of Ebola versus Marburg virus VP35s to prevent recognition of 5′-triphosphate-containing dsRNA by RIG-I (Ramanan et al., 2012).
VP35 IFN-antagonist function and virulence
Studies with recombinant filoviruses bearing mutations in VP35 demonstrate that VP35 innate immune suppressing functions are important for virus propagation and virulence (Prins et al., 2010). Ebola and Marburg virus mutants, where central basic patch residues have been mutated to alanine, have been recovered. These viruses grow well in cell culture, provided Vero cells are used, because these cells are defective for IFNα/β production. In cells that have an intact IFN-α/β response, the VP35 mutant viruses elicit strong antiviral responses and exhibit impaired replication, features absent following infection with wild-type VP35 viruses. In mice, a wild-type VP35 virus grew well in infected animals, but a VP35 mutant virus grew poorly. In guinea pigs, a wild-type VP35 control was lethal, but a VP35 mutant caused no illness or death and even protected animals from challenge with wild-type virus 17 days after the initial inoculation with the mutant. Therefore, VP35 IFN-antagonist function is critical for virulence and might serve as a target for antivirals. Remaining to be clarified is the contribution of the various VP35 inhibitory activities. Mutant viruses with specific defects in PACT interaction, IKKε/TBK1 interaction or PKR inhibition, for example, would help to answer such questions.
Ebola virus VP24 and Marburg virus VP40 proteins block IFN signaling
Filovirus infected cells fail to respond to exogenously added IFN-α/β or IFNγ (Harcourt et al., 1999; Kash et al., 2006). This may contribute to the failure of IFNs as therapeutics and the facilitation of virus spread in vivo. Ebola and Marburg viruses have each been demonstrated to impair the Jak-STAT signaling normally triggered by IFNs, however, the mechanisms differ (Figure 3). For Ebola viruses, the VP24 protein is sufficient to block IFN signaling (Reid et al., 2006). When cells are infected with Ebola virus or when eVP24 is expressed, IFN addition results in STAT1 tyrosine phosphorylation, as normally occurs. However, whereas tyrosine phosphorylated STAT1 (PY-STAT1) normally localizes rapidly to the nucleus following IFN addition, this fails to happen in Ebola virus-infected or VP24-expressing cells. This effect correlates with the interaction of eVP24 with select karyopherin alpha (KPNA: also known as importin alpha) proteins (Reid et al., 2006; Reid et al., 2007). The KPNA proteins are a family of nuclear import factors that in complex with importin beta shuttle cargo through the nuclear pore (McBride and Reich, 2003). The KPNA family is divided into subfamilies. eVP24 interacts specifically with members of one subfamily, the NPI-1 KPNAs (KPNA1, KPNA5 and KPNA6), and not with other KNPAs. PY-STAT1 has the same KPNA binding profile, and interaction with these KPNAs allows the IFN-induced nuclear accumulation of PY-STAT1 necessary for IFN induction of gene expression (Reid et al., 2006; Reid et al., 2007).
Figure 3. Filoviruses block IFN signaling.
Addition of IFNα or IFNβ to cells triggers a Jak-STAT signaling pathway which leads to tyrosine phosphorylation of STAT1 and STAT2. These dimerize and enter the nucleus through interactions with karyopherin alpha (KPNA) proteins. In the nucleus, a STAT1-STAT2-IRF9 complex activates IFN-stimulated response element (ISRE)-containing promoters. This leads to the upregulation of IFN stimulated genes such as MHC Class 1 and PKR. Ebola virus VP24 (eVP24) blocks interaction of phospho-STAT1 with KPNA proteins, preventing STAT1-STAT2 nuclear import. Marburg virus VP40 (mVP40) inhibits Jak1 function.
Co-immunoprecipitation and mapping experiments suggested that eVP24 and PY-STAT1 share a common binding site on the KPNAs and that eVP24 might compete with PY-STAT1 for KPNA to block IFN responses (Reid et al., 2006; Reid et al., 2007). Biochemistry and structural biology have confirmed this proposed competition model. The X-ray crystal structure of eVP24 revealed a pyramid-shaped protein in which multiple α-helices sit on a β-sheet “pedestal” (Edwards et al.; Zhang et al., 2012). To determine how this structure interacts with KPNAs, a minimal region of KPNA5 necessary to bind to eVP24 was defined, and a co-crystal structure of this domain and eVP24 was solved (Xu et al., 2014).
