Abstract
Although robust and highly effective anti-viral T cells contribute to the clearance of many acute infections, viral persistence is associated with the development of functionally inferior, exhausted, T cell responses. Exhaustion develops in a step-wise and progressive manner, ranges in severity, and can culminate in the deletion of the anti-viral T cells. This disarming of the response is consequential as it compromises viral control and potentially serves to dampen immune-mediated damage. Exhausted T cells are unable to elaborate typical anti-viral effector functions. They are characterized by the sustained upregulation of inhibitory receptors and display a gene expression profile that distinguishes them from prototypic effector and memory T cell populations. In this review we discuss the properties of exhausted T cells; the virological and immunological conditions that favor their development; the cellular and molecular signals that sustain the exhausted state; and strategies for preventing and reversing exhaustion to favor viral control.
Keywords: Cytokines, Immunity, Inhibitory receptors, Persistent infections, T cell exhaustion
Introduction
Anti-viral T cells are a key component of the adaptive immune response and contribute to the elimination of acute infections, restrict the reactivation of latent viral infections, and control viral loads at steady state levels during chronic infections. Prototypic acute viral infections result in the formation of short-lived, but highly functional, terminally differentiated effector T cell populations that eliminate infected target cells. In addition, a set of phenotypically distinct memory precursors are generated that are retained over time and mature to form the memory T cell pool which contributes to life-long immunity (Hand and Kaech, 2009; Mueller et al., 2013). The tempo and power of these responses can be remarkable, with activated T cells expanding over 50,000 fold by the peak of infection (Blattman et al., 2002), cell numbers doubling every 6-8 hours (Murali-Krishna et al., 1998), and the power to kill target cells with 5 minutes of engagement (Stinchcombe et al., 2001).
Although robust protective T cell responses which help achieve complete viral clearance are often elicited, exhausted T cell populations with reduced effector properties can emerge, especially during chronic infections (Klenerman and Hill, 2005; Wherry, 2011; Yi et al., 2010a). Exhausted T cells are unable to deploy the arrays of anti-viral activities associated with full-fledged effector and memory populations. This immunological austerity hinders the ability to control the infection and favors the foundation of persistence. While this rebalancing of the cell-mediated immune response is detrimental in terms of viral control, it may be beneficial as it limits the adverse effects of hyper-T cell activation including immunopathology and sustained cytokine synthesis. In this review we discuss the virological and immunological cues that favor the development of T cell exhaustion, the conditions that sustain the exhausted state within the chronically infected host, and proven and potential strategies for preventing and reversing exhaustion to favor immune-mediated viral control.
The exhausted state
Although T cell exhaustion has been most widely studied during chronic viral infections, it also develops more generally under conditions of antigen persistence that arise during certain non-viral infections such as malaria and Mycobacterium tuberculosis as well as during tumor outgrowth (Ahmadzadeh et al., 2009; Bhadra et al., 2011; Day et al., 2011). The first indications that anti-viral T cells became exhausted during persistent viral infections stemmed from studies of lymphocytic choriomeningitis virus (LCMV) infected mice (Zajac et al., 1998). Analyses using major histocompatibility multimers in combination with sensitive functional readouts revealed the presence of effector function-negative virus-specific CD8 T cells. Therefore, anti-viral T cells were not necessarily physically lost during chronic infections (Moskophidis et al., 1993) but instead could be maintained in a non-functional, or poorly functional, exhausted state (Zajac et al., 1998; Gallimore et al., 1998).
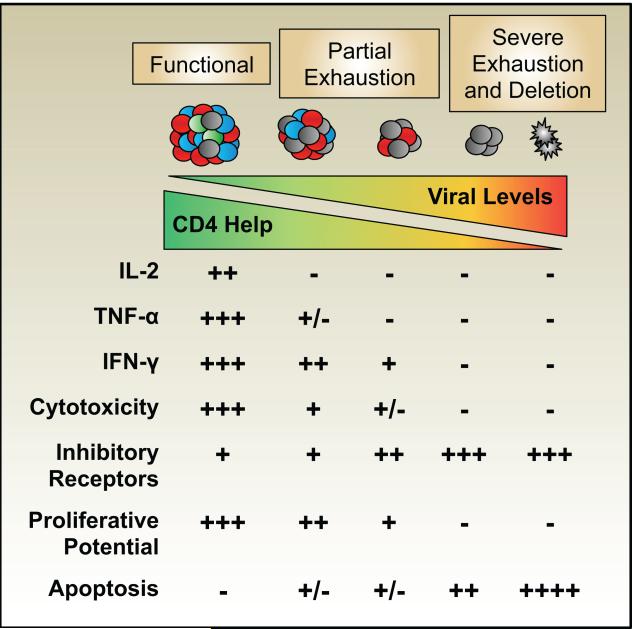
The exhausted state develops in a step-wise and progressive manner and is characterized by the inability to elaborate the arrays of effector functions associated with typical effector and memory T cells (Figure 1). Exhausted T cells also display altered proliferative properties and maintenance requirements; consequently, in the most extreme cases anti-viral T cells decay in number over time and may become undetectable. The loss of functional potential is not stochastic but occurs in a predictable manner as distinct effector modules are successively disabled. Loss of interleukin (IL)-2 production is one of the earliest signs of exhaustion (Fuller et al., 2004; Wherry et al., 2003). Subsequently, the production of other cytokines including tumor necrosis factor (TNF)-α is abolished. However, interferon (IFN)-γ and beta-chemokine production, and possibly cytolytic effector activities, are more resilient to inactivation, although these abilities are also extinguished in the most severely exhausted subsets (Fuller et al., 2004; Agnellini et al., 2007; Shin et al., 2009; Zhou et al., 2004; Mackerness et al., 2010). Thus, a spectrum of exhausted states with varying impacts on the ability to contain the infection can emerge. The extent of exhaustion varies depending upon the type of infection and usually correlates positively with the viral burden. In addition, the degree of exhaustion can differ depending upon the epitope-specificity of the responding cells, which can result in changes in immunodominance as the more severely impeded populations more rapidly succumb to deletion (Blattman et al., 2009; Fuller et al., 2004; Wherry et al., 2003; Zajac et al., 1998).
Figure 1. CD8 T cells can adopt a spectrum of exhausted states.
The levels of viral antigen and availability of CD4 T cells are key determinants of the extent of CD8 T cell exhaustion. CD4 T cells also succumb to exhaustion, which can result in further deterioration of the anti-viral CD8 T cell response. CD8 T cell exhaustion is characterized by the step-wise and progressive loss of effector capabilities, the sustained upregulation of inhibitory receptors, and the loss of self-renewal abilities. which compromise viral control. Severely exhausted T cells may undergo apoptosis and become deleted from the chronically infected host.
Transcriptional determinants of exhaustion
It has become clear that the transcriptional program of exhausted T cells differs dramatically from that of functional effector or memory T cells. Studies defining the genome-wide transcriptional signatures and underlying molecular circuitry of exhausted CD8 T cells, for example, have identified major changes in the expression of inhibitory and co-stimulatory receptors, transcription factors, signaling molecules, cytokine and chemokine receptors and genes involved in metabolism (Crawford et al., 2014; Doering et al., 2012; Wherry et al., 2007). Indeed, these studies originally identified the diverse immunoregulatory pathways operating in exhausted T cells such as programmed death-1 (PD-1) that negatively regulates T cell function (Barber et al., 2006). While there appears to be some shared features of an “activation” signature with functional effector T cells, exhausted T cells also have major transcriptional changes not found in effector T cells. These and other fate tracing experiments support the notion that exhausted T cells attain a unique state of differentiation (Angelosanto et al., 2012; Utzschneider et al., 2013).
A major question that arises from the distinct transcriptional program of exhausted T cells is, what are the central mechanisms that control this altered pattern of gene expression? Although, a number of important transcription factors including T-bet, Eomes, Blimp-1, NFAT, BATF and VHL have been implicated in T cell exhaustion, a master lineage specifying transcription factor has not been identified (Agnellini et al., 2007; Doedens et al., 2013; Kao et al., 2011; Paley et al., 2012; Quigley et al., 2010; Shin et al., 2009). Interestingly, several of these transcription factors can function in a context-specific manner in exhausted T cells that is different from their function in other T cell subsets (Kao et al., 2011; Kurachi et al., 2011; Paley et al., 2012; Quigley et al., 2010; Shin et al., 2009). For example, while T-bet is expressed by and plays a functional role in the formation of terminally differentiated CD8 T cells during acute infections (Intlekofer et al., 2005; Kaech and Cui, 2012), it also controls the population of non-terminal progenitor cells within the exhausted T cell pool (Paley et al., 2012). The related T-box transcription factor, Eomes, is involved in central memory CD8 T cells following acute infection and controls quiescence and homeostatic turnover (Banerjee et al., 2010; Paley et al., 2012; Zhou et al., 2010). However, during chronic infections this same transcription factor controls the terminally differentiated subset of exhausted T cells that are enriched in peripheral tissues (Paley et al., 2012). For exhausted CD8 T cells scale-free network analysis demonstrated that the same transcription factors can have dramatically different functions in memory versus exhausted CD8 T cells (Doering et al., 2012). In some cases, such as T-bet, the network analyses demonstrated a core of conserved functions and sets of context-specific activity found in either functional or exhausted T cells. In other cases, such as Eomes, essentially all transcriptional connections were unique to either functional or exhausted T cells, with essentially no common network connections in both settings. Thus, while exhausted CD8 T cells do not appear to possess a lineage defining transcription factor, these cells do express a distinct constellation of transcription factors. Moreover, many of the transcription factors function in an exhaustion specific manner, perhaps helping to define the exhausted T cell fate.
The epigenetic landscape of a cell is known to have a major influence on how and where transcription factors function, and recent studies suggest that the epigenome may provide a better definition of cell fate than transcription factors. For example, the epigenetic “fate” of a Th1 cell was largely preserved in the absence of T-bet, the transcription factor previously thought to be lineage-defining for Th1 cells (Vahedi et al., 2012). There is relatively little epigenetic information about exhausted T cells. Recent work indicates a global reduction in diacetylated histone H3 suggesting loss of epigenetically active genes (Zhang et al., 2014). Treatment with histone deacetylase inhibitors improved function of exhausted T cells consistent with this idea (Zhang et al., 2014). The details of how such epigenetic changes work for individual genes in exhausted T cells still remains poorly understood. However, as discussed later, studies at the Pdcd1 locus (encoding PD-1) have been informative.
Establishing and maintaining exhaustion
As T cells first respond to an infection they integrate successive cellular and molecular signals that direct their differentiation and fate decisions. During the early stages of persistent viral infections, antigenic signals, cellular environment, and inflammatory constituents guide the T cell response but subvert development of effector and memory pools. Under these conditions the overall magnitude of the T cell response may be lower due to reduced clonal expansion and the participating cells may fail to attain or maintain sufficient breadth and vigor of anti-viral effector functions. This sets the stage for viral persistence as the sub-optimal response fails to eliminate the infection. Parameters that influence the development and maintenance of exhaustion include antigen levels, which are determined by the extent and pace of viral replication; the availability of CD4 T cell help; the strength of the natural killer cell response; the quality and character of the antigen presenting cell (APC) populations; the engagement of co-inhibitory receptor pathways such as PD-1: PD-L1/2; and the levels and composition of the cytokine milieu.
Viral loads
The level and duration of antigen-exposure during persistent viral infections are primary factors that drive the development of T cell exhaustion. This is well illustrated by the observation that T cell exhaustion becomes most pronounced during high grade chronic infections such as persistent LCMV infection of adult mice and hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) infections of humans (Boni et al., 2007; El-Far et al., 2008; Goepfert et al., 2000; Radziewicz et al., 2007; Reignat et al., 2002; Shankar et al., 2000; Wherry et al., 2003; Zajac et al., 1998). Infections such as Epstein-Barr virus (EBV) and cytomegalovirus (CMV) that persist but do not result in such marked and prolonged antigenic activation are associated with intermediate T cell phenotypes (Appay et al., 2008, 2002; Tussey et al., 2003). By contrast, acute viral infections that resolve including influenza, yellow fever, and vaccinia result in the induction of robust effector and durable memory responses (Belz et al., 2000; Gaucher et al., 2008; Harrington et al., 2002; Miller et al., 2008). Collectively, these findings support the concept that the magnitude, duration, and repetitiveness of T cell activation by presented viral antigen dictates the differentiation state and, therefore, the phenotypic and functional quality of the response.
