Abstract
Cord blood transplantation, an alternative to traditional stem cell transplants (bone marrow or peripheral blood stem cell transplantation), is an attractive option for patients lacking suitable stem cell transplant donors. Cord blood units have also proven to be a valuable donor source for the development of cellular therapeutics. Virus-specific T cells and regulatory T cells are two cord blood derived products that have shown promise in early phase clinical trials to prevent and/or treat viral infections and graft-versus-host disease (GvHD), respectively. Here we describe how current strategies utilizing cord blood-derived regulatory T cells and virus-specific T cells have been developed to improve outcomes for cord blood transplant recipients.
Keywords: Regulatory T cells (Treg), cord blood, transplant, GvHD, T cell, immunotherapy, cell therapy, antiviral, virus
Introduction
Umbilical Cord blood (UCB) has been shown to be a valuable alternative donor graft source for allogeneic hematopoietic stem cell transplantation (HSCT). Worldwide, there are about 600,000 CB units stored for clinic use. While the main application of UCB is as an allogeneic stem cell source, these units may be also used as a donor source of cells (1)for the development of novel cell therapeutics. The unique immunological properties of UCB present both challenges and opportunities for these applications. The naiveté of the UCB immune system necessitates novel manipulations for the development of antigen specific T cells. In contrast, the unique properties linked to materno-fetal tolerance make UCB an excellent source of regulatory T cells. In this manuscript we review the utilization of UCB-derived cells as a source of both multi-virus-specific T cells (mTC), for the treatment and prevention of viral infections, and natural regulatory T cells (Treg), for the suppression and treatment of GVHD.
Adoptive Transfer of Regulatory T cells (nTregs)
Regulatory T cells (Treg) help modulate responses mediated by effector T cells to avoid an autoimmune response in vivo. (2) Individuals that are born with a functional deficiency of naturally occuring Tregs (nTreg) develop severe auto-immunity syndrome known as IPEX (immunodysregulation polyendocrinopathy enteropathy X-linked syndrome). (3) Tregs are CD4+ CD25hi T cells that express the FoxP3 transcription factor and more recently, have also be shown to express low levels of CD127, the interleukin (IL)-7 α-chain receptor. (4, 5) Notably, Tregs depend on IL-2 secreted by other T cells for survival and proliferation. (2) More recently, the results from several groups have improved our understanding of Treg biology as well as the potential clinical application of these cells not only to reduce the risk of acute graft versus host disease (GVHD) after allogeneic transplantation, (6–12) but also to suppress graft rejection after solid organ transplantation (13) and the treatment of auto immune diseases. (14)
The clinical application of Tregs requires approaches that have typically utilized CD25 positive selection from peripheral blood or umbilical cord blood (UCB) donor sources as follows: 1) Treg infusion with or without the administration of IL-2 to promote Treg expansion in vivo, 2) ex vivo expansion/activation of Tregs prior to infusion, and 3) ex vivo expansion/induction of the Treg (iTreg) phenotype followed by infusion. (15) Currently, in clinicaltrials.gov there are over 10 clinical trials evaluating the adoptive transfer of Tregs for the treatment or prevention of GVHD after HSCT or graft rejection after solid organ transplantation or for the treatment of autoimmune diseases (e.g. type 1 diabetes and Crohn’s disease). Among the numerous studies that have evaluated Tregs clinically, one study using UCB-derived Tregs has been reported with promising results. (16, 17)
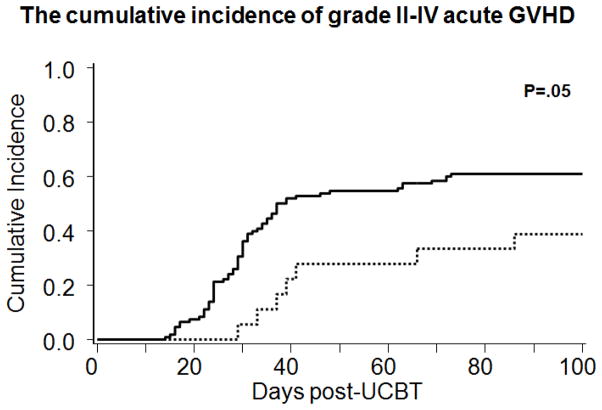
