Abstract
Recessive dystrophic epidermolysis bullosa (RDEB) is a severe blistering skin disease caused by mutations in the COL7A1 gene. These mutations lead to decreased or absent levels of collagen VII at the dermal-epidermal junction. Over the past decade, significant progress has been made in the treatment of RDEB, including the use of hematopoietic cell transplantation, but a cure has proven elusive. Patients still experience life-limiting and life-threatening complications as a result of painful and debilitating wounds. The continued suffering of these patients drives the need to improve existing therapies and develop new ones. In this review, we will discuss how recent advances in placenta-, umbilical cord blood- and amniotic membrane-based therapies may play a role in the both the current and future treatment of RDEB.
Keywords: epidermolysis bullosa, umbilical cord blood, bone marrow, hematopoietic cell transplantation, mesenchymal stromal/stem cells, induced pluripotent stem cells
1 – Introduction
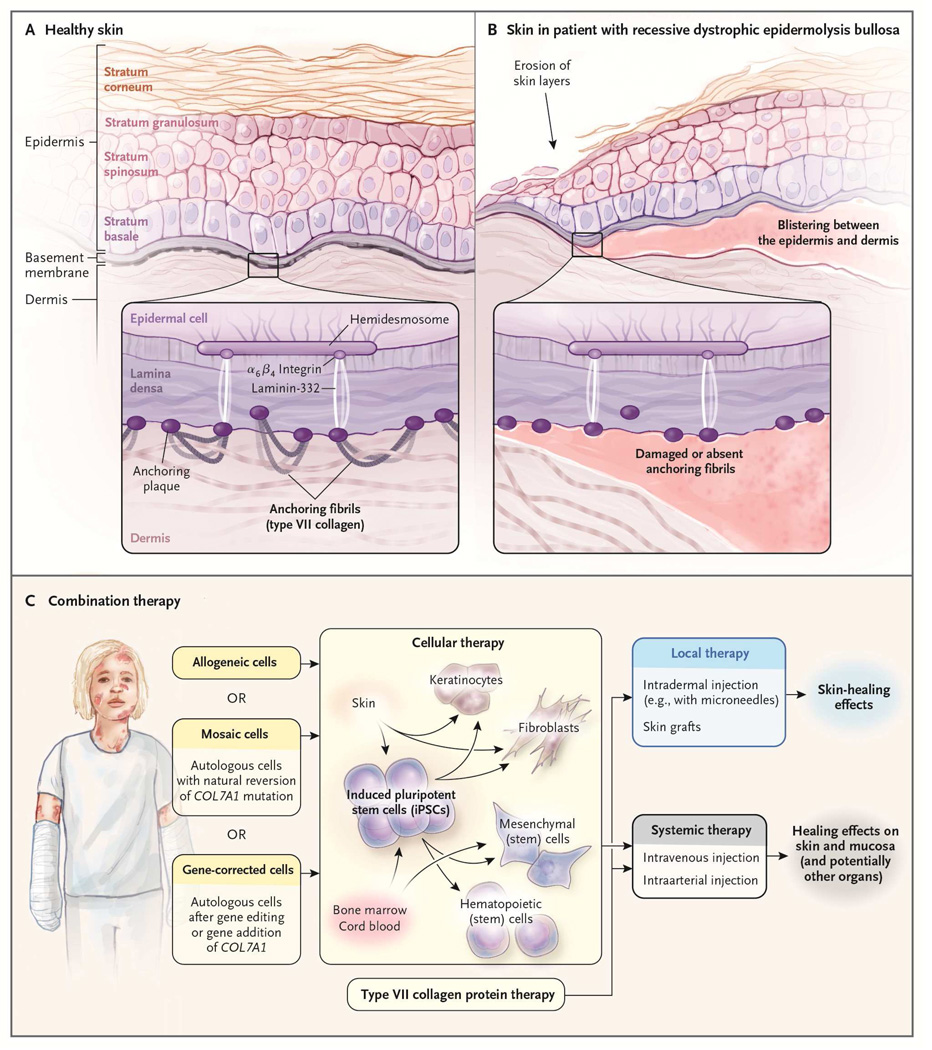
Epidermolysis bullosa (EB) is a heterogeneous group of predominantly genetically inherited blistering skin diseases. Generalized severe recessive dystrophic epidermolysis bullosa (RDEB), one of the major and most severe subtypes of EB, is caused by mutations in the gene COL7A1 that lead to decreased or absent levels of the extracellular matrix protein type VII collagen (C7) [1]. C7 is normally found near the dermal-epidermal junction (DEJ) and plays a role in the formation of anchoring fibrils that attach the epidermis to the dermis (Figure 1 A). Starting at birth, patients with RDEB experience severe, painful blistering of the skin from even minor trauma (Figure 1 B). Patients are also subject to mucosal lesions leading to esophageal strictures and difficulty maintaining proper nutrition. Additionally, as a likely result of the near constant inflammation associated with repeated cycles of blistering and healing, patients who survive beyond the first few years of life often experience aggressive and fatal forms of squamous cell carcinoma [2].