The importance of antigen abundance in pushing T cell exhaustion is exemplified by studies in which the presentation of viral antigens by MHC class I molecules to CD8 T cells is confined to either cells of hematopoietic origin or, more specifically, to dendritic cells (DC) and keratinocytes. LCMV-clone 13 infection of chimeric mice that only express MHC class I molecules on bone marrow-derived cells results in high viral loads (Mueller and Ahmed, 2009; Richter et al., 2012). Nevertheless, even though helper CD4 T cell responses remain intact, the onset of anti-viral CD8 T cell exhaustion is delayed due to limited antigen presentation. The persistently high levels of virus in these hosts do, however, drive eventual exhaustion of the T cell response. Restricting antigen-presentation to DCs and keratinocytes reduced the severity of CD8 T cell exhaustion that usually develops during chronic LCMV infection, but exacerbated immunopathology, highlighting the potential benefits of silencing strong immune responses to avoid morbidity and mortality. Bolstering the levels of antigen-presentation on non-hematopoietic cells or DCs promoted exhaustion. Thus, expanding the levels of antigen exposure weakened the anti-viral CD8 T cell response.
The concept that the abundance of viral antigen promotes exhaustion by driving strong T cell activation is further supported by studies in which viral levels change. During HIV infection the number of polyfunctional anti-viral CD8 T cells capable of producing multiple cytokines declines, and their expression of inhibitory receptors increases with viral loads (Betts, 2006; Day et al., 2006; Oxenius et al., 2002; Trautmann et al., 2006). Improvements in the polyfunctional qualities of HIV-specific T cells is associated with successful anti-retroviral therapy (ART) and is also apparent in non-progressors which are able to contain the infection (Rehr et al., 2008; Streeck et al., 2008). Improvements in the functional status of anti-viral CD8 T cells are also detected as viral levels drop during the spontaneous resolution of HCV infections in humans. In this case, IFN-γ production became detectable as populations of stunned virus-specific CD8 T cells that initially failed to produce this cytokine regained function as the infection resolved (Lechner et al., 2000).
Although influenza infection does not result in long-term viral persistence, reoccurring intraperitoneal inoculation of mice with this virus resulted in CD8 T cell exhaustion (Bucks et al., 2009). The antigen-specific responses were lower in magnitude, less capable of producing IFN-γ, and elicited reduced secondary proliferative responses. Interestingly, although the CD8 T cells generated under these conditions of sustained antigenic exposure expressed the inhibitory receptor PD-1, this did not appear to influence the quality of the response. Notably, the responsiveness of the exhausted CD8 T cells recovered if they were left to rest without successive antigenic exposures, suggesting that halting TCR signaling can lead to functional improvements (Bucks et al., 2009). Similarly, as the levels of LCMV-clone 13 naturally subside, thereby limiting the antigenic activation of the anti-viral T cell pool, some recovery of anti-viral T cell functions, especially IFN-γ production, and the attainment of a more resting phenotype is detected (Fuller et al., 2004; Ou et al., 2001).
The emergence of viral epitope escape mutations can also alter the phenotypic and functional status of the anti-viral T cell pools, demonstrating the potential plasticity of exhausted subsets. The emergence of an escape mutation within the LCMV GP33-41 epitope prevents the exhaustion of P14 CD8 T cells, which recognize this sequence (Blattman et al., 2009). Nevertheless, anti-viral CD8 T cells, specific for other unaltered epitopes continue to lose function, express high level of PD-1, and progress to exhaustion (Blattman et al., 2009; Shin et al., 2007). The emergence of viral escape mutants that influence the functionality and extent of T cell exhaustion are also observed during other chronic infections including HCV, HIV, and simian immunodeficiency virus (SIV) (Petrovas et al., 2007; Rutebemberwa et al., 2008; Streeck et al., 2008). During HIV infection, CD8 T cells specific for epitopes that have mutated over the course of the infection have been shown to regain functionality and decrease their levels of PD-1 (Streeck et al., 2008). In SIV infection models, Tat TL8 epitope-specific CD8 T cells initially express PD-1 early, but this decreases after escape mutants emerge (Petrovas et al., 2007). Similarly, during HCV infections decreases in PD-1 expression on CD8 T cells that recognize mutated epitopes have also been reported (Rutebemberwa et al., 2008).
The selection of viral escape mutations and the associated changes in the quality of the responding cells not only supports the concept that the magnitude and duration of antigenic activation is a principal driver of exhaustion but also demonstrates that the responses can be dynamic and possibly rejuvenated. Adoptive transfer studies have shown that anti-viral CD8 T cell responses can be rescued if they are transferred at early time points from the environment of an ascending chronic infection (LCMV-clone 13) into an acutely infected host (Angelosanto et al., 2012; Brooks et al., 2006a). This suggests that the exhausted fate decision is not preset, imprinted, or irreversible, at least during the initial phase of the infection. Conversely, virus-specific KLRG-1lo memory precursors primed during acute LCMV-infection become exhausted in chronically infected recipients (Angelosanto et al., 2012). Thus, the responding T cells exhibit a level of developmental flexibility especially during the early stages of infection, and high viral loads as well as other infection associated cues orchestrate the outcome of the response. Interestingly, in contrast to the KLRG-1lo memory precursors, KLRG-1hi effector cells were unable to give rise to exhausted T cells and were physically eliminated from chronically infected recipients. These observations indicate that exhausted T cells are derived from memory precursors and highlight a distinction between senescence (at least defined by KLRG1 expression) and exhaustion.
Intriguingly, although exhausted CD8 T cells are typically viewed as inept, there is evidence that they can exert anti-viral effector functions and contribute to infection control. The assessment of killing functions using in vivo CTL assays suggest that cytolytic effector activities are more resistant to exhaustion than cytokine production (Agnellini et al., 2007; Fuller et al., 2004; Graw et al., 2011; Zhou et al., 2004). Moreover, LCMV infection of recipients that had been seeded with exhausted CD8 T cells resulted in expansion of the donor cells and accelerated viral control, even though the signatures of exhaustion, including higher PD-1 expression and reduced polyfunctionality, remained detectable (Utzschneider et al., 2013). Ongoing immunosurviellance by CD8 T cells during chronic infections is further indicated by the findings that the depletion of CD8 T cells during chronic SIV infection results in increased viral loads (Jin et al., 1999; Schmitz et al., 1999). Therefore, although abundance of viral antigen is a key determinant of exhaustion, a range of sub-optimal states emerge, which may be functionally tuned to limit immunopathology but still allow a level of viral control.
CD4 T cell help
CD4 T cells play a vital role in supporting CD8 T cell responses during many chronic viral infections. Again, as with much of our understanding of exhaustion, this is arguably best exemplified following infection with strains of LCMV that are predisposed to persist. In these instances, the absence of CD4 T cells at the onset of the infection converts a prolonged, slowly contained infection into a high grade chronic infection associated with extreme exhaustion and eventual deletion of the virus-specific CD8 T cells (Battegay et al., 1994; Fuller et al., 2004; Matloubian et al., 1994).
CD4 T cells can influence all stages of the anti-viral CD8 T cell response including activation, expansion, and maintenance, as well as secondary recall responses; however, the necessity for CD4 T cell help varies extensively depending upon the pathogen (Wiesel and Oxenius, 2012). CD4 T cells can facilitate the activation of dendritic cells and other APCs. These licensing functions, commonly delivered by CD40:CD40L interactions, can promote the priming and expansion of T and B cell responses (Bennett et al., 1998; Ridge et al., 1998; Schoenberger et al., 1998; Wiesel et al., 2010). Nevertheless, the requirement for CD4 T cell-dependent maturation of professional APCs during the priming phase can be bypassed during many infections by direct activation via pattern recognition receptors as well as by inflammatory cytokines such as type I IFN (IFN-I; IFN-α/β) (Wiesel et al., 2011; Wiesel and Oxenius, 2012).
CD4 T cells are also major producers of the related common-γ chain receptor family cytokines IL-2 and IL-21, which influence the differentiation and properties of the CD8 T cells (Boyman and Sprent, 2012; Cox et al., 2011). IL-2 has been shown to serve as a differentiation factor that promotes the formation of short-lived effector CD8 T cells (Kalia et al., 2010; Pipkin et al., 2010) and also sustains effector populations in peripheral tissues (D’Souza et al., 2002; D’Souza and Lefrançois, 2003). In addition, IL-2 signals during the priming phase have also been implicated in programming the formation of memory T cells, which have the ability to mount rapid recall responses during secondary challenges (Bachmann et al., 2007; Williams et al., 2006). During chronic LCMV infections CD8 T cells that lack expression of the high affinity IL-2 receptor are rapidly lost, highlighting the potential importance of this cytokine in maintaining responses in the persistently infected host (Bachmann et al., 2007).
CD4 T cells are also the principal source of IL-21, which has multiple immunoregulatory roles (Leonard and Spolski, 2005; Yi et al., 2010b). The requirements for IL-21 are especially stringent during chronic LCMV infections, as the anti-viral CD8 T cell response collapses in the absence of this cytokine, and the virus persists at sustained high levels (Elsaesser et al., 2009; Fröhlich et al., 2009; Yi et al., 2009). This mirrors the findings from CD4-deficient mice and suggests that IL-21 is a vital CD4 T cell derived cytokine that helps to sustain CD8 T cell responses under conditions of continuous antigenic activation. The importance of IL-21 in maintaining anti-viral CD8 T cell responses has also been reported during HIV infections where the fraction of IL-21 producing T cells correlates with superior anti-viral CD8 T cell responses and viral control (Chevalier et al., 2011; Iannello et al., 2010; Williams et al., 2011; Yue et al., 2010). Moreover, higher levels of IL-21 are also associated with better containment of HBV and HCV infections (Feng et al., 2013; Kared et al., 2013; Spaan et al., 2014).
Mixed bone marrow chimeras have demonstrated that IL-21 acts directly on anti-viral CD8 T cells during chronic LCMV infections to sustain their functionality and to prevent full exhaustion (Elsaesser et al., 2009; Fröhlich et al., 2009; Yi et al., 2009). How IL-21 operates to support the response is less clear. Exhausted CD8 T cells downregulate the receptors for IL-7 and IL-15, two cytokines which usually support the proliferation and survival of the response (Fuller et al., 2005; Lang et al., 2005; Wherry et al., 2004). It is plausible that IL-21 acts when the signals from these homeostatic cytokines are limited and thus maintains the responding CD8 T cells, especially if they continue to receive strong antigenic signals. In addition, IL-21 has been suggested to limit the complete development of short-lived effectors and to promote the longevity of the responding T cells by preventing their full terminal differentiation and upregulating expression of the transcription factors, T cell factor 1 (TCF-1) and lymphoid enhancer binding factor 1 (LEF1), which are critical factors in the formation of functional memory (Hinrichs et al., 2008; Jeannet et al., 2010; Zhou et al., 2010; Zhou and Xue, 2012).
During acute viral infections IL-21 has limited impact on anti-viral CD8 T cell responses, although recall responses may be curtailed, which has been attributed to TNF-related apoptosis-inducing ligand (TRAIL)-dependent cell death (Barker et al., 2010). Interestingly, TRAIL-mediated apoptosis also accounts for the failure of LCMV-specific CD8 T cells primed in the absence of CD4 T cell help to accumulate following reactivation (Janssen et al., 2005). Moreover, TRAIL has also been implicated in the loss of CD8 T cell responses during repetitive sustained influenza infections, which cause prolonged antigenic activation (Bucks et al., 2009), suggesting that this may be a common mechanism for culling CD8 T cells that have differentiated under suboptimal conditions due to limited CD4 T cell help or IL-21 levels and are reencountering antigenic signals.
In addition to directly regulating anti-viral CD8 T cells during chronic infections, IL-21 may also indirectly affect the quality of the response. IL-21 can limit the formation of regulatory CD4 T cells (Tregs) (Attridge et al., 2012; Fantini et al., 2007; Korn et al., 2007; Schmitz et al., 2013), which may result in more pronounced effector CD8 T cell responses, in part by increasing the availability of IL-2 (de Goër de Herve et al., 2012; Kastenmuller et al., 2011; McNally et al., 2011). IL-21 also plays a prominent role in the formation and maintenance of anti-viral antibody responses by acting directly on follicular helper CD4 T (TFH) cells and B cells. Increases in the proportions of TFH CD4 T cells, which produce IL-21, have been reported during chronic LCMV, HBV, and HIV infections (Crawford et al., 2014; Fahey et al., 2011; Li et al., 2013; Streeck et al., 2008). IL-6 is another cytokine that, in collaboration with IL-21, contributes to the generation of TFH cells and the generation of robust humoral immunity (Dienz et al., 2009; Eto et al., 2011; Karnowski et al., 2012). Mice deficient in IL-6 fail to clear chronic LCMV infection and generate defective TFH and B cells responses. Similarly, blockade of IL-6 or its receptor during chronic LCMV infection impairs TFH and B cell responses and enhances viremia (Harker et al., 2011). These findings emphasize that aberrations in any aspect of the CD4 T cell, CD8 T cell, B cell, or cytokine response can result in less effective containment of the infection, with the resulting higher viral loads driving the development of exhaustion.