The choice to develop an UCB-derived Treg strategy was based on pre-clinical studies that demonstrated a distinct population of CD4+CD25hi T cells in UCB, responsible for maternal-fetal tolerance. (18) This population could be easily delineated and after expansion/activation in culture these cells were reproducibly suppressive. (19) In contrast to peripheral blood, only one selection step based on CD25 expression is required to expand Tregs from UCB and the expansion culture does not require sirolimus to prevent T effector outgrowth. After CD25 selection, the resultant cell population is ~60% CD4+CD25+FoxP3+CD127-. The expansion methodology has undergone an evolution over time. (16) Patients undergoing a double UCB transplant for hematological malignancies received partially HLA matched UCB derived Tregs obtained from a third unit (partially matched with the patient and hematopoietic stem cell graft). In the first 23 patients, CD25+ T cells were cultured in the presence of beads coated with anti-CD3/anti-CD28 and supplemental IL-2. After passing lot release UCB-derived Tregs were infused the day after UCB transplantation in order to monitor for infusion-related side effects. Important observations from this initial study were the favorable profile of ex vivo expanded UCB-derived Tregs with no infusion related severe adverse events. There were no deleterious effects clinically, and there was a reduction in the risk for the development of grade 2–4 acute GVHD (Figure 1). After a minimum follow up of 2 years, no adverse effects on treatment failure and mortality were identified in the Treg recipients compared to historical controls. (20) There was a suggestion of an increased risk of viral reactivation specifically within the first 30 days, but the historical comparison was limited as viral testing was not available during most of the time the historical controls were treated and could represent an observation bias. Nevertheless, cautious monitoring for viral reactivation in such Treg adoptive transfer studies is warranted.
Figure 1.
the cumulative incidence of garde II-IV acute GVHD by 100 for patients who received ex vivo expanded Tregs (---)and historical controls (⚊). (Adapted from Brunstein et al, 2011;117(3):1061–70).
In the initial clinical trial, albeit in small numbers, Tregs were detectable in the peripheral blood up to 14 days after infusion (21). This detection was based on flow cytometry for the expression of HLA antigens that were different between the Treg donor unit and the patient and two donor UCB units. For example, the Treg units were HLA-A2 positive, whereas the patient and the two donor units were HLA-A2 negative. The length of persistence in the peripheral blood was similar to what was observed in the murine models of GVHD. As the early contact between donor cells and recipient antigens are critical for the development of GVHD, the presence of Tregs early post infusion of the graft is desirable. However, long term persistence on Tregs may not be required to suppress GVHD and could potentially lead to an increased risk of relapse as seen after in vivo or ex vivo T-cell depletion. In mice, the presence of Tregs in lymphoid tissues has been shown (10). While it would be of great interest to document whether or not adoptively transferred Tregs persist long term in lymphoid tissues, we do not yet have a practical and medically appropriate way to do it as it would take a lymph node biopsy.
However, higher Treg doses are desired in order to achieve the target T effector to Treg ratio of 1:1. A modified methodology included expansion using K562-based artificial antigen presenting cells (aAPC) that express the high affinity receptor for the Fc portion, loaded with anti-CD3 antibody, and CD86, the natural ligand of CD28/CTL-4 (KT64/86). The use of these aAPC resulted in a greater expansion of Tregs in vitro compared to the bead-based methodology. (22, 23) In addition, higher doses of Treg were possible with a single restimulation with the KT64/86 aAPCs. This methodological advance ensures that Treg cell doses of 100 × 106/kg can be obtained. A phase I dose escalation trial is currently underway to test the safety and potential efficacy of high dose UCB-derived Treg expanded using aAPC with promising early results (NCT00602693).
Other promising adoptive Treg strategies are under investigation by several groups worldwide generally using adult peripheral blood as the donor source (17). However, another promising UCB strategy is under development at the MD Anderson Cancer Center applying fucosylation to ex vivo expanded UCB-derived Tregs in order to promote Treg engraftment to enhance the anti-GVHD effect (personal communication S. Parmar and E.J. Shpall). (24, 25) Their clinical trial is expected to open in 2015.
