Abstract
Melanin may interfere with immunohistochemical staining. The goal of this study was to investigate the effects of trichloroisocyanuric acid (TCCA) bleaching, potassium permanganate bleaching, and potassium dichromate bleaching on melanin, tissue antigen, and 3,3′-diaminobenzidine (DAB) using melanin-containing and melanin-free tissue samples. Our results demonstrated that all 3 bleaching methods efficiently bleached melanin and partially destroyed tissue antigen. In addition, potassium permanganate bleaching and potassium dichromate bleaching clearly destroyed DAB, whereas TCCA bleaching had no significant effect on DAB. Therefore, neither potassium permanganate nor potassium dichromate is an ideal solution, whereas TCCA might be an ideal solution for melanin bleaching after the immunohistochemical staining of melanin-containing tissues. After immunostaining followed by TCCA bleaching, the melanin could be completely removed in all 120 malignant melanoma tissue sections. Compared with the control, the DAB intensity was clear, and the tissue structure and cellular nuclei were well maintained. It is worth noting that TCCA should be freshly prepared before each experiment, and used within 2 hours of its preparation. In addition, sections should not be incubated with TCCA for over 30 minutes.
Key Words: melanin, immunohistochemistry, trichloroisocyanuric acid, potassium permanganate, potassium dichromate
Excessive amounts of melanin pigments may hamper histopathologic assessments of melanocytic lesions by obscuring cellular morphology.1,2 Melanin may interfere with immunohistochemical (IHC) staining, especially when 3,3′-diaminobenzidine (DAB) is used, as melanin is similar in color to DAB. Therefore, melanin must be removed from tissue sections before IHC staining. Melanins are sulfur-containing pigments. They do not contain iron, are insoluble in water and almost all are organic solvents, and can be found in paraffin-imbedded specimens after routine fixation. Melanin can be bleached by strong oxidants, including potassium permanganate and potassium dichromate solutions. Two common bleaching processes are potassium permanganate followed by oxalic acid treatment and dilute hydrogen peroxide (H2O2) process. The potassium permanganate/oxalic acid method is faster and more easily incorporated in conventional daily immunostaining protocols, whereas the dilute H2O2 method requires 24 hours.3 Permanganate/oxalate precluded the use of some antibodies.4 H2O2 can effectively bleach melanin in pigmented melanocytic lesions without significantly affecting MIB1-Ki67 immunolabelling.5 Complications of bleaching included tissue damage and loss of cytologic detail.6 Although some antigens were resistant to the effects of bleaching, some were not, and no other step in the IHC procedure could withstand bleaching.7 After conventional immunoperoxidase-DAB treatment, azure B melanin counterstaining can distinguish melanin granules from DAB chromogen. This method stains immunoproducts brown with DAB and stains melanin granules green-blue. However, the problem of physical masking remains because of the presence of excess melanin granules.3 The use of non-DAB [3-amino-9-ethylcarbazole (AEC)] IHC detection is an alternative approach for dealing with melanin-rich tissues. Because AEC is red, melanin may not interfere with IHC staining. AEC can be faded away in the time, so that the IHC-stained sections cannot be stored for a long time. Another non-DAB IHC detection using alkaline phosphatase and fast red is also an alternative approach for dealing with melanin-rich tissues. Fast red cannot be stored for a long time too. DAB cannot be faded away; it is the most widely used chromogen for demonstrating antigen-antibody reactions.3
Our study revealed that either potassium permanganate or potassium dichromate bleaching can partially destroy tissue antigen and efficiently bleach DAB. Hence, neither potassium permanganate nor potassium dichromate is an ideal solution for bleaching melanin-containing tissues in IHC studies. In an attempt to find an agent that would bleach melanin without affecting DAB, we treated melanin-containing tissue sections with another bleaching agent, trichloroisocyanuric acid (TCCA). Our results showed that this agent was able to completely bleach melanin and partially destroy tissue antigen, with limited effects on DAB. Therefore, TCCA may be an ideal solution for melanin bleaching after the IHC staining of melanin-containing tissues.
MATERIALS AND METHODS
Materials
A total of 120 malignant melanoma tissue samples were used in this study. An additional 120 uterine leiomyoma tissue samples were recruited as melanin-free samples.
