Abstract
Innate immune DNA sensing underpins many physiological and pathological responses to DNA, including anti-viral immunity to DNA viruses. Although it has been appreciated for many years that cytosolic DNA can evoke a type I interferon response, it is only within the past decade that the cellular mechanisms responsible for such a response have been defined. Here we review the discoveries that led to an appreciation of the existence of cytosolic DNA sensor proteins, and discuss two key such sensors, cGAS and IFI16, in detail. DNA sensors operate via STING, a protein shown to have a central role in controlling altered gene induction in response to DNA in vivo, and as such to be central to a rapidly expanding list of both protective and harmful responses to DNA. We also discuss recent insights into how and when DNA stimulates innate immunity, and highlight current outstanding questions in the DNA sensing field.
1. Introduction
Innate immunity has undergone a revolution in the past 15 years, with the uncovering of the molecular basis underpinning Charles Janeway's now validated hypothesis of the existence of germline encoded pattern recognition receptors (PRRs) capable of detecting and responding to pathogen-associated molecular patterns (PAMPs) on microbes [1]. With the discovery of the Toll-like receptors (TLRs), mechanisms for pathogen detection, leading in particular to induction of cytokines and type I interferons (IFN-Is), were revealed, and thus the innate immunity renaissance began [2]. In terms of viral detection, cell membrane bound TLRs were shown to recognize glycoproteins on the surface of viral capsids, while endosomally placed TLRs responded to viral nucleic acid, in particular viral RNA [2]. Rapid progress was made from 2004 in understanding how PRRs also survey and protect the cytosolic compartment from viruses, with the discovery and characterization of the RIG-I-like receptors (RLRs) RIG-I and MDA5, which recognize viral RNA genomes, replication intermediates and/or transcription products [3, 4]. Further structural studies of RLRs led to an appreciation of how these PRRs distinguish self from non-self RNA, in that RIG-I is activated by viral RNA with a 5’triphosphate, or a 5’diphosphate, moiety juxtaposed to a dsRNA panhandle, while MDA5 binds to long dsRNA [5, 6]. Both RLRs then transduce a signal via MAVS, leading to activation of transcription factors such as NF-κB and IFN regulatory factor 3 (IRF3) and subsequent cytokine and IFN-I induction.
In light of these discoveries and in the context of the paradigm of PRRs recognizing PAMPs, the questions of how, why and when DNA activates the innate immune system has received considerable attention in the past decade. The immunostimulatory and anti-viral potential of DNA introduced into mammalian cells had been reported more than 50 years ago [7, 8], but only recently have the cellular mechanisms of DNA detection leading to immune responses, and to IFN-I induction in particular, been identified. This has led to an understanding of the role of cytosolic DNA sensing in many protective and pathological responses to DNA, for example in detection of DNA viruses and in aberrant responses to self DNA leading to autoimmunity respectively.
Here we review the discovery of proposed cytosolic DNA sensors over the past decade (Figure 1), discuss the current understanding of how such sensors operate to respond to immune-stimulatory DNA to mobilize immune responses (as outlined in Figure 2), and highlight current issues that remain to be resolved in this rapidly evolving field.
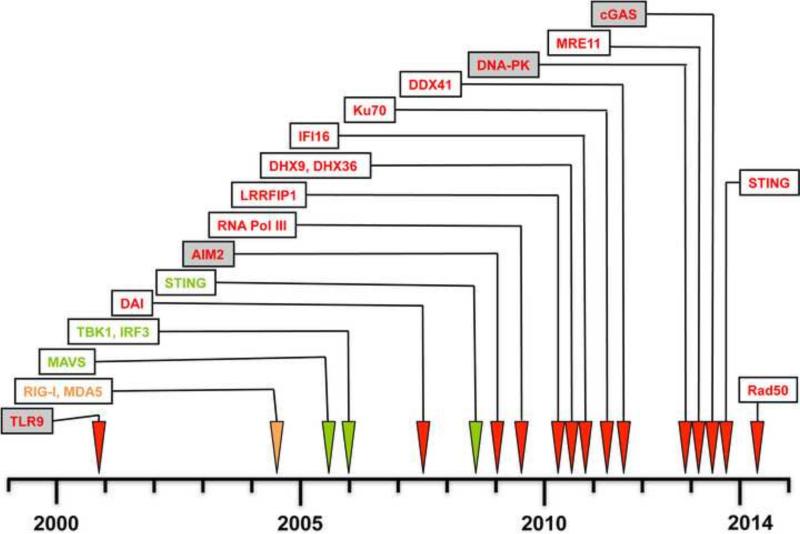
Figure 1. Timeline for discovery of putative cytosolic DNA sensors and associated signaling molecules.