As therapeutic strategies utilizing Tregs are translated to the clinic, the importance of HLA-matching (especially when using third party Tregs) and antigen specificity will need to be considered in the study design. Murine models of solid organ and skin allografts have shown better graft survival and less rejection when antigen-specific Tregs are administered (26, 27). In this setting, Treg recognition of antigen present on recipient cells resulted in more efficient suppression of the alloreactive response. (1) However, in a murine model of GVHD, both donor and recipient derived Tregs were able to suppress GVHD.(10) Current studies have used partially HLA-matched UCB-derived Treg from a third party donor. (21) In addition, a clinical trial for patients after haploidentical donor transplant used Tregs derived from the same donor, thus HLA identical to the hematopoietic cell graft (17). While there is a theoretical concern that HLA disparity between donor and/or recipient Tregs could result in rejection of the adoptively transferred Tregs, the clinical data utilizing partially HLA-matched UCB-derived Tregs does not suggest this occurs in vivo. Furthermore, the immunological naiveté of cord blood effector cells, the role of neonatal Tregs in feto-maternal tolerance, and the ease of access to cord blood units make cord blood an attractive donor source for Treg expansion and adoptive therapy. However, it still remains to be determined whether this “off the shelf” third party Treg approach will effectively prevent and/or treat GVHD in patients after HSCT.
In summary, the adoptive transfer of UCB-derived Tregs for the suppression of GVHD is promising. The ability to produce large numbers of Tregs from a single UCB unit by either bead or aAPC stimulation/expansion has the potential for the development of a cryopreserved Treg “off the shelf” product that would be readily available for clinical use. Ongoing and planned studies will further define the clinical efficacy of this cell therapy for the suppression of acute GVHD as well as other clinical contexts beyond GVHD.
Adoptive Transfer of Virus-specific T cells (mTC)
In addition to GvHD and relapse, one of the biggest risks of morbidity and mortality after stem cell transplant are viral infections, most notably from Cytomegalovirus (CMV), Epstein-Barr Virus (EBV), and adenovirus. (28) The risk for viral infections varies and is dependent upon the donor source, conditioning regimen and the degree of T cell depletion. More than 1/3 of deaths after alternative donor stem cell transplant are attributed to viral infection. (28) Antiviral pharmacotherapy drugs do exist but are associated with unacceptable side effect profiles and are not always effective. (29, 30) The adoptive transfer of ex vivo-expanded virus-specific T cells is an appealing alternative to patients at high risk for viral infection, or those who cannot tolerate or have failed conventional pharmacotherapies. (31–34)
The clinical use of virus-specific T cells after stem cell transplant was first reported in 1992 by Riddell, Greenberg, and colleagues. In this study, CMV-infected fibroblasts were used to expand donor-derived CMV-specific T cells prior to infusion. Infused T cells were safe, did not cause GvHD, and provided reconstitution of CD8+ CMV-specific T cells in patients after HSCT. (32) Over the last twenty years, advances in technology and manufacturing have led to less complex T cell expansion procedures, reduced the culture time, and eliminated or limited the use of live virus during production. (35) Notably, in some cases the virus specific T cells were immediately available as an off-the-shelf product. (36–39) Listed in Table 1 are current methods of T cell expansion or selection. However, the limitation of all these methodologies is that they all require prior viral exposure. Additional methodologies have been used to generate virus-specific T cells from naïve donors, especially for EBV, but these methodologies are more extensive than those listed in Table 1 and often involve multiple selections and manipulations. (40–42)
Table 1.