KPNA proteins can be divided into distinct domains: 1) an N-terminal domain that binds importin beta is important, as importin beta mediates transport of the cargo-containing complex through the nuclear pore; 2) ten armadillo (ARM) repeats mediate interactions with nuclear localization signals (NLSs); and 3) a carboxy-terminal domain. Proteins possessing classical nuclear localization signals (cNLSs), mono- or bi-partite stretches of basic residues, bind to the central ARM repeats; however, PY-STAT1 possesses a non-classical NLS (ncNLS) and binds to a region that includes ARMS 8–10. The eVP24-KPNA5 structure identified interactions between eVP24 and each of ARM repeats 8, 9, and 10 (Edwards et al.; Xu et al.). Mutations designed based on the structure and accompanying binding studies demonstrated that interaction of eVP24 with this region of KPNA5 is needed for inhibition of PY-STAT1 nuclear accumulation. Consistent with a competition model, the same region of KPNA5 is critical for PY-STAT1, and wild-type eVP24 can disrupt PY-STAT1 binding to KPNA5. However, eVP24 mutants that do not bind KPNA5 fail to compete for binding by PY-STAT1. The interaction of eVP24 with KPNA5 does not appear to affect the cNLS binding site on KPNA nor does it affect KPNA5 interaction with a monopartite cNLS cargo. Therefore eVP24 may be fairly selective in how it impacts nuclear trafficking. This selectivity might facilitate survival of the infected cell. It remains possible, however, that bipartite cNLSs and other ncNLS cargo will be affected by eVP24 (Shabman et al., 2011; Xu et al.). Additional characterization of nuclear trafficking will therefore be needed to further clarify the full impact of Ebola virus-infection or eVP24-expression on the host cell.
Instead of blocking PY-STAT1 nuclear import, Marburg virus infection prevents the tyrosine phosphorylation events that typically characterize IFN signaling (Valmas et al., 2010). Expression of the Marburg virus VP40 protein (mVP40), but not the Marburg virus VP24 protein (mVP24), could reproduce this effect. In the case of IFN-α/β, tyrosine phosphorylation was absent for the receptor associated kinases, Jak1, Tyk2, STAT1 and STAT2 (Valmas et al., 2010).. The impact on IFNγ signaling was the same; Jak and STAT tyrosine phosphorylation was disrupted (Rodig et al., 1998; Rodriguez et al., 2004). Jak1 knockout cells also have a phenotype for the IL-6 signaling pathway where IL-6-induced STAT1 phosphorylation becomes undetectable and STAT3 phosphorylation is reduced (Rodig et al., 1998). Expression of mVP40 reproduced this phenotype as well (Valmas et al., 2010). Indications are that mVP40 specifically targets Jak1 function: while over-expressing Jak1 results in phosphorylation of JAK1, STAT1 and STAT2, expression of mVP40 prevents this. However, in a similar experiment in which Jak family kinase Tyk2 is over-expressed, mVP40 had little to no impact on Tyk2, STAT1 or STAT2 phosphorylation.
How mVP40 inhibits Jak1 remains to be defined. However, evidence suggests that the function is relevant to virus host range and pathogenesis. Marburg virus is not lethal in immune competent mice, but serial passage in mice adapts the virus such that it becomes deadly following intraperitoneal inoculation (Lofts et al., 2011; Warfield et al., 2007). Whereas the mVP40 from a parental virus effectively blocks IFN signaling in human cell lines, no inhibition was detected in mouse cell lines (Valmas and Basler, 2011). However, following mouse-adaptation of two different Marburg virus strains, Ci67 and Ravn, mVP40 had acquired the ability to inhibit IFN signaling in mouse cells (Feagins and Basler, 2015; Valmas and Basler, 2011). Mapping studies implicated different amino acid changes as sufficient to allow each mVP40 to block IFN signaling in mouse cells, suggesting a significant genetic plasticity for mVP40 IFN-antagonist function. Interestingly, mVP40 is the viral matrix protein and it directs budding of virus particles at the plasma membrane. While the parental mVP40s efficiently bud from both human and mouse cell lines, the mouse-adapted mVP40s exhibit reduced budding from human cells, but not from mouse cells. The precise mechanism by which mouse-adaptation impacts budding is not yet fully elucidated, but an association was made to the function of the IFN-inducible antiviral protein tetherin (Feagins and Basler, 2014a). In assays performed in Hepa1.6 cells, a mouse-derived cell line, human, but not mouse, tetherin could impede budding of the mouse-adapted mVP40, but not the parental mVP40. These findings suggest that there are functional costs associated with adaptation of MARV to new hosts and they also provide an intriguing link between host range, innate immune evasion and virus assembly functions.
Marburg virus VP24 interacts with Keap1 and modulates antioxidant responses
Although Marburg virus VP24 (mVP24) does not interact with KPNAs or block IFN signaling, it does modulate host responses in novel ways. A proteomics study and a yeast two hybrid screen each identified as an mVP24 interaction partner the cellular protein kelch-like ech-associated protein 1 (Keap1) (Page et al., 2014; Pichlmair et al., 2012). Keap1 is best characterized for its role in directing the Cul3 ubiquitin ligase-mediated ubiquitination and degradation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (Copple et al., 2008; Taguchi et al., 2011). Nrf2 is a transcription factor that associates with small Maf proteins and binds to promoters possessing antioxidant response element (ARE) sequences. Under normal circumstances, Nrf2 is targeted for degradation by Keap1. However, under conditions of stress, such as oxidative stress, the Keap1-Nrf2 interaction is destabilized. This reduces Nrf2 degradation, allows Nrf2 levels to increase and results in the expression of ARE genes, the products of which confer a cytoprotective state that allows cells to recover from stress (Copple, 2012). There have been increasing associations between the antioxidant response and virus infection. For example, Nrf2 has been shown to be activated by influenza virus and respiratory syncytial virus infection, where it may exert antiviral effects (Cho et al., 2009; Kesic et al., 2011). However, stimulation of cytoprotective antioxidant responses may also promote survival of virus-infected cells and regulate expression of the immunoproteasome, thereby altering antigen presentation (Burdette et al., 2010; Ivanov et al., 2011; Lee et al., 2013b; Schaedler et al., 2010). What distinguished interaction of mVP24 with Keap1 from other examples of viral engagement of the antioxidant response is the direct engagement of a viral protein with a key component of this pathway.
