Abstract
Objectives
We examined the outcomes of a cognitive-behavioral therapy (CBT) intervention for pain in pediatric sickle cell disease (SCD) using smartphones as a novel delivery method.
Methods
Forty-six children with SCD received CBT coping skills training using a randomized, waitlist control design. The intervention involved a single-session of CBT training and home-based practice using smartphones for eight weeks. Pre-post questionnaires between the randomized groups were used to evaluate changes in active psychological coping and negative thinking using the Coping Strategies Questionnaire. Daily diaries completed by the full sample during the treatment period were used to assess if CBT skill use was related to reductions in next day pain intensity and increases in same day functional activity.
Results
The pre-post group comparison suggested that youth increased active psychological coping attempts with the intervention. Daily diary data indicated that when children used CBT skills on days with higher pain, there were reductions in next day pain intensity. There was no such association between skill use and functional activity.
Discussion
CBT coping skills training supported via smartphones can increase coping and reduce pain intensity for children with SCD; however, additions to the study protocols are recommended in future studies. Advantages and caveats of using smartphones are also discussed.
Keywords: sickle cell disease, technology, cognitive-behavioral, intervention, pain
Pediatric sickle cell disease (SCD) is a group of inherited blood disorders characterized by recurrent vaso-occlusive pain episodes. Pain in SCD can have multiple effects on quality of life for children, including increased health care utilization, frequent school absences, reduced opportunities for social recreation, reductions in daily activities, and increased symptoms of anxiety and depression [1-4]. Research suggests that the majority of pain episodes in children with SCD are managed in the home environment [5]. In addition, current medical guidelines for pediatric SCD suggest that home-based medical management (e.g., fluids, oral analgesics) is the first line of care for uncomplicated pain episodes [6].
Recent efforts have focused on the provision of psychological interventions for pediatric pain as an adjunct to standard medical care. The use of cognitive-behavioral therapy (CBT) skills, in particular, has demonstrated efficacy for other pediatric recurrent and chronic pain conditions [7, 8]. In SCD, research suggests that children may benefit from an approach that allows for practicing CBT skills in the home environment to complement existing medical strategies. For example, one previous study by Gil and colleagues [9, 10] implemented a CBT intervention for pain in 46 youth with SCD using a clinician-delivered primary CBT training session and a brief booster session followed by a one-month period consisting of home-based practice with audio files and minimal therapist contact. The authors found that the intervention was effective at increasing active psychological coping in response to pain, provided transient reductions in negative thinking in response to pain, and that skill use on days with pain during the one-month follow-up period led to reduced health care utilization for pain and less activity disruption.
Although the use of CBT in pediatric SCD is promising, systematic reviews indicate that this intervention approach does not yet meet the criteria for a well-established intervention for this population [11, 12]. These reviewers have noted concerns about an overall small number of studies that have been conducted with this population, study design concerns, and incomplete data reporting across all studies examined. Intervention studies specifically focused on SCD may be needed because this population differs from other pediatric pain populations in terms of the underlying sources of pain, the typical age of onset of pain, co-morbid medical complications, and potentially in culturally-influenced preferences for pain coping [13, 14]. In addition, children with SCD may have limited access to skilled clinicians or may be unable to attend frequent intervention visits to acquire CBT skills; thus, interventions that require fewer demands in terms of therapist contact and that facilitate home-based learning of skills and practice have been encouraged [15, 16].
The use of technology may help to facilitate home-based approach for pain management in pediatric SCD. Research suggests that technology-based interventions are appealing to youth and may enhance involvement and learning in interventions [17-19]. In terms of methodology, the use of electronic diaries has also been shown to improve the accuracy of pain diaries and to increase rates of reporting in children [20]. Although a variety of modalities have been used in previous pediatric pain management studies [21-25], the use of smartphones may hold particular promise as a tool for home-based care. Specifically, smartphones are portable devices capable of both delivering coping strategies remotely through programmable applications and tracking intervention outcomes through electronic diaries and wireless technology [26, 27].
The primary aim of the present study was to describe the primary outcomes of a pain management intervention for pediatric SCD that involved a single session of CBT skills training followed by home-based practice using smartphones. Outcomes were chosen to replicate the findings of Gil and colleagues [9, 10], who found pre-post changes in active psychological coping and transient decreases in negative thinking, as well as changes in health care use and activity that were dependent on skill use. Specifically, for our pre-post analysis, we hypothesized that the intervention would result in increases in active psychological coping and decreases in negative thinking in response to pain. In addition, for our daily diary analysis, we hypothesized that children who practiced skills on days with higher pain would experience improvements in next day pain and same day activity.
Materials and Methods
Participants
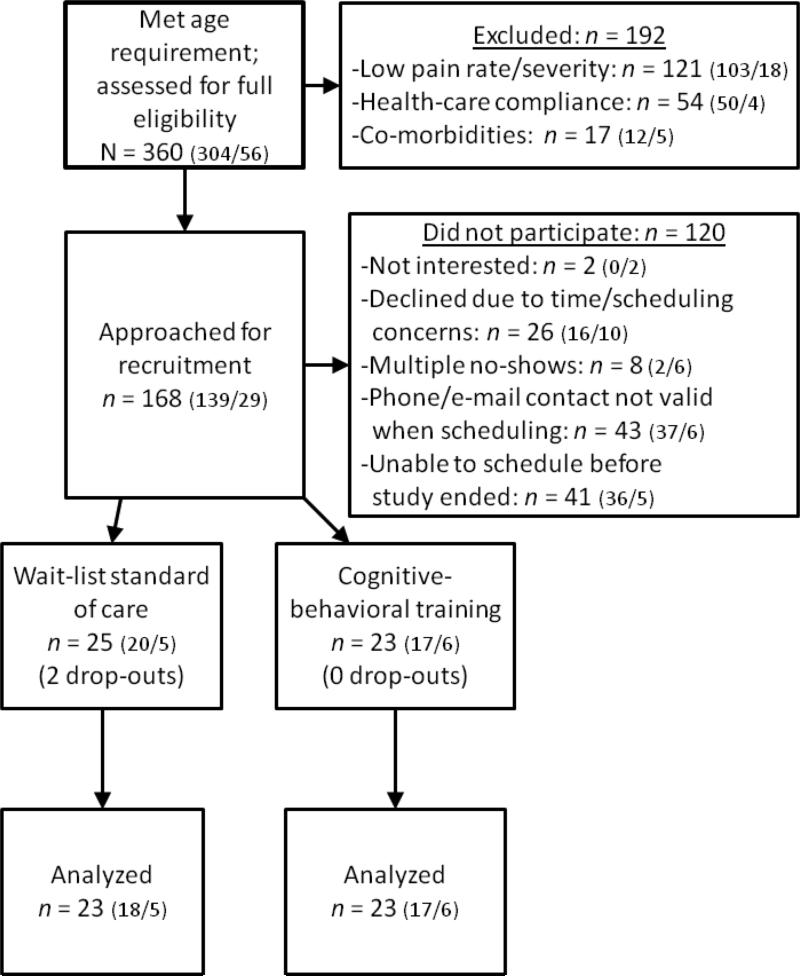
Participants were 46 children and adolescents with SCD aged 8-21 years and their primary caregivers recruited from two regional SCD specialty clinics in the Southeastern United States (see Table 1 and Figure 1). The first 17 of these participants were described in a prior report, which provided information on implementation issues for these participants, but did not report on primary treatment outcomes [26]. As shown in Figure 1, 48 children and adolescents were initially enrolled in the trial; however, two children subsequently dropped out during the waitlist period. As shown in Table 1, children in the intervention condition were significantly older than children in the waitlist condition, t(44) = 2.16, p < .05; however, there were no other significant differences between groups, including for recent laboratory data (a marker of disease status) and recent pain history. Children were recruited in two waves, the first of which occurred from January of 2007 to November of 2008 and the second of which occurred from May of 2010 to December of 2011. The recruitment gap was primarily caused by changes in project staffing and new study personnel were trained before the next wave was initiated. During the first wave, 23 children were recruited from Site 1 and three were recruited from Site 2. During the second wave, 14 were recruited from Site 1 and eight children were recruited from Site 2.