The chronology of the identification of intracellular DNA sensors is shown. DNA sensors are in red, key signaling proteins in green, and the RNA sensors Rig-I and MDA5 shown for comparison (orange). As detailed in the text, certain DNA sensors (boxed in grey here) have been confirmed to have a role in DNA- and/or DNA-virus dependent cytokine and IFN-I production in vivo, by using knockout mice.
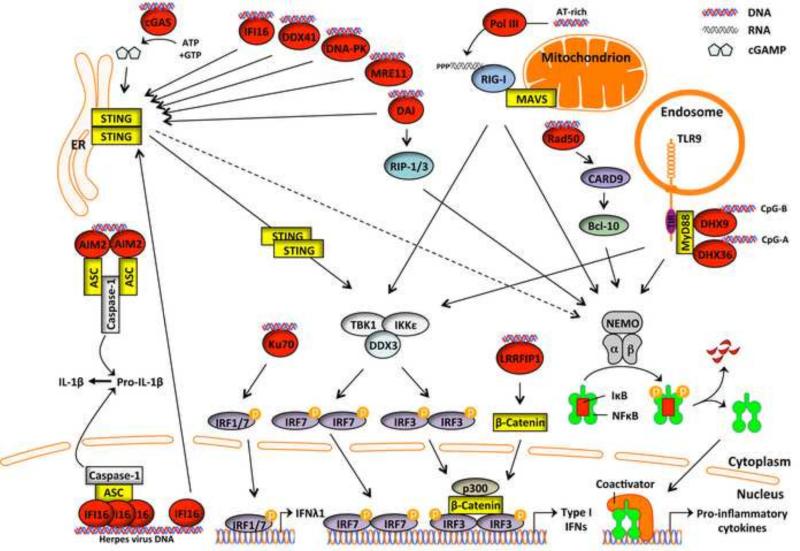
Figure 2. Cytosolic DNA sensors and their signaling pathways.
Upon recognition of dsDNA in the cytosol or nucleus, DNA sensors signal via adaptor proteins to altered gene induction via transcription factors (IRFs and NF-κB), and to pro-IL-1β processing, via Caspase 1. DNA sensors described in the main text are shown in red.
2. Early studies in defining cytosolic DNA sensor signaling (2006 – 2009)
The first PRR implicated in detection of DNA was TLR9 (Figure 1), shown in 2000 to be responsible for the ability of mice to respond to CpG DNA [9], leading to cytokine and IFN-I induction via NF-κB and IRF7 respectively [2]. This provided an elegant example of self versus non-self immune discrimination, since TLR9 recognized under methylated DNA (i.e. CpG DNA), which is enriched in microbial genomes compared to mammalian cells. In contrast, it is now clear that if either self or pathogen dsDNA accumulates in the cytosol, TLR9-independent cytokine and IFN-I induction is elicited in many cell types (reviewed in [10]). TANK-binding kinase (TBK1) was identified in 2006 as being required for IFN-β production in response to transfected synthetic dsDNA (poly(dA:dT)) [11] (Figure 1). After activation of upstream PRR signaling, TBK1 directly phosphorylates IRF3 leading to IFN-β induction, and the TBK1-IRF3 signaling axis was quickly established as fundamental to the cytosolic DNA response to other forms of synthetic dsDNA [12], to DNA viruses and for the immune response to DNA vaccines (reviewed in [13]). Thus by 2006 the search was on to discover one or more DNA sensors that would directly bind to cytosolic DNA and mediate IFN-I induction via a signaling pathway involving TBK1 and IRF3.