Cell based and in vitro studies confirmed the interaction of VP24 with the six-bladed beta sheet propeller Kelch domain of Keap1, the same domain of Keap1 that interacts with Nrf2 (Edwards et al., 2014; Page et al., 2014). On the mVP24 side, interaction requires an acid motif that is present within a loop dubbed the K-loop (Edwards et al., 2014). This motif included the amino acid residues “GE,” similar with additional acidic residues nearby, but upstream. This is similar to Keap1 interacting motifs found on other proteins including Nrf2. This motif sits at the end of extended beta-strands that project from the body of mVP24 and constitute the major structural difference between eVP24 and mVP24 (Zhang et al., 2014). Sticking out from the rest of mVP24 would allow insertion of the acidic motif into the Kelch β-propeller. That this structural motif is critical for the interaction was supported by transfer of the mVP24 acidic motif onto eVP24, which allowed eVP24 to bind Keap1, and by mutation studies where mutation of the K-loop sequence disrupted mVP24-Keap1 binding (Edwards et al., 2014).
Functionally, disruption of Keap1-Nrf2 interaction should activate ARE gene expression. mVP24 expression can interrupt binding of Keap1 to Nrf2 and turn on ARE gene expression (Edwards et al., 2014). This correlates with binding to Keap1, as eVP24 or mVP24 mutants unable to interact with Keap1 did not activate ARE gene expression. However, the eVP24-mVP24 chimera that binds to Keap1 also activates ARE genes. Expression of mVP24 could also render cells resistant to killing by menadione, a chemical that causes oxidative damage. Relevance to Marburg virus infection was suggested by a comparison of host gene expression following infection of THP-1 cells with Ebola virus or Marburg virus. Whereas Ebola virus did not cause an upregulation of ARE genes, Marburg virus infection did. One hypothesis as to why Marburg viruses evolved this interaction is that the upregulation of cytoprotective antioxidant responses may prolong the life of infected cells. In fact, Keap1 has been reported to interact with and regulate additional pathways that also influence cell survival including phosphoglycerate mutase family member 5 (pgam5), the kinase IKKβ, and autophagy factor p62 (Kim et al., 2010; Komatsu et al., 2010; Lau et al., 2010; Lee et al., 2009; Lo and Hannink, 2006). Nonetheless, the definitive consequences of mVP24-Keap1 interaction for Marburg virus remain to be defined. It is intriguing that heme oxygenase 1 (HO-1), one well-characterized ARE gene, inhibits EBOV replication (Hill-Batorski et al., 2013). Therefore, in addition to differences in terms of whether or not Ebola and Marburg viruses activate the antioxidant response, there may be differences in the consequences of such activation as well.
Upstream open reading frames in filoviral mRNAs regulate viral protein synthesis in response to cell stress
A major mechanism of stress-induced translation inhibition is the phosphorylation of translation initiation factor eIF-2α, a target of several stress-activated kinases including PKR, a long recognized IFN-α/β induced antiviral protein (Gale and Katze, 1998). However, translation of a number of cellular mRNAs is upregulated in response to cell stress that triggers eIF-2α phosphorylation. One strategy employed by several cellular mRNAs (including those encoding the ATF4, CHOP and GCN2 proteins) to overcome translation inhibition due to eIF-2α phosphorylation involves upstream AUGs (uAUGs) and upstream open reading frames (uORFs) located within the 5′-untranslated regions (UTRs)(Hinnebusch, 1997; Palam et al., 2011; Vattem and Wek, 2004; Wek et al., 2006). Under normal conditions, a complex consisting of eIF2-GTP-Met-tRNA binds to the 40S subunit forming the 43S preinitiation complex (Wong et al.). The 43S subunit scans the mRNA to a start codon where translation initiation occurs. When eIF2α~P is increased, eIF2-GTP levels decrease, and translation initiation is less efficient. In the presence of eIF2α~P, ribosome initiation at uAUGs is reduced, allowing scanning and initiation at the downstream primary ORF (pORF; referred to as the leaky scanning model).