Figure 1.
Combination therapy for epidermolysis bullosa.
From The New England Journal of Medicine, Jakub Tolar and John Wagner, A Biological Velcro Patch; 372;4, 383. Copyright© 2015 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
The devastating impact of RDEB on patients and their families has inspired intensive research efforts, but there is still no definitive cure for the disease. Several promising therapies have been developed to treat skin wounds by using intradermal injection or cutaneous application of fibroblasts, mesenchymal stromal/stem cells (MSCs), and recombinant C7. The limitation of these therapies is that they are unable to address the mucosal lesions and other systemic complications [3]. The need for a therapy that could address these challenges is what led to the first human trial of hematopoietic cell transplantation (HCT) for the treatment of RDEB [4]. Results from RDEB patients treated with HCT thus far are encouraging, but outcomes are still not perfect. Ultimately, the most effective approach to treating RDEB will probably require a combination of the local and systemic therapies being investigated (Figure 1 C) [5].
Recent advancements in the field of placenta-based therapies may be useful in refining and improving our current treatment strategies for RDEB. For example, in HCT umbilical cord blood (UCB) has several potential advantages over bone marrow (BM), including decreased collection risk to the donor compared to the harvesting of BM, decreased risk of infection transmission from donor to patient, a need for less stringent human leukocyte antigen (HLA)-matching requirements, and an overall lower risk of graft-versus-host disease (GvHD). Additionally, UCB is becoming more readily available as cord blood banks grow and techniques for ex vivo expansion of hematopoietic cells improve [6; [7]. Likewise, the amount of research being done on non-HCT UCB-based therapies is increasing [8; [9; [10]. In this review, we will discuss these advances as they relate to both the current and future treatment of RDEB.
2 – Hematopoietic cell transplantation for epidermolysis bullosa
2.1 Preclinical studies
For many years it was widely believed that the use of BM transplantation in the setting of a protein deficiency would only be feasible if the deficient protein was soluble, e.g., iduronidase deficiency in mucopolysacharidosis type I [11]. This notion was challenged when Chino et al. [12] demonstrated that an in utero BM transplant could be used to improve survival in a murine model of RDEB. In a simultaneous and independent study, Tolar and colleagues performed HCT on a murine model of RDEB using various populations of stem cells and found that 15% of mice that received a transplant of signaling lymphocyte activating molecule-positive (SLAM+) (CD150+) cells survived long term compared to untreated pups, which typically died within the first days of life. Furthermore, an immunohistochemical examination of the skin of these transplanted mice showed that donor cells homed to the skin and produced C7 [13]. The ability to use hematopoietic stem cell therapy to treat an extracellular matrix disease was confirmed again by Fujita et al., who demonstrated that BM transplantation improved survival in a murine model of a related genodermatosis, junctional EB [14].