In 2007, the first reported cytosolic DNA receptor was identified as DNA-dependent activator of IRFs (DAI) [14]. DAI (or ZBP-1), encoded by an IFN-inducible gene, was found to be capable of upregulating the expression of IFN-I via NFκB and IRF3 in response to poly(dA:dT), and to bind to DNA [14]. DAI knockdown in mouse L929 cells reduced HSV-1-stimulated IFN-β production, while subsequent studies showed a role for DAI in IFN-I and cell death responses to CMV in fibroblasts [15-17]. However, no role for DAI in macrophage responses to DNA was observed [18], while the in vivo response to DNA vaccination was TBK1-dependent, but DAI-independent [19]. This indicated the existence of other as of yet unidentified cytosolic DNA sensors, especially for myeloid cells. A further DNA sensing pathway was identified in 2009, which, surprisingly for a DNA response, involved RIG-I and MAVS [20, 21]. It was shown that RNA polymerase III (RNA Pol III) is capable of transcribing AT-rich dsDNA, such as poly(dA:dT), into an RNA-containing 5’-triphosphate moiety, which can then be sensed by RIG-I leading to IFNβ induction [20, 21]. This provided an explanation for the previously reported involvement of MAVS in some DNA responses [11], and certainly accounts for the ability of some human cell types to respond to poly(dA:dT). However the physiological role of RNA Pol III in detection of pathogens has remained controversial, and RNA Pol III could not account for known IFN-I responses to non-AT-rich dsDNA [22]. Thus, given that the role of DAI is cell type-specific, and that the Pol III-RIG-I pathway only detects AT-rich DNA, clearly additional cytosolic DNA PRRs remained to be discovered.
3. The centrality of STING in controlling DNA sensing
In parallel to these early studies identifying putative cytosolic DNA sensors, a new signaling adaptor protein called STING (also called MITA, MPYS and ERIS) was discovered in 2008 [23-26]. STING was shown to be essential for IFN-β induction by DNA and also by HSV-1, and STING knock out mice were subsequently shown to be impaired in their ability to respond to DNA viruses [27]. STING is now known to have a role in vivo in responses to viral and bacterial pathogens, to self-DNA during autoimmune disease, and in DNA adjuvancy [28]. The mechanism whereby STING directly engages with TBK1 to direct IRF3 activation has also been determined [29] although how exactly STING mediates NFκB activation (presumably via IKKβ) is to date still an open question (Figure 2) [30]. In addition to its adaptor function for DNA sensing pathways, STING was shown to directly recognize and induce a type I IFN response to the bacterial second messenger cyclic dinucleotide (CDN) diguanylate monophosphate (c-di-GMP) [31], and more recently to the structurally related novel mammalian second messenger cGAMP (described below). The bacterial CDN could be being detected by STING as a PAMP to sense the presence of a bacterial infection, or alternatively bacterial CDN stimulation of STING may be utilized as an immune escape mechanism when induction of type I IFN may benefit the bacterial pathogen.
Work since 2009 up to the present has now linked STING to many physiological and pathological responses involving innate and adaptive immunity, and placed this innate immune adaptor at the center of both protective and detrimental processes in vivo. For example, a protective role for STING during cancer immunotherapy has been revealed in mouse tumor models whereby STING in dendritic cells is activated by the recognition of tumor cell DNA, leading to IFN-β induction which renders DCs competent to present tumor antigens and prime CD8+ T lymphocytes [32-34]. In contrast, STING has a detrimental role in driving inflammatory disease when self-DNA accumulates due to defective or absent DNase activity [35]. Importantly, STING has been directly implicated in human disease since a new monogenic disorder termed STING-associated vasculopathy with onset in infancy (SAVI) has been recently characterized as an autoinflammatory disease caused by gain-of-function mutations in TMEM173 (the gene encoding STING) [36].
4. Discovery of multiple putative DNA sensors
Since 2009 at least 10 further proteins have been proposed as cytosolic DNA sensors: AIM2, IFI16, LRRFIP1, DHX9, DHX36, DDX41, Ku70, DNA-PKcs, MRE11, cGAS, STING itself and Rad50 (Figure 1 and Figure 2). AIM2 and IFI16 are pyrin and HIN200 domain (PYHIN) proteins that have been shown to directly bind to DNA and mediate inflammasome and transcription factor activation respectively (discussed in detail in the next section).
In 2010 an siRNA screen identified LRRFIP1 as required for IFN-β gene induction in response to Listeria monocytogenes or dsDNA in macrophages [37]. LRRFIP1 was found to not be required for NFκB nor IRF3 activation but rather promoted phosphorylation of the co-activator, β-catenin, leading to recruitment of p300 to the IFNβ enhanceosome via IRF3 [38]. LRRFIP1 was proposed to be a DNA sensor since it co-immunoprecipitated with dsDNA, however direct binding to DNA was not conclusively demonstrated, and in fact LRRFIP1 also had a role in dsRNA-stimulated IFN-β induction. Hence, by promoting β-catenin phosphorylation following nucleic acid detection, LRRFIP1 is capable of indirectly up regulating IFNβ [37].