Table 1.
Descriptive Information for Study Sample
| Variable | Randomization Group | Total (N = 46) | |
|---|---|---|---|
| WLSC (n = 23) | ICBT (n = 23) | ||
| Age (M, SD) | 12.3 (2.2) | 13.8 (2.7) | 13.04 (2.5) |
| Gender (Male/Female) | 10/13 | 9/14 | 19/27 |
| Race/Ethnicity (n) | |||
| Black/African-American, non-Hispanic | 22 | 21 | 43 |
| African International | 0 | 2 | 2 |
| Biracial | 1 | 0 | 1 |
| Parental education (n) | |||
| No high school diploma | 2 | 1 | 3 |
| High school diploma | 4 | 5 | 9 |
| Some college | 9 | 9 | 18 |
| College degree | 5 | 6 | 11 |
| College degree plus post-graduate education | 3 | 2 | 5 |
| Insurance status (n) | |||
| Medicaid only | 12 | 16 | 28 |
| Medicaid plus private insurance | 3 | 3 | 6 |
| Private insurance only | 8 | 4 | 12 |
| Sickle cell genotype (n) | |||
| HbSS | 18 | 17 | 35 |
| HbSC | 2 | 2 | 4 |
| HbSβ+ thalassemia | 1 | 2 | 3 |
| HbSβ0 thalassemia | 1 | 1 | 2 |
| HbSD | 1 | 0 | 1 |
| Not specified in medical record | 0 | 1 | 1 |
| Routine CBC data (M, SD) | |||
| Hematocrit | 26.1 (3.9) | 26.5 (3.8) | 26.3 (3.9) |
| White blood cells | 10.1 (4.3) | 11.1 (4.4) | 10.6 (4.4) |
| Platelets | 363.0 (157.3) | 392.9 (96.6) | 378.0 (127.0) |
| Pain episodes during last year, parent report; (M, SD) | |||
| Resulting in inpatient and/or ER visit | 4.7 (4.8) | 3.9 (4.2) | 4.3 (4.5) |
| Managed as outpatient | 12.9 (13.0) | 16.2 (21.3) | 14.5 (17.2) |
| Pain episodes during last year, youth report, (M, SD) | |||
| Resulting in inpatient and/or ER visit | 3.8 (2.7) | 5.4 (7.4) | 4.6 (5.0) |
| Managed as outpatient | 20.9 (42.4) | 25.4 (33.9) | 23.2 (38.2) |
| Treatment history (n) | |||
| Currently taking hydroxycarbamide | 10 | 10 | 20 |
| History of transfusion(s) for acute complications | 15 | 12 | 27 |
Note. WLSC = waitlist standard of care; ICBT = immediate cognitive-behavioral training
Figure 1.
Flow of participants in randomized trial (Site 1 / Site 2)
Eligible participants had to: a) display adherence to standard SCD medical care, as evidenced by at least one annual health maintenance hematological visit and 50% attendance to scheduled specialist visits over a 24-month period; b) have had at least one major pain episode (those requiring an emergency room visit or hospitalization) or three other pain episodes in the previous six months that resulted in functional impairment; c) not be receiving chronic transfusion therapy; and d) not have cognitive limitations that would limit the validity of self-report measures. Children receiving hydroxycarbamide (a.k.a. hydroxyurea) were not automatically excluded as long as they met the study criteria. While both transfusion therapy and hydroxycarbamide can reduce pain severity, the latter treatment was much more prevalent in our clinic population and is frequently recommended for children with greater pain complications. In addition, research has shown that children continue to experience pain even with therapeutic doses of hydroxycarbamide [28, 29]. Participants taking hydroxycarbamide were excluded only if they had recently started hydroxycarbamide therapy such that the onset of a therapeutic dose, which typically takes six to eight months, might occur during study participation [30].
Procedures
Recruitment and Assignment to Study Conditions
Institutional review board (IRB) approval was obtained from each site and the investigators’ institution prior to participant recruitment. Potential participants were identified by examining the child's medical chart with the treating hematologist at each clinic. Eligible families were approached at routine hematological visits to determine interest in the study. An intake session was scheduled for that day or the earliest convenient date. As shown in Figure 1, many families were initially approached for the study, particularly for site 1; however, not all families were able to participate. One particular challenge to enrollment was that many families preferred to participate at their child's next hematological visit, which could range from 3 to 6 months later. During the interim, contact information may have changed or the family may have encountered logistical (i.e., time/transportation) barriers to participation. In addition, we had a fixed number of smartphones and resources available, so only a certain number of families could participate at any one time.
All procedures, including consent/assent, completion of questionnaires and the CBT training session (described below) for both the waitlist and intervention conditions were conducted in a private room at the child's SCD specialty clinic. Parent informed consent and child assent was obtained from families who agreed to participate. At the intake session, the parent and child completed baseline measures and were then randomly assigned to an immediate CBT intervention (ICBT) or waitlist standard of care (WLSC) condition using random assignment without replacement. Randomization was achieved by drawing colored marbles out of an opaque bag (wave 1) or by computer software using blocks of 10 (wave 2) [31]. A researcher not involved in study data collection prepared sequentially numbered, opaque, sealed envelopes that assigned each participant and were opened by the youth. Participants in the intervention condition received the CBT coping skills training immediately following intake procedures and returned for post-intervention measures eight weeks after the initial skills training session. Participants in the waitlist control group received the skills training approximately eight weeks after intake procedures and completed post-intervention measures after another eight weeks (i.e., 16 total weeks in the study). Children and caregivers received incentives for completion of baseline ($15 for caregivers, $10 for children) and follow-up measures ($10 for caregivers, $5 for children).
CBT Coping Skills Training
Our intervention approach was adapted from those described by Gil and colleagues for providing single-session training in CBT methods for pain management [9, 10]. An overview of the intervention elements are shown in Table 2. Our intervention primarily differed from that used by Gil and colleagues in two ways. First, we did not use a brief, in-person booster session described by Gil and colleagues to review the CBT methods 1-2 weeks after the training session. Second, we used smartphone technology (rather than paper-and-pencil diaries and audiotapes) as a method to make the intervention more appealing to youth, facilitate home-based practice and skill acquisition, collect daily pain diaries in real time, and monitor study engagement in real time.
Table 2.
Overview of the Study Intervention
| Intervention Elements |
|---|
| CBT Coping Skills Session |
| • Psychoeducation using participatory activities |
| ○ Pain in sickle cell disease |
| ○ Gate control theory |
| ○ Active versus passive coping |
| • Rationale and demonstration of distraction, deep breathing, progressive muscle relaxation, and guided imagery using participatory activities |
| • Demonstration of deep breathing, progressive muscle relaxation, and guided imagery using the smartphone |
| • Demonstration of how to use the daily diary using the smartphone |
| • “Show What You Know Quiz” |
|
Smartphone Applications |
| • Daily diary application to complete ratings of pain and activity |
| • Skill practice/use application to access audio files for deep breathing, progressive muscle relaxation, and guided imagery |
|
Written Materials |
| • Pain Management Flowchart |
| • Child's usual medications and how to take them (completed by family with guidance from hematology team) |
| • Reminders to use distraction, deep breathing, progressive muscle relaxation, and guided imagery |
| • Information about when to call the doctor (developed by hematologist) |
| • Coping Skills Handout |
| • Brief rationale and instructions for skills |
| • Description of the child's favorite places for guided imagery |
| • List of best activities the child generated for implementing distraction |
| • Information about the technical aspects of the smartphone |
|
Telephone Calls |
| • Weekly check-in calls with study staff (once per week) |
| • Calls to address missing diaries (after 3 consecutive days of missing diaries) |
| • Technical support for smartphone issues (as requested) |
The in-person CBT training session typically took 45-60 minutes and the instructions on the smartphone typically took 30-40 minutes. The training was delivered by a licensed clinical psychologist or a doctoral student with master's level graduate training in clinical psychology and one year of experience in delivering CBT to youth (CBM, SH, JS). The majority of participants (70%) elected to have their parent present during the skills training. Those who did not were in the older range of ages (15 to 20 years). Standardized procedures, including step-by-step guides for intake procedures and a script for the skills training, were used to ensure treatment integrity for this and all other study components. A second interventionist also intermittently observed the intervention (~20% of sessions) to monitor consistency in the intervention across study personnel, which was discussed after the session. No formal data on interventionist adherence were collected.