Current selection or expansion strategies for virus-specific T cells
| Method | Culture period (including release testing) | Intermediaries | Reference(s) |
|---|---|---|---|
| Rapid multi-virus T cells | 13–20 days | None | Papadopoulou et al 2014 (29); Gerdemann et al 2013 (58); Gerdemann et al 2012 (35) |
| Peptide-specific expansion | 21–30 days | Dendritic cells | Micklethwaite et al 2007 (59) |
| Gamma-selection | 1–2 days | None | Peggs et al 2011 (34) |
| Tetramer selection | 1–2 days | none | Cobbold et al. 2005 (60); Mackinnon et al 2008 (61); Luo et al 2010 (62) |
| Multi-virus T cells with engineered adenoviral vector | 1–3 months (including LCL generation) | EBV-LCL, monocytes or dendritic cells | Leen et al 2006 (31), Micklethwaite et al 2008 (33); Hanley et al 2010 (63) |
Significant advantages as well as challenges exist when trying to utilize UCB as a source for adoptive cellular immunotherapy. A major advantage is that UCB is a readily available “off the shelf” donor source. However, since the units are already collected and cryopreserved, any manipulation of the cells will likely need to be done upon thawing – saving aliquots of the cells for later use is not an option unless the bags are partitioned into 20% and 80% fractions; even still, using both fractions of the unit is only an option in the third party setting because in CBT, the 80% fraction would need to be transferred to the recipient as part of their cord blood transplant. For this reason, UCB donor-derived donor lymphocyte infusion is not an option after cord blood transplantation (CBT) unless it is ex vivo expanded at the time of thaw. (43, 44) Another challenge is the naiveté of the cord blood immune cells. (45) The majority of UCB T cells express naïve T cell markers such as CCR7, CD62L, and CD45RA. Further, UCB denditric cells (DC) were reported to be less potent, (46, 47) and to secrete less IL-12, which is critical cytokine for T cell priming. It has also been suggested that UCB T cells are less cytotoxic than peripheral blood derived T cells. (48)
For these reasons, the majority of the GMP-compliant expansion and selection methods for virus-specific T cells from virus-experienced donors are not an option when virus naïve donors (e.g. UCB) are used– at least not yet. The rapid expansion of T cells using mononuclear cells is not currently an option because the naïve T cells with T cell receptors recognizing viral epitopes are at a lower frequency than memory virus-specific T cells in the peripheral blood. In fact, antigen-specific T cells from the naïve population require optimized priming conditions, such as the use of professional antigen presenting cells like dendritic cells, as well as cytokines that favor the priming of naïve T cells such as IL-7. (49, 50)
The first report of a virus-specific T cell line derived from UCB came from Sun et al in 1999 (50) where EBV-specific T cells were expanded from UCB using autologous EBV-transformed lymphoblastoid cell lines (LCL) in the presence of IL-2. In 2006, Park et al reported the in vitro priming of CMV-specific cord blood T cells using UCB dendritic cells pulsed with CMV lysate as stimulators in the presence of IL-7 and IL-12. In this study, after 4 weeks ex vivo expansion, the majority of the T cell product was comprised of CD45RO+ memory T cells that secreted IFN-g, IL-2, and TNF-a. However, expansion was relatively limited (2–5 fold) which was problematic for clinical translation. (49) This seminal report led us to later report the GMP-applicable expansion of UCB-derived multi-virus T cells (mTC) recognizing CMV, EBV, and adenovirus to the numbers required for clinical use. (51) Moreover, by utilizing the Grex culture device, these mTC could be expanded to clinically relevant numbers (>6×107) using only the 20% fraction of thawed UCB units providing a donor-specific mTC product for patients after CBT. (51–53) mTC required the priming of naïve T cells with dendritic cells in addition to IL-7 and IL-12, (49) as well as the addition of IL-15 to prevent activation-induced cell death (54).