Many DEAD/H-box helicases, such as RIG-I and MDA5, have been implicated in innate nucleic acid sensing, but DHX9 and DHX36 were the first such proteins proposed to sense cytosolic DNA. These two helicases were identified in plasmacytoid DCs (pDCs) by mass spectroscopy as CpG binding proteins, and surprisingly, shown to signal to altered gene expression via the TLR adaptor MyD88 [39]. DHX36 was associated with IFNα production and IRF7 nuclear translocation in response to CpG-A DNA, while DHX9 was found to be important for TNFα and IL-6 production and NFκB activation in response to CpG-B DNA [39]. Knockdown of TLR9 in these cells was also found to almost completely abrogate CpG DNA-dependent responses [39], consistent with the possibility that DHX9 and DHX36 may be required for the TLR9 pathway in pDCs, rather than acting as DNA sensors themselves. DHX9 has also been proposed as a dsRNA sensor in myeloid DCs since knockdown of DHX9 by shRNA showed DHX9 to be required for the production of type I IFNs and pro-inflammatory cytokines in response to poly(I:C), influenza A and reovirus. [40]. DHX9 was shown to bind poly(I:C) via its dsRNA-binding domain and signal via MAVS to trigger activation of NFκB and IRF3 [40]. How exactly DHX9 might activate MyD88 and MAVS for signaling after DNA and RNA sensing respectively has not been resolved to date.
In order to identify the potential involvement of other DExD/H family members in innate immunity, Yong-Jun Liu's group carried out a siRNA screen with 59 members of the family. In 2011 they identified DDX41 as being required for NF-κB and IRF3 activation, and for cytokine induction, following stimulation of mDCs or human monocytes with dsDNA or HSV-1 [41]. DDX41 was shown to bind DNA and to also associate with both STING and TBK1, in keeping with its role as a proposed DNA sensor [41]. More recently, DDX41 was reported to directly bind the bacterial CDNs, cyclic-di-GMP and cyclic di-AMP [42]. Upon binding to CDNs, DDX41 was shown to induce an IFN-I response via STING, TBK1 and IRF3 [42]. As DDX41 was shown to bind bacterial CDNs, it may also have a role in cGAMP sensing (see below) which could contribute to the mounting of an effective response to DNA virus infection.
Unlike other PRRs such as TLRs and RIG-I, DExD/H-box proteins implicated in DNA sensing do not have clearly defined signaling domains that are distinct from their DNA-binding domains. This makes it difficult to explain how the binding of dsDNA (and of CDNs in the case of DDX41) would lead to the recruitment of signaling adaptors such as STING, and there have been no follow-on studies to date addressing this issue or providing clarity on the signaling mechanism. Further, in vivo studies demonstrating an essential role for DExD/H-box proteins in cytosolic dsDNA sensing have yet to emerge.
Apart from the DExD/H-box family of proteins, proteins known previously to have a role in responding to DNA damage in the nucleus have also been implicated as cytosolic DNA sensors. Such proteins might act independently of their role in the DNA damage response (DDR) as direct sensors of viral DNA, and/or they may be mobilized to stimulate innate immune signaling in response to virus-induced cellular DNA damage in the nucleus (discussed in [10]). DNA dependent protein kinase (DNA-PK) is a heterotrimeric protein complex consisting of Ku70, Ku80 and the catalytic subunit DNA-PKcs. Ku70 is mainly known for its involvement in the DNA repair process [43]. However, in 2011 it was also described as having a role in the cytoplasmic detection of dsDNA of greater than 500 bp in length, triggering the production of type III IFN (IFNλ1), rather than IFN-I, via IRF1 and IRF7 activation [44]. A year after identification of Ku70 as a DNA sensor [44], the DNA-PK complex was identified as a DNA sensor with a particular role in fibroblasts, where it associated with DNA in the cytoplasm and signaled via the STING-TBK1-IRF3 axis to IFN-I induction [45]. DNA-PK colocalised with sites of vaccinia virus replication and both cells and mice lacking DNA-PKcs showed attenuated cytokine responses to both DNA and DNA viruses, but not to RNA or RNA viruses. Interestingly, although crucial for IRF3 activation in response to immunostimulatory DNA or vaccinia virus infection, DNA-PK was not required for DNA-dependent NFκB activation [45]. Further investigations on how DNA-PK signals via STING for IRF3 activation without NF-κB activation may shed light on differential mechanisms for DNA-stimulated transcription factor activation.