The training session involved participatory activities and demonstrations using materials to engage the youth in understanding pain, gate control theory, and how CBT methods help one to cope with pain via relaxation and attention control. The interventionist provided information on the importance of pain medication use, explained active versus passive coping methods, and introduced the CBT techniques that would be practiced at home, including progressive muscle relaxation, deep breathing, guided imagery, and distraction.
After the skills training, children were provided with a smartphone and were shown how to navigate the phone's features, including the coping skills program and the electronic diary. Smartphones were provided to all youth due to variable access to technology among our patient population (e.g., variability in access to the internet and/or smartphones at home). The coping skills program was designed to facilitate the practice of deep breathing, progressive muscle relaxation, and guided imagery. The program using an application that provided icons that the child would click on to start the audio file. For example, clicking on a picture of a balloon would initiate the audio file for deep breathing, and the balloon icon had been incorporated into demonstrations and written materials provided at the training session. The audiofiles were unique to the study and developed by one of the coauthors (CBM), but were modeled after widely available scripts for these activities as found in numerous CBT manuals and commercial recordings for teaching these skills. Details of how to practice the skill were reviewed and written instructions were provided in the participants’ packet of take home materials. Youth were encouraged to practice CBT techniques daily.
Participants were also taught how to complete the daily pain diary on the smartphone and how it was transmitted to the researcher instantly via electronic mail. Children, parents, and the researcher worked together to select a time of day that allowed the participant to have enough time to practice the skills and complete the diary. All children chose a priori to complete the diary in the evening, though some children occasionally reported diaries in the morning.
Handheld Smartphones
For the first wave of the study, children used a Motorola Q smartphone to practice skills and complete daily diaries. Due to changes in technology, the Motorola Q was no longer available by the second wave of the study; thus, children in the second wave used a Samsung Saga smartphone, similar in appearance and dimensions to the Motorola Q. The Samsung Saga primarily differed by having a touch screen capability. The calling capability of the phone and for-fee game downloading was disabled for the study, but children were able to access all other features of the smartphone (e.g., internet browsing). The smartphone was programmed to compile data into text files that were sent via a wireless Internet connection to a secure server. The files were date-stamped with the time that the diary was completed (not the time the file was sent). In some cases, the wireless capability malfunctioned on the phone and diaries were not immediately sent. These diaries were transmitted once the malfunction was resolved; thus, children still received credit for the diaries and they were not included in missing data calculations. During the study, upon completion of 5 diaries, each child was mailed a $5 gift card to a local department store ($1 credit per diary). Participants were specifically instructed that the incentive was provided for pain diary completion and not for skills practice. In addition, children could only receive credit for one pain diary per day.
Weekly Check-In Calls and Adherence
Within three days after the initial visit, families were contacted by phone to address questions and ensure implementation of skills. Families received a weekly telephone contact at a pre-arranged time to address any new difficulties with the protocol. Any reports of medical difficulties were relayed to clinical staff for follow-up per the consent document. If participants did not complete a pain diary for three consecutive days, they were contacted to assess barriers to adherence using either telephone, e-mail, or text messages based on family preference. The researcher recorded reasons for non-completion of pain diaries, including whether non-completion was due to pain or a pain-related health care visit (e.g., hospitalization), which allowed us to determine reasons for missing data.
Measures
Descriptive Measures
Background Information Questionnaire
Caregivers completed a questionnaire to provide basic demographic information (e.g., age and gender).
Pain History Interview
At the beginning of the study, youth and caregivers completed a pain history interview, which is a modified version of the Structured Pain Interview [32]. For the purposes of this study, the questionnaire was used to provide descriptive information about the participants’ pain history, including number of pain episodes in the previous year (both those treated at home and via medical encounter), emergency room visits, and hospitalizations for pain. This information is described in Table 1 to aid in determining generalizability of the findings to other children with SCD. Gil and colleagues [32] demonstrated temporal stability of the Structured Pain Interview in a sample of 44 caregivers of children with SCD over a period of 9 and 12 months. These authors also established that caregiver reports of health care utilization were consistent with medical records.
Quality of the CBT Training Session: Interventionist rating of child learning
The interventionist completed a rating at the end of the CBT training session evaluating “How well did the participant appear to understand the skills presented” with “very well”, “okay”, or “concerns” as the choices. Qualitative comments about the session were also logged.
Quality of the CBT Training Session: Show-what-you-know quiz
At the end of the CBT training and instruction on the smartphone, youth completed an 11-item multiple choice test regarding the content of the training session. Incorrect answers were discussed with the youth to clarify misunderstandings about the content of the CBT training.
Pre-Post Measures
Coping Strategies Questionnaire for Sickle Cell Disease (CSQ)
The CSQ is an 80-item self-report measure assessing the frequency of using different strategies to cope with pain that was administered to the youth with SCD [4]. The CSQ has three primary scales (Coping Attempts, Negative Thinking, and Passive Adherence) that are summed from 7-point ratings from “never do that” to “always do that”. In addition, there are two questions at the end of the scale that ask about beliefs about Pain Controllability. The Coping Attempts and Negative Thinking scales were central to our study hypotheses.
The Coping Attempts scale reflects the frequency of coping with CBT strategies and ranges from 0 to 180, with higher scores indicating more frequent coping attempts. The Negative Thinking Scale reflects the frequency of coping responses involving negative, pessimistic, and/or distressing thoughts and ranges from 0 to 144, with higher scores indicating higher rates of negative thinking. Although not central to our study hypotheses, the Passive Adherence scale and Pain Controllability outcomes were also evaluated. The Passive Adherence scale assesses the frequency of using coping behaviors that are prescribed through medical advice using non-psychological methods (e.g., apply heat to painful area). Scores range from 0 to 144, with higher scores indicating more frequent use of these methods. Although Pain Controllability is not a formal scale, the two items were totaled to produce a score ranging from 0 to 12, with higher scores indicating stronger beliefs that one can control and reduce pain through coping responses. CSQ items were read out loud to all children to reduce reading requirements.
The Coping Attempts and Negative Thinking scales have previously shown sensitivity to change in the context of a brief CBT intervention [9, 10]. Previous reliability data has been reported for subscales, but not at the scale-level. In the present sample the internal consistency values for the scales were .92, .91, .85, and .73 for Coping Attempts, Negative Thinking, Passive-adherence, and Pain Controllability, respectively.
Daily Diary Measures
Electronic Daily Pain and Activity Diary (DPAD)
The DPAD represents a combination of the Daily Pain Diary [33] and the Daily Home Diary [34]. The DPAD has been used successfully in SCD intervention studies with children as young as 9 years of age [35]. Participants reported morning and evening pain, participation in daily activities, and coping skill use each day. The diary could typically be completed in two to three minutes.
