Abstract
To prevent the spread of infection, an invading pathogen must first be recognized by the innate immune system. Host pattern recognition receptors detect distinct pathogen-associated molecules and induce the transcription and release of interferon and inflammatory molecules to resolve infection. Unlike infections with pathogens that replicate autonomously from the host, viral infections blur the boundaries of self and non-self. Differentiation of host from virus is achieved by restricting localization of host nucleic acids and by placing pattern recognition receptors in specific subcellular compartments. Within this review, we discuss how several families of pattern recognition receptors act to provide a comprehensive surveillance network that has the potential to induce interferon expression in response to any viral infection.
Keywords: Interferon, Toll-like Receptors, RIG-I-like Receptors, cGAS, STING, innate immunity
Introduction
The production of inflammatory cytokines and interferons (IFNs) is triggered after detection of potentially infectious pathogens and is central to the innate immune response. Type-I IFNs signal in an autocrine and paracrine fashion for the production of hundreds of IFN stimulated genes (ISGs), many of which are still uncharacterized [1,2]. These ISGs play an important role in restricting viral replication and stopping the spread of infection to other cells. Thus, detection of a virus is a vital first step towards immunity.
The innate immune system relies on its ability to recognize general pathogen associated molecular patterns (PAMPs). PAMPs are pathogen-derived molecules unique from the host and difficult to mutate or sequester from detection without a fitness cost [3]. These PAMPs are recognized by a limited number of germline encoded pattern recognition receptors (PRRs). The PAMPs detected are produced by a wide variety of pathogens, thus allowing a single PRR to report on many infectious encounters. In the case of viral pathogens, the innate immune system often detects the most intrinsic part of a virus, its nucleic acid genome. Viral genomes exist as RNA or DNA, are single or double-stranded, and can be partitioned into one or more segments.
Nucleic acids are not unique to viruses since they are essential to all cellular life. To differentiate between host and viral nucleic acids, PRRs use two criteria to detect viral nucleic acids specifically. The first criteria is based on the intrinsic ability of PRRs to recognize unusual biochemical features present in viral, but not host, nucleic acids. For example, the PRR retinoic acid-inducible gene 1 (RIG-I) detects RNAs that are tri- (or di-) phosphorylated, and lack a 7-methylguanosine cap [4–7]. Host mRNAs are also tri-phosphorylated, but the presence of the cap minimizes recognition by RIG-I. The second criteria that PRRs use to detect viral nucleic acids specifically is based on the ability of the host to restrict its own nucleic acids to specific locations within the cell. For example, host DNA should not be found in the cytoplasm of uninfected cells, and the PRR cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) synthase (cGAS) detects cytoplasmic DNA, which is presumably of viral origin [8,9].