Yet another DNA damage sensor protein, Mre11 has also been shown to mediate STING-dependent cytosolic responses to dsDNA (but not to DNA virus) [46]. Most recently, Rad50, which is part of a complex together with Mre11 and Nbs1 that senses and responds to double-strand breaks in nuclear DNA, has also been implicated in innate immune DNA sensing [47]. In that case, after vaccinia virus infection, Rad50 translocated to the cytosol, engaged with viral DNA and coupled to a STING-independent signaling axis involving CARD9 and Bcl10. This led to NF-κB activation, and in particular induction of pro-IL-1β mRNA. Thus in the context where DNA viruses cause inflammasome activation, the Rad50-CARD9-Bcl10-NF-κB pathway contributes to virus-stimulated IL-1β release [47]. The discovery of the role of Rad50 in DNA sensing suggests that in certain contexts STING-independent DNA response pathways to gene induction exist.
Apart from its key role as a signaling adaptor in cytosolic DNA sensing, it has recently been reported that STING binds directly to DNA both in vitro and in intact cells, possibly leading to direct activation of the STING signaling pathway [48]. However, further work will need to be carried out in order to establish whether STING can directly sense foreign DNA without the aid of other DNA sensors in certain contexts, especially since stimulation of HEK293 cells stably expressing STING can induce IFN I in response to CDNs but not DNA [31].
5. Mechanisms of innate DNA sensing: lessons from PYHIN proteins
As well as triggering altered gene induction via transcription factor activation, cytosolic DNA is also capable of stimulating inflammasome activity leading to production of the mature active forms of IL-1β and IL-18. Many inflammasome activators work via stimulation of an NLRP3-containing inflammasome complex that involves ASC recruitment and subsequent caspase 1 activation, however cytosolic DNA was shown to stimulate an NLRP3-independent inflammasome complex. In 2009, the human PYHIN family member absent in melanoma 2 (AIM2) was shown to detect viral dsDNA in the cytoplasm by direct binding via the AIM2 HIN200 domain. This facilitated recruitment of ASC to AIM2, presumably via homotypic PYRIN domain interactions, and subsequent caspase 1 activation [49-52]. Importantly, since there is an ortholog of human AIM2 in the mouse, the generation of AIM2 knock out mice allowed demonstration of a role for AIM2 in DNA- and DNA virus-stimulated IL-1β and IL-18 production in vivo [53, 54].
After the discovery of the role of AIM2 in DNA sensing, a second human PYHIN protein was implicated as a PRR for intracellular DNA. Interferon-γ-inducible protein 16 (IFI16) was identified as a novel PRR for DNA based on its association with, and requirement for IFN-I responses to, a 70 base pair dsDNA derived from the vaccinia virus genome [18]. Upon DNA stimulation [18] or infection of cells with herpes viruses [55], IFI16 associated with STING while siRNA knockdown of IFI16 or its presumed mouse counterpart, p204 (IFI204), inhibited gene induction induced by DNA or HSV-1, but not by RNA or RNA virus [18].
The structure of the PYHIN proteins is consistent with their proposed role as cytosolic DNA sensors, as they possess a defined signaling (pyrin) and DNA binding (HIN200) domain. IFI16, p204 and AIM2 form a new family of PRRs, termed the AIM2-like receptors (ALRs) [13], and whether other PYHIN protein family members also function as ALRs remained to be determined. Ongoing study of the ALRs has revealed structural and mechanistic insights into how innate immune DNA sensing operates. The crystal structure of the AIM2 and IFI16 HIN200 domains bound to dsDNA provided a rationale for sequence-independent sensing of DNA, since the contacts between the protein receptors and the DNA were primarily electrostatic interactions with the phosphate-sugar DNA backbone [56]. This is consistent with the fact that DNA of any sequence, when transfected into monocytic cells, is capable of eliciting cell death and IFN-I responses. Thus self-DNA is not discriminated from pathogen DNA based on nucleotide composition.
Alternatively, self:non-self discrimination might be based on the aberrant appearance of DNA in the cytosol, and hence the nucleus would be ‘immune privileged’. However this is also not the case since IFI16 is mainly nuclear in its expression, and has now been demonstrated to detect multiple nuclear herpes viruses (Reviewed in [57]). Thus innate DNA sensing can also initiate from the nucleus, and not just the cytosol. In fact IFI16 has been shown to shuttle between the cytosol and nucleus for DNA sensing in both compartments, depending on the acetylation status of its nuclear localization sequence [58]. The mechanism by which IFI16 discriminates between pathogen and cellular DNA in the nucleus is not yet clear. However insights into this puzzle come from recent work showing that in vitro IFI16 assembles on naked dsDNA strands in a cooperative manner, which is dependent on homotypic pyrin domain interactions [59]. This leads to the formation of oligomeric IFI16 foci, reminiscent of the assembly of inflammasomes, which could be expected to be signaling-competent. Optimal IFI16 polymerization required approximately 150 bp of dsDNA. Although these observations remain to be shown in intact cells, this work potentially provides an elegant mechanism for IFI16 to discriminate between self and non-self DNA based on ‘measuring’ the length of naked DNA, since host nuclear DNA should normally be complexed with histones. Thus the true ligand for DNA sensing by PYHIN proteins is likely long naked DNA.