For pain, a yes/no format question asked about the presence or absence of morning and evening pain. A follow-up question about pain severity asked children to rate pain from 0 to 10, with higher ratings indicating more intense pain. The scale included two anchors at the 0 and 10 point marks labeled “no pain” and “worst pain”, respectively. These questions were combined to produce morning and evening pain intensity ratings that ranged from 0 to 10, which were averaged to produce a single average daily pain intensity rating. For daily activities, children were asked about their participation in six activities (playing, eating dinner, eating lunch, spending time with friends, helping around the house, and going to school) by noting less than usual, as usual, or more than usual activity. Because some children participated in the summer months, the item regarding school participation was dropped for the primary analyses. Thus, five items were tallied to produce a total activity score ranging from 5 (less activity on all activities measured) to 15 (more activity on all activities measured). We also conducted a supplemental analysis using the school participation item for participants who had at least six weeks of school attendance during the study. For this supplemental analysis, six items were tallied to produce a total activity score ranging from 6 to 18. Internal consistency for the activity scale suggested adequate reliability (α = .79 without school participation item; α = .77 with school participation item). For skill use, children were asked to record whether they practiced each of the skills in a yes/no format.
Smartphone-Recorded Logs of Skill Practice
Electronic tallies of the frequency of accessing the coping skills audio files on the handheld smartphone were sent with the daily diary text file as an unobtrusive measure of skill practice (i.e., participants were not aware that the audio files were monitored). In this study, we focused on whether children did or did not practice or use skills in a particular day, rather than total tallies of the measures. This approach was chosen to make the self-report and unobtrusive measures more directly comparable and to be consistent with our intervention approach (which emphasized the skills as a flexible menu of tools to use when in pain, rather than a prescribed series of activities). In addition, for the data analysis below, we only focused on those skills that could be practiced both with and without the device (i.e., progressive muscle relaxation, deep breathing, and guided imagery). Please note that the term “skill practice” is used to describe all skill engagement, whereas “skill use” is used to specifically denote engagement in skills on days with pain. These terms will be used throughout the analysis, results, and discussion sections for both the self-reported and smartphone-recorded outcome; however, it is acknowledged that we did not have direct observation of actual skill use.
Data Analytic Approach
Pre-Post Analysis
Pre-post data were analyzed using SPSS (version 21). Mixed factor repeated measures multivariate analysis of variance (MANOVA) was used to evaluate treatment effects (group differences over time). Time was the within-subjects factor (baseline, 8-week follow-up) and Group (WLSC, ICBT) was the between-subjects factor. A significant TimeXGroup interaction for Coping Attempts and Negative Thinking was expected according to our study hypotheses. Alpha was set to p < .05. Pillai's trace was used to calculate the multivariate F statistic. Post hoc analyses were also run with age as a covariate to address potential confounds because the ICBT group was older, on average, than the waitlist group.
Analysis of Daily Diaries
Daily diary analyses were conducted using R (version 2.15). Diaries were analyzed for all 46 participants during the intervention period of the study, which for 23 participants occurred after the 8-week waitlist period. Due to clustering of observations within children as well as serial dependency in the data, multilevel modeling was used. An auto-regressive, moving average (ARMA) structure was used to allow for dependence across time for within-person residuals. During this process, we were required to add in a time variable, in order for the error structure to estimate serial dependency according to days in the intervention. This variable (noted as Day) was not of substantive interest, but is reported in the results. We examined two models, one for next day pain intensity and one for same day activity. We were specifically interested in the interaction of pain intensity and skill use (self-reported and smartphone-recorded) predicting next day pain intensity (a lagged model) and same day activity, which would provide support for our hypothesis. To interpret the interactions, we calculated simple slopes and predicted values for a child with a moderate pain episode (e.g., pain rated as a 5 out of 10) to better understand the clinical meaningfulness of the effects. This level of pain was chosen because it is typical of a moderate pain episode that would be managed at home [36, 37].
Predictors for pain intensity (continuous), skill use (self-report, and smartphone-recorded; dichotomous), and the interaction of pain intensity and skill use (self-report and smartphone-recorded) were added to the models in hierarchical steps and evaluated using a log-likelihood method. All continuous variables were person-mean centered and dichotomous variables were centered using effects coding (e.g., 1, −1). Self-reported and smartphone-recorded skill use were included in the same model because they were only modestly associated (r = .20). In order to control for average person-level effects of pain intensity on activity, an additional variable was added at step two that contained the average levels of pain intensity for each child over the intervention period, consistent with prior studies by Gil and colleagues [9, 36]. Similar to the day variable, this component of the model is reported in the results, but is not of substantive interest.
Results
Preliminary Analyses
Quality Measures of the CBT Session
Summary data for these measures is found in Table 3. There was a moderate correlation between interventionist ratings and the child's performance on the post-intervention quiz indicating convergent validity (r = −.32, p = .030). Most youth showed evidence of learning across both quality measures; however, there was a slight trend toward lower interventionist ratings and statistically significant lower scores on the Show-what-you-know quiz for the WLST group as compared to the ICBT group.
Table 3.
Descriptive Data for Intervention Process Variables
| Variable | Randomization Group | Combined (n = 46) | |
|---|---|---|---|
| WLSC (n = 23) | ICBT (n = 23) | ||
| Interventionist rating of child's skill learning (n) | |||
| Very well | 9 | 12 | 21 |
| Okay (occasional misunderstandings or poor focus) | 12 | 11 | 23 |
| Concerns about learning of skills | 2 | 0 | 2 |
| “Show-what-you-know” post-training knowledge quiz (M, SD, in % correct; Range of raw scores) | 77.5 (12.2)* | 85.7 (11.6)* | 81.6 (11.9) |
| (6 to 11) | (7 to 11) | (6 to 11) | |
| Diary Characteristics (M, SD, Range, in days) | |||
| Diaries completed | 35.5 (12.9) | 34.6 (12.9) | 33.6 (12.9) |
| (4 to 54) | (7 to 53) | (4 to 54) | |
| Self-reported pain | 17.7 (14.9) | 16.4 (14.3) | 15.2 (13.6) |
| (0 to 53) | (0 to 37) | (0 to 53) | |
| Self-reported practice of skills | 31.8 (14.3) | 29.5 (14.4) | 27.3 (14.6) |
| (1 to 53) | (6 to 52) | (1 to 53) | |
| Smartphone-recorded practice of skills | 7.3 (8.5) | 7.0 (8.6) | 6.8 (8.7) |
| (0 to 34) | (0 to 45) | (0 to 45) | |
| Self-reported pain and self-reported use of skills | 17.0 (14.7) | 15.3 (14.0) | 13.6 (13.3) |
| (0 to 53) | (0 to 37) | (0 to 53) | |
| Self-reported pain and smartphone-recorded skill use | 4.0 (5.0) | 3.7 (5.4) | 3.4 (5.7) |
| (0 to 21) | (0 to 9) | (0 to 26) | |
Notes: WLSC = wait-list standard of care; ICBT = immediate cognitive-behavioral training
scores differed across groups, t(44) = 2.36, p = .022; possible range of scores for diary variables range from 0 to 55 days
Descriptive Information on Diary Data
For the full sample evaluated over the intervention period, children completed diaries on 1547 days (61%) out of a possible 2530 days for a missing data rate of 39%. Our rate of diary completion was higher than Gil and colleagues [9], who reported a rate of 55%, though the range of completion rates was similar (from 7 to 98% for individual child reports in the present study). Children self-reported high engagement in skills on days with pain, with skill use reported on 81.6% of days with pain (an average of individual rates for each child). According to smartphone records, children had lower engagement in smartphone-assisted skill use, with skill use recorded on 19.4% of days with pain.