2.2 Clinical trials
Based on the encouraging results of the preclinical experiments described above, a clinical trial of HCT for EB was initiated by Wagner et al. [4]. As of 2014, 26 individuals with severe RDEB have been treated with allogeneic HCT. Stem cell sources have varied, with 15 individuals receiving HLA-matched or partially HLA-matched, related BM cells; six receiving HLA-matched or partially HLA-matched unrelated BM cells; and five receiving partially HLA-matched UCB cells. The pre-transplantation conditioning regimen has also varied, with half of the patients receiving standard myeloablative conditioning (MAC) and half receiving reduced intensity conditioning (RIC). In an abstract by Tolar et al. submitted to the Society of Investigative Dermatology Annual Meeting 2015, it is reported that a significant majority of the engrafted individuals have shown marked biochemical (increased C7 expression at the DEJ), ultrastructural (increased number of anchoring fibrils, albeit with immature architecture), and/or clinical improvements (decreased body surface area effected by disease and increased resistance to blistering).
2.3 Explaining what works
Although there is clear evidence suggesting that HCT may be an effective treatment for RDEB, the exact mechanism behind the observed therapeutic effect remains a mystery. Initially it was thought that improved outcomes might be related either to some aspect of the preconditioning regimen used to prepare patients for HCT or to the higher level of care patients received during and after transplant. However, many of the treated RDEB patients have shown symptomatic improvement for several years following HCT, long after immunosuppressive medications used for preconditioning (typically six months after HCT) or increased care during transplant would be expected to have an effect. It seems more likely that the therapeutic effect of HCT for RDEB is truly due to characteristics of the transplanted donor cells [15]. Supporting this theory is the identification of donor cell chimerism in the skin of several RDEB patients following transplant [4].
Several previous studies showed donor cell chimerism in the epithelium of transplant recipients (Table 1), so the observation that donor cells home to and engraft in the skin of RDEB patients after HCT was not unexpected. In 2007, Murata et al. had noted the presence of both donor-derived keratinocytes and endothelial cells in the skin of 18 patients who experienced GvHD following sex-mismatched HCT [16]. This study also observed a higher number of donor cells where the skin experienced the greatest wounding due to GvHD, leading Murata et al. to hypothesize that donor cells were playing a role in epidermal and microvessel repair [16].
Table 1.
Human studies demonstrating donor cell chimerism following transplant.
| Subject | Chimerism | Additional Findings | Reference |
|---|---|---|---|
| Patients with GvHD following sex-mismatched HCT | Donor-derived keratinocytes and endothelial cells found in the skin | Increased level of chimerism in regions with severe GvHD | Murata et al. 2007 [16] |
| Patients who received HCT | Donor-derived epithelial cells found in nasal scrapings | Engraftment occurred most rapidly during first few months following transplant | Khan et al. 2010 [17] |
| Patients with severe RDEB | Donor-cell chimerism detected in the skin | Hematopoietic and nonhematopoietic donor cells identified | Wagner et al. 2010 [4] |
A few years later, Khan et al. identified the presence of donor-derived epithelial cells in nasal scrapings taken from 60 patients who had received allogeneic HCT. The median percentage of donor epithelial cells increased from 0% on day seven post transplant, to 2.8% at three months post transplant, to 8.5% at 12–22 years post-transplant, suggesting that donor cell migration to the epithelium and engraftment therein occurs most rapidly during the first few months following transplant [17].
A study by Tamai et al. found that, when given a green fluorescent protein (GFP) BM transplant, mice with skin grafts had a high number of donor-derived epithelial cells near the grafts. These epithelial cells were found to be derived from Lin-/PDGFRα+ cells and were believed to home to sites of skin grafting due to the release of high mobility box group 1 protein by skin grafts. In the same study, donor cell chimerism was not identified in the skin of wounded mice that did not receive a skin graft [18]. Expanding on this work, Iinuma et al. used flow cytometry to identify donor-derived cells that migrated to RDEB mouse skin grafted onto a wild-type mouse following a GFP BM transplant. Non-hematopoietic BM cells, including Lin-/PDGFRα+ mesenchymal cells, were found in the RDEB skin graft and via real-time PCR were shown to highly express C7. Interestingly, when mice were given a systemic administration of the CXCR4 antagonist AMD3100, migration of PDGFRα+ cells to skin grafts was significantly reduced and deposition of C7 along the DEJ of the RDEB skin grafts, observed in the absence of AMD3100, was interrupted suggesting that PDGFRα+ cells may play a crucial role in the wound healing observed after transplant [19].