Reagents and Antibodies
TCCA (Aiershi Pai Disinfectant; Shanghai Likang Disinfectant Hi-Tech Co. Ltd), potassium permanganate, and potassium dichromate (AR; Xilong Shantou Chemical Industrial Company Limited) were used in bleaching methods. Vimentin (V9), Ki-67 (GM001), Melanosome (HMB45) (Ready-to-use, Dako), and UltraView Universal DAB Detection Kit (Roche 760-500) were used in IHC staining.
Potassium Permanganate Bleaching Methods
Sections were incubated in 2 g/L potassium permanganate solution for 30 minutes at 65°C. After 3 rinses in water, sections were immersed in 10 g/L oxalic acid for 1 minute at room temperature. Samples were dipped up and down 10 times during incubation. After 3 washes, sections were incubated in phosphate buffered saline (PBS), pH 7.2 to 7.6, for 10 minutes at room temperature.
Potassium Dichromate Bleaching Methods
Sections were incubated in 100 g/L potassium dichromate solution containing 5% sulfate for 30 minutes at 65°C. After 3 rinses in water, sections were immersed in 75% ethanol for 10 minutes at room temperature. After 3 washes, sections were incubated in PBS for 10 minutes at room temperature.
TCCA Bleaching Methods
Sections were incubated in 10 g/L TCCA solution for 30 minutes at room temperature. Samples were dipped up and down 10 times every 10 minutes. After 3 rinses in water, sections were incubated in PBS for 10 minutes at room temperature.
Immunohistochemistry
Immunohistochemistry was performed on the Roche/Ventana (BenchMark XT) IHC/ISH Automatic Staining Module. The Ultraview DAB Detection Kit was used. Sections (4-μm-thick) from each case were mounted on SuperFrost Plus charged glass slides (Menzel-Glaser, Braunschweig, Germany). The IHC Staining was automatically performed in the Roche/Ventana (BenchMark XT) IHC/ISH Automatic Staining Module. The consecutive steps were performed with the following: (1) EZ prep deparaffinization, 16 minutes; (2) CC1, 30 minutes; (3) H2O2, 8 minutes; (4) incubation with antivimentin, Ki-67, or HMB45 antibody, 32 minutes; (5) secondary antibody, 8 minutes; (6) DAB, 4 minutes; (7) copper sulfate solution to enhance the DAB color, 4 minutes; (8) counterstaining with Mayer hematoxylin, 4 minutes; (9) postcounterstaining with bluing reagent, 4 minutes.
Finally, dehydration was performed manually through 95% ethanol, 100% ethanol, cleared in xylene, and then mounted.
Melanin Bleaching
Samples containing melanin were treated according to the aforementioned potassium permanganate, potassium dichromate, or TCCA bleaching methods. Finally, the sections were counterstained with Mayer hematoxylin for 4 minutes. Dehydration was performed through 95% ethanol, 100% ethanol, cleared in xylene, and then mounted.
Control sections of melanin-containing tissues were stained with Mayer hematoxylin for 4 minutes. Dehydration was performed through 95% ethanol, 100% ethanol, cleared in xylene, and then mounted.
Antigen Analysis
Samples without melanin were treated manually with the aforementioned potassium permanganate, potassium dichromate, or TCCA bleaching methods. The bleached sections were placed in the Roche/Ventana (BenchMark XT) IHC/ISH Automatic Staining Module, covered with PBS, and automatically immunostained using antivimentin antibody.
Control sections of melanin-free samples were also automatically immunostained with BenchMark using antivimentin antibody.
DAB Bleaching
Melanin-free samples were automatically immunostained with BenchMark using antivimentin antibody. The IHC-stained sections were bleached manually by potassium permanganate, potassium dichromate, or TCCA bleaching methods and counterstained with Mayer hematoxylin for 4 minutes. Dehydration was performed through 95% ethanol, 100% ethanol, cleared in xylene, and then mounted.
Control sections of melanin-free samples were also automatically immunostained with BenchMark using antivimentin antibody.
Confirmatory Experiment
Samples containing melanin were automatically immunostained with BenchMark using anti-HMB45 and anti-Ki-67 antibodies, respectively. The IHC-stained sections were bleached manually by TCCA bleaching methods and counterstained with Mayer hematoxylin for 4 minutes. Dehydration was performed through 95% ethanol, 100% ethanol, cleared in xylene, and then mounted.