Notably, the 5′-UTRs of several EBOV mRNAs (for VP35, VP30, VP24 and L) possess uAUGs and uORFs prior to the pORF that encodes the viral protein. When examined in transfection assays in which viral 5′UTRs were placed upstream of reporter genes, the L 5′UTR was notable because it suppressed translation of the reporter by about 10-fold, and this suppression required the presence of the uAUG within the 5′UTR. The L 5′UTR resembles the CHOP mRNA, where a single uORF modulates translation in response to cell stress (Geisbert; Palam et al., 2011). Like the situation for CHOP, under conditions of cell stress where eIF2α~P levels are increased, the EBOV L uORF promotes enhanced expression of the pORF (L) (Shabman et al., 2013). Specifically, when eIF-2α phosphorylation was induced by thapsigargin, the L 5′UTR was resistant to translation inhibition and expression increased modestly. Further, when the L uAUG was mutated in the context of a recombinant Ebola virus, virus replication was attenuated and the mutant virus was more sensitive to inhibition by thapsigargin than was wild-type virus. These data point to the L uAUG as a mechanism by which the virus can sustain the appropriate level of its polymerase even in the face of an innate immune response.
Similar to the L uORF, uORFs in VP35, VP30 and VP24 also sustain viral protein expression in the face of cell stress, such as stresses which are due to virus activation of either PKR or ER stress (which also induces eIF2α~P). Given the role of VP35 and VP24 in suppressing innate antiviral responses, it may be that their uAUGs help preserve expression of these proteins under conditions where innate immune responses have activated the PKR pathway. It is also notable that VP35 and VP30 are, along with L, essential for viral RNA synthesis (Muhlberger et al., 1998; Muhlberger et al., 1999). Perhaps, induction of eIF2α~P would result in upregulation of VP35, VP30 and L expression to sustain replication, while upregulation of VP35 and VP24 would also enhance viral counter-defenses against innate immunity.
GP inhibition of tetherin
The host protein tetherin, also known at BST2, is an IFN-induced protein that can inhibit budding and release of enveloped viruses (Neil, 2013). It also plays a role in stimulating immune responses by activating NF-kB signaling (Cocka and Bates, 2012; Tokarev et al., 2013). Tetherin expression was demonstrated to antagonize budding of the Ebola virus VP40 protein in transfection studies (Jouvenet et al., 2009). However, tetherin did not inhibit Ebola virus replication (Radoshitzky et al., 2010). Ebola virus GP was found to account for this apparent discrepancy in that expression of GP could rescue VP40 budding in the presence of tetherin (Kaletsky et al., 2009). Marburg virus GP also counteracts tetherin (Kaletsky et al., 2009). Precisely how GP exerts its anti-tetherin effects remains ambiguous; it does not seem to affect tetherin stability, remove tetherin from the plasma membrane or disrupt tetherin localization to lipid rafts (Lopez et al., 2012; Lopez et al., 2010). In addition to these mechanistic questions, it will be of interest to define the contribution of tetherin antagonism to filoviral pathogenesis. The Marburg virus VP40 variants noted above, which exhibit enhanced sensitivity to restriction by tetherin, may be useful in this regard (Feagins and Basler, 2014b). However, identification of GP mutants that lose the ability to block tetherin function may be needed to fully address this question.
Future Directions
As evidenced by the information summarized above, much is now known about filoviral innate immune evasion. Nonetheless, much remains to be learned. For example, the fact that VP35 is part of the viral RNA replication machinery and that its function as part of the viral RDRP complex is modulated by interaction with PACT suggests connections between viral innate immune evasion and function and viral replication. Similarly, the fact that uORFs modulate L expression in response to cell stress provides another link between immune escape and viral RNA synthesis. That an RNA virus might be able to adjust its genome replication rate according to the innate immune status of the host cell would make sense, as viral RNA synthesis reactions create the RNAs that activate innate immune responses. Therefore, further investigation into how these functions are integrated is warranted. Because filoviruses are zoonotic pathogens that presumably have adapted to bats, it will be important to determine how the innate immune evasion functions that work so completely in humans and non-human primates act in bats and whether the lower virulence of filoviruses in their reservoir hosts is due to less potent innate immune suppression. Animal studies demonstrate the critical role played by VP35 IFN-antagonist function in terms of causing disease. Yet, it remains unclear to what extent the filoviral innate immune evasion functions shape the adaptive immune response and to what extent factors other than VP35 contribute. Finally, with detailed mechanistic insights now available, it will be important to use this information to inform novel therapeutic approaches for these terrible pathogens.