Natural Killer Cells
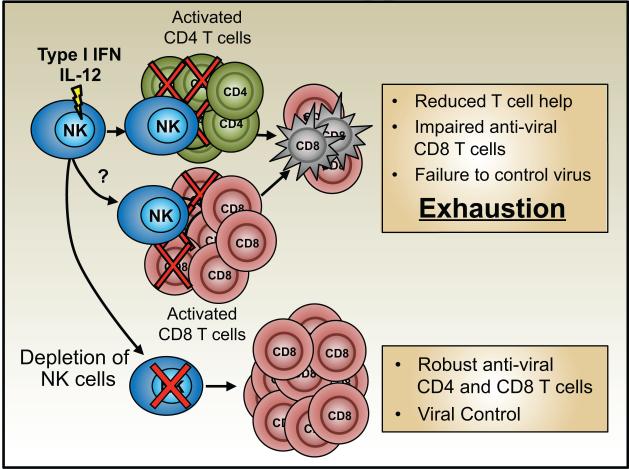
Natural killer (NK) cells are a subset of innate lymphoid cells that function as early controllers of viral infections (Welsh and Waggoner, 2013). Recently the roles of NK cells in restricting the induction of adaptive anti-viral T cell responses and abetting the development of exhaustion have been highlighted (Figure 2). The pronounced induction of NK cell responses during the early stages of chronic LCMV infections suppresses the development of anti-viral CD8 T cells resulting in more extreme exhaustion and greater levels of viral replication. This is profoundly consequential for the infected host, as although immunological intervention by NK cells slows or prevents the resolution of the infection it also dampens the adaptive response sufficiently to stop immunopathology and lethal disease (Cook and Whitmire, 2013; Lang et al., 2012; Waggoner et al., 2014, 2012).
Figure 2. NK cells can promote T cell exhaustion during chronic infections.
NK cells can target and kill activated CD4 T cells during the early stages of viral infections, removing vital helper functions, thereby fostering the development of CD8 T cell exhaustion. NK cells may also directly target anti-viral CD8 T cells, reducing their availability to control the infection and thus also promoting exhaustion. The targeted depletion of NK cells can save the anti-viral T cell responses and permit viral clearance.
Mechanistically, NK cells may curtail the anti-viral CD8 T cell response by direct killing, by limiting the activating ability of APCs, by the production of immunoreguatory cytokines such as IL-10, and by culling CD4 T cells that help the response (Crome et al., 2013; Waggoner et al., 2012). The perforin-dependent killing of CD4 T cells by NK cells has been shown in the LCMV system, and this likely feeds back to promote the exhaustion of the anti-viral CD8 T cell response (Waggoner et al., 2012). The targeting of activated T cells by NK cells occurs not only during LCMV infections but more broadly following vaccinia virus (VV), murine cytomegalovirus, Pichinde, and mouse hepatitis virus infections and may also contribute to poor T cell responses during HBV and HCV infections (Waggoner et al., 2012). Although suppression of anti-viral T cells by activated NK cells is observed, especially during the initial phases of certain chronic infections, some level of response is usually elicited. IFN-I signals have been shown to render activated T cells resistant to NK cell-mediated attack by causing an elevation of NK cell inhibitory ligands including the non-classical MHC class I molecule Qa-1b and downregulation of the putative NCR1 (NKp46) activatory ligand (Crouse et al., 2014; Xu et al., 2014). This interplay between IFN-I levels, NK cells, and qualitative and quantitative changes in the anti-viral T cell pool is more complex, as although IFN-I may shield the T cells from death by NK cells, it also promotes T cell expansion and terminal differentiation. Moreover, extended high levels of IFN-I can dampen the overall effectiveness of the T cell response (Teijaro et al., 2013; Wilson et al., 2013).
Immunosuppressive cytokines
As persistent viral infections become established the levels of stimulatory and immunosuppressive cytokines recalibrate (Cox et al., 2013). The production of suppressive cytokines such as IL-10 and transforming growth factor (TGF)-β can act to quell potentially pathogenic responses but consequently limit immunological protection and delay viral control. High levels of inflammatory cytokines, including IFN-I can also have unanticipated effects in attenuating T cell responses to both the ongoing persistent viral infection as well as newly encountered pathogens (Stelekati et al., 2014).
IL-10 is an immunosuppressive cytokine that has a wide range of regulatory effects including suppression of T cell proliferation, APC functions, and modulation of cytokine and chemokine synthesis (Saraiva and O’Garra, 2010). It is expressed at elevated levels during several persistent infections including EBV, HBV, HCV, HIV, and LCMV (Brockman et al., 2009; Clerici et al., 1994; Hyodo et al., 2004; Kaplan et al., 2008; Ohga et al., 2004). Furthermore, certain viruses including CMV and EBV encode IL-10 homologs with both structural and functional similarities to human IL-10, which aid in immune-evasion and viral persistence (Fiorentino et al., 1990; Nachtwey and Spencer, 2008; Slobedman et al., 2009). The initial production of IL-10 is similar during the earliest stages of acute and chronic LCMV infections but levels remain elevated if the infection persists, suggesting a role for IL-10 in restricting immune mediated viral control and in the development of exhaustion (Brooks et al., 2006b; Ejrnaes et al., 2006; Maris et al., 2007).
During chronic LCMV infection exhausted CD4 and CD8 T cells are more capable of synthesizing IL-10, which is driven by the transcription factor Blimp-1 (Parish et al., 2014). Ablation of T cell-derived IL-10 promotes viral control and improves the functionality of the anti-viral T cells (Richter et al., 2013a). Immune deviation resulting in the production of IL-10 by anti-viral CD8 T cells occurs during murine γ-herpesvirus 68 infection in the absence of CD4 T cell help and compromises viral control (Molloy et al., 2011). T cells, however, may not be the only significant source of IL-10 during chronic infections, as macrophages as well as a distinct set of immunoregulatory APCs have also been shown to produce this cytokine and contribute to the immunosuppressive cellular environment that favors virus persistence and promotes exhaustion (Richter et al., 2013a; Wilson et al., 2012).
TGF-β is an immunosuppressive cytokine that modulates cell proliferation, survival, and differentiation (Li et al., 2006). Certain viruses including HBV and HCV directly promote TGF-β synthesis, most likely to take advantage of these properties. Notably, elevated levels of TGF-β have been reported during several persistent viral infections including EBV, HBV, HCV, HIV and LCMV (Alatrakchi et al., 2007; Kekow et al., 1990; Li et al., 2012; Ohga et al., 2004; Tinoco et al., 2009a). The administration of TGF-β inhibits the generation of VV and LCMV-specific cytotoxic T cells and TGF-β has been shown to drive the apoptosis of short-lived effector CD8 T cells (Fontana et al., 1989). Moreover, the function of HCV-specific CD4 and CD8 T cells is improved by blocking TGF-β in vitro, further implicating its role in regulating the efficacy of the anti-viral T cell response (Alatrakchi et al., 2007). Nevertheless, contrasting findings have been observed in the LCMV system. During chronic LCMV infection selective inhibition of TGF-β signaling on CD8 T cells increased size and function of the response and was associated with viral clearance (Garidou et al., 2012; Tinoco et al., 2009b). By contrast, when TGF-β levels were depleted using TGF-β antagonists or antibodies no significant differences in viral clearance were detected despite somewhat increased anti-viral T cell responses (Garidou et al., 2012). Thus modest improvements in T cell function may be insufficient to improve viral control.
Studies of chronic LCMV infections have shown that, paradoxically, the number one enemy of many viral infections, IFN-I, enhances CD4 T cell exhaustion and promotes viral persistence, possibly as a tactic for avoiding immunopathology (Teijaro et al., 2013; Wilson et al., 2013). During SIV infection of macaques sustained administration of IFN-I also dampens the anti-viral response leading to accelerated disease progression (Sandler et al., 2014). Exhausted T cells also recalibrate their responsiveness to other proinflammatory cytokines. They downregulate expression of the IL-18 receptor and this is associated with an inability to react to cytokines such as IL-12 and IL-18, as well as IFN-I which can drive the antigen-independent activation of effector and memory T cells (Haining et al., 2008; Ingram et al., 2011; Raué et al., 2013, 2004). Thus, exhausted T cells adjust their reactivity to cytokines which usually kick-start the response and are therefore further impeded by infection associated shifts in cytokine levels.
Inhibitory receptors
A prominent trait of exhausted T cells is the sustained upregulation of arrays of inhibitory receptors that negatively regulate their responsiveness (Chen and Flies, 2013; McMahon et al., 2002; Odorizzi and Wherry, 2012). These receptors are induced as the anti-viral T cells first become activated and likely operate as a safety mechanism to restrain the response. Their expression subsides as acute infections resolve. By contrast, the presence of persisting viral antigen drives their continuous expression, limiting the ability of anti-viral T cells to control the infection and enforcing their state of exhaustion.
In the context of T cell exhaustion, the most widely studied inhibitory receptor is the CD28 family member PD-1. PD-1 is upregulated on virus-specific T cells during many chronic viral infections, including HBV, HCV, HIV, LCMV and SIV, and restricts their function and proliferative capacity (Barber et al., 2006; Day et al., 2006; Golden-Mason et al., 2007; Peng et al., 2008; Trautmann et al., 2006; Velu et al., 2009). The engagement of PD-1 with it ligands, PD-L1 and PD-L2, can induce the transcription factor BATF in CD8 T cells, which feeds back to limit proliferation and IFN-γ production (Quigley et al., 2010). PD-1 can also suppress the immune response by reducing the duration of APC:T cell interactions and increasing the threshold of TCR derived signals required for activation and development of effector functions (Fife et al., 2009; Wei et al., 2013).
The expression of PD-1 is induced by the transcription factor NFATc1, which translocates to the nucleus upon TCR activation (Oestreich et al., 2008). Conversely, PD-1 is repressed by the transcription factors Blimp-1 and T-bet, both of which are expressed in activated T cells and are associated with effector T cell differentiation (Kao et al., 2011; Lu et al., 2014). Intriguingly, although NFATc1 and Blimp-1 are positive and negative regulators of PD-1, respectively, their roles in coordinating expression of this inhibitory receptor during chronic infections are more complex. In exhausted cells, higher PD-1 levels directly correlate with Blimp-1 levels, and ablation of Blimp-1 leads to decreased PD-1 expression (Shin et al., 2009). Moreover, the nuclear translocation of NFATc1, which drives expression of PD-1, has been shown to be compromised in exhausted cells. Consequently, in these circumstances NFATc1 may not be the primary driver of PD-1 (Agnellini et al., 2007). Epigenetic modifications may predispose exhausted T cells to express PD-1. During chronic infections including LCMV, HIV, CMV, and EBV the PD-1 gene locus is demethylated as anti-viral T cells undergo activation and succumb to exhaustion (Youngblood et al., 2011). These epigenetic changes are retained in exhausted T cells even if antigen levels drop, which differs from the pattern in conventional memory populations which remethylate the PD-1 regulatory regions (Youngblood et al., 2013, 2011). It is therefore likely that distinct transcriptional scaffolds differentially regulate PD-1 expression in effector, memory, and exhausted T cells.
Although sustained upregulation of PD-1 is a prominent trait of exhausted T cells, other inhibitory receptors which negatively regulate T cell functions are also expressed. One such molecule is T-cell immunoglobulin and mucin domain containing molecule-3 (Tim-3). Like PD-1, Tim-3 is transiently upregulated on virus-specific CD8 T cells during acute infections and remains elevated on exhausted T cells (Fujita et al., 2014; Jones et al., 2008; McMahan et al., 2010). During chronic LCMV infections anti-viral T cells that co-express Tim-3 and PD-1 are less functional than their PD-1+ Tim-3− counterparts (Jin et al., 2010). Nevertheless, Tim-3 expression by Mycobacterium tuberculosis-specific CD4 and CD8 T cells correlates with stronger IFN-γ and perforin production (Qiu et al., 2012). These potentially contradictory observations may reflect a role of Tim-3 in enhancing proximal antigenic signals received via the T cell receptor (Ferris et al., 2014; Lee et al., 2011). This may positively regulate transiently activated responses, but may help to drive exhaustion under conditions of constant antigen exposure that occurs during chronic infections.