Control sections of melanin-containing samples were also automatically immunostained with BenchMark using anti-HMB45 and anti-Ki-67 antibodies, respectively.
RESULTS
A total of 15 images were captured at ×400 and grouped together into Figures 1 and 2 using Photoshop. All these images had not been used in Color Balance.
FIGURE 1.
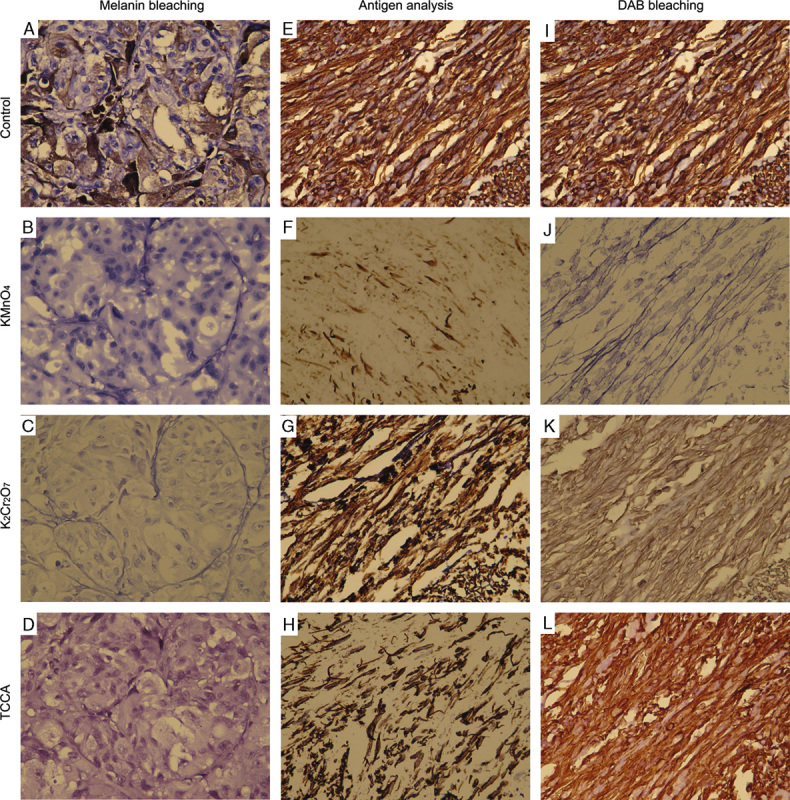
Melanin bleaching, antigen analysis, and DAB bleaching. A, Control sections of melanin-containing tissues were stained with hematoxylin. B, Samples containing melanin were treated with potassium permanganate (KMnO4) bleaching method. C, Samples containing melanin were treated with potassium dichromate (K2Cr2O7) bleaching method. D, Samples containing melanin were treated with TCCA bleaching method. E, Control sections of melanin-free samples were stained with antivimentin. F, Samples without melanin were treated with potassium permanganate (KMnO4) bleaching method followed by immunostaining using antivimentin antibody. G, Samples without melanin were treated with potassium dichromate (K2Cr2O7) bleaching method followed by immunostaining using antivimentin antibody. H, Samples without melanin were treated with TCCA bleaching method followed by immunostaining using antivimentin antibody. I, Control sections of melanin-free samples were stained with antivimentin. J, Samples without melanin were treated with potassium permanganate (KMnO4) bleaching method after immunostaining using antivimentin antibody. K, Samples without melanin were treated with potassium dichromate (K2Cr2O7) bleaching method after immunostaining using antivimentin antibody. L, Samples without melanin were treated with TCCA bleaching method after immunostaining using antivimentin antibody.
FIGURE 2.
