Abstract
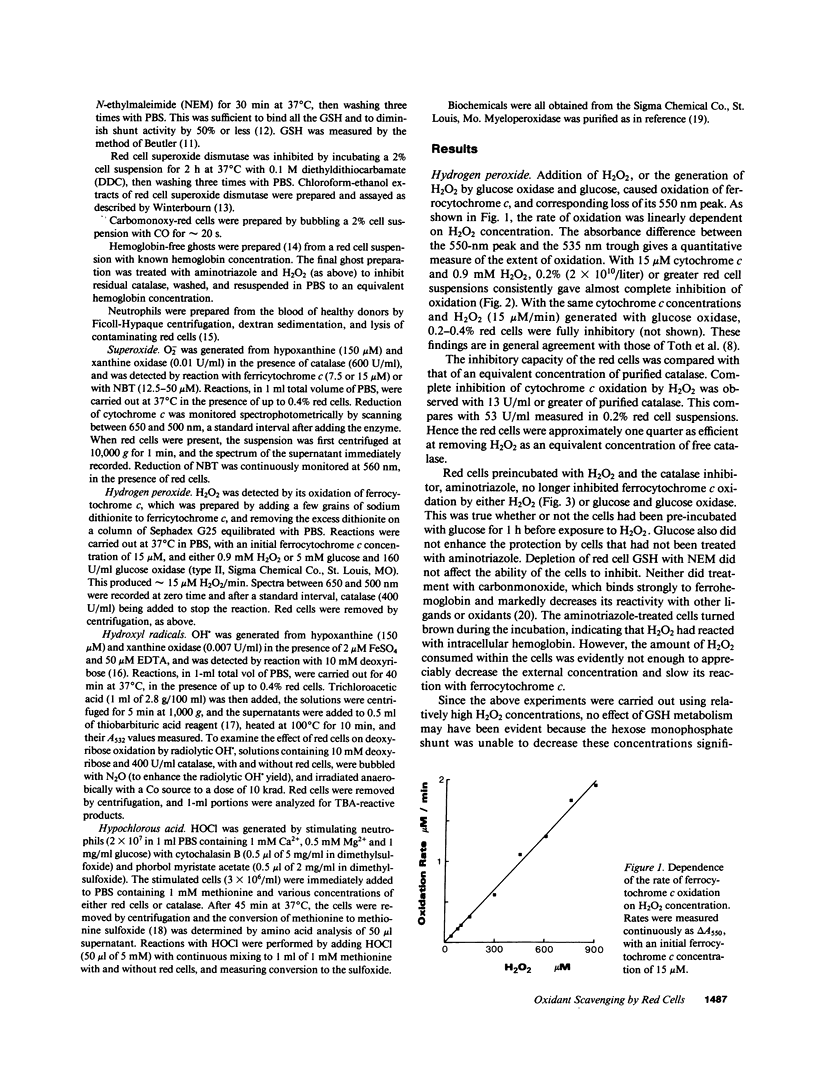
The ability of intact human red cells to scavenge extracellularly generated H2O2 and O2-, and to prevent formation of hydroxyl radicals and hypochlorous acid has been examined. Red cells inhibited oxidation of ferrocytochrome c by H2O2. Cells treated with aminotriazole no longer inhibited, indicating that protection was almost entirely due to intracellular catalase. Contribution by the GSH system was slight, and apparent only with low H2O2 concentrations when catalase was inhibited by aminotriazole. The cells were about a quarter as efficient at inhibiting cytochrome c oxidation as an equivalent concentration of purified catalase. No inhibition of O2(-)-dependent reduction of ferricytochrome c or nitroblue tetrazolium was observed, although extracted red cell superoxide dismutase inhibited nitroblue tetrazolium reduction at one fortieth the concentration of that in the cells. Red cells efficiently inhibited deoxyribose oxidation by hydroxyl radicals generated from H2O2, O2- and Fe(EDTA), and myeloperoxidase-dependent oxidation of methionine to methionine sulfoxide by stimulated neutrophils. Most of the red cell inhibition of hydroxyl radical production, and all the inhibition of methionine oxidation, was prevented by blocking intracellular catalase with aminotriazole. Thus red cells are able to efficiently scavenge H2O2, but not O2-, produced in their environment, and to inhibit formation of hydroxyl radicals and hypochlorous acid. They may therefore have an important role in extracellular antioxidant defense.