Lymphocyte activating gene-3 (LAG-3) (CD223) is an MHC class-II ligand that is structurally similar to CD4 and is expressed on activated and exhausted T cells. LAG-3 has been shown to inhibit calcium fluxes associated with TCR signaling, and dampen cytokine production and proliferation (Hannier et al., 1998). Interest in the roles of LAG-3 in T cell exhaustion grew after it was discovered to be upregulated on anti-viral CD8 T cells during chronic LCMV infection and is heavily associated with the co-expression of PD-1 (Blackburn et al., 2008b). However, despite the expression of LAG-3 by exhausted T cells, viral clearance and T cell functions are similar in wild-type and LAG-3 deficient mice during chronic LCMV infection (Richter et al., 2010). Thus, LAG-3 alone may not drive T cell exhaustion but may cooperate with other inhibitory receptors to influence the extent of T cell exhaustion (Richter et al., 2010).
Consistent with the expression patterns of many inhibitory receptors, the CD28 family member CD160 is also transiently upregulated upon activation and remains elevated as exhaustion ensues (Blackburn et al., 2008b; Peretz et al., 2012; Tsujimura et al., 2006). The regulatory roles of CD160 are complex as it has been shown that crosslinking CD160 both promotes and inhibits T cell responses (Cai et al., 2008; Nikolova et al., 2002). These divergent outcomes may reflect the different avidities of CD160 for its ligands, MHC I and herpesvirus entry mediator (HVEM), which result in distinct downstream signaling (Cai and Freeman, 2009). CD160 is upregulated by subsets of exhausted LCMV-specific CD8 T cells, and its expression by CD8 T cells correlates with lower cytokine production and disease progression during HIV infection (Blackburn et al., 2008b; Peretz et al., 2012). Blocking CD160 interactions with HVEM in vitro increases the proliferation of HIV and CMV-specific CD8 T cells (Peretz et al., 2012). The cytotoxicity of exhausted LCMV-specific CD8 T cells is also enhanced by abrogating CD160 interactions (Blackburn et al., 2008b). Although CD160 can modulate T cell functions independently of PD-1 expression, T cell subsets which co-express these inhibitory receptors are more dysfunctional, illustrating the cooperative and additive impact of multiple inhibitory receptors in controlling exhaustion.
An additional regulatory molecule expressed by exhausted T cells is 2B4 (CD244), which binds to CD48. 2B4 is upregulated on activated T cells during many infections including HIV, HBV, CMV, EBV, and LCMV (Aldy et al., 2011; Blackburn et al., 2008b; Peritt et al., 1999; Raziorrouh et al., 2010; Schlaphoff et al., 2011) and has both positive and negative effects which depend upon its levels of expression (Chlewicki et al., 2008). Crosslinking 2B4 in conjunction with TCR activation of either bulk or HCV-specific CD8 T cells increased the proliferation of 2B4lo but not 2B4hi subsets (Schlaphoff et al., 2011). Similarly, the production of IFN-γ by LCMV-specific CD8 T cells which expressed intermediate levels of 2B4 was impeded when the interaction with CD48 was blocked, but IFN-γ production by exhausted 2B4hi CD8 T cells was enhanced (Blackburn et al., 2008b). This highlights how variations in the levels of 2B4 expression have contrasting regulatory influences on the anti-viral T cell response.
CD4 T cell exhaustion
While much of the information we have about exhaustion is regarding CD8 T cells, CD4 T cells also lose effector function during chronic viral infections (Brooks et al., 2005; Crawford et al., 2014; Fuller et al., 2004; Oxenius et al., 1998). Exhausted CD4 T cells display poor production of effector cytokines (e.g. TNF and IFN-γ) and express high levels of PD-1 (Crawford et al., 2014). While these general features are similar to what occurs for CD8 T cells certain aspects of CD4 T cell exhaustion are distinct. For example, when carefully analyzed early in infections that will become chronic, virus-specific CD4 T cells appear to lose effector function earlier than CD8 T cells (Brooks et al., 2006a, 2005; Crawford et al., 2014). Moreover, exhausted CD4 T cells can often produce IL-10 and/or IL-21 (Brooks et al., 2006b; Crawford et al., 2014; Fahey et al., 2011) both of which may be important for negatively regulating (IL-10) and/or supporting the persistence (IL-21) of CD8 T cell and B cell responses. In addition to these functional changes virus-specific CD4 T cell populations during chronic infections can become highly enriched in cells that express CXCR5 and other markers of TFH cells that may be dependent on chronic IFN-I signaling (Osokine et al., 2014). These observations are intriguing and may provide insights for cryoglobulinemia and altered antibody production during some human chronic infections (Charles and Dustin, 2009; Haas et al., 2011; Hunziker et al., 2003) and also for the importance of IL-21 signaling to sustain exhausted CD8 T cells (Elsaesser et al., 2009; Fröhlich et al., 2009; Yi et al., 2009). Thus, there appear to be several key cellular differences between exhaustion of CD4 and CD8 T cells during chronic viral infections.
Direct comparison of the transcriptional programs of exhausted CD4 and CD8 T cells demonstrate a conserved core transcriptional signature of T cell exhaustion common to both lineages. This common molecular profile includes inhibitory receptors and an IFN-I inducible gene signature. There are also major features of the transcriptional program of CD4 and CD8 T cell exhaustion that are distinct. For example, while high and sustained PD-1 expression was common to both lineages, the pattern of other inhibitory receptors expressed was distinct (Crawford et al., 2014; Kaufmann et al., 2007). A key difference between exhausted CD4 and CD8 T cells is the expression of transcription factors. For example, exhausted CD4 T cells had altered expression of GATA-3, Bcl-6, Helios and other transcription factors that was not observed for exhausted CD8 T cells. As discussed above, Eomes is a major transcription factor for exhausted CD8 T cells, but only a minor subset of exhausted CD4 T cells expresses this molecule. Single cell transcription factor protein co-expression patterns in exhausted CD4 T cells reveal substantial heterogeneity in this population (Crawford et al., 2014) suggesting perhaps more complexity and diversity in this population than observed for exhausted CD8 T cells. Thus, current observations suggest a distinct pattern of differentiation for exhausted CD4 T cells that may include enrichment for TFH-like cells, altered immunoregulatory pathways and a distinct network of transcription factors (Crawford et al., 2014).
Preventing and reversing T cell exhaustion
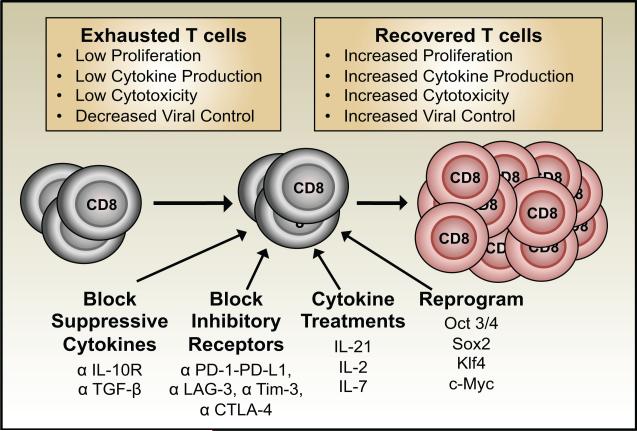
Advancements in our understanding of the cellular and molecular mechanisms that regulate T cell exhaustion have helped to identify potential pathways that can be targeted to prevent and reverse functional unresponsiveness and improve infection control. These strategies include impeding inhibitory receptor interactions, altering the availability of activatory and suppressive cytokines, and molecularly reprogramming exhausted T cells (Figure 3). Other potential approaches include the targeted removal of regulatory T cells as well as vaccinations to bolster T cell responses.
Figure 3. Strategies for rejuvenating exhausted T cells.
Inhibiting suppressive cytokines, blocking inhibitory receptors, cytokine treatments, and molecular reprogramming have all been shown to help restore the functions of exhausted T cells. Combination approaches are also successful.
Seminal studies inhibiting PD-1 established that blocking this receptor can rejuvenate certain subsets of exhausted anti-viral T cells. Anti-PD-L1 antibody treatment of mice chronically infected with LCMV boosted the numbers of virus-specific CD8 T cells and restored their ability to produce IFN-γ and TNF-α, proliferate, and kill infected target cells (Barber et al., 2006). Most importantly viral loads were reduced. Similarly, studies of exhausted HIV-specific CD8 T cells also demonstrated restoration of proliferation, cytokine production and killing activities following PD-1 blockade (Day et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006). Moreover, in vivo treatments of SIV infected macaques with anti-PD-1 antibodies increased the numbers of polyfunctional virus-specific CD8 T cells, augmented neutralizing antibody titers, and lowered viral loads (Velu et al., 2009).
Blockade of PD-1 during HCV infection has been shown to restore anti-viral CD4 and CD8 T cell responses and control viral replication. Nevertheless, the efficacy was variable and likely influenced by the severity of exhaustion and abundance of anti-viral T cells present before the initiation of treatment (Fuller et al., 2013). The extent of exhaustion can vary in a tissue dependent manner due to differences in the local levels of viral antigen. These compartmental differences are detected during HCV infections where virus-specific CD8 T cells that encounter higher levels of antigen in the liver adopt a more severely exhausted phenotype than their counterparts in the peripheral blood (Nakamoto et al., 2008; Radziewicz et al., 2007). Accordingly, not all exhausted T cells can be rescued by PD-1 blockade, as liver-resident HCV-specific CD8 T cells and LCMV-specific CD8 T cells that express the highest levels of PD-1 are less sensitive to reactivation (Blackburn et al., 2008a; Nakamoto et al., 2008).
Inhibitory receptors other than PD-1 have also been targeted in order to restore the functions of anti-viral T cell populations. The ex vivo blocking of Tim-3 can enhance cytokine production and the proliferation of HIV and HCV-specific CD8 T cells (Jones et al., 2008; McMahan et al., 2010). In the LCMV system, Tim-3 blockade alone has minimal effects on the recovery of the response; however, the combined blockade of Tim-3 and PD-1 is more successful than impeding either receptor alone. Thus, targeting multiple inhibitory receptors may be more efficacious at rejuvenating functionally inferior T cell responses. This is further illustrated by studies of LAG-3, as blocking LAG-3 alone fails to rescue exhausted LCMV-specific T cells or accelerate viral clearance. Nevertheless, co-blockade of PD-1 and LAG-3 acts synergistically to improve T cell functions and aid viral clearance (Blackburn et al., 2008b; Richter et al., 2010).
Although blocking inhibitory receptors can have significant impacts on improving cellular immune responses, adverse effects can also occur (Frebel and Oxenius, 2013). These can result from immunopathology caused by enhanced T cell responses, which does develop if PD-1 treatment is applied early during the course of chronic LCMV infection. In the case of SIV infection of macaques blockade of CTLA-4, another inhibitory molecule that can attenuate lymphocyte responses, resulted in the loss of CD4 T cells and increased viral loads, as well as diminished the efficacy of anti-viral treatments (Cecchinato et al., 2008). Thus, therapeutic approaches to improve cell-mediated immunity to infections need to be carefully calibrated to ensure that potentially damaging effects are mitigated.
Cytokines are attractive therapeutic targets for modulating immune responses as their actions can be altered by cytokine administration, receptor blockade, or by inhibiting signaling pathways. Blocking the IL-10 receptor can improve viral control and reduce the extent of exhaustion during chronic LCMV infections (Brooks et al., 2006b; Ejrnaes et al., 2006; Maris et al., 2007). Although inhibition of IL-10 can enhance anti-viral T cell responses these positive effects can be overcome by rapid viral replication and dissemination (Maris et al., 2007; Richter et al., 2013b). This supports the concept that increasing levels of viral antigen production can outpace and corrupt the responding virus-specific T cells, resulting in exhaustion and impaired viral control. Combination strategies to block PD-1 and IL-10 have been shown to be efficacious, and IL-10 inhibition together with therapeutic DNA vaccination also improves immunity and facilitates viral clearance (Brooks et al., 2008). One effect of high IL-10 levels on T cell responses is the induction of Socs3, which negatively regulates cytokine signals delivered though the gp130 receptor as well as others (Cui et al., 2011; Rottenberg and Carow, 2014). Interestingly, IL-7 treatments have been shown to reverse the establishment of exhaustion and decrease Socs3 expression, resulting in improved viral control (Nanjappa et al., 2011; Pellegrini et al., 2011). Notably, effector and exhausted CD8 T cells downregulate IL-7Rα thus the positive effects may be due to the indirect actions of IL-7, including upregulation of IL-6 which may boost T cell responses, as well as by the synthesis of IL-22 which limits immunopathology (Nanjappa et al., 2011; Pellegrini et al., 2011). In the case of HIV infections, IL-7 therapies expanded the overall numbers of CD4 and CD8 T cells but viral control was not improved (Levy et al., 2009; Sereti et al., 2009).