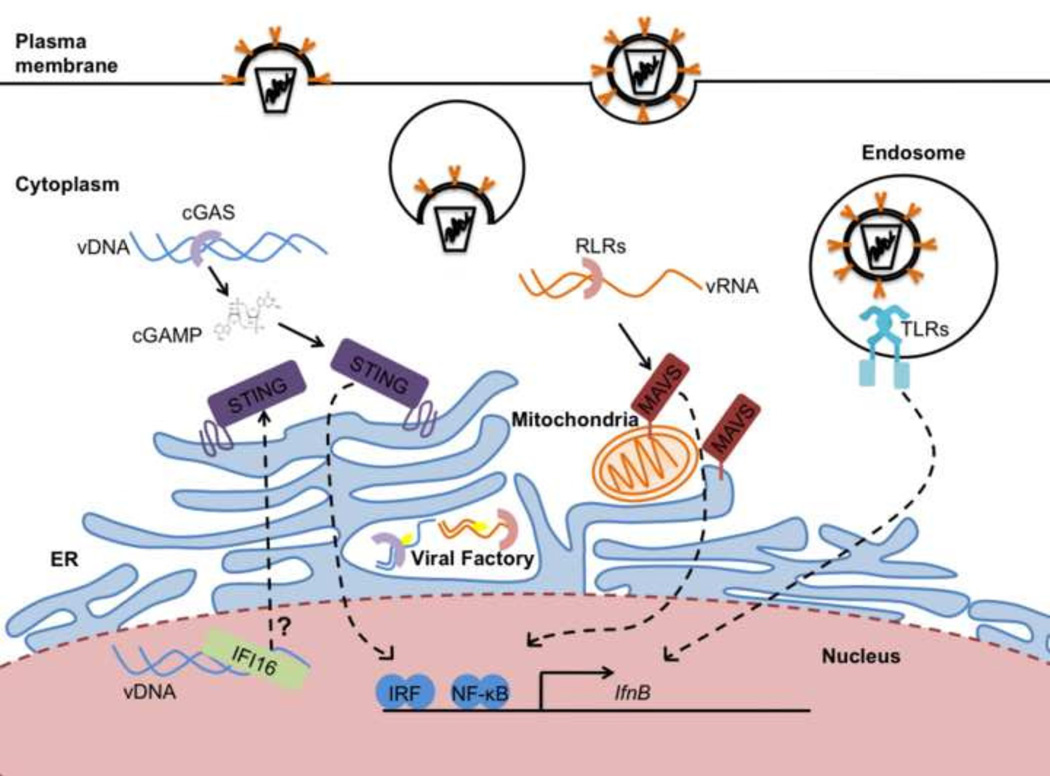
In this review, we expand on the ideas described above as we describe the PRRs of the innate immune system that function to produce type-I IFNs. We focus on the three major IFN-inducing families of PRRs in mammals, the Toll-like receptors (TLRs), RIG-I-like receptors (RLRs) and cGAS (Figure 1). We highlight how differences in the subcellular localization of host and viral nucleic acids, and the PRRs that detect them, can regulate the ability of the host to activate an IFN response during a viral infection.
Figure 1. Multiple organelles initiate signal transduction that lead to type I IFN expression.
Viruses can enter most cells through the endocytic pathway or by crossing the plasma membrane. TLRs detect viral genomes in endosomes. Once in the cytoplasm, genomic viral RNA (vRNA) can be detected by the RLRs and activate type-I IFN through MAVS, located on mitochondria and ER membranes. Genomic viral DNA (vDNA) is detected in the cytoplasm by cGAS or in the nucleus by IFI16. Both sensors activate STING to induce type-I IFNs. cGAS and RLRs can also detect viral replication intermediates in viral factories. All pathways activate the IRF and NF-kB transcription factors to stimulate IFNB transcription.
Toll-like Receptors
TLRs are PRRs that detect PAMPs derived from various microbes and initiate the transcription of inflammatory cytokines and type-I IFNs. These receptors are transmembrane proteins that contain an extracellular/lumenal domain that consists of a series of leucine rich repeats (LRRs). The LRR-containing domain detects PAMPs either directly or through interactions with accessory proteins that have high affinity PAMP-binding activities. Several TLRs recognize nucleic acids and have an important role in antiviral defense. For example, TLR3 recognizes viral double stranded RNA [10], TLR7 and TLR8 recognize viral single stranded RNA [11,12], and TLR9 recognizes DNA containing unmethylated CpG motifs present in numerous viral and non-viral pathogens [13–15]. In addition, recent work identified murine TLR13 as a nucleic acid sensor, but the ligand is a specific sequence present in bacterial ribosomal RNA [16]. Thus, while most nucleic acid sensing TLRs have an important role in detecting viruses, at least one (TLR13) has a specific anti-bacterial function.
The nucleic acid sensing TLRs are localized to endolysosomes. During an infection, viral particles are endocytosed. The acidification of endosomes allow for some viruses to uncoat and gain entry into the cytoplasm. However, not all viral particles are successful in the process. In the acidic environment of the lysosome, viral particles are degraded and the genomic nucleic acids are released, allowing for detection by TLRs. Thus, the nucleic acid sensing TLRs are localized to compartments where viral genomes can be exposed.