To date, 9 patients have been treated at the Center for Cell and Gene Therapy (Baylor College of Medicine and Texas Children’s Hospital) with UCB-derived mTC. All infusions were well tolerated and not associated with the development of GvHD. (55) Importantly, reserving the 20% fraction of the UCB unit for mTC manufacture did not result in delayed engraftment, with a median time-to-neutrophil engraftment of 21 days. Clinically, three patients had viral reactivation or infection: 1 patient had CMV reactivation and adenovirus infection, and 2 patients had EBV reactivation. All three patients resolved their viral infections and we were able to detect the adoptively transferred virus-specific T cells in the peripheral blood by interferon gamma ELISPOT assay and/or deep T cell receptor sequencing. (55)
To further extend the application of CB-derived mTC as a therapeutic, these cells were transduced with a retrovirus vector expressing a chimeric antigen receptor (CAR) targeting CD19, present on many B cell malignancies including ALL and CLL. The resultant T cell product had antiviral specificity through the endogenous T cell receptor and anti-leukemic specificity through the CD19-CAR. (56) This approach is now being used clinically in the peripheral blood setting to prevent and treat virus infection and leukemia relapse after HSCT, and a similar study for patients after CBT is planned. (57)
In summary, the adoptive transfer of UCB-derived mTC to prevent and treat viral infection after CBT is feasible and early phase studies suggest that the approach has an excellent safety profile. A subsequent study is currently evaluating the administration of UCB-derived mTC generated without gene-modified autologous APC for expansion (NCT01923766), as well as strategies to decrease the mTC manufacturing time and to extend the virus panel beyond CMV, EBV, and adenovirus.
Conclusions
Adoptive immunotherapy from cord blood cells has gained momentum in recent years due to new technological advances as well as the increased use of cord blood as a graft source. While UCB has some disadvantages, the fact that this donor source is immediately available, well characterized, and contains mostly naïve lymphocytes also makes it an ideal candidate for immunotherapy. In the case of Tregs, the high expression of CD25 makes UCB an ideal starting cell population, and in the case of virus-specific T cells, the ability to manufacture antiviral therapies personalized to the recipients of cord blood transplants, including minorities, is extremely beneficial. There is continued interest in UCB as a unique donor source and with the results from current as well as planned clinical studies using UCB-derived Tregs and mTC (Table 2) it will be possible to better define the clinical efficacy profile and application of these novel cell therapies even beyond the HSCT/CBT setting.
Table 2.
UCB Adoptive T cell and Treg therapies in clinical trial
| Cord Blood Therapy | Method | Intermediaries | Cord Blood Source | Clinical Trial Number |
|---|---|---|---|---|
| Tregs (15,19,20,21) | CD25 selection, CD3/CD28 bead and KT64/86 stimulation | Genetically modified K562 | Third party | NCT00376519 |
| Tregs (20,21) | CD25 selection, KT64/86 stimulation | Genetically modified K562 | Third party | NCT02118311 |
| mTC (55) | T cell stimulation with Ad5f35pp65-transduced APCs | DCs, EBV-LCL | Donor-derived (20% fraction) | NCT00880789 |
| mTC | T cell stimulation with overlapping peptide-pulsed APCs | DCs, EBV-LCL | Donor-derived (20% fraction) | NCT01923766 |
| DLI (64) | CD3 selection, CD3/CD28 bead stimulation | None | 5% of donor UCB | Not available |
| DLI (65) | CD3 selection, CD3/CD28 bead stimlulation | None | UCB Donor-derived | NCT01630564 |
Acknowledgments
The authors acknowledge Dr. John E. Wagner for review and comments. This work was supported in part by NCI PO1 CA148600-02 (C.M.B.), PF-13-046-01-LIB from the American Cancer Society (P.J.H), NCI P01 CA65493 (C.G.B.), and Leukemia and Lymphoma Society Scholar in Clinical Research Award, grant R6029-07 (C.G.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanchez-Fueyo A, Domenig CM, Mariat C, Alexopoulos S, Zheng XX, Strom TB. Influence of direct and indirect allorecognition pathways on CD4+CD25+ regulatory T-cell function in transplantation. Transpl Int. 2007;20(6):534–41. doi: 10.1111/j.1432-2277.2007.00470.x. Epub 2007/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Regulatory T cells: history and perspective. Methods Mol Biol. 2011;707:3–17. doi: 10.1007/978-1-61737-979-6_1. Epub 2011/02/03. [DOI] [PubMed] [Google Scholar]