Another important observation to include in this discussion is that the degree of donor chimerism observed in the skin of RDEB patients who received HCT was high (on average 20%) [4] suggesting that migration and/or engraftment of donor derived cells is enhanced by some characteristic of actively wounded skin. One possible explanation is that the epidermal stem cell niches in RDEB patients are depleted of cells due to rapid skin cell turnover caused by frequent blistering/healing and are therefore more suitable for the engraftment of donor cells. Another possibility is that the active wounds of RDEB patients secrete homing molecules that actively recruit donor cells to sites of injured tissue [15].
The donor cells found in the epidermis of RDEB patients receiving HCT were of both hematopoietic and non-hematopoietic origin [4]. Since hematopoietic cells have not been shown to express significant amounts of C7, it is reasonable to hypothesize that these non-hematopoietic cells are at least partially responsible for the increased deposition of C7 at the DEJ [15]. It is possible that HSCs are capable of transdifferentiation into epidermal stem cells, however it is the view of the authors that a population of stem cells contained within transplanted cells are intrinsically capable of both homing to wounded skin and secreting C7. Additional preclinical research will help to elucidate the answers to these mechanistic questions and, most importantly, will allow us to improve upon what has turned out to be a promising therapy for RDEB.
3 – Placenta-based therapies and epidermolysis bullosa
3.1 Mesenchymal stromal/stem cells
MSCs can be found in multiple adult and fetal tissues, and have been shown to have extensive regenerative and immunomodulatory properties. Although strictly defined by criteria set forth by the International Society of Cellular Therapy in 2006 [20], recent studies are refining our working definition of MSCs [21]. Much of our current knowledge is based on MSCs isolated from BM, adipose tissue, or peripheral blood (PB), however, interest in MSCs derived from UCB is increasing [22; [23; [24], partly due to the fact that isolation of UCB MSCs is easier than isolation of MSCs from other sources [25; [26]. Additionally, UCB MSCs have been shown to have a faster population doubling time in vitro [27] and are more readily available than other sources of MSCs due to the increasing prevalence of cord blood banking.In addition to UCB, MSCs can also be isolated from the placenta, umbilical artery or vein, cord lining and Wharton’s jelly [28]. It is important to note that although MSCs from these various sources are often collectively referred to as UCB MSCs, differences between them have been demonstrated [29].
With regards to the treatment of skin diseases, it has now been well documented that MSCs play a role in the normal physiological process of wound healing [29; [30] and can be used therapeutically to accelerate wound healing [31; [32]. At least two studies have specifically evaluated the effect of cutaneous MSC transplantation in the context of RDEB. In a study by Alexeev et al., autologous MSCs were transplanted into the skin of a murine model of RDEB. Immunofluorescence analysis of the skin of pups that survived three weeks post-transplant revealed patchily distributed C7, estimated to be about 15% that of wild type C7 levels. Remarkably, when the skin of these transplanted pups was subjected to a mechanical stress test, separation along the DEJ did not occur suggesting that even a partial restoration of C7 could provide clinically meaningful benefits [33]. Around the same time, Congent et al. injected BM MSCs from an unrelated donor intradermally near the wounds of two patients with RDEB. At one week post-treatment, the DEJ was observed to be intact and at 12 weeks post-treatment, C7 was identified in MSC treated skin. However, the therapeutic effect was transient, as re-ulceration occurred approximately four months following treatment in both patients [34].