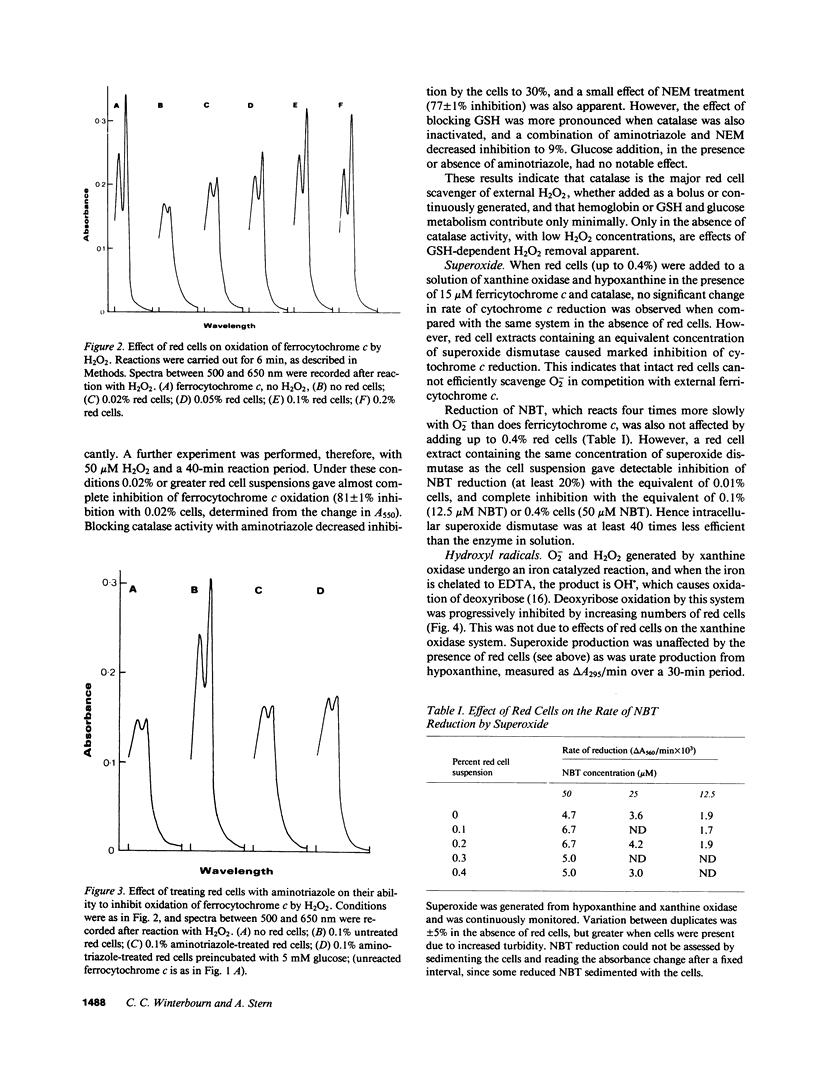
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agar N. S., Sadrzadeh S. M., Hallaway P. E., Eaton J. W. Erythrocyte catalase. A somatic oxidant defense? J Clin Invest. 1986 Jan;77(1):319–321. doi: 10.1172/JCI112294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Jefferson M. M., Melton D. F., Thomas E. L. Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J Biol Chem. 1984 Aug 25;259(16):10404–10413. [PubMed] [Google Scholar]
- Grisham M. B., Jefferson M. M., Thomas E. L. Role of monochloramine in the oxidation of erythrocyte hemoglobin by stimulated neutrophils. J Biol Chem. 1984 Jun 10;259(11):6757–6765. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Kelman S. N., Sullivan S. G., Stern A. Primaquine-mediated oxidative metabolism in the human red cell. Lack of dependence on oxyhemoglobin, H2O2 formation, or glutathione turnover. Biochem Pharmacol. 1982 Jul 15;31(14):2409–2414. doi: 10.1016/0006-2952(82)90537-8. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978 Jul 10;253(13):4697–4699. [PubMed] [Google Scholar]
- Marklund S. L., Holme E., Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982 Nov 24;126(1):41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Sutton H. C., Roberts P. B., Winterbourn C. C. The rate of reaction of superoxide radical ion with oxyhaemoglobin and methaemoglobin. Biochem J. 1976 Jun 1;155(3):503–510. doi: 10.1042/bj1550503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Test S. T., Lampert M. B., Ossanna P. J., Thoene J. G., Weiss S. J. Generation of nitrogen-chlorine oxidants by human phagocytes. J Clin Invest. 1984 Oct;74(4):1341–1349. doi: 10.1172/JCI111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Test S. T., Weiss S. J. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J Biol Chem. 1984 Jan 10;259(1):399–405. [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Melton D. F., Jefferson M. M. Evidence for a role of taurine in the in vitro oxidative toxicity of neutrophils toward erythrocytes. J Biol Chem. 1985 Mar 25;260(6):3321–3329. [PubMed] [Google Scholar]
- Toth K. M., Clifford D. P., Berger E. M., White C. W., Repine J. E. Intact human erythrocytes prevent hydrogen peroxide-mediated damage to isolated perfused rat lungs and cultured bovine pulmonary artery endothelial cells. J Clin Invest. 1984 Jul;74(1):292–295. doi: 10.1172/JCI111414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Chen J. W. Oxidation of methionine by human polymorphonuclear leukocytes. J Clin Invest. 1980 May;65(5):1041–1050. doi: 10.1172/JCI109756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Denison R. C. Oxidation of amino acids by human neutrophils. Inflammation. 1981 Dec;5(4):379–386. doi: 10.1007/BF00911101. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Winterbourn C. C. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985 Jun 18;840(2):204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Free-radical production and oxidative reactions of hemoglobin. Environ Health Perspect. 1985 Dec;64:321–330. doi: 10.1289/ehp.8564321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Sutton H. C. Iron and xanthine oxidase catalyze formation of an oxidant species distinguishable from OH.: comparison with the Haber-Weiss reaction. Arch Biochem Biophys. 1986 Jan;244(1):27–34. doi: 10.1016/0003-9861(86)90090-1. [DOI] [PubMed] [Google Scholar]
- van Asbeck B. S., Hoidal J., Vercellotti G. M., Schwartz B. A., Moldow C. F., Jacob H. S. Protection against lethal hyperoxia by tracheal insufflation of erythrocytes: role of red cell glutathione. Science. 1985 Feb 15;227(4688):756–759. doi: 10.1126/science.2982213. [DOI] [PubMed] [Google Scholar]