Binding to nucleic acids promotes interactions between two TLRs, which consequently dimerize the Toll/Interleukin-1 receptor (TIR) domains present in the cytoplasmic tails of the receptors [17]. The dimerized TIRs serve as a platform to recruit cytosolic adaptor proteins that also contain TIR domains. Two distinct sets of adaptors are thought to be recruited to dimerized TLRs. The most common set of adaptors, utilized by all TLRs except TLR3, is that of TIRAP and MyD88. The recruitment of these adaptors initiates the formation of a large helical oligomer called the myddosome, which is functionally considered a supramolecular organizing center (SMOC) [18]. This SMOC mediates kinase- and ubiquitin ligase-dependent events that activate inflammatory transcription factors such as NF-κB and IRF7. NF-κB and IRF7 then translocate into the nucleus to promote the expression of IFNs and other inflammatory mediators. The second set of adaptors that control TLR signaling consists of TRAM and TRIF, but these adaptors are utilized by a small set of TLRs. Whether TRAM and TRIF assemble a SMOC similar to the myddosome that activates inflammatory transcription factors is unclear, although the use of SMOCs as signaling platforms in multiple PRR pathways suggests that this may be the case [18].
In order to minimize detection of host-encoded nucleic acids, the nucleic acid-sensing TLRs are restricted to endolysosomal compartments, by a process mediated by UNC93B1. UNC93B1 is a multipass membrane protein localized to the endoplasmic reticulum (ER) that was identified because the H412R mutation in one of its transmembrane domains abrogates IFN expression elicited by TLR3, 7, and 9 agonists [19]. This protein was originally proposed to not have a function in TLR transport throughout the cell [19]. However, subsequent work demonstrated that bone marrow derived dendritic cells with the H412R mutation in UNC93B1 were defective in trafficking TLR9 to endolysosomes [20]. UNC93B1 and nucleic acid sensing TLRs bind to each other in the ER, and the H412R mutation ablates those interactions [21]. UNC93B1 loads the nucleic acid sensing TLRs into COPII vesicles in order to bud from the ER and travel through the Golgi complex, and TLR9 additionally requires the adaptor protein-2 complex in order to traffic to endolysosomes [22].
In addition to being regulated by UNC93B1 trafficking, TLR3, 7, 8, and 9 need to be cleaved by various proteases such as cathepsins, asparagine endopeptidase, and furin-like proprotein convertases [23–29]. The proteolysis occurs as the TLRs are transported through acidic endosomal compartments where these enzymes are active. Although nucleic acids are capable of binding to the unprocessed TLRs, downstream signaling cannot occur until after proteolysis [23]. This proteolytic regulation is particularly important to prevent autoimmunity. In the case of systemic lupus erythromatitis, host nucleic acids are immunostimulatory [30]. Having nucleic acid sensing TLRs mislocalized to the plasma membrane increases the chances of an autoimmune reaction [31]. By designing TLRs to remain incapable of being activated until they reach their proper destination, the risk of autoimmunity is reduced.
Once in endosomes, nucleic acid sensing TLRs do not remain static. TLR9 can traffic to phagosomes that have been initiated by FcγR signaling irrespective of DNA content [32]. Upon interaction with CpG DNA, TLR9 initiates a signaling pathway that activates NF-κB-dependent cytokine and IFN expression [32]. Recent work has indicated that TLR9 activates cytokine expression and IFN expression from distinct endosomal populations, with the latter requiring non-canonical autophagy components to traffic to a distinct vesicle [32]. Non-canonical autophagy does not form a double membrane. Instead, the autophagy regulator LC3 binds TLR9-containing phagosomes and somehow induces transport to distinct organelles where the IFN-inducing transcription factor IRF7 can become activated [32]. This requirement for moving TLR9 to induce IFNs is not unique to phagocytosed cargo. Cargo that has been endocytosed instead of phagocytosed also requires movement of TLR9 to induce type-I IFNs [33]. However, in the case of endosomes, the movement of TLR9 depends upon activator protein-3 (AP-3) which traffics TLR9 to lysosomal related organelles (LROs) where IRF7 can be activated for IFN induction. Disruption of TLR9 movement in either of these two trafficking pathways by eliminating LC3 or AP-3 results in blocking IFN induction without blocking the production of inflammatory cytokines [33]. Although the work published primarily focused on TLR9, some work with TLR3 and 7 ligands in the endocytic pathway suggest that they may be regulated similarly [33].