- 3.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Frontiers in immunology. 2012;3:211. doi: 10.3389/fimmu.2012.00211. Epub 2012/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, et al. CD4(+)CD25(+)CD127(low/−) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131(1):109–18. doi: 10.1016/j.clim.2008.11.010. Epub 2009/01/21. [DOI] [PubMed] [Google Scholar]
- 5.Yu N, Li X, Song W, Li D, Yu D, Zeng X, et al. CD4(+)CD25 (+)CD127 (low/−) T cells: a more specific Treg population in human peripheral blood. Inflammation. 2012;35(6):1773–80. doi: 10.1007/s10753-012-9496-8. Epub 2012/07/04. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196(3):401–6. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193(11):1311–8. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 10.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas PJ, Gress RE, Levine BL, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104(12):3804–12. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 11.Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112(11):1688–96. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannon M, Lechanteur C, Lucas S, Somja J, Seidel L, Belle L, et al. Infusion of clinical-grade enriched regulatory T cells delays experimental xenogeneic graft-versus-host disease. Transfusion. 2014;54(2):353–63. doi: 10.1111/trf.12279. Epub 2013/06/19. [DOI] [PubMed] [Google Scholar]
- 13.Singer BD, King LS, D’Alessio FR. Regulatory T cells as immunotherapy. Frontiers in immunology. 2014;5:46. doi: 10.3389/fimmu.2014.00046. Epub 2014/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187(5):2061–6. doi: 10.4049/jimmunol.1003224. Epub 2011/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11(6):1148–57. doi: 10.1111/j.1600-6143.2011.03558.x. Epub 2011/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–8. doi: 10.1182/blood-2010-10-311894. Epub 2011/02/05. [DOI] [PubMed] [Google Scholar]
- 18.Leber A, Teles A, Zenclussen AC. Regulatory T cells and their role in pregnancy. Am J Reprod Immunol. 2010 doi: 10.1111/j.1600-0897.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 19.Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, et al. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105(2):750–8. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 20.Brunstein CG, Blazar BR, Miller JS, Cao Q, Hippen KL, McKenna DH, et al. Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol Blood Marrow Transplant. 2013;19(8):1271–3. doi: 10.1016/j.bbmt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–70. doi: 10.1182/blood-2010-07-293795. Epub 2010/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3(83):83ra41. doi: 10.1126/scitranslmed.3001809. Epub 2011/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112(7):2847–57. doi: 10.1182/blood-2008-01-132951. Epub 2008/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmar S, Liu X, Tung SS, Robinson SN, Rodriguez G, Cooper LJ, et al. Third-party umbilical cord blood-derived regulatory T cells prevent xenogenic graft-versus-host disease. Cytotherapy. 2014;16(1):90–100. doi: 10.1016/j.jcyt.2013.07.009. Epub 2014/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmar S, Liu X, Najjar A, Shah N, Yang H, Yvon E, et al. Ex vivo fucosylation of 3rd party human regulatory T cells enhances anti-graft versus host disease potency in vivo. Blood. 2014 doi: 10.1182/blood-2014-10-603449. Epub 2014/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109(2):827–35. doi: 10.1182/blood-2006-05-025460. Epub 2006/09/28. [DOI] [PubMed] [Google Scholar]