IFI16 has also been identified as having a role in the detection of HIV DNA [60, 61]. In HIV-infected macrophages, IFI16 bound HIV ssDNA in duplex structure due to long stem and terminal loop regions that form during the HIV lentiviral life cycle [60]. Thus in macrophages IFI16 contributed to the early control of HIV. IFI16 also has a HIV detection role in abortively infected CD4+ T cells. In that case, the accumulation of viral DNA replication intermediates caused IFI16-dependent pyroptosis [61], leading to depletion of T cells. Thus similar to AIM2, in certain contexts IFI16 can promote inflammasome activation. This is also true for IFI16 sensing of the herpes viruses KSHV, HSV-1 and EBV [62-64].
Currently the molecular details as to how in response to viruses IFI16 signals to the inflammasome in some contexts, and also to STING for IFN-I induction remain unclear. Activated AIM2, like NLRP3, has recently been shown to mediate inflammasome signaling by nucleating pyrin domain filaments of ASC, leading to clustering of the ASC CARD domains and activation of Caspase-1 [65, 66]. Whether IFI16-containing inflammasomes would function in a similar manner remains to be tested.
Notwithstanding the lack of information as to how IFI16 transmits a downstream signal, the importance of IFI16 in viral DNA sensing is underscored by the fact that viral immune evasion strategies have already been identified that target IFI16 function. Specifically, the HSV-1 protein ICP0 targets IFI16 for proteasomal degradation in order to suppress IRF3 activation [67], while the HCMV protein pUL83 interacts with the pyrin domain of IFI16 in order to prevent it forming an oligomer, likely blocking receptor activation [68].
6. Discovery of cGAS as a critical DNA sensor acting via STING
In 2013, the discovery of a novel signaling pathway upstream of STING involving cGAMP synthase (cGAS) represented a hugely significant advance in our understanding of the signaling mechanisms underpinning innate DNA sensing. Zhijian Chen's group identified a factor synthesized by mammalian cells following stimulation with DNA that was capable of activating STING [69]. This factor was cyclic-GMP-AMP (cGAMP) and it was shown to directly bind STING and lead to IRF3 activation [69, 70]. cGAMP is very similar in structure to the bacterial CDNs that can bind and activate STING, suggesting that such CDNs are actually mimicking host cGAMP as a means of stimulating STING-dependent type I IFN-Induction to benefit the bacteria [5, 31]. Chen's group also identified the enzyme that generates cGAMP upon DNA stimulation of cells, from the substrates ATP and GTP, as cGAS [71]. This novel cGAS-cGAMP signaling pathway is reminiscent of the pathway whereby adenylate cyclase generates the second messenger cAMP from ATP in response to G protein-coupled receptors. The crystal structures of both free cGAS and of cGAS bound to DNA have already been solved. This revealed that cGAS resembles the dsRNA binding protein OAS1 in overall structure, with modifications in the nucleotide binding region that specify affinity for dsDNA rather than dsRNA [70, 72, 73]. In the case of cGAS, enzyme activation occurs in response to direct binding of DNA to cGAS, which causes a conformational change allowing access of the nucleotide substrates into the active site, and subsequent synthesis of cGAMP (reviewed in [74]). Similar to the case for DNA recognition by AIM2 and IFI16, cGAS contacts dsDNA exclusively via the DNA phosphate backbone, again leading to nucleotide sequence-independent DNA sensing [74].
Importantly, the role of cGAS in DNA sensing in vivo has been confirmed since cGAS knock out mice are now available. Cells from such mice (fibroblasts and BMDMs) were unable to induce IFN and cytokines in response to DNA or live DNA viruses (vaccinia virus, HSV-1 and MHV68), while cGAS−/− mice were more susceptible to lethal infection with HSV-1 or vaccinia virus compared to wild type mice [75, 76]. In addition, similar to IFI16, cGAS has been shown to have a role in sensing HIV-1 since HIV-1-infected cells produced cGAMP, and HIV-1 induced IFN was cGAS-dependent [77].