TLR3, 7, 8 and 9 all induce type-I IFNs in plasmacytoid dendritic cells where they are expressed, but the signaling molecules downstream differ between TLR3 and the others. TLR7, 8 and 9 utilize MyD88, and were recently found to also depend on TIRAP for their signaling functions [34]. The requirement of TIRAP for signaling from TLR7 and TLR9 was originally missed because experimental conditions utilizing synthetic nucleic acids rather than viruses masked the requirement for TIRAP [35,36]. TIRAP has a lipid-binding domain that interacts promiscuously with acidic phosphoinositides, allowing it to survey the plasma and endosomal membranes for TLRs bound to ligand. TLR activation causes TIRAP to oligomerize with MyD88 and IRAK kinases, thus creating a SMOC called the myddosome that promotes the expression of inflammatory cytokines and IFNs. In contrast, TLR3 engages only the cytosolic adaptor TRIF to induce cytokine and IFN expression [37,38]. It is unclear why TLR3 evolved to utilize TRIF instead of MyD88 to induce IFNs. One possibility is that TLR3 resides in an endosomal population that differs from TLR7 and TLR9, and thus may have access to different sets of adaptor proteins. In support of this idea, TLR3, TLR7, and TLR9 traffic to endosomes differently. TLR7 proceeds directly from the trans-Golgi network to endosomes, whereas TLR3 is delivered to the plasma membrane before reaching endosomes [22,39]. However, TLR9, which signals via MyD88, can also traffic to the plasma membrane before its proteolytic processing in endosomes [22]. Additional studies that identify the precise endosomal populations inhabited by nucleic acid sensing TLRs would greatly benefit this discussion.
In addition to the nucleic acid sensing TLRs, two TLRs localized to the plasma membrane sense viral pathogens. Although TLR2 and TLR4 are better known to recognize bacterial lipoproteins and lipopolysaccharides, respectively, these PRRs are activated by viral ligands. TLR4 can initiate TRIF-dependent signaling after binding the glycoprotein of vesicular stomatitis virus [40]. In a subset of inflammatory monocytes, TLR2 is activated by mouse cytomegalovirus and vaccinia virus [41]. TLR2 is activated by additional viruses in other cell types [42,43]. Although these PRRs are located at the plasma membrane, IFN-inducing signal transduction occurs from endosomal compartments [41,44]. Whether TLR2 and TLR4 recognize common molecular motifs among the viruses is currently unknown.
RIG-I-like Receptors
TLRs are limited in their ability to detect viruses because they only survey the extracellular space and are expressed in a limited number of cell types. In contrast, the RLRs, RIG-I and melanoma differentiation-associated protein 5 (MDA5), are expressed in almost all cells. RIG-I and MDA5 are cytoplasmic RNA helicases that recognize non-self RNA motifs. RIG-I binds 5’ tri- and di- phosphates present on short double stranded (ds) RNA whereas MDA5 binds long dsRNA molecules [4,5,7,45–50]. RIG-I and MDA5 activate IRF3 and NF-κB through the adaptor, mitochondrial antiviral signaling protein (MAVS). RIG-I and MDA5 form filaments upon RNA binding which oligomerizes the RLR caspase recruitment domain (CARD), and enhances interactions with the MAVS CARD [51–53]. The interaction between RLRs and MAVS CARDs leads to the formation of a SMOC with prion-like properties on the surface of mitochondria. The MAVS SMOC then functions to activate transcription factors that induce type-I IFNs [54].
RNA viruses enter the cytoplasm after breaching plasma or endosomal membranes. Unlike many DNA viruses, RNA viruses must rely on their own enzymes for genome replication. Replication intermediates formed by these enzymes contain uncapped RNA or have dsRNA character and can be recognized by RLRs. In order to catalyze RNA replication, RNA viruses may set up organelle-like replication complexes in the cytoplasm. These viral “factories” are composed of cellular membranes, usually ER-derived, but examples can be found for viruses utilizing the Golgi and lysosomal compartment as well [55]. Mitochondria are also recruited to sites of RNA virus replication presumably to take advantage of mitochondrial ATP production during RNA replication [56].
Intriguingly, MAVS is situated at the same membranes that many RNA viruses exploit for their replication, namely the mitochondria, peroxisomes and mitochondrial-associated ER membranes (MAM), which are tethered to mitochondria and peroxisomes by the protein mitofusin 2 (MFN2) [57]. MAVS on the mitochondria and peroxisomes induce different types of IFN. Mitochondrial MAVS induces both type-I and type-III, whereas peroxisomal MAVS induces type-III IFN [58,59]. It has been proposed that the MAM coordinates MAVS-dependent responses from mitochondria and peroxisomes [60]. Viral antagonists that target MAVS can lead to a profound loss of signaling while only inactivating a small pool of MAVS in a cell [60–62]. These observations suggest that the distribution of MAVS on multiple organelles is not redundant, but is rather a functionally important aspect of the RLR network that promotes antiviral immunity [60,62].