- 27.Callaghan CJ, Rouhani FJ, Negus MC, Curry AJ, Bolton EM, Bradley JA, et al. Abrogation of antibody-mediated allograft rejection by regulatory CD4 T cells with indirect allospecificity. J Immunol. 2007;178(4):2221–8. doi: 10.4049/jimmunol.178.4.2221. Epub 2007/02/06. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy-Nasser AA, Bollard CM, Myers GD, Leung KS, Gottschalk S, Zhang Y, et al. Comparable outcome of alternative donor and matched sibling donor hematopoietic stem cell transplant for children with acute lymphoblastic leukemia in first or second remission using alemtuzumab in a myeloablative conditioning regimen. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(11):1245–52. doi: 10.1016/j.bbmt.2008.08.010. Epub 2008/10/23. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulou A, Gerdemann U, Katari UL, Tzannou I, Liu H, Martinez C, et al. Activity of Broad-Spectrum T Cells as Treatment for AdV, EBV, CMV, BKV, and HHV6 Infections after HSCT. Science translational medicine. 2014;6(242):242ra83. doi: 10.1126/scitranslmed.3008825. Epub 2014/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanley PJ, Bollard CM. Controlling cytomegalovirus: helping the immune system take the lead. Viruses. 2014;6(6):2242–58. doi: 10.3390/v6062242. Epub 2014/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nature medicine. 2006;12(10):1160–6. doi: 10.1038/nm1475. Epub 2006/09/26. [DOI] [PubMed] [Google Scholar]
- 32.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–41. doi: 10.1126/science.1352912. Epub 1992/07/10. [DOI] [PubMed] [Google Scholar]
- 33.Micklethwaite KP, Clancy L, Sandher U, Hansen AM, Blyth E, Antonenas V, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112(10):3974–81. doi: 10.1182/blood-2008-06-161695. Epub 2008/09/05. [DOI] [PubMed] [Google Scholar]
- 34.Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(1):49–57. doi: 10.1093/cid/ciq042. Epub 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 35.Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20(8):1622–32. doi: 10.1038/mt.2012.130. Epub 2012/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–23. doi: 10.1182/blood-2013-02-486324. Epub 2013/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116(23):5045–9. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qasim W, Derniame S, Gilmour K, Chiesa R, Weber M, Adams S, et al. Third-party virus-specific T cells eradicate adenoviraemia but trigger bystander graft-versus-host disease. British journal of haematology. 2011;154(1):150–3. doi: 10.1111/j.1365-2141.2011.08579.x. Epub 2011/04/20. [DOI] [PubMed] [Google Scholar]
- 39.Uhlin M, Okas M, Gertow J, Uzunel M, Brismar TB, Mattsson J. A novel haplo-identical adoptive CTL therapy as a treatment for EBV-associated lymphoma after stem cell transplantation. Cancer immunology, immunotherapy : CII. 2010;59(3):473–7. doi: 10.1007/s00262-009-0789-1. Epub 2009/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savoldo B, Cubbage ML, Durett AG, Goss J, Huls MH, Liu Z, et al. Generation of EBV-specific CD4+ cytotoxic T cells from virus naive individuals. J Immunol. 2002;168(2):909–18. doi: 10.4049/jimmunol.168.2.909. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 41.Metes D, Storkus W, Zeevi A, Patterson K, Logar A, Rowe D, et al. Ex vivo generation of effective Epstein-Barr virus (EBV)-specific CD8+ cytotoxic T lymphocytes from the peripheral blood of immunocompetent Epstein Barr virus-seronegative individuals. Transplantation. 2000;70(10):1507–15. doi: 10.1097/00007890-200011270-00019. Epub 2000/12/16. [DOI] [PubMed] [Google Scholar]
- 42.Jedema I, van de Meent M, Pots J, Kester MG, van der Beek MT, Falkenburg JH. Successful generation of primary virus-specific and anti-tumor T-cell responses from the naive donor T-cell repertoire is determined by the balance between antigen-specific precursor T cells and regulatory T cells. Haematologica. 2011;96(8):1204–12. doi: 10.3324/haematol.2010.039099. Epub 2011/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazur MA, Davis CC, Szabolcs P. Ex vivo expansion and Th1/Tc1 maturation of umbilical cord blood T cells by CD3/CD28 costimulation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(10):1190–6. doi: 10.1016/j.bbmt.2008.07.016. Epub 2008/09/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis CC, Marti LC, Sempowski GD, Jeyaraj DA, Szabolcs P. Interleukin-7 permits Th1/Tc1 maturation and promotes ex vivo expansion of cord blood T cells: a critical step toward adoptive immunotherapy after cord blood transplantation. Cancer research. 2010;70(13):5249–58. doi: 10.1158/0008-5472.CAN-09-2860. Epub 2010/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanley PJ, Cruz CR, Shpall EJ, Bollard CM. Improving clinical outcomes using adoptively transferred immune cells from umbilical cord blood. Cytotherapy. 2010;12(6):713–20. doi: 10.3109/14653249.2010.517518. Epub 2010/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, et al. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166(3):2141–6. doi: 10.4049/jimmunol.166.3.2141. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 47.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clinical and experimental immunology. 2002;128(1):118–23. doi: 10.1046/j.1365-2249.2002.01817.x. Epub 2002/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YJ, Stringfield TM, Chen Y, Broxmeyer HE. Modulation of cord blood CD8+ T-cell effector differentiation by TGF-beta1 and 4-1BB costimulation. Blood. 2005;105(1):274–81. doi: 10.1182/blood-2003-12-4343. Epub 2004/09/09. [DOI] [PubMed] [Google Scholar]