Acknowledgments
The author thanks Christine Schwall for critical reading of the manuscript and the National Institutes of Health (R01AI05953, U19AI109945 and U19AI109664) and the Department of the Defense, Defense Threat Reduction Agency (HDTRA1-12-1-0051 and HDTRA1-14-1-0013) for financial support. The content of the information does not necessarily reflect the position or the policy of the federal government, and no official endorsement should be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ebola Situation Report. World Health Organization; Jan 21, 2015. [Google Scholar]
- 2.Amman BR, Carroll SA, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, Kemp A, Erickson BR, Comer JA, Campbell S, Cannon DL, Khristova ML, Atimnedi P, Paddock CD, Crockett RJ, Flietstra TD, Warfield KL, Unfer R, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Rollin PE, Ksiazek TG, Nichol ST, Towner JS. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 2012;8:e1002877. doi: 10.1371/journal.ppat.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keita S, De Clerck H, Tiffany A, Dominguez G, Loua M, Traore A, Kolie M, Malano ER, Heleze E, Bocquin A, Mely S, Raoul H, Caro V, Cadar D, Gabriel M, Pahlmann M, Tappe D, Schmidt-Chanasit J, Impouma B, Diallo AK, Formenty P, Van Herp M, Gunther S. Emergence of Zaire Ebola Virus Disease in Guinea - Preliminary Report. N Engl J Med. 2014 doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 4.Bale S, Julien JP, Bornholdt ZA, Kimberlin CR, Halfmann P, Zandonatti MA, Kunert J, Kroon GJ, Kawaoka Y, MacRae IJ, Wilson IA, Saphire EO. Marburg virus VP35 can both fully coat the backbone and cap the ends of dsRNA for interferon antagonism. PLoS Pathog. 2012;8:e1002916. doi: 10.1371/journal.ppat.1002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bale S, Julien JP, Bornholdt ZA, Krois AS, Wilson IA, Saphire EO. Ebolavirus VP35 coats the backbone of double-stranded RNA for interferon antagonism. J Virol. 2013;87:10385–10388. doi: 10.1128/JVI.01452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basler CF, Amarasinghe GK. Evasion of interferon responses by Ebola and Marburg viruses. J Interferon Cytokine Res. 2009;29:511–520. doi: 10.1089/jir.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basler CF, Wang X, Muhlberger E, Volchkov V, Paragas J, Klenk HD, Garcia-Sastre A, Palese P. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci U S A. 2000;97:12289–12294. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray M, Geisbert TW. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int J Biochem Cell Biol. 2005;37:1560–1566. doi: 10.1016/j.biocel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annual review of immunology. 2015 doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdette D, Olivarez M, Waris G. Activation of transcription factor Nrf2 by hepatitis C virus induces the cell-survival pathway. J Gen Virol. 2010;91:681–690. doi: 10.1099/vir.0.014340-0. [DOI] [PubMed] [Google Scholar]
- 11.Cardenas WB, Loo YM, Gale M, Jr, Hartman AL, Kimberlin CR, Martinez-Sobrido L, Saphire EO, Basler CF. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol. 2006;80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med. 2009;179:138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocka LJ, Bates P. Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 2012;8:e1002931. doi: 10.1371/journal.ppat.1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copple IM. The keap1-nrf2 cell defense pathway - a promising therapeutic target? Adv Pharmacol. 2012;63:43–79. doi: 10.1016/B978-0-12-398339-8.00002-1. [DOI] [PubMed] [Google Scholar]
- 15.Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology. 2008;246:24–33. doi: 10.1016/j.tox.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Edwards MR, Johnson B, Mire CE, Xu W, Shabman RS, Speller LN, Leung DW, Geisbert TW, Amarasinghe GK, Basler CF. The Marburg virus VP24 protein interacts with Keap1 to activate the cytoprotective antioxidant response pathway. Cell Rep. 2014;6:1017–1025. doi: 10.1016/j.celrep.2014.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabozzi G, Nabel CS, Dolan MA, Sullivan NJ. Ebolavirus proteins suppress the effects of small interfering RNA by direct interaction with the mammalian RNA interference pathway. J Virol. 2011;85:2512–2523. doi: 10.1128/JVI.01160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feagins AR, Basler CF. The VP40 protein of Marburg virus exhibits impaired budding and increased sensitivity to human tetherin following mouse-adaptation. J Virol. 2014a doi: 10.1128/JVI.02069-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feagins AR, Basler CF. The VP40 protein of Marburg virus exhibits impaired budding and increased sensitivity to human tetherin following mouse adaptation. J Virol. 2014b;88:14440–14450. doi: 10.1128/JVI.02069-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feagins AR, Basler CF. Amino acid residue 79 of Marburg virus VP40 confers interferon-antagonism in mouse cells. Journal of Infectious Diseases. 