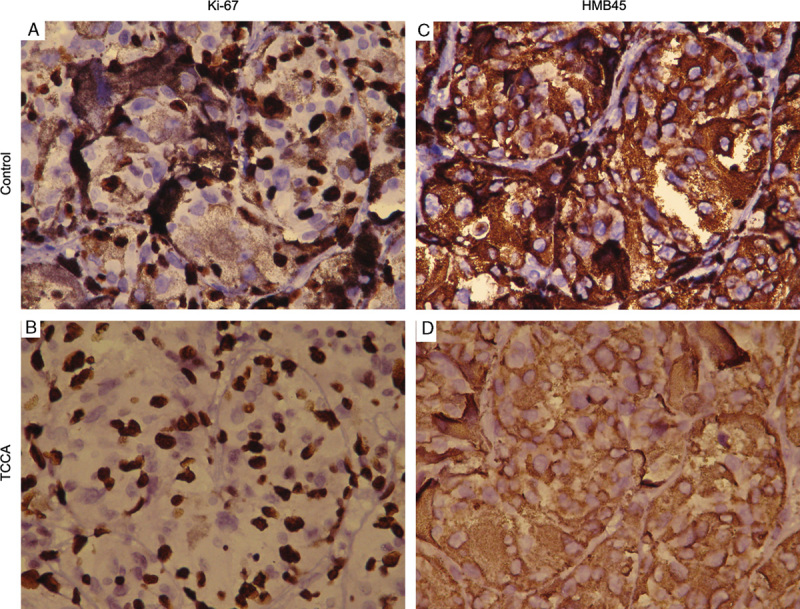
Confirmatory experiment. A, Samples containing melanin were immunostained with anti-Ki-67 antibody. B, Samples containing melanin were immunostained with anti-Ki-67 antibody followed by TCCA bleaching method. C, Samples containing melanin were immunostained with anti-HMB45 antibody. D, Samples containing melanin were immunostained with anti-HMB45 antibody followed by TCCA bleaching method.
Melanin Bleaching
Our results showed that after potassium permanganate (Fig. 1B), potassium dichromate (Fig. 1C), or TCCA bleaching (Fig. 1D), melanin could be completely removed compared with the control (Fig. 1A) in all 120 malignant melanoma tissue sections. The tissue structure and cellular nuclei could be clearly observed after potassium permanganate or TCCA bleaching; however, they could not be clearly seen after potassium dichromate bleaching.
Antigen Analysis
After potassium permanganate (Fig. 1F), potassium dichromate (Fig. 1G), or TCCA bleaching (Fig. 1H) followed by immunostaining using antivimentin antibody, the DAB intensities in the 120 uterine leiomyoma tissue samples were significantly reduced compared with the control (Fig. 1E). After treatment with strong antioxidant, followed by antigen retrieval, the adhesive tissues, especially in the potassium permanganate–bleached group, tended to detach from the slides.
DAB Bleaching
After immunostaining using antivimentin antibody, followed by potassium permanganate (Fig. 1J) or potassium dichromate (Fig. 1K) bleaching, the DAB intensities in the 120 uterine leiomyoma tissue samples were significantly reduced compared with the control (Fig. 1I). Nonetheless, DAB intensity was not greatly altered after TCCA bleaching (Fig. 1L). Moreover, the tissue structure and cellular nuclei were well maintained in TCCA-bleached samples.
Confirmatory Experiment
After immunostaining using anti-Ki-67 and anti-HMB45 antibodies, respectively, followed by TCCA bleaching (Figs. 2B, D), the melanin could be completely removed in all 120 malignant melanoma tissue sections. Compared with the control (Figs. 2A, C), the DAB intensity was clear, and the tissue structure and cellular nuclei were well maintained (Figs. 2A, B were for anti-Ki-67 staining and Figs. 2C, D were for anti-HMB45 staining).
DISCUSSION
In our preliminary experiments, we found that incubation with 10% H2O2 for 30 minutes at 65°C did not completely remove melanin. When the incubation temperature was changed to room temperature, potassium permanganate bleaching or potassium dichromate bleaching was still ineffective in bleaching melanin. However, when samples were treated with 10 g/L TCCA for 4 hours at room temperature, DAB could be clearly removed.
Routine immunohistochemistry procedures, in which sections were bleached with melanin followed by immunostaining, can easily cause tissue detachment and loss of antigen. Here, we provide a novel strategy for IHC analysis whereby sections were immunostained first, followed by melanin bleaching. This method allowed the avoidance of possible tissue detachment and antigen loss.