Rates of accessing the skill-practice programs on the device were relatively low compared with self-report. However, 93% (43/46) of participants used the device for at least some of their CBT practice with an average of 12.9 total instances of device-assisted practice over the eight-week period (SD = 24.6, range 0 to 131). At the eight-week follow-up visit, 87% (40/46) of participants reported practicing without using the sound files on the device some of the time. Qualitative comments about why youth switched away from using the smartphone to independent practice suggested variability in beliefs as to how important the device was for skill practice and many youth reported it was easier to practice the skills without the smartphone.
Missing Diary Data
Phone records for non-completion of diaries were used to determine reasons for missing data. As noted previously, these calls were made only when three consecutive days passed with missing data; thus, records do not document periods of time without data that were less than three days long. We were able to document reasons for 34% of the missing data; for an additional 8% of the missing data, calls were made to determine the reason for the missing data, but there was no response. Of the missing data with documentation, the following reasons were reported in order of frequency: smartphone malfunction/smartphone missing (56%), life interference/life stressors (18%), pain/hospitalization (14%), forgot (10%), and no reason given (2%). In total, the amount of missing data documented due to pain represents 2% of the total amount of data possible (i.e., 2530 days). Since this amount represents about a third of all missing data, it can be estimated that the overall amount was around 6% for missing data due to pain.
Primary Analyses
Pre-Post Analysis of Coping Strategies Outcomes
The results supported the hypothesized effect for one of two CSQ outcomes. Summary data are presented in Table 4. The two groups appeared similar for baseline scores with all t-values less than one. For the primary outcome of CSQ Coping Attempts, there was a two-way interaction between Time and Group (F (1,44) = 4.91, p = .032), whereas Negative Thinking did not show significant effects for TimeXGroup (F (1,44) = 0.74, p = .394). Follow-up analyses indicated the ICBT group showed increases in Coping Attempts, t (22) = −2.64, p = .015, whereas the WLSC group did not change over time, t (22) = 0.97, p = .923.
Table 4.
Raw scores and treatment-related effect sizes for the Coping Skills Questionnaire (CSQ).
| Outcome | WLSC (n = 23) | ICBT (n = 23) | η 2 | ||
|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | ||
| CSQ (M, SD) | |||||
| Coping Attempts | 73.0 (32.2) | 72.6 (46.6) | 74.0 (31.5) | 90.8 (39.9) | .100* |
| Negative Thinking | 54.8 (22.9) | 52.9 (27.7) | 51.6 (32.7) | 45.9 (32.3) | .017 |
| Passive Adherence | 96.9 (22.6) | 93.0 (26.5) | 102.2 (19.0) | 99.8 (26.2) | .001 |
| Pain Controllability | 5.5 (1.7) | 6.0 (2.0) | 6.1 (2.5) | 7.9 (1.9) | .103* |
Notes. Eta-squared (η2) values represent pre-post repeated measures MANOVA results comparing waitlist and intervention groups over time. WLSC = waitlist standard of care; ICBT = standard of care plus immediate cognitive-behavioral training; Scales listed in bold font were a priori primary outcomes.
p < .05.
Lower order main effects were evident for CSQ Coping Attempts for Time, F (1,44) = 4.42, p = .041, η2 = .091, but not for Group, F (1,44) = 0.83, p = .367, η2 = .018. CSQ Negative Thinking did not show significant main effects for either Time, F (1,44) = 3.11, p = .085, η2 = .066, or Group, F (1,44) = 0.37, p = .545, η2 = .008.
Analyses of the other CSQ scales suggested group differences over time for Pain Controllability, but not for Passive Adherence. For CSQ Pain Controllability, there was a two-way interaction between Time and Group (F (1,44) = 5.06, p = .029). CSQ Passive Adherence showed no Time X Group intervention effect (F (1,44) = 0.65, p = .801. Follow-up analyses indicated the ICBT group showed increases in Pain Controllability, t (22) = −4.28, p = .000, whereas the WLSC group did not change over time, t (22) = −1.01, p = .323.
Lower order effects were also present for CSQ Pain Controllability. The main effects for Time, F (1,44) = 13.70, p = .001, η2 = .237, and Group, F (1,44) = 5.58, p = .023, η2 = .113, showed significant effects. CSQ Passive Adherence did not demonstrate significant main effects for Time, F (1,44) = 1.11, p = .298, η2 = .025, or for Group, F (1,44) = 0.93, p = .341, η2 = .021.
Post hoc testing examining the influence of other study factors did not change the study outcomes. Covarying age did not change the statistical significance of any CSQ findings. Exploratory analyses also indicated similar intervention effect sizes across both study sites and waves of recruitment.
Daily Diary Analysis
Next Day Pain
Results for multilevel modeling of next day pain and same day activity can be found in Table 5. For next day pain, our hypothesis for the interaction of skill use and pain intensity was supported for smartphone-recorded skill use, x2 (12) = 3.91, p = .048, but not for self-reported skill use, x2 (11) = 0.36, p = .546. Simple slopes suggested that the use of smartphone-assisted skills on days with pain attenuated next day pain (B = .37) versus no use of skills (B = .50). In other words, a child with a moderate pain episode would be predicted to have a next day pain rating of 4.40 with skill use versus 4.94 without skill use.
Table 5.
Multilevel Modeling Analysis of Next Day Pain Intensity and Same Day Activity
| Next Day Pain | B | SE | 95% CI | t |
|---|---|---|---|---|
| Step 1 | ||||
| Intercept | 2.50 | .35 | 1.81, 3.19 | 7 19*** |
| Day | .01 | .01 | −.01, .03 | 1.62 |
| Step 2 | ||||
| Pain Intensity | .49 | .03 | .43, .55 | 19.33*** |
| Person-Level Pain Intensity | 1.02 | .03 | .99, 1.05 | 40.67*** |
| Step 3 | ||||
| Self-Reported Skills | −.08 | .07 | −.22, .06 | −1.07 |
| Step 4 | ||||
| Smartphone-Recorded Skills | .03 | .07 | −.11, .17 | 0.40 |
| Step 5 | ||||
| Pain Intensity X Self-Reported Skills | .02 | .04 | −.06, .10 | 0.60 |
| Step 6 | ||||
| Pain Intensity X Smartphone-Recorded Skills | −.06 | .03 | .00, −.12 | −1.97* |
| Same Day Activity | B | SE | 95% CI | t |
|---|---|---|---|---|
| Step 1 | ||||
| Intercept | 9.41 | .19 | 9.04, 9.76 | 49.67*** |
| Day | .00 | .01 | −.02, .02 | −0.46 |
| Step 2 | ||||
| Pain Intensity | −.21 | .02 | −.17, −.24 | −11.44*** |
| Person-Level Pain Intensity | −.10 | .08 | −.26, .06 | −1.15 |
| Step 3 | ||||
| Self-Reported Skills | .02 | .07 | −.12, .16 | 0.26 |
| Step 4 | ||||
| Smartphone-Recorded Skills | −.10 | .06 | −.22, .02 | −1.66 |
| Step 5 | ||||
| Pain Intensity X Self-Reported Skills | −.04 | .03 | −.10, .02 | −1.56 |
| Step 6 | ||||
| Pain Intensity X Smartphone-Recorded Skills | .01 | .02 | −.03, .05 | 0.58 |
p < .05
**p < .01
p < .001
For main effects, same day pain intensity predicted significantly higher next day pain levels. The addition of same day pain intensity and the person-level average pain intensity significantly improved model fit, x2 (8) = 186.44, p < .001. The addition of self-reported, x2 (9) = 1.15, p = .283, and smartphone-recorded skill use, x2 (10) = 0.16, p = .691, did not improve fit.