DNA detection by cGAS also provides a mechanism for the spread of intrinsic innate immunity to DNA viruses from infected cells into neighboring cells, since Ablasser et al recently showed that cGAMP produced in virally-infected cells can be transferred into uninfected neighboring cells through gap junctions, leading to direct activation of STING and induction of IFNs [78]. Thus antiviral immunity can be rapidly conferred on surrounding uninfected cells independent of the need for the infected cell to first produce IFNs.
7. Conclusion
A vast array of cytosolic DNA sensors have been described in the past decade, leading to many new insights into when, how and why innate immune responses to DNA occur. In particular in the case of IFI16 and cGAS, many studies have now demonstrated key roles for these PRRs in sensing multiple types of DNA viruses and of retroviruses, while structural studies with these proteins have confirmed that innate DNA sensors respond to dsDNA in a sequence-independent manner. Many questions remain to be answered and given the rapid rate of progress in this field we can expect further clarification on many of these in the near future. A key issue is why so many DNA sensors exist, and what is their relative role in DNA sensing in vivo, and in different cell types? In particular it is interesting that IFI16 and cGAS have been shown to both be required for detection of HIV-1 and HSV-1 so whether these sensors actually cooperate to signal to STING in a given cell type, and/or operate in distinct cell types is unclear. Interestingly, Storek et al recently showed an essential role for both IFI16 (p204) and cGAS in the same cell type for IFN I induction following detection of Francisella novicida DNA during a bacterial infection, by using the CRISPR/Cas9 system to generate cGAS and p204 single- and double-knock out cells [79]. In the case of HIV-1 sensing, the role of IFI16 and cGAS in different cells examined to date correlates well with the relative expression of these proteins in such cells. Furthermore, how cytosolic cGAS might be so crucial for sensing nuclear HSV-1 and for protection against HSV-1 in vivo is unclear, as are the mechanisms whereby nuclear IFI16 would signal to the STING-TBK1-IRF3 signaling axis in the cytosol.
What is clear to date is that innate immune DNA sensing underpins many physiological and pathological responses and so further insights and developments in this area will likely yield new approaches to treatment of infectious, autoimmune and inflammatory disease.
Innate immune sensing of DNA leads to both beneficial and harmful immune responses
Intracellular DNA sensing is critical for the detection of DNA viruses
The adaptor STING is essential for DNA-dependent interferon induction
Many DNA sensors upstream of STING have been proposed, including IFI16 and cGAS
cGAS senses DNA in vitro and in vivo, and synthesizes the STING activator cGAMP
Acknowledgements
Work in the authors laboratory is funded by grants from the National Institutes of Health (AI093752), Science Foundation Ireland (11/PI/1056) and the Irish Health Research Board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13(6):453–60. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 3.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 4.Andrejeva J, et al. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-(beta) promoter. PNAS. 2004;101(49):1726417269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurtler C, Bowie AG. Innate immune detection of microbial nucleic acids. Trends Microbiol. 2013;21(8):413–20. doi: 10.1016/j.tim.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goubau D, et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5'-diphosphates. Nature. 2014;514(7522):372–5. doi: 10.1038/nature13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ISAACS A, COX RA, ROTEM Z. Foreign nucleic acids as the stimulus to make interferon. Lancet. 1963;2(7299):113–6. doi: 10.1016/s0140-6736(63)92585-6. [DOI] [PubMed] [Google Scholar]
- 8.Rotem Z, Cox RA, Isaacs A. Inhibition of virus multiplication by foreign nucleic acid. Nature. 1963;197:564–6. doi: 10.1038/197564a0. [DOI] [PubMed] [Google Scholar]
- 9.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 10.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38(5):870–80. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7(1):40–8. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 12.Stetson DB, Medzhitov R. Recognition ofcytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24(1):93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Keating SE, Baran M, Bowie AG. Cytosolic DNA sensors regulating type I interferon induction. Trends Immunol. 2011;32(12):574–81. doi: 10.1016/j.it.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448(7152):501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 15.DeFilippis VR, et al. Human cytomegalovirus induces the interferon response via the DNA sensorZBP1. J Virol. 2010;84(1):585–98. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11(3):290–7. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–13. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451(7179):725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 20.Ablasser A, et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10(10):1065–72. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138(3):576–91. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkins C, Gale M., Jr. Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22(1):41–7. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008 doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29(4):538–50. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Jin L, et al. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28(16):5014–26. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun W, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci USA. 2009;106(21):8653–8. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–92. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14(1):19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5(214):ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe T, Barber GN. Cytosolic DNA-Mediated, STING-Dependent Pro-Inflammatory Gene Induction Necessitates canonical NF-kappaB activation Through TBK1. J Virol. 2014 doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdette DL, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011 doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klarquist J, et al. STING-Mediated DNA Sensing Promotes Antitumor and Autoimmune Responses to Dying Cells. J Immunol. 