Despite the fact that melanomas contain melanin, not all melanin-containing tissues are classified as melanoma. For instance, pigment neurofibroma, clear cell sarcoma, and medulloblastoma are melanin-containing masses, but are not melanoma. Thus, these tumors should be carefully diagnosed. Immunohistochemistry is generally used for diagnosis. When the melanin in tissue sections is bleached with strong oxidants followed by immunostaining, it is difficult to correctly interpret the data as these oxidants can destroy tissue antigen. If sections are immunostained first, followed by melanin bleaching, the correct analysis of the results depends upon whether or not the strong oxidant destroys DAB.
TCCA is a strong oxidant, with an active chlorine concentration of >90%. The solubility of TCCA is 12 g/L in water at 25°C. It can be degraded in acidic or alkaline solutions. Furthermore, TCCA is an antimicrobial agent and decolorizer with broad spectrum, high efficiency, and low toxicity, and is thus widely used in food hygiene. The TCCA disinfection tablet is now inexpensive and commercially available.8 As a laboratory reagent, it is relatively safe and can be conveniently purchased and prepared. As a result of potassium permanganate or potassium dichromate bleaching can bleach DAB and TCCA bleaching does not, TCCA might be an ideal solution for melanin bleaching after the IHC staining of melanin-containing tissues. It is worth noting that TCCA should be freshly prepared before each experiment, and used within 2 hours of its preparation. In addition, sections should not be incubated with TCCA for over 30 minutes.
After conventional immunoperoxidase-DAB treatment, azure B melanin counterstaining can distinguish melanin granules from DAB chromogen. This method stains immunoproducts brown with DAB and stains melanin granules green-blue. However, the problem of physical masking remains because of the presence of excess melanin granules.3 The use of non-DAB (AEC) IHC detection is an alternative approach for dealing with melanin-rich tissues. Because AEC is red, melanin may not interfere with IHC staining. AEC can be faded away in the time, so that the IHC-stained sections cannot be stored for a long time. Another non-DAB IHC detection using alkaline phosphatase and fast red is also an alternative approach for dealing with melanin-rich tissues. Fast red cannot be stored for a long time too. DAB cannot be faded away; it is the most widely used chromogen for demonstrating antigen-antibody reactions.3 In conclusion, TCCA might be an ideal solution for melanin bleaching after the IHC staining of melanin-containing tissues.
ACKNOWLEDGMENT
The authors thank Medjaden Bioscience Limited for editing and proofreading the manuscript.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Momose M, Ota H, Hayama M. Re-evaluation of melanin bleaching using warm diluted hydrogen peroxide for histopathological analysis. Pathol Int. 2011;61:345–350. [DOI] [PubMed] [Google Scholar]
- 2.Orchard GE, Calonje E. The effect of melanin bleaching on immunohistochemical staining in heavily pigmented melanocytic neoplasms. Am J Dermatopathol. 1998;20:357–361. [DOI] [PubMed] [Google Scholar]
- 3.Liu CH, Lin CH, Tsai MJ, et al. Melanin bleaching with dilute hydrogen peroxide: a simple and rapid method. Appl Immunohistochem Mol Morphol. 2013;21:275–279. [DOI] [PubMed] [Google Scholar]
- 4.Orchard GE. Heavily pigmented melanocytic neoplasms: comparison of two melanin-bleaching techniques and subsequent immunohistochemical staining. Br J Biomed Sci. 1999;56:188–193. [PubMed] [Google Scholar]
- 5.Li LX, Crotty KA, Kril JJ, et al. Method of melanin bleaching in MIB1-Ki67 immunostaining of pigmented lesions: a quantitative evaluation in malignant melanomas. Histochem J. 1999;31:237–240. [DOI] [PubMed] [Google Scholar]
- 6.Kligora CJ, Fair KP, Clem MS, et al. A comparison of melanin bleaching and azure blue counterstaining in the immunohistochemical diagnosis of malignant melanoma. Mod Pathol. 1999;12:1143–1147. [PubMed] [Google Scholar]
- 7.Foss AJ, Alexander RA, Jefferies LW, et al. Immunohistochemical techniques: the effect of melanin bleaching. Br J Biomed Sci. 1995;52:22–25. [PubMed] [Google Scholar]
- 8.Basu N, Maity SK, Chaudhury A, et al. Trichloroisocyanuric acid (TCCA): an efficient green reagent for activation of thioglycosides toward hydrolysis. Carbohydr Res. 2013;22:10–13. [DOI] [PubMed] [Google Scholar]