Strategies to rejuvenate exhausted T cells using IL-2 or IL-21 therapies also have mixed outcomes. During chronic LCMV infections the administration of IL-2 has been shown to boost the size of the virus-specific CD8 T cell response and reduce viral loads, especially in conjunction with PD-1 blockade (Blattman et al., 2003; West et al., 2013). By contrast, IL-2 therapy has also been shown to expand Tregs, augment exhaustion, and impede viral control (Schmitz et al., 2013). The benefits of IL-2 treatments during HIV infections are also uncertain, as even though CD4 T cell numbers are increased, greater viral control is not observed and CD8 T cells are largely unaffected (Abrams et al., 2009; Caggiari et al., 2001; Marchetti et al., 2004). Treatments with IL-21 during the initial phase of chronic LCMV infections have been shown to expand the numbers and functional quality of anti-viral CD8 T cells as well as lower viral loads. Nevertheless, this was associated with exacerbated immunopathology (Yi et al., 2009). The treatment of SIV infected macaques with IL-21 is well-tolerated and enhances the expression of cytotoxic effector molecules and IFN-γ by virus-specific T cells, and boosts the titers of anti-SIV antibodies but does not reduce viremia (Pallikkuth et al., 2011). Notably, the loss of IL-21 signaling increases the numbers of Tregs (Schmitz et al., 2013), and in separate studies it was shown that during chronic LCMV infection depletion of Tregs increases numbers of anti-viral CD8 T cells; however, viral loads are not affected unless PD-1 is also blocked (Penaloza-MacMaster et al., 2014). Thus, as discussed above, IL-21 may both directly and indirectly augment anti-viral CD8 T cells responses.
New technological approaches that potentially allow antigen-specific T cell populations to be molecularly reprogrammed into induced pluripotent stem cells (iPS) and redifferentiated into functionally effective T cells for adoptive cell therapy are now being developed. The key to success is being able to isolate sufficient numbers of T cells that are receptive to the introduction of the transcription factors Oct3/4, Sox2, Klf4, and c-Myc, which rejuvenate the cells and allow for the formation of iPS cells (Crompton et al., 2014; Nishimura et al., 2013; Vizcardo et al., 2013). These progenitors can then be redifferentiated into functional antigen-specific T cells for infusion. This strategy requires that the original antigen-specificity is retained as the mature T cells develop. Nevertheless, clonal mono-specific populations of anti-viral T cells may be rendered irrelevant at infection control if epitope escape mutations arise. They will also be subject to antigenic, cellular, and cytokine signals that may favor the reestablishment of exhaustion. If these obstacles are encountered then further optimization of these methods will be necessary, perhaps by using poly-clonal, multi-specific pools or by using differentiated subsets that are most likely to be sustained over time and least prone to functional inactivation.
Concluding remarks
Over the last two decades there have been many exciting developments in our understanding of T cell exhaustion. We now appreciate that both CD4 and CD8 T cells are susceptible to exhaustion and that this commonly occurs during persistent infections. It is now also recognized that exhaustion ranges in severity, and that this functional tuning of the response may have a positive impact by limiting the likelihood of immune mediated damage. The intricate relationships between innate cellular immune responses, T and B cells, cytokine levels, and the extent of viral replication in regulating the development of exhaustion are being deciphered, but more studies are necessary given the complexities of these interactions. Our understanding of the transcriptional regulation of exhaustion is advancing, but it remains to be determined whether context-specific transcription factor functions in exhausted T cells result from the use of distinct co-factors, concentration-dependent binding to different genomic sites, or altered genomic accessibility due to epigenetic changes. Importantly, it has been shown that exhausted responses can be functionally reincarnated, leading to improvements in infection control. Further research to customize these approaches in an infection specific manner to restore durable long-term infection control while avoiding immunopathology will be key next steps.
Highlights.
Persistent viral infections can result in the exhaustion of anti-viral T cells.
Excessive and sustained levels of viral antigen drive T cell exhaustion.
Exhausted T cells are distinct from typical effector and memory subsets.
Exhausted T cells are functionally ineffective and compromise viral clearance.
Blocking inhibitory receptors and modifying cytokine levels can alleviate exhaustion.
Acknowledgements
We wish to thank Jennifer Ingram, Yuan Tian and Ian McWilliams for critical reading of this review. Some of the findings described were supported in part by grants AI049360, AI082966, and AI109962 (to A.J.Z.), T32 AI007051 (to S.M.K.), and AI105343, AI112521, AI082630, AI095608, HHSN266200500030C (to E.J.W) from the National Institutes of Health. As a result of the space constraints, we apologize that we were unable to cite all our colleagues who have advanced our understanding of T cell exhaustion during viral infections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams D, Lévy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, Fox L, Gordin F, Lane HC, Lundgren JD, Mitsuyasu R, Neaton JD, Phillips A, Routy JP, Tambussi G, Wentworth D, INSIGHT-ESPRIT Study Group, SILCAAT Scientific Committee Interleukin-2 therapy in patients with HIV infection. N. Engl. J. Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. doi:10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnellini P, Wolint P, Rehr M, Cahenzli J, Karrer U, Oxenius A. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc. Natl. Acad. Sci. 2007;104:4565–4570. doi: 10.1073/pnas.0610335104. doi:10.1073/pnas.0610335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. doi:10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatrakchi N, Graham CS, Vliet H.J.J. van der, Sherman KE, Exley MA, Koziel MJ. Hepatitis C Virus (HCV)-Specific CD8+ Cells Produce Transforming Growth Factor β That Can Suppress HCV-Specific T-Cell Responses. J. Virol. 2007;81:5882–5892. doi: 10.1128/JVI.02202-06. doi:10.1128/JVI.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldy KN, Horton NC, Mathew PA, Mathew SO. 2B4+ CD8+ T cells play an inhibitory role against constrained HIV epitopes. Biochem. Biophys. Res. Commun. 2011;405:503–507. doi: 10.1016/j.bbrc.2011.01.062. doi:10.1016/j.bbrc.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive Loss of Memory T Cell Potential and Commitment to Exhaustion during Chronic Viral Infection. J. Virol. 2012;86:8161–8170. doi: 10.1128/JVI.00889-12. doi:10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GMA, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002;8:379–385. doi: 10.1038/nm0402-379. doi:10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Appay V, van Lier RAW, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: Consensus and issues. Cytometry A. 2008;73A:975–983. doi: 10.1002/cyto.a.20643. doi:10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- Attridge K, Wang CJ, Wardzinski L, Kenefeck R, Chamberlain JL, Manzotti C, Kopf M, Walker LSK. IL-21 inhibits T cell IL-2 production and impairs Treg homeostasis. Blood. 2012;119:4656–4664. doi: 10.1182/blood-2011-10-388546. doi:10.1182/blood-2011-10-388546. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur. J. Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. doi:10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting Edge: The Transcription Factor Eomesodermin Enables CD8+ T Cells To Compete for the Memory Cell Niche. J. Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. doi:10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. doi:10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Barker BR, Gladstone MN, Gillard GO, Panas MW, Letvin NL. Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur. J. Immunol. 2010;40:3085–3096. doi: 10.1002/eji.200939939. doi:10.1002/eji.200939939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Xie W, Altman JD, Doherty PC. A Previously Unrecognized H-2Db-Restricted Peptide Prominent in the Primary Influenza A Virus-Specific CD8+T-Cell Response Is Much Less Apparent following Secondary Challenge. J. Virol. 2000;74:3486–3493. doi: 10.1128/jvi.74.8.3486-3493.2000. doi:10.1128/JVI.74.8.3486-3493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. doi:10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Betts MR. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. doi:10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1–PDL-1 blockade. Proc. Natl. Acad. Sci. 2011;108:9196–9201. doi: 10.1073/pnas.1015298108. doi:10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl. Acad. Sci. 2008a;105:15016–15021. doi: 10.1073/pnas.0801497105. doi:10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2008b;10:29–37. doi: 10.1038/ni.1679. doi:10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Antia R, Sourdive DJD, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the Precursor Frequency of Naive Antigen-specific CD8 T Cells. J. Exp. Med. 2002;195:657–664. doi: 10.1084/jem.20001021. doi:10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 2003;9:540–547. doi: 10.1038/nm866. doi:10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- Blattman JN, Wherry EJ, Ha S-J, Most R.G. van der, Ahmed R. Impact of Epitope Escape on PD-1 Expression and CD8 T-Cell Exhaustion during Chronic Infection. J. Virol. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. doi:10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of Hepatitis B Virus (HBV)-Specific T-Cell Dysfunction in Chronic HBV Infection. J. Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. doi:10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012;12:180–190. doi: 10.1038/nri3156. doi:10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. doi:10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MBA. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J. Exp. Med. 2008;205:533–541. doi: 10.1084/jem.20071948. doi:10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, McGavern DB, Oldstone MBA. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 2006a;116:1675–1685. doi: 10.1172/JCI26856. doi:10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Teyton L, Oldstone MBA, McGavern DB. Intrinsic Functional Dysregulation of CD4 T Cells Occurs Rapidly following Persistent Viral Infection. J. Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. doi:10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MBA. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006b;12:1301–1309. doi: 10.1038/nm1492. doi:10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic Antigen Stimulation Alone Is Sufficient to Drive CD8+ T Cell Exhaustion. J. Immunol. 2009;182:6697–6708. doi: 10.4049/jimmunol.0800997. doi:10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiari L, Zanussi S, Crepaldi C, Bortolin MT, Caffau C, D’Andrea M, De Paoli P. Different rates of CD4+ and CD8+ T-cell proliferation in interleukin-2–treated human immunodeficiency virus-positive subjects. Cytometry. 2001;46:233–237. doi: 10.1002/cyto.1132. doi:10.1002/cyto.1132. [DOI] [PubMed] [Google Scholar]
- Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat. Immunol. 2008;9:176–185. doi: 10.1038/ni1554. doi:10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol. Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. doi:10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai W-P, Petrovas C, Fuchs D, Heraud J-M, Venzon D, Shearer GM, Koup RA, Lowy I, Miller CJ, Franchini G. Immune Activation Driven by CTLA-4 Blockade Augments Viral Replication at Mucosal Sites in Simian Immunodeficiency Virus Infection. J. Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. doi:10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ED, Dustin LB. Hepatitis C virus–induced cryoglobulinemia. Kidney Int. 2009;76:818–824. doi: 10.1038/ki.2009.247. doi:10.1038/ki.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. doi:10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier MF, Jülg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, Cutler S, McAndrew E, Jessen H, Pereyra F, Rosenberg ES, Altfeld M, Walker BD, Streeck H. HIV-1-Specific Interleukin-21+ CD4+ T Cell Responses Contribute to Durable Viral Control through the Modulation of HIV-Specific CD8+ T Cell Function. J. Virol. 2011;85:733–741. doi: 10.1128/JVI.02030-10. doi:10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular Basis of the Dual Functions of 2B4 (CD244) J. Immunol. 2008;180:8159–8167. doi: 10.4049/jimmunol.180.12.8159. doi:10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J. Clin. Invest. 1994;93:768–775. doi: 10.1172/JCI117031. doi:10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook KD, Whitmire JK. The Depletion of NK Cells Prevents T Cell Exhaustion to Efficiently Control Disseminating Virus Infection. J. Immunol. 2013;190:641–649. doi: 10.4049/jimmunol.1202448. doi:10.4049/jimmunol.1202448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MA, Harrington LE, Zajac AJ. Cytokines and the inception of CD8 T cell responses. Trends Immunol. 2011;32:180–186. doi: 10.1016/j.it.2011.01.004. doi:10.1016/j.it.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MA, Kahan SM, Zajac AJ. Anti-viral CD8 T cells and the cytokines that they love. Virology. 