Same Day Activity
For same day activity, our hypothesis for the interaction of skill use and activity engagement was not supported for either self-reported, x2 (13) = 2.43, p = .119, or smartphone-recorded skill use, x2 (14) = 0.33, p = .563. Of note is that the interaction of same day pain intensity and self-reported skill use was influenced by outlying values, such that the deletion of a single case resulted in the effect approaching significance; however, the beta coefficient was not in the hypothesized direction and suggested that children who self-reported using skills had greater reductions in activity on days with higher pain (B = −.05).
For main effects, the addition of same day pain intensity and the person-level average pain intensity significantly improved model fit, x2 (10) = 121.50, p < .001. The addition of self-reported, x2 (11) = 0.07, p = .798, and smartphone-recorded skill use, x2 (12) = 2.74, p = .098, did not improve model fit.
For the subset of children with data on school attendance (N = 38, 1333 diaries), the results were largely unchanged. The interaction of skill use and same day pain intensity did not significantly improve model fit for self-reported, x2 (13) = 0.72, p = .395, or smartphone-recorded skill use, x2 (14) = 0.21, p = .649. For main effects, the addition of same day pain intensity and the person-level average pain intensity significantly improved model fit, x2 (10) = 115.57, p < .001. The addition of self-reported, x2 (11) = 0.00, p = .958, and smartphone-recorded skill use, x2 (12) = 0.49, p = .452, did not improve model fit. Post hoc analyses examining for differential effects by recruitment site or recruitment wave did not demonstrate any significant differences for next day pain or same day activity based on these factors.
Discussion
This study evaluated the primary outcomes of a randomized clinical trial for pain management in pediatric SCD using smartphones. The impact of the intervention was evaluated through both pre-post questionnaire data comparing wait-list to immediate treatment groups as well as through daily diary data from the treatment period for the full sample. The results of the pre-post, randomized comparison indicated that youth increased their self-reported use of CBT skills with the intervention and reported increased beliefs in pain controllability; however, negative thinking in response to pain, a primary outcome for the study, was unchanged. Results from the daily diary data further suggested that children who used smartphone-guided CBT skills on days when they reported higher pain had overall reductions in next day pain intensity relative to similar days when they did not use CBT skills. These specific findings, along with limitations and recommendations for future studies, are described below.
The pre-post comparison of WLSC to ICBT indicated an overall increase in self-reported active psychological coping in the ICBT group, which is consistent with a prior study by Gil and colleagues [9]. We did not observe significant changes in self-reported negative thinking; however, prior studies by these authors found an immediate, transient effect after the coping skills sessions that was no longer present after home-based practice [9, 10]. Cumulatively, these findings suggest that additional strategies and/or assistance from clinicians may be necessary to evidence long-term improvements in negative thinking. In the present study, we did not provide formal training in cognitive restructuring or instruction on using specific skills to alter negative thinking. These modifications are recommended in future studies, particularly those involving adolescents, who tend to exhibit higher levels of negative thinking in relation to pain versus younger children [38]. Although pain controllability was not the focus of the present study, we did observe positive changes in this outcome in the ICBT group. This component of the CSQ has not been the focus of previous studies, but may be worth including in future studies as a measure of beliefs about pain.
For the daily dairy data, we found that smartphone-assisted skill use was associated with a beneficial effect on next day pain intensity, which we used as an alternative measure to Gil and colleagues’ [9] findings for skill use dependent reductions in health care utilization for pain. This effect was not supported with the self-reported measure of skill use. These results may indicate that smartphone-assisted use of coping skills (rather than independent skill use) was necessary to achieve reductions in pain. Our predicted values also suggested that the effect of smartphone-assisted skill use on next day pain was fairly small (the equivalent of about a half-point change on a 10-point scale for a child with a moderate pain episode). Future studies should consider methods for increasing smartphone-assisted skill use as this method was used to practice skills on less than one fourth of study days with pain. It may also be useful to determine which characteristics of the participants or facets of the intervention are associated with higher rates of skill use in order to inform future intervention protocols.
In terms of activity, we did not observe a general benefit of skill practice, which stands in contrast to Gil and colleagues, who found reduced activity disruption for school and household activities [9]. Both the present study and that of Gil and colleagues specifically targeted pain, but not activity. In addition, children with SCD are often taught to reduce activity and to rest during acute episodes [6]. In the absence of specific education about how to safely improve activity, children in the present study may not have used the coping skills as a way to achieve higher engagement in functional activities. Studies aimed at improving daily activity may consider additions to the treatment protocol that specifically address activity or that provide additional skills for managing activity, such as pleasant events scheduling or activity pacing, which have been used in previous pain management studies with other populations [21, 39].
A particular strength of the current study was the use of self-reported and smartphone-recorded skill practice and use, which provided both subjective and unobtrusive indices of engagement in the CBT skills. We noticed a large discrepancy in self-reported and smartphone-recorded skill practice, with children self-reporting approximately four times more skill use than what the smartphone recorded. This discrepancy indicates that self-report and smartphone records may be capturing different aspects of skill practice. For example, in our post-intervention questions about study participation, children typically reported practicing skills independently from the smartphone before the end of the eight-week intervention period and the discrepancy between self-report and smartphone-recorded skill use varied widely across participants. Thus, some of the discrepancy may be due to children's preferences to practice with or without the device. It is also possible that children reported using skills out of social desirability or wanting to complete the diary quickly. Future studies may consider communicating stronger messages about the importance of device use for skill practice/use, alterations to the smartphone programs to make guided-practice more attractive, or working with caregivers to more actively promote skill practice and use. In addition, future work should consider using proxy measures (e.g., parent report) of skill practice for additional information about study engagement.
The findings of this study should be interpreted in the context of limitations. First, there are potential issues related to statistical power and the meaningfulness of the observed effect sizes. We attempted to balance Type I and Type II errors by using a test-wise alpha level of .05 rather than a more conservative criteria. Inadequate power and Type II errors are of potential concern given the small sample size the testing of interaction effects to address study hypotheses. The null results for the primary outcome of negative thinking on the CSQ showed a very small effect size (equivalent of less than 2% variance) that is unlikely to be clinically meaningful. We were able to detect intervention effects that accounted for approximately 10% of explained variance for the CSQ Coping Attempts scale. This effect size is similar that found with other outcomes in interventions for pediatric pain [21, 23]; however, this is in the range of “medium-size” effects using Cohen's conventions and it is unclear whether this degree of change in self-reported coping is clinically meaningful [40]. Likewise, for decreases in pain intensity with skill use it is unclear if the estimated improvement of approximately 0.5 points on the 11-point pain scale for a moderate pain episode is a meaningful decrease in pain intensity. For the daily diary analyses, the models were able to detect very small effects due to the large number of observations (equivalent of less than 1% variance); thus, it is unlikely that we were underpowered in these analyses: The predicted findings for self-reported skill use in the models for pain intensity and activity were in the wrong direction and the predicted finding for smartphone-recorded skill use for activity was in the correct direction for activity, but the effect was very small (B = −.20 for skill use versus B = −.22 for no skill use). Overall, the meaningful of the observed intervention effects is open to interpretation, but we are not concerned about Type II errors for these data.
Second, there was a missing diary data rate of 39%. Previous studies have noted the possibility that children with SCD are missing data due to pain or a pain-related health care visit (e.g., hospitalization). This type of missing data is systematic and can pose problems for making inferences about models focused on the effects of pain [9]. Our estimate of 6% missing data due to pain suggests that this issue may not have been a problem in the present study; however, this estimate was extrapolated from records that documented reasons for only 34% of missing data. It is also notable that the majority of documented missing data was due to reported smartphone malfunction or children losing track of the smartphone. Future studies may consider using forms of electronic technology that have greater flexibility (e.g., internet-based access not dependent on a single device) to improve rates of diary completion. Strengths of the current study included the use of electronic diaries and wireless technology, which allowed us to immediately determine when children were not completing diaries and document reasons for missing data. Future studies should consider implementing similar procedures in order to better understand the frequency of missing data due to pain.