2014;193(12):6124–34. doi: 10.4049/jimmunol.1401869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woo SR, et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng L, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn J, Ruiz P, Barber GN. Intrinsic self-DNA triggers inflammatory disease dependent on STING. J Immunol. 2014;193(9):4634–42. doi: 10.4049/jimmunol.1401337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371(6):507–18. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang P, et al. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 2010;11(6):487–94. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 38.Wathelet MG, et al. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol Cell. 1998;1(4):507–18. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 39.Kim T, et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107(34):15181–6. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, et al. DHX9 pairs with IPS-1 to sense double-stranded RNA in myeloid dendritic cells. J Immunol. 2011;187(9):4501–8. doi: 10.4049/jimmunol.1101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12(10):959–65. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parvatiyar K, et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13(12):1155–61. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412(6847):607–14. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, et al. Cutting edge: Ku70 is a novel cytosolic DNA sensor that induces type III rather than type IIFN. J Immunol. 2011;186(8):4541–5. doi: 10.4049/jimmunol.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson BJ, et al. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. elife. 2012;1:e00047. doi: 10.7554/eLife.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kondo T, et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci USA. 2013;110(8):2969–74. doi: 10.1073/pnas.1222694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roth S.e.a. Rad50/Card9 interactions link cytosolic DNA sensing to IL-1beta production. Nature Immunology. 2014 doi: 10.1038/ni.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abe T, et al. STING recognition of cytoplasmic DNA instigates cellular defense. Mol Cell. 2013;50(1):5–15. doi: 10.1016/j.molcel.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts TL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323(5917):1057–60. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 50.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–8. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10(3):266–72. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes-Alnemri T, et al. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–13. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11(5):385–93. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horan KA, et al. Proteasomal degradation of herpes simplex virus capsids in macrophages releases DNA to the cytosol for recognition by DNA sensors. J Immunol. 2013;190(5) doi: 10.4049/jimmunol.1202749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jinjf, et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36(4):561–71. doi: 10.1016/j.immuni.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orzalli MH, Knipe DM. Cellular sensing of viral DNA and viral evasion mechanisms. Annu Rev Microbiol. 2014;68:477–92. doi: 10.1146/annurev-micro-091313-103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li T, et al. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc Natl Acad Sci USA. 2012;109(26):10558–63. doi: 10.1073/pnas.1203447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrone SR, et al. Cooperative assembly of IFI16 filaments on dsDNA provides insights into host defense strategy. Proc Natl Acad Sci USA. 2014;111(1):E62–71. doi: 10.1073/pnas.1313577111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jakobsen MR, et al. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci USA. 2013;110(48):E4571–80. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monroe KM, et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343(6169):428–32. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerur N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9(5):363–75. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson KE, Chikoti L, Chandran B. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol. 2013;87(9):5005–18. doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ansari MA, et al. Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J Virol. 2013;87(15):8606–23. doi: 10.1128/JVI.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu A, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156(6):1193–206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai X, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156(6):1207–22. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orzalli MH, DeLuca NA, Knipe DM. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation ofIFI16 by the viral ICP0 protein. Proc NI%Acad Sci U S A. 2012;109(44):E3008–17. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li T, Chen J, Cristea IM. Human Cytomegalovirus Tegument Protein pUL83 Inhibits IFI16-Mediated DNA Sensing for Immune Evasion. Cell Host Microbe. 2013;14(5):591–9. doi: 10.1016/j.chom.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao P, et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153(5):1094–107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun L, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Civril F, et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498(7454):332–7. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kranzusch PJ, et al. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Rep. 2013;3(5):1362–8. doi: 10.1016/j.celrep.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014;54(2):289–96. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 75.Li XD, et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341(6152):1390–4. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoggins J, et al. Pan--viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505(7485):691–5. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao D, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341(6148):903–6. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ablasser A, et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503(7477):530–4. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Storek KM, et al. cGAS and Ifi204 Cooperate To Produce Type I IFNs in Response to Francisella Infection. J Immunol. 2015 doi: 10.4049/jimmunol.1402764. [DOI] [PMC free article] [PubMed] [Google Scholar]