2013;435:157–169. doi: 10.1016/j.virol.2012.09.012. doi:10.1016/j.virol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. Molecular and Transcriptional Basis of CD4+ T Cell Dysfunction during Chronic Infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. doi:10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crome SQ, Lang PA, Lang KS, Ohashi PS. Natural killer cells regulate diverse T cell responses. Trends Immunol. 2013;34:342–349. doi: 10.1016/j.it.2013.03.002. doi:10.1016/j.it.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Crompton JG, Clever D, Vizcardo R, Rao M, Restifo NP. Reprogramming antitumor immunity. Trends Immunol. 2014;35:178–185. doi: 10.1016/j.it.2014.02.003. doi:10.1016/j.it.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, Kalinke U, Vivier E, Jonjic S, Oxenius A. Type I Interferons Protect T Cells against NK Cell Attack Mediated by the Activating Receptor NCR1. Immunity. 2014;40:961–973. doi: 10.1016/j.immuni.2014.05.003. doi:10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An Interleukin-21-Interleukin-10-STAT3 Pathway Is Critical for Functional Maturation of Memory CD8+ T Cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. doi:10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Abrahams DA, Lerumo L, Rensburg E.J. van, Stone L, O’rie T, Pienaar B, Kock M. de, Kaplan G, Mahomed H, Dheda K, Hanekom WA. Functional Capacity of Mycobacterium tuberculosis-Specific T Cell Responses in Humans Is Associated with Mycobacterial Load. J. Immunol. 2011;187:2222–2232. doi: 10.4049/jimmunol.1101122. doi:10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJR, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. doi:10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- De Goër de Herve MG, Jaafoura S, Vallée M, Taoufik Y. FoxP3(+) regulatory CD4 T cells control the generation of functional CD8 memory. Nat. Commun. 2012;3:986. doi: 10.1038/ncomms1992. doi:10.1038/ncomms1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, Teuscher C, Haynes L, Rincon M. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009;206:69–78. doi: 10.1084/jem.20081571. doi:10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. doi:10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network Analysis Reveals Centrally Connected Genes and Pathways Involved in CD8+ T Cell Exhaustion versus Memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. doi:10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza WN, Lefrançois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J. Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Schluns KS, Masopust D, Lefrançois L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J. Immunol. 2002;168:5566–5572. doi: 10.4049/jimmunol.168.11.5566. [DOI] [PubMed] [Google Scholar]
- Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. doi:10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Far M, Halwani R, Said E, Trautmann L, Doroudchi M, Janbazian L, Fonseca S, van Grevenynghe J, Yassine-Diab B, Sékaly R-P, Haddad EK. T-cell exhaustion in HIV infection. Curr. HIV/AIDS Rep. 2008;5:13–19. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- Elsaesser H, Sauer K, Brooks DG. IL-21 Is Required to Control Chronic Viral Infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. doi:10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 Are Critical for Different Aspects of B Cell Immunity and Redundantly Induce Optimal Follicular Helper CD4 T Cell (Tfh) Differentiation. PLoS ONE. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. doi:10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J. Exp. Med. 2011;208:987–999. doi: 10.1084/jem.20101773. doi:10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini MC, Rizzo A, Fina D, Caruso R, Becker C, Neurath MF, MacDonald TT, Pallone F, Monteleone G. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur. J. Immunol. 2007;37:3155–3163. doi: 10.1002/eji.200737766. doi:10.1002/eji.200737766. [DOI] [PubMed] [Google Scholar]
- Feng G, Zhang J-Y, Zeng Q-L, Jin L, Fu J, Yang B, Sun Y, Jiang T, Xu X, Zhang Z, Yuan J, Wu L, Wang F-S. HCV-Specific Interleukin-21+CD4+ T Cells Responses Associated with Viral Control through the Modulation of HCV-Specific CD8+ T Cells Function in Chronic Hepatitis C Patients. Mol. Cells. 2013;36:362–367. doi: 10.1007/s10059-013-0181-z. doi:10.1007/s10059-013-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RL, Lu B, Kane LP. Too Much of a Good Thing? Tim-3 and TCR Signaling in T Cell Exhaustion. J. Immunol. 2014;193:1525–1530. doi: 10.4049/jimmunol.1400557. doi:10.4049/jimmunol.1400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR–induced stop signal. Nat. Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. doi:10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A, Frei K, Bodmer S, Hofer E, Schreier MH, Palladino MA, Zinkernagel RM. Transforming growth factor-beta inhibits the generation of cytotoxic T cells in virus-infected mice. J. Immunol. 1989;143:3230–3234. [PubMed] [Google Scholar]
- Frebel H, Oxenius A. The risks of targeting co-inhibitory pathways to modulate pathogen-directed T cell responses. Trends Immunol. 2013;34:193–199. doi: 10.1016/j.it.2012.12.002. doi:10.1016/j.it.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T Cells Is Critical for Sustained Functionality and Control of Chronic Viral Infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. doi:10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- Fujita T, Burwitz BJ, Chew GM, Reed JS, Pathak R, Seger E, Clayton KL, Rini JM, Ostrowski MA, Ishii N, Kuroda MJ, Hansen SG, Sacha JB, Ndhlovu LC. Expansion of Dysfunctional Tim-3–Expressing Effector Memory CD8+ T Cells during Simian Immunodeficiency Virus Infection in Rhesus Macaques. J. Immunol. 2014 doi: 10.4049/jimmunol.1400961. 1400961. doi:10.4049/jimmunol.1400961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, Sharpe AH, Dustin LB, Rice CM, Grakoui A, Ahmed R, Walker CM. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1) Proc. Natl. Acad. Sci. 2013;110:15001–15006. doi: 10.1073/pnas.1312772110. doi:10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MJ, Hildeman DA, Sabbaj S, Gaddis DE, Tebo AE, Shang L, Goepfert PA, Zajac AJ. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 2005;174:5926–5930. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J. Immunol. 2004;172:4204–4214. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- Gallimore A, Glithero A, Godkin A, Tissot AC, Plückthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and Exhaustion of Lymphocytic Choriomeningitis Virus–specific Cytotoxic T Lymphocytes Visualized Using Soluble Tetrameric Major Histocompatibility Complex Class I–Peptide Complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. doi:10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garidou L, Heydari S, Gossa S, McGavern DB. Therapeutic Blockade of Transforming Growth Factor Beta Fails To Promote Clearance of a Persistent Viral Infection. J. Virol. 2012;86:7060–7071. doi: 10.1128/JVI.00164-12. doi:10.1128/JVI.00164-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, Castro E, Akondy R, Rinfret A, Yassine-Diab B, Said EA, Chouikh Y, Cameron MJ, Clum R, Kelvin D, Somogyi R, Greller LD, Balderas RS, Wilkinson P, Pantaleo G, Tartaglia J, Haddad EK, Sékaly R-P. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. doi:10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert PA, Bansal A, Edwards BH, Ritter GD, Tellez I, McPherson SA, Sabbaj S, Mulligan MJ. A Significant Number of Human Immunodeficiency Virus Epitope-Specific Cytotoxic T Lymphocytes Detected by Tetramer Binding Do Not Produce Gamma Interferon. J. Virol. 2000;74:10249–10255. doi: 10.1128/jvi.74.21.10249-10255.2000. doi:10.1128/JVI.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 Expression on Circulating and Intrahepatic Hepatitis C Virus-Specific CD8+ T Cells Associated with Reversible Immune Dysfunction. J. Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. doi:10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw F, Richter K, Oxenius A, Regoes RR. Comparison of cytotoxic T lymphocyte efficacy in acute and persistent lymphocytic choriomeningitis virus infection. Proc. R. Soc. B Biol. Sci. 2011;278:3395–3402. doi: 10.1098/rspb.2011.0453. doi:10.1098/rspb.2011.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Zimmermann K, Oxenius A. Antigen-Dependent and -Independent Mechanisms of T and B Cell Hyperactivation during Chronic HIV-1 Infection. J. Virol. 2011;85:12102–12113. doi: 10.1128/JVI.05607-11. doi:10.1128/JVI.05607-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining WN, Ebert BL, Subrmanian A, Wherry EJ, Eichbaum Q, Evans JW, Mak R, Rivoli S, Pretz J, Angelosanto J, Smutko JS, Walker BD, Kaech SM, Ahmed R, Nadler LM, Golub TR. Identification of an Evolutionarily Conserved Transcriptional Signature of CD8 Memory Differentiation That Is Shared by T and B Cells. J. Immunol. 2008;181:1859–1868. doi: 10.4049/jimmunol.181.3.1859. doi:10.4049/jimmunol.181.3.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand T, Kaech S. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol. Res. 2009;45:46–61. doi: 10.1007/s12026-008-8027-z. doi:10.1007/s12026-008-8027-z. [DOI] [PubMed] [Google Scholar]
- Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR Complex-Associated Lymphocyte Activation Gene-3 Molecules Inhibit CD3/TCR Signaling. J. Immunol. 1998;161:4058–4065. [PubMed] [Google Scholar]
- Harker JA, Lewis GM, Mack L, Zuniga EI. Late Interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. doi:10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Most R. van der, Whitton JL, Ahmed R. Recombinant Vaccinia Virus-Induced T-Cell Immunity: Quantitation of the Response to the Virus Vector and the Foreign Epitope. J. Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. doi:10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. doi:10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker L, Recher M, Macpherson AJ, Ciurea A, Freigang S, Hengartner H, Zinkernagel RM. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat. Immunol. 2003;4:343–349. doi: 10.1038/ni911. doi:10.1038/ni911. [DOI] [PubMed] [Google Scholar]
- Hyodo N, Nakamura I, Imawari M. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin. Exp. Immunol. 2004;135:462–466. doi: 10.1111/j.1365-2249.2003.02376.x. doi:10.1111/j.1365-2249.2003.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Boulassel M-R, Samarani S, Debbeche O, Tremblay C, Toma E, Routy J-P, Ahmad A. Dynamics and Consequences of IL-21 Production in HIV-Infected Individuals: A Longitudinal and Cross-Sectional Study. J. Immunol. 2010;184:114–126. doi: 10.4049/jimmunol.0901967. doi:10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- Ingram JT, Yi JS, Zajac AJ. Exhausted CD8 T cells downregulate the IL-18 receptor and become unresponsive to inflammatory cytokines and bacterial co-infections. PLoS Pathog. 2011;7:e1002273. doi: 10.1371/journal.ppat.1002273. doi:10.1371/journal.ppat.1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. doi:10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. doi:10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- Jeannet G, Boudousquié C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Natl. Acad. Sci. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. doi:10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H-T, Anderson AC, Tan WG, West EE, Ha S-J, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. doi:10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS, Ho DD. Dramatic Rise in Plasma Viremia after CD8+ T Cell Depletion in Simian Immunodeficiency Virus–infected Macaques. J. Exp. Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. doi:10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun T-W, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. doi:10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012;12:749–761. doi: 10.1038/nri3307. doi:10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. doi:10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali M-AA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. doi:10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DE, Ikeda F, Li Y, Nakamoto N, Ganesan S, Valiga ME, Nunes FA, Reddy KR, Chang K-M. Peripheral virus-specific T-cell Interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. J. Hepatol. 2008;48:903–913. doi: 10.1016/j.jhep.2008.01.030. doi:10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kared H, Fabre T, Bédard N, Bruneau J, Shoukry NH. Galectin-9 and IL-21 Mediate Cross-regulation between Th17 and Treg Cells during Acute Hepatitis C. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003422. doi:10.1371/journal.ppat.1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, D’Costa K, Tarlinton DM, Kallies A, Corcoran LM. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J. Exp. Med. 2012;209:2049–2064. doi: 10.1084/jem.20111504. doi:10.1084/jem.20111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller W, Gasteiger G, Subramanian N, Sparwasser T, Busch DH, Belkaid Y, Drexler I, Germain RN. Regulatory T Cells Selectively Control CD8+ T Cell Effector Pool Size via IL-2 Restriction. J. Immunol. 2011;187:3186–3197. doi: 10.4049/jimmunol.1101649. doi:10.4049/jimmunol.1101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. doi:10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- Kekow J, Wachsman W, McCutchan JA, Cronin M, Carson DA, Lotz M. Transforming growth factor beta and noncytopathic mechanisms of immunodeficiency in human immunodeficiency virus infection. Proc. Natl. Acad. Sci. 1990;87:8321–8325. doi: 10.1073/pnas.87.21.8321. doi:10.1073/pnas.87.21.