Third, the methods of the current study were not an exact replication of previous research. Although the coping skills taught to children were consistent with a previous study in pediatric SCD [9, 10], the use of smartphones to deliver skills may have resulted in different effects on pain and activity. For example, children may have been more focused on using the smartphone for enjoyment rather than to practice skills. We also did not include the brief booster session and increased the practice period to eight weeks (versus a six-to-seven weeks post CBT training follow-up in the prior study). In addition, our measure of activity differed from the previous study. Health care utilization was not included as an outcome because our clinics, consistent with the national trend, focus more on home-based pain management than in decades past [6]. We also chose only two primary outcomes within the diary because we wanted to limit the time and effort needed to complete the diary. Other outcomes may be more responsive to the effects of CBT skills, such as changes in self-efficacy for managing pain, mood, or beliefs about pain [9, 41-43]. The PedIMMPACT initiative also provides suggestions for additional outcome measures that may be adapted for electronic diaries in future studies [44].
Finally, children recruited for this study met specific criteria that may not generalize to the overall population of children with SCD pain. For example, all children recruited for the study had to have a history of recent pain. Children in our study and their caregivers generally reported a history of more frequent pain than reported in more general samples from similar clinic settings, which show approximate averages of one-to-two episodes per year of pain treated in a hospital setting and ten episodes per year of pain treated at home [45-47]. In addition, children had to be adherent with medical care and must have received treatment through specialized SCD clinics.
This study also highlights specific advantages and caveats of using smartphone technology. Advantages include the portability of the smartphone for remote, home-based pain management, higher rates of diary completion, the ability to closely monitor diary completion and skill use, and the ability to obtain both self-report and an unobtrusive measures of skill practice. Caveats include potential issues with youth losing track of the device, potential limitations in the number of outcome measures that are feasible with a smartphone-based electronic diary, and the attractiveness of the smartphone's features becoming a distraction from the intervention. For this project we provided smartphones due to concerns about availability; however, since initiating this project there is a marked increase in access to smartphones among families at our clinics, which is consistent with national trends [48]. In addition, race/ethnicity differences in smartphone access appear to be very modest compared to the discrepancies found for internet access via personal computers [48]. A more critical barrier than e-technology access that we encountered in recruitment was arranging for the in-person consent, completion of study questionnaires, and CBT training session. Future intervention attempts may need to consider measuring outcomes strictly via diary data and other steps that could reduce the length of the initial in-person session.
In conclusion, the present study suggests that a single session of CBT skills followed by home-based practice with smartphones can have beneficial effects in terms of increasing active psychological coping and reducing next day pain; however, modifications to the protocol are recommended for future studies. Specifically, future studies should consider additional training in cognitive restructuring to target negative thinking, identify methods for improving engagement in smartphone-assisted use of CBT skills, and should consider the addition of information about how to safely increase activity when in pain.
Supplementary Material
Acknowledgements
This study is registered at ClinicalTrials.gov (ID: NCT00386048). The data analysis for this paper was conducted with the assistance of Multilevel Modeling, an advanced quantitative course taught by Dr. Lee Van Horn, Associate Professor, in the Department of Psychology at the University of South Carolina.
Funding
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (R21HL0923365 to J.S. and C.B.M and T32 GM081740 and F31HL108582 to A.M.S.).
Footnotes
Conflicts of Interests
The authors declare no conflicts of interest.
References
- 1.Anie KA. Psychological complications in sickle cell disease. Br J Haematol. 2005;129:723–729. doi: 10.1111/j.1365-2141.2005.05500.x. [DOI] [PubMed] [Google Scholar]
- 2.Edwards CL, Scales MT, Loughlin C, Bennett GG, Harris-Peterson S, De Castro LM, Whitworth E, Abrams M, Feliu M, Johnson S, Wood M, Harrison O, Killough A. A brief review of the pathophysiology, associated pain, and psychosocial issues in sickle cell disease. Int J Beh Med. 2005;12:171–179. doi: 10.1207/s15327558ijbm1203_6. [DOI] [PubMed] [Google Scholar]
- 3.Fuggle P, Shand PA, Gill LJ, Davies SC. Pain, quality of life, and coping in sickle cell disease. Arch Dis Child. 1996;75:199–203. doi: 10.1136/adc.75.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gil KM, Williams DA, Thompson RJ, Kinney TR. Sickle cell disease in children and adolescents: the relation of child and parent pain coping srategies to adjustment. J Pediatr Psychol. 1991;16:643–663. doi: 10.1093/jpepsy/16.5.643. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro BS, Dinges DF, Orne EC, Bauer N, Reilly LB, Whitehouse WG, Ohene-Frempong K, Orne MT. Home management of sickle cell-related pain in children and adolescents: natural history and impact on school attendance. Pain. 1995;61:139–44. doi: 10.1016/0304-3959(94)00164-A. [DOI] [PubMed] [Google Scholar]
- 6.AAP Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526–35. doi: 10.1542/peds.109.3.526. [DOI] [PubMed] [Google Scholar]
- 7.Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain. 2010;148:387–397. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eccleston C, Palermo TM, Williams AC, Lewandowski A, Morley S. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2009:CD003968. doi: 10.1002/14651858.CD003968.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Gil KM, Anthony KK, Carson JW, Redding-Lallinger R, Daeschner CW, Ware RE. Daily coping practice predicts treatment effects in children with sickle cell disease. J Pediatr Psychol. 2001;26:163–73. doi: 10.1093/jpepsy/26.3.163. [DOI] [PubMed] [Google Scholar]
- 10.Gil KM, Wilson JJ, Edens JL, Workman E, Ready J, Sedway J, Redding-Lallinger R, Daeschner CW. Cognitive coping skills training in children with sickle cell disease pain. Int J Beh Med. 1997;4:364–77. doi: 10.1207/s15327558ijbm0404_7. [DOI] [PubMed] [Google Scholar]
- 11.Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database Syst Rev. 2012;2:CD001916. doi: 10.1002/14651858.CD001916.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Chen E, Cole SW, Kato PM. A review of empirically supported psychosocial interventions for pain and adherence outcomes in sickle cell disease. J Pediatr Psychol. 2004;29:197–209. doi: 10.1093/jpepsy/jsh021. [DOI] [PubMed] [Google Scholar]