2015 doi: 10.1093/infdis/jiv010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2010;377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gale M, Jr, Katze MG. Molecular mechanisms of interferon resistance mediated by viral- directed inhibition of PKR, the interferon-induced protein kinase. Pharmacology & therapeutics. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 23.Geisbert TW. Medical research: Ebola therapy protects severely ill monkeys. Nature. doi: 10.1038/nature13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Scott DP, Kagan E, Jahrling PB, Davis KJ. Pathogenesis of Ebola Hemorrhagic Fever in Cynomolgus Macaques: Evidence that Dendritic Cells Are Early and Sustained Targets of Infection. The American journal of pathology. 2003;163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haasnoot J, de Vries W, Geutjes EJ, Prins M, de Haan P, Berkhout B. The Ebola virus VP35 protein is a suppressor of RNA silencing. PLoS Pathog. 2007;3:e86. doi: 10.1371/journal.ppat.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harcourt BH, Sanchez A, Offermann MK. Ebola virus selectively inhibits responses to interferons, but not to interleukin-1beta, in endothelial cells. J Virol. 1999;73:3491–3496. doi: 10.1128/jvi.73.4.3491-3496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill-Batorski L, Halfmann P, Neumann G, Kawaoka Y. The cytoprotective enzyme heme oxygenase-1 suppresses Ebola virus replication. J Virol. 2013;87:13795–13802. doi: 10.1128/JVI.02422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinnebusch AG. Translational regulation of yeast GCN4. A window on factors that control initiator-trna binding to the ribosome. J Biol Chem. 1997;272:21661–21664. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov AV, Smirnova OA, Ivanova ON, Masalova OV, Kochetkov SN, Isaguliants MG. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS One. 2011;6:e24957. doi: 10.1371/journal.pone.0024957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci U S A. 2009;106:2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kash JC, Muhlberger E, Carter V, Grosch M, Perwitasari O, Proll SC, Thomas MJ, Weber F, Klenk HD, Katze MG. Global suppression of the host antiviral response by Ebola- and Marburgviruses: increased antagonism of the type I interferon response is associated with enhanced virulence. J Virol. 2006;80:3009–3020. doi: 10.1128/JVI.80.6.3009-3020.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kesic MJ, Simmons SO, Bauer R, Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic Biol Med. 2011;51:444–453. doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JE, You DJ, Lee C, Ahn C, Seong JY, Hwang JI. Suppression of NF-kappaB signaling by KEAP1 regulation of IKKbeta activity through autophagic degradation and inhibition of phosphorylation. Cell Signal. 2010;22:1645–1654. doi: 10.1016/j.cellsig.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Kimberlin CR, Bornholdt ZA, Li S, Woods VL, Jr, MacRae IJ, Saphire EO. Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression. Proc Natl Acad Sci U S A. 107:314–319. doi: 10.1073/pnas.0910547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kok KH, Lui PY, Ng MH, Siu KL, Au SW, Jin DY. The double-stranded RNA-binding protein PACT functions as a cellular activator of RIG-I to facilitate innate antiviral response. Cell Host Microbe. 2011;9:299–309. doi: 10.1016/j.chom.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 39.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y, Shen J, Chen CT, Huo L, Hsu MC, Li CW, Ding Q, Liao TL, Lai CC, Lin AC, Chang YH, Tsai SF, Li LY, Hung MC. KEAP1 E3 ligase-mediated downregulation of NF-kappaB signaling by targeting IKKbeta. Mol Cell. 2009;36:131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HY, Zhou K, Smith AM, Noland CL, Doudna JA. Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 2013a;41:6568–6576. doi: 10.1093/nar/gkt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, Koh K, Kim YE, Ahn JH, Kim S. Upregulation of Nrf2 expression by human cytomegalovirus infection protects host cells from oxidative stress. J Gen Virol. 2013b;94:1658–1668. doi: 10.1099/vir.0.052142-0. [DOI] [PubMed] [Google Scholar]
- 43.Leung DW, Prins KC, Borek DM, Farahbakhsh M, Tufariello JM, Ramanan P, Nix JC, Helgeson LA, Otwinowski Z, Honzatko RB, Basler CF, Amarasinghe GK. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat Struct Mol Biol. 2010a;17:165–172. doi: 10.1038/nsmb.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung DW, Shabman RS, Farahbakhsh M, Prins KC, Borek DM, Wang T, Muhlberger E, Basler CF, Amarasinghe GK. Structural and functional characterization of Reston Ebola VP35 Interferon Inhibitory Domain. J Mol Biol. 2010b doi: 10.1016/j.jmb.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leung LW, Park MS, Martinez O, Valmas C, Lopez CB, Basler CF. Ebolavirus VP35 suppresses IFN production from conventional but not plasmacytoid dendritic cells. Immunol Cell Biol. 2011 doi: 10.1038/icb.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo SC, Hannink M. PGAM5, a Bcl-XL-interacting protein, is a novel substrate for the redox-regulated Keap1-dependent ubiquitin ligase complex. J Biol Chem. 2006;281:37893–37903. doi: 10.1074/jbc.M606539200. [DOI] [PubMed] [Google Scholar]