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 2005;6:873–879. doi: 10.1038/ni1241. doi:10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. doi:10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi M, Kurachi J, Suenaga F, Tsukui T, Jun Abe, Ueha S, Tomura M, Sugihara K, Takamura S, Kakimi K, Matsushima K. Chemokine receptor CXCR3 facilitates CD8+ T cell differentiation into short-lived effector cells leading to memory degeneration. J. Exp. Med. 2011;208:1605–1620. doi: 10.1084/jem.20102101. doi:10.1084/jem.20102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang KS, Recher M, Navarini AA, Harris NL, Löhning M, Junt T, Probst HC, Hengartner H, Zinkernagel RM. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. doi:10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brüstle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Häussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc. Natl. Acad. Sci. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. doi:10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner F, Wong DKH, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of Successful Immune Responses in Persons Infected with Hepatitis C Virus. J. Exp. Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. doi:10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, Smithgall TE, Kuchroo VK, Kane LP. Phosphotyrosine-Dependent Coupling of Tim-3 to T-Cell Receptor Signaling Pathways. Mol. Cell. Biol. 2011;31:3963–3974. doi: 10.1128/MCB.05297-11. doi:10.1128/MCB.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat. Rev. Immunol. 2005;5:688–698. doi: 10.1038/nri1688. doi:10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- Levy Y, Lacabaratz C, Weiss L, Viard J-P, Goujard C, Lelièvre J-D, Boué F, Molina J-M, Rouzioux C, Avettand-Fénoêl V, Croughs T, Beq S, Thiébaut R, Chêne G, Morre M, Delfraissy J-F. Enhanced T cell recovery in HIV-1–infected adults through IL-7 treatment. J. Clin. Invest. 2009 doi: 10.1172/JCI38052. doi:10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson A-KL, Flavell RA. Transforming Growth Factor-β Regulation of Immune Responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. doi:10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Li S, Vriend LEM, Nasser IA, Popov Y, Afdhal NH, Koziel MJ, Schuppan D, Exley MA, Alatrakchi N. Hepatitis C virus-specific T-cell-derived transforming growth factor beta is associated with slow hepatic fibrogenesis. Hepatology. 2012;56:2094–2105. doi: 10.1002/hep.25951. doi:10.1002/hep.25951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ma S, Tang L, Li Y, Wang W, Huang X, Lai Q, Zhang M, Sun J, Li CK, Abbott WGH, Naoumov NV, Zhang Y, Hou J. Circulating chemokine (C-X-C Motif) receptor 5+CD4+ T cells benefit hepatitis B e antigen seroconversion through IL-21 in patients with chronic hepatitis B virus infection. Hepatology. 2013;58:1277–1286. doi: 10.1002/hep.26489. doi:10.1002/hep.26489. [DOI] [PubMed] [Google Scholar]
- Lu P, Youngblood BA, Austin JW, Mohammed AUR, Butler R, Ahmed R, Boss JM. Blimp-1 represses CD8 T cell expression of PD-1 using a feed-forward transcriptional circuit during acute viral infection. J. Exp. Med. 2014;211:515–527. doi: 10.1084/jem.20130208. doi:10.1084/jem.20130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackerness KJ, Cox MA, Lilly LM, Weaver CT, Harrington LE, Zajac AJ. Pronounced virus-dependent activation drives exhaustion but sustains IFN-γ transcript levels. J. Immunol. 2010;185:3643–3651. doi: 10.4049/jimmunol.1000841. doi:10.4049/jimmunol.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti G, Meroni L, Molteni C, Bandera A, Franzetti F, Galli M, Moroni M, Clerici M, Gori A. Interleukin-2 immunotherapy exerts a differential effect on CD4 and CD8 T cell dynamics. AIDS. 2004 Jan 23;18:211–216. doi: 10.1097/00002030-200401230-00010. 2004. [DOI] [PubMed] [Google Scholar]
- Maris CH, Chappell CP, Jacob J. Interleukin-10 plays an early role in generating virus-specific T cell anergy. BMC Immunol. 2007;8:8. doi: 10.1186/1471-2172-8-8. doi:10.1186/1471-2172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J. Clin. Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. doi:10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CW, Zajac AJ, Jamieson AM, Corral L, Hammer GE, Ahmed R, Raulet DH. Viral and Bacterial Infections Induce Expression of Multiple NK Cell Receptors in Responding CD8+ T Cells. J. Immunol. 2002;169:1444–1452. doi: 10.4049/jimmunol.169.3.1444. doi:10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc. Natl. Acad. Sci. 2011;108:7529–7534. doi: 10.1073/pnas.1103782108. doi:10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human Effector and Memory CD8+ T Cell Responses to Smallpox and Yellow Fever Vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. doi:10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Molloy MJ, Zhang W, Usherwood EJ. Suppressive CD8+ T Cells Arise in the Absence of CD4 Help and Compromise Control of Persistent Virus. J. Immunol. 2011;186:6218–6226. doi: 10.4049/jimmunol.1003812. doi:10.4049/jimmunol.1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. doi:10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. doi:10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T Cell Subsets, Migration Patterns, and Tissue Residence. Annu. Rev. Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. doi:10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting Antigen-Specific CD8 T Cells: A Reevaluation of Bystander Activation during Viral Infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. doi:10.1016/S1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang K. Functional Restoration of HCV-Specific CD8 T Cells by PD-1 Blockade Is Defined by PD-1 Expression and Compartmentalization. Gastroenterology. 2008;134:1927–1937. doi: 10.1053/j.gastro.2008.02.033. e2. doi:10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa SG, Kim EH, Suresh M. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood. 2011;117:5123–5132. doi: 10.1182/blood-2010-12-323154. doi:10.1182/blood-2010-12-323154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova M, MarieDCardine A, Boumsell L, Bensussan A. BY55/CD160 acts as a coDreceptor in TCR signal transduction of a human circulating cytotoxic effector T lymphocyte subset lacking CD28 expression. Int. Immunol. 2002;14:445–451. doi: 10.1093/intimm/14.5.445. doi:10.1093/intimm/14.5.445. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, Nakayama-Hosoya K, Iriguchi S, Uemura Y, Shimizu T, Takayama N, Yamada D, Nishimura K, Ohtaka M, Watanabe N, Takahashi S, Iwamoto A, Koseki H, Nakanishi M, Eto K, Nakauchi H. Generation of Rejuvenated Antigen-Specific T Cells by Reprogramming to Pluripotency and Redifferentiation. Cell Stem Cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. doi:10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Odorizzi PM, Wherry EJ. Inhibitory Receptors on Lymphocytes: Insights from Infections. J. Immunol. 2012;188:2957–2965. doi: 10.4049/jimmunol.1100038. doi:10.4049/jimmunol.1100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 Regulates PD-1 Expression upon T Cell Activation. J. Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. doi:10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga S, Nomura A, Takada H, Tanaka T, Furuno K, Takahata Y, Kinukawa N, Fukushima N, Imai S, Hara T. Dominant expression of interleukin-10 and transforming growth factor-β genes in activated T-cells of chronic active epstein–barr virus infection. J. Med. Virol. 2004;74:449–458. doi: 10.1002/jmv.20197. doi:10.1002/jmv.20197. [DOI] [PubMed] [Google Scholar]
- Osokine I, Snell LM, Cunningham CR, Yamada DH, Wilson EB, Elsaesser HJ, Torre J.C. de la, Brooks D. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. Proc. Natl. Acad. Sci. 2014;111:7409–7414. doi: 10.1073/pnas.1401662111. doi:10.1073/pnas.1401662111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou R, Zhou S, Huang L, Moskophidis D. Critical Role for Alpha/Beta and Gamma Interferons in Persistence of Lymphocytic Choriomeningitis Virus by Clonal Exhaustion of Cytotoxic T Cells. J. Virol. 2001;75:8407–8423. doi: 10.1128/JVI.75.18.8407-8423.2001. doi:10.1128/JVI.75.18.8407-8423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenius A, Sewell AK, Dawson SJ, Günthard HF, Fischer M, Gillespie GM, Rowland-Jones SL, Fagard C, Hirschel B, Phillips RE, Price DA. Functional Discrepancies in HIV-Specific CD8+ T-Lymphocyte Populations Are Related to Plasma Virus Load. J. Clin. Immunol. 2002;22:363–374. doi: 10.1023/a:1020656300027. doi:10.1023/A:1020656300027. [DOI] [PubMed] [Google Scholar]
- Oxenius A, Zinkernagel RM, Hengartner H. Comparison of Activation versus Induction of Unresponsiveness of Virus-Specific CD4+ and CD8+ T Cells upon Acute versus Persistent Viral Infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. doi:10.1016/S1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and Terminal Subsets of CD8+ T Cells Cooperate to Contain Chronic Viral Infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. doi:10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallikkuth S, Rogers K, Villinger F, DosterII M, Vaccari M, Franchini G, Pahwa R, Pahwa S. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine. 2011;29:9229–9238. doi: 10.1016/j.vaccine.2011.09.118. doi:10.1016/j.vaccine.2011.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish IA, Marshall HD, Staron MM, Lang PA, Brüstle A, Chen JH, Cui W, Tsui Y-C, Perry C, Laidlaw BJ, Ohashi PS, Weaver CT, Kaech SM. Chronic viral infection promotes sustained Th1-derived immunoregulatory IL-10 via BLIMP-1. J. Clin. Invest. 2014;124:3455–3468. doi: 10.1172/JCI66108. doi:10.1172/JCI66108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik J, DePinho RA, Basta S, Ohashi PS, Mak TW. IL-7 Engages Multiple Mechanisms to Overcome Chronic Viral Infection and Limit Organ Pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. doi:10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O’Mara L, Yang S, Konieczny BT, Sharpe AH, Freeman GJ, Rudensky AY, Ahmed R. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J. Exp. Med. 2014 doi: 10.1084/jem.20132577. jem.20132577. doi:10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol. Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. doi:10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Peretz Y, He Z, Shi Y, Yassine-Diab B, Goulet J-P, Bordi R, Filali-Mouhim A, Loubert J-B, El-Far M, Dupuy FP, Boulassel MR, Tremblay C, Routy J-P, Bernard N, Balderas R, Haddad EK, Sékaly R-P. CD160 and PD-1 Co-Expression on HIV-Specific CD8 T Cells Defines a Subset with Advanced Dysfunction. PLoS Pathog. 2012;8:e1002840. doi: 10.1371/journal.ppat.1002840. doi:10.1371/journal.ppat.1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peritt D, Sesok-Pizzini DA, Schretzenmair R, Macgregor RR, Valiante NM, Tu X, Trinchieri G, Kamoun M. C1.7 Antigen Expression on CD8+ T Cells Is Activation Dependent: Increased Proportion of C1.7+CD8+ T Cells in HIV-1-Infected Patients with Progressing Disease. J. Immunol. 1999;162:7563–7568. [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. doi:10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, Douek DC, Morgan SH, Davis SJ, Franchini G, Koup RA. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. doi:10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. doi:10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Chen J, Liao H, Zhang Y, Wang H, Li S, Luo Y, Fang D, Li G, Zhou B, Shen L, Chen CY, Huang D, Cai J, Cao K, Jiang L, Zeng G, Chen ZW. Tim-3-Expressing CD4+ and CD8+ T Cells in Human Tuberculosis (TB) Exhibit Polarized Effector Memory Phenotypes and Stronger Anti-TB Effector Functions. PLoS Pathog. 2012;8:e1002984. doi: 10.1371/journal.ppat.1002984. doi:10.1371/journal.ppat.1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, Russell K, Toth I, Piechocka-Trocha A, Dolfi D, Angelosanto J, Crawford A, Shin H, Kwon DS, Zupkosky J, Francisco L, Freeman GJ, Wherry EJ, Kaufmann DE, Walker BD, Ebert B, Haining WN. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. doi:10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. Liver-Infiltrating Lymphocytes in Chronic Human Hepatitis C Virus Infection Display an Exhausted Phenotype with High Levels of PD-1 and Low Levels of CD127 Expression. J. Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. doi:10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raué H-P, Beadling C, Haun J, Slifka MK. Cytokine-Mediated Programmed Proliferation of Virus-Specific CD8+ Memory T Cells. Immunity. 2013;38:131–139. doi: 10.1016/j.immuni.2012.09.019. doi:10.1016/j.immuni.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raué H-P, Brien JD, Hammarlund E, Slifka MK. Activation of Virus-Specific CD8+ T Cells by Lipopolysaccharide-Induced IL-12 and IL-18. J. Immunol. 2004;173:6873–6881. doi: 10.4049/jimmunol.173.11.6873. [DOI] [PubMed] [Google Scholar]
- Raziorrouh B, Schraut W, Gerlach T, Nowack D, Grüner NH, Ulsenheimer A, Zachoval R, Wächtler M, Spannagl M, Haas J, Diepolder HM, Jung M-C. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52:1934–1947. doi: 10.1002/hep.23936. doi:10.1002/hep.23936. [DOI] [PubMed] [Google Scholar]
- Rehr M, Cahenzli J, Haas A, Price DA, Gostick E, Huber M, Karrer U, Oxenius A. Emergence of Polyfunctional CD8+ T Cells after Prolonged Suppression of Human Immunodeficiency Virus Replication by Antiretroviral Therapy. J. Virol. 2008;82:3391–3404. doi: 10.1128/JVI.02383-07. doi:10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reignat S, Webster GJM, Brown D, Ogg GS, King A, Seneviratne SL, Dusheiko G, Williams R, Maini MK, Bertoletti A. Escaping High Viral Load Exhaustion CD8 Cells with Altered Tetramer Binding in Chronic Hepatitis B Virus Infection. J. Exp. Med. 2002;195:1089–1101. doi: 10.1084/jem.20011723. doi:10.1084/jem.20011723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int. Immunol. 2010;22:13–23. doi: 10.1093/intimm/dxp107. doi:10.1093/intimm/dxp107. [DOI] [PubMed] [Google Scholar]