STING Pathway
While RLRs and MAVS produce IFN in response to viral RNA, a parallel pathway detects intracellular viral DNA. Stimulator of IFN Genes (STING) is an ER-localized protein that coordinates the type-I IFN response to viral DNA [63,64]. While many DNA sensors have been proposed to activate STING [65], only cGAS has a clear mechanism of STING activation. cGAS is a cytoplasmic protein that recognizes B-form DNA. Upon binding to these ligands, cGAS synthesizes the cyclic dinucleotide cGAMP, which binds and activates STING to promote IFN gene expression [8,9]. Intermediates of the retroviral replication cycle, namely RNA:DNA hybrids, have also been proposed to activate cGAS. In vitro, cGAS can synthesize cGAMP in response to synthetic RNA:DNA ligands [66–68], but it is unclear if cGAS-dependent signaling can occur and if cGAS is activated in response to natural RNA:DNA ligands. Some bacteria also synthesize cyclic dinucleotides through the actions of a prokaryotic cGAS-like enzyme. These bacterial dinucleotides can also activate STING [69]. While both cGAMP and bacterial dinucleotides bind STING and activate type-I IFN, cGAMP binds STING at a higher affinity due to its noncanonical 2’–5’ phosphodiester linkage [70–73].
In order to induce IFNs, STING must traffic from the ER to vesicles [74,75]. This movement appears to be orchestrated by proteins involved in the autophagy pathway [74,75]. Upon binding dinucleotides, STING moves from the ER to the Golgi via a mechanism dependent on VPS34, a Class III PI3K that regulates many pathways including endocytic trafficking [75,76]. STING then leaves the Golgi and associates with TBK1 on vesicles to activate IRF3 and NF-κB [64]. The specific identity of these IFN-inducing vesicles remains undefined. After ATG9 depletion, STING and TBK-1 still traffic to vesicles, but more type-I IFN is induced than wild-type cells, implying that ATG9 or autophagy negatively regulates STING activation of TBK-1 [74]. ULK1, another kinase involved in autophagy, phosphorylates human and mouse STING at serine 366 and 365 respectively to negatively regulate cGAMP-induced STING signaling [75]. Knockdown of ULK1 leads to loss of phosphorylation of serine 366/365, which ultimately prevents STING degradation and sustains IFN expression [75]. STING is likely degraded by autophagy in the autolysosome because after activation, STING associates with LC3 and p62/SQSTM1, proteins that target cargo for autophagy [74]. In addition, treatment of cells with chloroquine, which inhibits acidification of endolysosomes, also prevents STING degradation [75].
STING is not the only pathway member to be regulated by autophagy. When cGAS is bound to DNA in the cytoplasm, Beclin-1 binds cGAS and inhibits its enzymatic activity [77]. Upon cGAS binding, Beclin-1 releases the negative regulator of autophagy, Rubicon, which allows for autolysosome maturation. This autophagic response may be directly antiviral as opposed to negatively regulating the cGAS/STING pathway. Knockdown of Beclin-1 or cGAS reduces LC3 association and clearance of foreign DNA.
Although the identification of cGAS has contributed to our understanding of innate immune sensing, a major conundrum still exists in our understanding of how viral DNA is detected. Unlike RNA viruses, many DNA viruses replicate in the nucleus of a cell. One notable exception to this observation is poxvirus replication, which occurs in the cytoplasm [78]. Without a lifecycle in the cytoplasm, how are DNA viruses detected? One hypothesis is that cGAS may bind incoming DNA viral genomes as they traverse the cytoplasm. However, many DNA virus genomes are protected by a viral capsid or travel to the nucleus through membrane bound compartments [79,80], so this mechanism of sensing may only occur during an abortive infection.