- 49.Park KD, Marti L, Kurtzberg J, Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006;108(5):1770–3. doi: 10.1182/blood-2005-10-006536. Epub 2006/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Q, Burton RL, Pollok KE, Emanuel DJ, Lucas KG. CD4(+) Epstein-Barr virus-specific cytotoxic T-lymphocytes from human umbilical cord blood. Cellular immunology. 1999;195(2):81–8. doi: 10.1006/cimm.1999.1514. Epub 1999/08/17. [DOI] [PubMed] [Google Scholar]
- 51.Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114(9):1958–67. doi: 10.1182/blood-2009-03-213256. Epub 2009/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanley PJ, Lam S, Shpall EJ, Bollard CM. Expanding cytotoxic T lymphocytes from umbilical cord blood that target cytomegalovirus, Epstein-Barr virus, and adenovirus. Journal of visualized experiments : JoVE. 2012;(63):e3627. doi: 10.3791/3627. Epub 2012/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33(3):305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. Epub 2010/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11445–50. doi: 10.1073/pnas.200363097. Epub 2000/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanley PJMC, Leung K, Savoldo B, Dotti G, Brenner MK, et al. Improving Immune Reconstitution after cord blood transplantation using ex vivo expanded virus-specific T cells: a phase I clinical study. ASH annual Meeting Abstracts; 2012; [Google Scholar]
- 56.Micklethwaite KP, Savoldo B, Hanley PJ, Leen AM, Demmler-Harrison GJ, Cooper LJ, et al. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood. 2010;115(13):2695–703. doi: 10.1182/blood-2009-09-242263. Epub 2010/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–73. doi: 10.1182/blood-2013-06-506741. Epub 2013/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(11):2113–21. doi: 10.1038/mt.2013.151. Epub 2013/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Micklethwaite K, Hansen A, Foster A, Snape E, Antonenas V, Sartor M, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(6):707–14. doi: 10.1016/j.bbmt.2007.02.004. Epub 2007/05/29. [DOI] [PubMed] [Google Scholar]
- 60.Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. The Journal of experimental medicine. 2005;202(3):379–86. doi: 10.1084/jem.20040613. Epub 2005/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mackinnon S, Thomson K, Verfuerth S, Peggs K, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood cells, molecules & diseases. 2008;40(1):63–7. doi: 10.1016/j.bcmd.2007.07.003. Epub 2007/09/18. [DOI] [PubMed] [Google Scholar]
- 62.Luo XH, Huang XJ, Liu KY, Xu LP, Liu DH. Protective immunity transferred by infusion of cytomegalovirus-specific CD8(+) T cells within donor grafts: its associations with cytomegalovirus reactivation following unmanipulated allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(7):994–1004. doi: 10.1016/j.bbmt.2010.02.007. Epub 2010/02/20. [DOI] [PubMed] [Google Scholar]
- 63.Hanley PJ, Shaffer DR, Cruz CR, Ku S, Tzou B, Liu H, et al. Expansion of T cells targeting multiple antigens of cytomegalovirus, Epstein-Barr virus and adenovirus to provide broad antiviral specificity after stem cell transplantation. Cytotherapy. 2011;13(8):976–86. doi: 10.3109/14653249.2011.575356. Epub 2011/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berglund S, Gertow J, Uhlin M, Mattsson J. Expanded umbilical cord blood T cells used as donor lymphocyte infusions after umbilical cord blood transplantation. Cytotherapy. 2014;16(11):1528–36. doi: 10.1016/j.jcyt.2014.08.001. Epub 2014/09/19. [DOI] [PubMed] [Google Scholar]
- 65.Parmar S, Robinson SN, Komanduri K, St John L, Decker W, Xing D, et al. Ex vivo expanded umbilical cord blood T cells maintain naive phenotype and TCR diversity. Cytotherapy. 2006;8(2):149–57. doi: 10.1080/14653240600620812. Epub 2006/05/16. [DOI] [PubMed] [Google Scholar]