- Richter K, Brocker T, Oxenius A. Antigen amount dictates CD8+T-cell exhaustion during chronic viral infection irrespective of the type of antigen presenting cell. Eur. J. Immunol. 2012 doi: 10.1002/eji.201142275. n/a–n/a. doi:10.1002/eji.201142275. [DOI] [PubMed] [Google Scholar]
- Richter K, Perriard G, Behrendt R, Schwendener RA, Sexl V, Dunn R, Kamanaka M, Flavell RA, Roers A, Oxenius A. Macrophage and T Cell Produced IL-10 Promotes Viral Chronicity. PLoS Pathog. 2013a;9:e1003735. doi: 10.1371/journal.ppat.1003735. doi:10.1371/journal.ppat.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Perriard G, Oxenius A. Reversal of chronic to resolved infection by IL-10 blockade is LCMV strain dependent. Eur. J. Immunol. 2013b;43:649–654. doi: 10.1002/eji.201242887. doi:10.1002/eji.201242887. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. doi:10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Rottenberg ME, Carow B. SOCS3, a major regulator of infection and inflammation. Mol. Innate Immun. 2014;5:58. doi: 10.3389/fimmu.2014.00058. doi:10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A, Sidney J, Pardoll DM, Cox AL. High-Programmed Death-1 Levels on Hepatitis C Virus-Specific T Cells during Acute Infection Are Associated with Viral Persistence and Require Preservation of Cognate Antigen during Chronic Infection. J. Immunol. 2008;181:8215–8225. doi: 10.4049/jimmunol.181.12.8215. doi:10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Bosinger SE, Estes JD, Zhu RTR, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, Hill BJ, Timmer JK, Reiss E, Yarden G, Darko S, Contijoch E, Todd JP, Silvestri G, Nason M, Norgren RB, Jr, Keele BF, Rao S, Langer JA, Lifson JD, Schreiber G, Douek DC. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. doi:10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. doi:10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Schlaphoff V, Lunemann S, Suneetha PV, Jaroszewicz J, Grabowski J, Dietz J, Helfritz F, Bektas H, Sarrazin C, Manns MP, Cornberg M, Wedemeyer H. Dual Function of the NK Cell Receptor 2B4 (CD244) in the Regulation of HCV-Specific CD8+ T Cells. PLoS Pathog. 2011;7:e1002045. doi: 10.1371/journal.ppat.1002045. doi:10.1371/journal.ppat.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz I, Schneider C, Fröhlich A, Frebel H, Christ D, Leonard WJ, Sparwasser T, Oxenius A, Freigang S, Kopf M. IL-21 Restricts Virus-driven Treg Cell Expansion in Chronic LCMV Infection. PLoS Pathog. 2013;9:e1003362. doi: 10.1371/journal.ppat.1003362. doi:10.1371/journal.ppat.1003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of Viremia in Simian Immunodeficiency Virus Infection by CD8+ Lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. doi:10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. doi:10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, Asmuth DM, Tenorio AR, Altman JD, Fox L, Moir S, Malaspina A, Morre M, Buffet R, Silvestri G, Lederman MM. IL-7 administration drives T cell–cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–6314. doi: 10.1182/blood-2008-10-186601. doi:10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–3101. [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 2007;204:941–949. doi: 10.1084/jem.20061937. doi:10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A Role for the Transcriptional Repressor Blimp-1 in CD8+ T Cell Exhaustion during Chronic Viral Infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. doi:10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan M, Kreefft K, de Graav GN, Brouwer WP, de Knegt RJ, ten Kate FJW, Baan CC, Vanwolleghem T, Janssen HLA, Boonstra A. CD4+CXCR5+ T-cells in chronic HCV infection produce less IL-21, yet are efficient at supporting B-cell responses. J. Hepatol. 2014 doi: 10.1016/j.jhep.2014.09.024. doi:10.1016/j.jhep.2014.09.024. [DOI] [PubMed] [Google Scholar]
- Stelekati E, Shin H, Doering TA, Dolfi DV, Ziegler CG, Beiting DP, Dawson L, Liboon J, Wolski D, Ali M-AA, Katsikis PD, Shen H, Roos DS, Haining WN, Lauer GM, Wherry EJ. Bystander Chronic Infection Negatively Impacts Development of CD8+ T Cell Memory. Immunity. 2014;40:801–813. doi: 10.1016/j.immuni.2014.04.010. doi:10.1016/j.immuni.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The Immunological Synapse of CTL Contains a Secretory Domain and Membrane Bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. doi:10.1016/S1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, Brumme CJ, Rosenberg ES, Alter G, Allen TM, Walker BD, Altfeld M. Antigen Load and Viral Sequence Diversification Determine the Functional Profile of HIV-1–Specific CD8+ T Cells. PLoS Med. 2008;5:e1001. doi: 10.1371/journal.pmed.0050100. doi:10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KCF, Welch M, Schreiber RD, Torre J.C. de la, Oldstone MBA. Persistent LCMV Infection Is Controlled by Blockade of Type I Interferon Signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. doi:10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-Intrinsic Transforming Growth Factor-β Signaling Mediates Virus-Specific CD8+ T Cell Deletion and Viral Persistence In Vivo. Immunity. 2009a;31:145–157. doi: 10.1016/j.immuni.2009.06.015. doi:10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. TGF-? Signaling in T cells is Essential for CD8 T Cell Suppression and Viral Persistence In Vivo. Immunity. 2009b;31:145–157. doi: 10.1016/j.immuni.2009.06.015. doi:10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel M-R, Delwart E, Sepulveda H, Balderas RS, Routy J-P, Haddad EK, Sekaly R-P. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. doi:10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Tsujimura K, Obata Y, Matsudaira Y, Nishida K, Akatsuka Y, Ito Y, Demachi-Okamura A, Kuzushima K, Takahashi T. Characterization of murine CD160+ CD8+ T lymphocytes. Immunol. Lett. 2006;106:48–56. doi: 10.1016/j.imlet.2006.04.006. doi:10.1016/j.imlet.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Tussey LG, Nair US, Bachinsky M, Edwards BH, Bakari J, Grimm K, Joyce J, Vessey R, Steigbigel R, Robertson MN, Shiver JW, Goepfert PA. Antigen Burden Is a Major Determinant of Human Immunodeficiency Virus–Specific CD8+ T Cell Maturation State: Potential Implications for Therapeutic Immunization. J. Infect. Dis. 2003;187:364–374. doi: 10.1086/367707. doi:10.1086/367707. [DOI] [PubMed] [Google Scholar]
- Utzschneider DT, Legat A, Fuertes Marraco SA, Carrié L, Luescher I, Speiser DE, Zehn D. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 2013;14:603–610. doi: 10.1038/ni.2606. doi:10.1038/ni.2606. [DOI] [PubMed] [Google Scholar]
- Vahedi G, Takahashi H, Nakayamada S, Sun H, Sartorelli V, Kanno Y, O’Shea JJ. STATs Shape the Active Enhancer Landscape of T Cell Populations. Cell. 2012;151:981–993. doi: 10.1016/j.cell.2012.09.044. doi:10.1016/j.cell.2012.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. doi:10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, Koseki H, Kawamoto H. Regeneration of Human Tumor Antigen-Specific T Cells from iPSCs Derived from Mature CD8+ T Cells. Cell Stem Cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. doi:10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. doi:10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Daniels KA, Welsh RM. Therapeutic Depletion of Natural Killer Cells Controls Persistent Infection. J. Virol. 2014;88:1953–1960. doi: 10.1128/JVI.03002-13. doi:10.1128/JVI.03002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc. Natl. Acad. Sci. 2013;110:E2480–E2489. doi: 10.1073/pnas.1305394110. doi:10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Waggoner SN. NK cells controlling virus-specific T cells: Rheostats for acute vs. persistent infections. Virology. 2013;435:37–45. doi: 10.1016/j.virol.2012.10.005. 2013 Reviews Issue. doi:10.1016/j.virol.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EE, Jin H-T, Rasheed A-U, Penaloza-MacMaster P, Ha S-J, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Invest. 2013;123:2604–2615. doi: 10.1172/JCI67008. doi:10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. doi:10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, Most R. van der, Ahmed R. Viral Persistence Alters CD8 T-Cell Immunodominance and Tissue Distribution and Results in Distinct Stages of Functional Impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. doi:10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ha S-J, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular Signature of CD8+ T Cell Exhaustion during Chronic Viral Infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. doi:10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wiesel M, Joller N, Ehlert A-K, Crouse J, Spörri R, Bachmann MF, Oxenius A. Th Cells Act Via Two Synergistic Pathways To Promote Antiviral CD8+ T Cell Responses. J. Immunol. 2010;185:5188–5197. doi: 10.4049/jimmunol.1001990. doi:10.4049/jimmunol.1001990. [DOI] [PubMed] [Google Scholar]
- Wiesel M, Kratky W, Oxenius A. Type I IFN Substitutes for T Cell Help during Viral Infections. J. Immunol. 2011;186:754–763. doi: 10.4049/jimmunol.1003166. doi:10.4049/jimmunol.1003166. [DOI] [PubMed] [Google Scholar]
- Wiesel M, Oxenius A. From crucial to negligible: functional CD8+ T-cell responses and their dependence on CD4+ T-cell help. Eur. J. Immunol. 2012;42:1080–1088. doi: 10.1002/eji.201142205. doi:10.1002/eji.201142205. [DOI] [PubMed] [Google Scholar]
- Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, Zajac AJ, Goepfert PA. Interleukin-21-Producing HIV-1-Specific CD8 T Cells Are Preferentially Seen in Elite Controllers. J. Virol. 2011;85:2316–2324. doi: 10.1128/JVI.01476-10. doi:10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. doi:10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Kidani Y, Elsaesser H, Barnard J, Raff L, Karp CL, Bensinger S, Brooks DG. Emergence of Distinct Multiarmed Immunoregulatory Antigen-Presenting Cells during Persistent Viral Infection. Cell Host Microbe. 2012;11:481–491. doi: 10.1016/j.chom.2012.03.009. doi:10.1016/j.chom.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of Chronic Type I Interferon Signaling to Control Persistent LCMV Infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. doi:10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, Deenen R, Köhrer K, Rahbar R, Diefenbach A, Gibbert K, Löhning M, Höcker L, Waibler Z, Häussinger D, Mak TW, Ohashi PS, Lang KS, Lang PA. Type I Interferon Protects Antiviral CD8+ T Cells from NK Cell Cytotoxicity. Immunity. 2014;40:949–960. doi: 10.1016/j.immuni.2014.05.004. doi:10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010a;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. doi:10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Cox MA, Zajac AJ. Interleukin-21: a multifunctional regulator of immunity to infections. Microbes Infect. Inst. Pasteur. 2010b;12:1111–1119. doi: 10.1016/j.micinf.2010.08.008. doi:10.1016/j.micinf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. doi:10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Noto A, Porichis F, Akondy RS, Ndhlovu ZM, Austin JW, Bordi R, Procopio FA, Miura T, Allen TM, Sidney J, Sette A, Walker BD, Ahmed R, Boss JM, Sékaly R-P, Kaufmann DE. Cutting edge: Prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J. Immunol. Baltim. Md 1950. 2013;191:540–544. doi: 10.4049/jimmunol.1203161. doi:10.4049/jimmunol.1203161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Oestreich KJ, Ha S-J, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL, Boss JM, Ahmed R. Chronic Virus Infection Enforces Demethylation of the Locus that Encodes PD-1 in Antigen-Specific CD8+ T Cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. doi:10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, Liu J, Song H, Jones RB, Sheth P, Chege D, Kaul R, Ostrowski MA. HIV-Specific IL-21 Producing CD4+ T Cells Are Induced in Acute and Chronic Progressive HIV Infection and Are Associated with Relative Viral Control. J. Immunol. 2010;185:498–506. doi: 10.4049/jimmunol.0903915. doi:10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJD, Suresh M, Altman JD, Ahmed R. Viral Immune Evasion Due to Persistence of Activated T Cells Without Effector Function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. doi:10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhou X, DiSpirito JR, Wang C, Wang Y, Shen H. Epigenetic Manipulation Restores Functions of Defective CD8+ T Cells From Chronic Viral Infection. Mol. Ther. 2014 doi: 10.1038/mt.2014.91. doi:10.1038/mt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Ou R, Huang L, Price GE, Moskophidis D. Differential Tissue-Specific Regulation of Antiviral CD8+ T-Cell Immune Responses during Chronic Viral Infection. J. Virol. 2004;78:3578–3600. doi: 10.1128/JVI.78.7.3578-3600.2004. doi:10.1128/JVI.78.7.3578-3600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Xue H-H. Cutting Edge: Generation of Memory Precursors and Functional Memory CD8+ T Cells Depends on T Cell Factor-1 and Lymphoid Enhancer-Binding Factor-1. J. Immunol. 2012;189:2722–2726. doi: 10.4049/jimmunol.1201150. doi:10.4049/jimmunol.1201150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao D-M, Harty JT, Badovinac VP, Xue H-H. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. doi:10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]