Before the discovery of cGAS, gamma-IFN-inducible protein 16 (IFI16) was identified as a nuclear DNA sensor necessary for the induction of IFN during herpes simplex virus-1 (HSV-1) infection [81,82]. IFI16 can traffic between the cytoplasm and nucleus, but at steady-state IFI16 localizes to the nucleus, allowing IFI16 to bind HSV-1 DNA and activate IRF3 [83]. IFI16 also induces IFN expression in response to HIV-1 single-stranded DNA and double-stranded DNA replication intermediates [66]. Although IFI16-induced IFN is STING-dependent, no direct activation of the STING pathway by IFI16 has been demonstrated. Interestingly, IFN induction during HSV-1 and HIV-1 infection is also dependent on STING and cGAS [8,84]. Given the dual dependence on cGAS and IFI16, the sensors could potentially cooperate to detect viral DNA where IFI16 could bind DNA in the nucleus, traffic to the cytoplasm, and subsequently interact with cGAS to activate signaling through STING.
cGAS may also have a role in controlling RNA virus replication. cGAS-deficient mice display enhanced mortality to lethal challenges of the RNA virus West Nile virus (WNV) compared to infections in wild-type mice [85]. Additionally, WNV infection of cGAS-deficient macrophages leads to modestly increased viral titers compared to wild-type controls. This control of viral replication is not due to direct recognition of RNA viruses by cGAS because this enzyme is not activated by double-stranded or single-stranded RNA [8,86,87]. Rather, the role of cGAS in controlling RNA virus infections may be linked to the observation that cGAS deficient cells exhibit decreased basal levels of ISGs. Low basal ISGs may allow for a viral replication advantage from which the host cannot recover, even if a robust IFN response is mounted later in infection.
Conclusion
Identifying innate immune sensors has been crucial to the understanding of how a host defends itself from viral infection. However, identification is insufficient, and these sensors must be placed in a cell biological context. As such, the subcellular location of these signaling pathways plays a major role in their function. The localization of PRRs helps differentiate between host and foreign nucleic acids, and the localization of downstream signaling molecules can also have major effects on the signaling outcomes that result. One theme slowly developing is that innate immune sensing is not static and the movement of the sensors and their immediate downstream effectors are of consequence. Understanding how this dynamic nature affects the function of sensors and downstream signaling proteins will be important for our understanding of how the type-I IFN response is achieved.
Highlights.
Multiple families of pattern recognition receptors detect viruses.
Viral infection causes receptor movement to diverse organelles.
Multiple organelles are sites of antiviral signal transduction.
Distinct antiviral responses are activated from distinct organelles.
Acknowledgements
J.C.K. is supported by NIH grants AI093589, AI072955, AI113141-01, and an unrestricted gift from Mead Johnson & Company. Dr. Kagan holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. K. Franz is supported through the Herchel Smith Graduate Fellowship Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams BR. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 2.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann K-K, Schlee M, et al. 5’-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 5.Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5’-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 6.Rehwinkel J, Tan CP, Goubau D, Schulz O, Pichlmair A, Bier K, Robb N, Vreede F, Barclay W, Fodor E, et al. RIG-I Detects Viral Genomic RNA during Negative-Strand RNA Virus Infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Goubau D, Schlee M, Deddouche S, Pruijssers AJ, Zillinger T, Goldeck M, Schuberth C, Van der Veen AG, Fujimura T, Rehwinkel J, et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5’-diphosphates. Nature. 2014 doi: 10.1038/nature13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexopoulou L, Holt a C, Medzhitov R, Flavell Ra. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 11.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 12.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 13.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redecke V, Hausmann S, Akira S, Bauer S, Kirschning CJ, Ha H, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaisho T, Sato S, Sanjo H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:5–10. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 16.Oldenburg M, Krüger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, Bathke B, Lauterbach H, Suter M, Dreher S, et al. TLR13 Recognizes Bacterial 23 S rRNA Devoid of Erythromycin Resistance–Forming Modification. Science. 2012;337:1111–1115. doi: 10.1126/science.1220363. [DOI] [PubMed] [Google Scholar]