- 47.Lofts LL, Wells JB, Bavari S, Warfield KL. Key genomic changes necessary for an in vivo lethal mouse marburgvirus variant selection process. J Virol. 2011;85:3905–3917. doi: 10.1128/JVI.02372-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez LA, Yang SJ, Exline CM, Rengarajan S, Haworth KG, Cannon PM. Anti-tetherin activities of HIV-1 Vpu and Ebola virus glycoprotein do not involve removal of tetherin from lipid rafts. J Virol. 2012;86:5467–5480. doi: 10.1128/JVI.06280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez LA, Yang SJ, Hauser H, Exline CM, Haworth KG, Oldenburg J, Cannon PM. Ebola virus glycoprotein counteracts BST-2/Tetherin restriction in a sequence-independent manner that does not require tetherin surface removal. J Virol. 2010;84:7243–7255. doi: 10.1128/JVI.02636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luthra P, Ramanan P, Mire CE, Weisend C, Tsuda Y, Yen B, Liu G, Leung DW, Geisbert TW, Ebihara H, Amarasinghe GK, Basler CF. Mutual antagonism between the Ebola virus VP35 protein and the RIG-I activator PACT determines infection outcome. Cell Host Microbe. 2013;14:74–84. doi: 10.1016/j.chom.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McBride KM, Reich NC. The ins and outs of STAT1 nuclear transport. Sci STKE. 2003;2003:RE13. doi: 10.1126/stke.2003.195.re13. [DOI] [PubMed] [Google Scholar]
- 52.Muhlberger E, Lotfering B, Klenk HD, Becker S. Three of the four nucleocapsid proteins of Marburg virus, NP, VP35, and L, are sufficient to mediate replication and transcription of Marburg virus-specific monocistronic minigenomes. J Virol. 1998;72:8756–8764. doi: 10.1128/jvi.72.11.8756-8764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muhlberger E, Weik M, Volchkov VE, Klenk HD, Becker S. Comparison of the transcription and replication strategies of marburg virus and Ebola virus by using artificial replication systems. J Virol. 1999;73:2333–2342. doi: 10.1128/jvi.73.3.2333-2342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neil SJ. The antiviral activities of tetherin. Curr Top Microbiol Immunol. 2013;371:67–104. doi: 10.1007/978-3-642-37765-5_3. [DOI] [PubMed] [Google Scholar]
- 55.Page A, Volchkova VA, Reid SP, Mateo M, Bagnaud-Baule A, Nemirov K, Shurtleff AC, Lawrence P, Reynard O, Ottmann M, Lotteau V, Biswal SS, Thimmulappa RK, Bavari S, Volchkov VE. Marburgvirus hijacks nrf2-dependent pathway by targeting nrf2-negative regulator keap1. Cell Rep. 2014;6:1026–1036. doi: 10.1016/j.celrep.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 56.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286:10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel RC, Sen GC. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pichlmair A, Kandasamy K, Alvisi G, Mulhern O, Sacco R, Habjan M, Binder M, Stefanovic A, Eberle CA, Goncalves A, Burckstummer T, Muller AC, Fauster A, Holze C, Lindsten K, Goodbourn S, Kochs G, Weber F, Bartenschlager R, Bowie AG, Bennett KL, Colinge J, Superti-Furga G. Viral immune modulators perturb the human molecular network by common and unique strategies. Nature. 2012;487:486–490. doi: 10.1038/nature11289. [DOI] [PubMed] [Google Scholar]
- 59.Pourrut X, Souris M, Towner JS, Rollin PE, Nichol ST, Gonzalez JP, Leroy E. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect Dis. 2009;9:159. doi: 10.1186/1471-2334-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prins KC, Delpeut S, Leung DW, Reynard O, Volchkova VA, Reid SP, Ramanan P, Cardenas WB, Amarasinghe GK, Volchkov VE, Basler CF. Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. J Virol. 2010;84:3004–3015. doi: 10.1128/JVI.02459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radoshitzky SR, Dong L, Chi X, Clester JC, Retterer C, Spurgers K, Kuhn JH, Sandwick S, Ruthel G, Kota K, Boltz D, Warren T, Kranzusch PJ, Whelan SP, Bavari S. Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin. J Virol. 2010;84:10569–10580. doi: 10.1128/JVI.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramanan P, Edwards MR, Shabman RS, Leung DW, Endlich-Frazier AC, Borek DM, Otwinowski Z, Liu G, Huh J, Basler CF, Amarasinghe GK. Structural basis for Marburg virus VP35-mediated immune evasion mechanisms. Proc Natl Acad Sci U S A. 2012;109:20661–20666. doi: 10.1073/pnas.1213559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reid SP, Cardenas WB, Basler CF. Homo-oligomerization facilitates the interferon-antagonist activity of the ebolavirus VP35 protein. Virology. 2005;341:179–189. doi: 10.1016/j.virol.2005.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol. 2006;80:5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reid SP, Valmas C, Martinez O, Sanchez FM, Basler CF. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J Virol. 2007;81:13469–13477. doi: 10.1128/JVI.01097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D, Johnson EM, Jr, Schreiber RD. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez JJ, Cruz CD, Horvath CM. Identification of the nuclear export signal and STAT-binding domains of the Nipah virus V protein reveals mechanisms underlying interferon evasion. J Virol. 2004;78:5358–5367. doi: 10.1128/JVI.78.10.5358-5367.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola Viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 1410–1448. [Google Scholar]