- 13.Schlenz AM, Schatz J, McClellan CB, Sweitzer SM, Roberts CW. Information-seeking coping behaviors during painful procedures in African-American children with sickle cell disease. Pain Manag Nurs. 2013;14:e54–e58. doi: 10.1016/j.pmn.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekkers OM, von Elm E, Algra A, Romijn JA, Vandenbroucke JP. How to assess the external validity of therapeutic trials: a conceptual approach. Int J Epidemiol. 2010;39:89–94. doi: 10.1093/ije/dyp174. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz LA, Radcliffe J, Barakat LP. The development of a culturally sensitive pediatric pain management intervention for african american adolescents with sickle cell disease. Child Health Care. 2007;36:267–283. doi: 10.1080/02739610701377954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barakat LP, Schwartz LA, Salamon KS, Radcliffe J. A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease. J Pediatr Hematol Oncol. 2010;32:540–7. doi: 10.1097/MPH.0b013e3181e793f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan DS, Callahan CW, Hatch-Pigott VB, Lawless A, Proffitt HL, Manning NE, Schweikert M, Malone FJ. Internet-based home monitoring and education of children with asthma is comparable to ideal office-based care: results of a 1-year asthma in-home monitoring trial. Pediatrics. 2007;119:569–78. doi: 10.1542/peds.2006-1884. [DOI] [PubMed] [Google Scholar]
- 18.Kumar VS, Wentzell KJ, Mikkelsen T, Pentland A, Laffel LM. The DAILY (Daily Automated Intensive Log for Youth) trial: a wireless, portable system to improve adherence and glycemic control in youth with diabetes. Diabetes Technol Ther. 2004;6:445–53. doi: 10.1089/1520915041705893. [DOI] [PubMed] [Google Scholar]
- 19.Shegog R, Bartholomew LK, Parcel GS, Sockrider MM, Masse L, Abramson SL. Impact of a computer-assisted education program on factors related to asthma self-management behavior. J Am Med Inform Assoc. 2001;8:49–61. doi: 10.1136/jamia.2001.0080049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palermo TM, Valenzuela D, Stork PP. A randomized trial of electronic versus paper pain diaries in children: impact on compliance, accuracy, and acceptability. Pain. 2004;107:213–9. doi: 10.1016/j.pain.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an Internet-delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146:205–13. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato AF, Clifford LM, Silverman AH, Davies WH. Cognitive-behavioral interventions via telehealth: applications to pediatric functional abdominal pain. Child Health Care. 2009;38:1–22. [Google Scholar]
- 23.Connelly M, Rapoff MA, Thompson N, Connelly W. Headstrong: a pilot study of a CDROM intervention for recurrent pediatric headache. J Pediatr Psychol. 2006;31:737–47. doi: 10.1093/jpepsy/jsj003. [DOI] [PubMed] [Google Scholar]
- 24.Stinson JN, McGrath PJ, Hodnett ED, Feldman BM, Duffy CM, Huber AM, Tucker LB, Hetherington CR, Tse SM, Spiegel LR, Campillo S, Gill NK, White ME. An internet-based self-management program with telephone support for adolescents with arthritis: a pilot randomized controlled trial. J Rheumatol. 2010;37:1944–52. doi: 10.3899/jrheum.091327. [DOI] [PubMed] [Google Scholar]
- 25.Hicks CL, von Baeyer CL, McGrath PJ. Online psychological treatment for pediatric recurrent pain: a randomized evaluation. J Pediatr Psychol. 2006;31:724–36. doi: 10.1093/jpepsy/jsj065. [DOI] [PubMed] [Google Scholar]
- 26.McClellan CB, Schatz JC, Puffer E, Sanchez CE, Stancil MT, Roberts CW. Use of handheld wireless technology for a home-based sickle cell pain management protocol. J Pediatr Psychol. 2009;34:564–73. doi: 10.1093/jpepsy/jsn121. [DOI] [PubMed] [Google Scholar]
- 27.Luxton DD, McCann RA, Bush NE, Mishkind MC, Reger GM. mHealth for mental health: integrating smartphone technology in behavioral healthcare. Prof Psychol - Res Pr. 2011;42:505–512. [Google Scholar]
- 28.Ballas SK, Barton FB, Waclawiw MA, Swerdlow P, Eckman JR, Pegelow CH, Koshy M, Barton BA, Bonds DR. Hydroxyurea and sickle cell anemia: effect on quality of life. Health Qual Life Outcomes. 2006;4:59. doi: 10.1186/1477-7525-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koren A, Segal-Kupershmit D, Zalman L, Levin C, Abu Hana M, Palmor H, Luder A, Attias D. Effect of hydroxyurea in sickle cell anemia: a clinical trial in children and teenagers with severe sickle cell anemia and sickle cell beta-thalassemia. Pediatr Hematol Oncol. 1999;16:221–32. doi: 10.1080/088800199277272. [DOI] [PubMed] [Google Scholar]
- 30.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–11. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piantadosi S. Randomization [ computer software] 2008 Available from: http://cshspccweb.csmc.edu/biostats/
- 32.Gil KM, Abrams MR, Phillips G, Keefe FJ. Sickle cell disease pain: relation of coping strategies to adjustment. J Consult Clin Psych. 1989;57:725–731. doi: 10.1037//0022-006x.57.6.725. [DOI] [PubMed] [Google Scholar]
- 33.Gil KM. Behavioral assessment of sickle cell disease pain. J Health Soc Policy. 1994;5:19–38. doi: 10.1300/J045v05n03_03. [DOI] [PubMed] [Google Scholar]
- 34.Dinges DF, Whitehouse WG, Orne EC, Bloom PB, Carlin MM, Bauer NK, Gillen KA, Shapiro BS, Ohene-Frempong K, Dampier C, Orne MT. Self-hypnosis training as an adjunctive treatment in the management of pain associated with sickle cell disease. Int J Clin Exp Hypn. 1997;45:417–32. doi: 10.1080/00207149708416141. [DOI] [PubMed] [Google Scholar]
- 35.Powers SW, Mitchell MJ, Graumlich SE, Byars KC, Kalinyak KA. Longitudinal assessment of pain, coping, and daily functioning in children with sickle cell disease receiving pain management skills training. J Clin Psychol Med S. 2002;9:109–119. [Google Scholar]
- 36.Gil KM, Porter LS, Ready J, Workman E, Sedway J, Anthony KK. Pain in children and adolescents with sickle cell disease: an analysis of daily pain diaries. Child Health Care. 2000;29:225–241. [Google Scholar]
- 37.Schwartz JE, Stone AA. Strategies for analyzing ecological momentary assessment data. Health Psychol. 1998;17:6–16. doi: 10.1037//0278-6133.17.1.6. [DOI] [PubMed] [Google Scholar]
- 38.Gil KM, Thompson RJ, Keith BR, Tota-Fauccette M, Noll S, Kinney TR. Sickle cell disease pain in children and adolescents: change in pain frequency and coping strategies over time. J Pediatr Psychol. 1993;18:621–637. doi: 10.1093/jpepsy/18.5.621. [DOI] [PubMed] [Google Scholar]
- 39.Nielson WR, Jensen MP, Karsdorp PA, Vlaeyen JW. Activity pacing in chronic pain: concepts, evidence, and future directions. Clin J Pain. 2013;29:461–468. doi: 10.1097/AJP.0b013e3182608561. rther development of the multidimensional pain readiness to change questionnaire: the MPRCQ2. J Pain 2008;9:552-65.
- 40.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition. Lawrence Erlbaum Associates; Hillsdale, JN: 1988. [Google Scholar]
- 41.Gil KM, Carson JW, Sedway JA, Porter LS, Schaeffer JJ, Orringer E. Follow-up of coping skills training in adults with sickle cell disease: analysis of daily pain and coping practice diaries. Health Psychol. 2000;19:85–90. doi: 10.1037//0278-6133.19.1.85. [DOI] [PubMed] [Google Scholar]
- 42.Gil KM, Carson JW, Porter LS, Scipio C, Bediako SM, Orringer E. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol. 2004;23:267–74. doi: 10.1037/0278-6133.23.3.267. [DOI] [PubMed] [Google Scholar]
- 43.Turk DC, Okifuji A. Psychological factors in chronic pain: Evolution and revolution. J Consult Clin Psychol. 2002;70:678–690. doi: 10.1037//0022-006x.70.3.678. [DOI] [PubMed] [Google Scholar]
- 44.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9:771–83. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 46.Nottage KA, Hankins JS, Smeltzer M, Mzayek F, Wang WC, Aygun B, Gurney JG. Hydroxyurea use and hospitalization trends in a comprehensive pediatric sickle cell program. PloS one. 2013;8:e72077. doi: 10.1371/journal.pone.0072077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dampier C, Ely B, Brodecki D. Characteristics of pain managed at home in children and adolescents with sickle cell disease by using diary self-reports. J Pain. 2002;3:461–470. doi: 10.1054/jpai.2002.128064. [DOI] [PubMed] [Google Scholar]
- 48.File T. Computer and internet use in the United States. Current Population Survey Reports, P20-568. U.S. Census Bureau; Washington, D.C.: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.