- 17.Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DCG, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, et al. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat. Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 18.Kagan JC, Magupalli VG, Wu H. SMOCs: supramolecular organizing centres that control innate immunity. Nat. Rev. Immunol. 2014;14:821–826. doi: 10.1038/nri3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y-M, Brinkmann MM, Paquet M-E, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 21.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim Y-M. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, Barton GM. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi G-P, Chapman Ha, Barton GM. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park B, Brinkmann MM, Spooner E, Lee CC, Kim Y-M, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepulveda FE, Maschalidi S, Colisson R, Heslop L, Ghirelli C, Sakka E, Lennon-Duménil A-M, Amigorena S, Cabanie L, Manoury B. Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling in dendritic cells. Immunity. 2009;31:737–748. doi: 10.1016/j.immuni.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J. Exp. Med. 2011;208:643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Cattaneo A, Gobert F, Müller M, Toscano F, Flores M, Lescure A, Del Nery E, Benaroch P. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc. Natl. Acad. Sci. 2012;109:9053–9058. doi: 10.1073/pnas.1115091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maschalidi S, Hässler S, Blanc F, Sepulveda FE, Tohme M, Chignard M, van Endert P, Si-Tahar M, Descamps D, Manoury B. Asparagine endopeptidase controls anti-influenza virus immune responses through TLR7 activation. PLoS Pathog. 2012;8:e1002841. doi: 10.1371/journal.ppat.1002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hipp MM, Shepherd D, Gileadi U, Aichinger MC, Kessler BM, Edelmann MJ, Essalmani R, Seidah NG, Reis E Sousa C, Cerundolo V. Processing of Human Toll-like Receptor 7 by Furin-like Proprotein Convertases Is Required for Its Accumulation and Activity in Endosomes. Immunity. 2013;39:711–721. doi: 10.1016/j.immuni.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin – IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;3:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 31.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 32.Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, et al. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–997. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-Like Receptor 9 Signaling by Adaptor Protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonham KS, Orzalli MH, Hayashi K, Wolf AI, Glanemann C, Weninger W, Iwasaki A, Knipe DM, Kagan JC. A Promiscuous Lipid-Binding Protein Diversifies the Subcellular Sites of Toll-like Receptor Signal Transduction. Cell. 2014;156:705–716. doi: 10.1016/j.cell.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, et al. Essential role of TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 36.Horng T, Barton GM, Flavell Ra, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. LPS-TLR4 Signaling to IRF-3/7 and NF-kB Involves the Toll Adapters TRAM and TRIF. J. Exp. Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 39.Pohar J, Pirher N, Bencina M, Mancek-keber M, Jerala R. The Role of UNC93B1 Protein in Surface Localization of TLR3 Receptor and in Cell Priming to Nucleic Acid Agonists. J. Biol. Chem. 2013;288:442–454. doi: 10.1074/jbc.M112.413922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Oldstone MB, Beutler B. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 41.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann M, Zeisel B, Paranhos-baccalà G. Toll-Like Receptor 2 Senses Hepatitis C Virus Core Protein but Not Infectious. J. Innate Immun. 2009;1:446–454. doi: 10.1159/000226136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kriz J, Gosselin J. Involvement of TLR2 in Recognition of Acute Gammaherpesvirus-68 Infection. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietrich N, Lienenklaus S, Weiss S, Gekara NO. Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Ludwig J, Schuberth C, Goldeck M, Schlee M, Li H, Juranek S, Sheng G, Micura R, Tuschl T, et al. Structural and functional insights into 5’-ppp RNA pattern recognition by the innate immune receptor RIG-I. Nat. Struct. Mol. Biol. 2010;17:781–787. doi: 10.1038/nsmb.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu C, Xu H, Ranjith-Kumar CT, Brooks MT, Hou TY, Hu F, Herr AB, Strong RK, Kao CC, Li P. The structural basis of 5′ triphosphate double-stranded RNA recognition by RIG-I C-terminal domain. Structure. 2010;18:1032–1043. doi: 10.1016/j.str.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo D, Ding SC, Vela A, Kohlway A, Lindenbach BD, Pyle AM. Structural insights into RNA recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peisley A, Wu B, Yao H, Walz T, Hur S. RIG-I Forms Signaling-Competent Filaments in an ATP-Dependent, Ubiquitin-Independent Manner. Mol. Cell. 2013;51:573–583. doi: 10.1016/j.molcel.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 52.Peisley A, Lin C, Wu B, Orme-Johnson M, Liu M, Walz T, Hur S. Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc. Natl. Acad. Sci. 2011;108:21010–21015. doi: 10.1073/pnas.1113651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, Hur S. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152:276–289. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 54.Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Den Boon JA, Diaz A, Ahlquist P. Cytoplasmic viral replication complexes. Cell Host Microbe. 2010;8:77–85. doi: 10.1016/j.chom.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novoa RR, Calderita G, Arranz R, Fontana J, Granzow H, Risco C. Virus factories: associations of cell organelles for viral replication and morphogenesis. Biol. Cell. 2005;97:147–172. doi: 10.1042/BC20040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 58.Odendall C, Dixit E, Stavru F, Bierne H, Franz KM, Durbin AF, Boulant S, Gehrke L, Cossart P, Kagan JC. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat. Immunol. 2014;15:717–728. doi: 10.1038/ni.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dixit E, Boulant S, Zhang Y, Lee ASY, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, et al. Peroxisomes Are Signaling Platforms for Antiviral Innate Immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horner SM, Liu HM, Park HS, Briley J, Gale M. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14590–14595. doi: 10.1073/pnas.1110133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 62.Li X-D, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goubau D, Deddouche S, Reis C. Cytosolic Sensing of Viruses. Immunity. 2013;38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jakobsen MR, Bak RO, Andersen A, Berg RK, Jensen SB, Tengchuan J, Jin T, Laustsen A, Hansen K, Ostergaard L, et al. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E4571–E4580. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mankan AK, Schmidt T, Chauhan D, Goldeck M, Höning K, Gaidt M, Kubarenko AV, Andreeva L, Hopfner K-P, Hornung V. Cytosolic RNA:DNA hybrids activate the cGAS–STING axis. EMBO J. 2014;33:2937–2946. doi: 10.15252/embj.201488726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rigby RE, Webb LM, Mackenzie KJ, Li Y, Leitch A, Reijns MAM, Lundie RJ, Revuelta A, Davidson DJ, Diebold S, et al. RNA:DNA hybrids are a novel molecular pattern sensed by TLR9. EMBO J. 2014;33:542–558. doi: 10.1002/embj.201386117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, Vance RE. The Innate Immune DNA Sensor cGAS Produces a Noncanonical Cyclic Dinucleotide that Activates Human STING. Cell Rep. 2013;3:1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Röhl I, Hopfner K-P, Ludwig J, Hornung V. cGAS produces a 2′–5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen Z. Cyclic GMP-AMP containing mixed Phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, et al. Cyclic [G(2',5')pA(3',5')p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saitoh T, Fujita N, Hayashi T, Takahara K, Satoh T, Lee H, Matsunaga K, Kageyama S, Omori H, Noda T, et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konno H, Konno K, Barber GN. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell. 2013;155:688–698. doi: 10.1016/j.cell.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jaber N, Dou Z, Chen J-S, Catanzaro J, Jiang Y-P, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang Q, Seo GJ, Choi YJ, Kwak MJ, Ge J, Rodgers MA, Shi M, Leslie BJ, Hopfner KP, Ha T, et al. Crosstalk between the cGAS DNA sensor and beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15:228–238. doi: 10.1016/j.chom.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cairns J. The initiation of vaccinia infection. Virology. 1960;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- 79.Whittaker GR, Kann M, Helenius A. Viral entry into the nucleus. Annu. Rev. Cell Dev. Biol. 2000;16:627–651. doi: 10.1146/annurev.cellbio.16.1.627. [DOI] [PubMed] [Google Scholar]
- 80.Tsai B, Qian M. Cellular entry of polyomaviruses. Curr. Top. Microbiol. Immunol. 2010;343:177–194. doi: 10.1007/82_2010_38. [DOI] [PubMed] [Google Scholar]
- 81.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orzalli MH, DeLuca NA, Knipe DM. PNAS Plus: Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc. Natl. Acad. Sci. 2012;109:E3008–E3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li T, Diner BA, Chen J, Cristea IM. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc. Natl. Acad. Sci. 2012;109:10558–10563. doi: 10.1073/pnas.1203447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao D, Wu J, Wu Y-T, Du F, Aroh C, Yan N, Sun L, Chen ZJ. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X, Wu J, Du F, Xu H, Sun L, Chen Z, Brautigam CA, Zhang X, Chen ZJ. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Rep. 2014;6:421–430. doi: 10.1016/j.celrep.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, Hornung V, Hopfner K-P. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498:332–337. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]