- 69.Schaedler S, Krause J, Himmelsbach K, Carvajal-Yepes M, Lieder F, Klingel K, Nassal M, Weiss TS, Werner S, Hildt E. Hepatitis B virus induces expression of antioxidant response element-regulated genes by activation of Nrf2. J Biol Chem. 2010;285:41074–41086. doi: 10.1074/jbc.M110.145862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schumann M, Gantke T, Muhlberger E. Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain. J Virol. 2009;83:8993–8997. doi: 10.1128/JVI.00523-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shabman RS, Gulcicek EE, Stone KL, Basler CF. The Ebola virus VP24 protein prevents hnRNP C1/C2 binding to karyopherin alpha1 and partially alters its nuclear import. J Infect Dis. 2011;204(Suppl 3):S904–910. doi: 10.1093/infdis/jir323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shabman RS, Hoenen T, Groseth A, Jabado O, Binning JM, Amarasinghe GK, Feldmann H, Basler CF. An upstream open reading frame modulates ebola virus polymerase translation and virus replication. PLoS Pathog. 2013;9:e1003147. doi: 10.1371/journal.ppat.1003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strong JE, Wong G, Jones SE, Grolla A, Theriault S, Kobinger GP, Feldmann H. Stimulation of Ebola virus production from persistent infection through activation of the Ras/MAPK pathway. Proc Natl Acad Sci U S A. 2008;105:17982–17987. doi: 10.1073/pnas.0809698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 75.Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. Stimulation of NF-kappaB activity by the HIV restriction factor BST2. J Virol. 2013;87:2046–2057. doi: 10.1128/JVI.02272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Towner JS, Amman BR, Sealy TK, Carroll SA, Comer JA, Kemp A, Swanepoel R, Paddock CD, Balinandi S, Khristova ML, Formenty PB, Albarino CG, Miller DM, Reed ZD, Kayiwa JT, Mills JN, Cannon DL, Greer PW, Byaruhanga E, Farnon EC, Atimnedi P, Okware S, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Ksiazek TG, Nichol ST, Rollin PE. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5:e1000536. doi: 10.1371/journal.ppat.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valmas C, Basler CF. Marburg virus VP40 antagonizes interferon signaling in a species-specific manner. J Virol. 2011;85:4309–4317. doi: 10.1128/JVI.02575-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valmas C, Grosch MN, Schumann M, Olejnik J, Martinez O, Best SM, Krahling V, Basler CF, Muhlberger E. Marburg virus evades interferon responses by a mechanism distinct from ebola virus. PLoS Pathog. 2010;6:e1000721. doi: 10.1371/journal.ppat.1000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warfield KL, Alves DA, Bradfute SB, Reed DK, VanTongeren S, Kalina WV, Olinger GG, Bavari S. Development of a model for marburgvirus based on severe-combined immunodeficiency mice. Virol J. 2007;4:108. doi: 10.1186/1743-422X-4-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 82.Wong G, Audet J, Fernando L, Fausther-Bovendo H, Alimonti JB, Kobinger GP, Qiu X. Immunization with vesicular stomatitis virus vaccine expressing the Ebola glycoprotein provides sustained long-term protection in rodents. Vaccine. doi: 10.1016/j.vaccine.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu W, Edwards MR, Borek DM, Feagins AR, Mittal A, Alinger JB, Berry KN, Yen B, Hamilton J, Brett TJ, Pappu RV, Leung DW, Basler CF, Amarasinghe GK. Ebola Virus VP24 Targets a Unique NLS Binding Site on Karyopherin Alpha 5 to Selectively Compete with Nuclear Import of Phosphorylated STAT1. Cell Host Microbe. 16:187–200. doi: 10.1016/j.chom.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu W, Edwards MR, Borek DM, Feagins AR, Mittal A, Alinger JB, Berry KN, Yen B, Hamilton J, Brett TJ, Pappu RV, Leung DW, Basler CF, Amarasinghe GK. Ebola virus VP24 targets a unique NLS binding site on karyopherin alpha 5 to selectively compete with nuclear import of phosphorylated STAT1. Cell Host Microbe. 2014;16:187–200. doi: 10.1016/j.chom.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yen B, Mulder LC, Martinez O, Basler CF. Molecular Basis for Ebola Virus VP35 Suppression of Human Dendritic Cell Maturation. J Virol. 2014 doi: 10.1128/JVI.02163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang AP, Bornholdt ZA, Abelson DM, Saphire EO. Crystal structure of Marburg virus VP24. J Virol. 2014 doi: 10.1128/JVI.03565-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang AP, Bornholdt ZA, Liu T, Abelson DM, Lee DE, Li S, Woods VL, Jr, Saphire EO. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog. 2012;8:e1002550. doi: 10.1371/journal.ppat.1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu Y, Cherukuri NC, Jackel JN, Wu Z, Crary M, Buckley KJ, Bisaro DM, Parris DS. Characterization of the RNA silencing suppression activity of the Ebola virus VP35 protein in plants and mammalian cells. J Virol. 86:3038–3049. doi: 10.1128/JVI.05741-11. [DOI] [PMC free article] [PubMed] [Google Scholar]