While the studies referenced and described above help demonstrate both the potential and limitations of MSC-based therapies for wound healing, they all used BM MSCs. A study by Luo et al. specifically evaluated the potential of UCB MSCs to promote cutaneous wound healing. In their study, they isolated MSCs from human UCB, labeled them with 5-bromodeoxyuridine, and then injected them into a murine cutaneous wounding model. Skin wound healing was accelerated compared to controls and labeled epidermal cells derived from the injected UCB MSCs were identified in the skin near the wound site [35]. While this study showed that UCB MSCs are similar to BM MSCs in their capacity to accelerate wound healing, it did not provide any data suggesting whether one source of MSCs might be superior to the other.
Fortunately, a recent study by Kim et al. directly compared the wound healing potential of MSCs isolated from UCB, BM, and PB. They injected 3 × 106 cells from each MSC source subcutaneously into a murine excisional skin wounding model and then used a photometric analysis to evaluate the healing response for 14 days. On day 8, average relative wound closure for UCB, BM and PB MSC treated wounds was significantly different at 58%, 42%, and 22%, respectively. Based on a histological analysis, the authors also noted that both granulation tissue and re-epithelialization of UCB MSC-treated wounds appeared to be thicker and larger than those treated with BM or PB MSCs [36]. Cumulatively, these studies (summarized in Table 2) provide evidence to support the use of MSC-based therapies for the treatment of RDEB and, although less definitively, suggest that UCB MSCs may have an advantage over MSCs isolated from other tissues.
Table 2.
Studies supporting the use of UCB MSC-based therapies for the treatment of RDEB.
| Purpose of Study | Subject | Results | Reference |
|---|---|---|---|
| Determine the effect of cutaneous BM MSC transplantation on wound healing in RDEB | Murine model of RDEB | Patchily distributed C7 at DEJ 3 weeks post transplant; increased DEJ stability at 12 weeks | Alexeev et al. 2011 [33] |
| Determine the effect of cutaneous BM MSC transplant on wound healing in RDEB | 2 patients with RDEB | Accelerated wound healing compared to controls at 1 week post transplant; increased C7 at DEJ at 12 weeks; recurrent ulceration at 16 weeks | Conget et al. 2010 [34] |
| Determine the effect of cutaneous UCB MSC transplantation on wound healing | Murine wounding model | Significantly increased wound healing compared to controls; identification of donor derived epidermal cells near wound sites | Luo et al. 2010 [35] |
| Compare the effect of UCB, BM, and PB MSC transplantation on cutaneous wound healing | Murine wounding model | Significantly greater wound healing UCB MSC treated wounds compared to BM or PB MSCs; increased granulation tissue and repithelialization in UCB MSC treated wounds | Kim et al. 2012 [36] |
While in general the molecular characteristics of MSCs isolated from UCB are very similar to those isolated from other sources [13; [37], differences have been identified. In the Kim et al. study described above, a proteomic analysis of UCB, BM, and PB-derived MSCs revealed several differentially regulated proteins. Specifically, cytoskeletal proteins were found to be upregulated in PB MSCs (tubulin alpha 1 C chain, annexin A2, laminin B1, PDZ domain-containing protein GIPC1 and actin) and BM MSCs (tubulin alpha 1 C chain and annexin A2), and antioxidant and detoxification proteins were found to be upregulated in both BM MSCs (carbonyl reductase, heat shock protein beta 1, glutathione S-transferase Mu3 and glutathione S-transferase omega-1) and UCB MSCs (heat shock protein beta 1, glutathione S-transferase Mu3, glutathione S-transferase omega-1, S-formylglutathione hydrolase, annexin A1 and chloride intracellular channel protein 4) (35). Likewise, an earlier study used serial analysis of gene expression to compare cultured UCB MSCs isolated from the umbilical vein to cultured BM MSCs and identified several genes related to matrix remodeling and the tumor necrosis factor alpha angiogenesis related pathways that were expressed either exclusively or at significantly greater levels in UCB MSCs. These genes included CXCL6, interleukin (IL)-8, IL-1 receptor-like ligand, matrix metalloproteinase-1, integrin alpha-3, CXCL1, and pentraxin 3 [38]. A recent reanalysis of this data combined with a proteomic analysis identified 67 differentially expressed proteins, most of which were upregulated in UCB MSCs when compared to BM MSCs [39].
Finally, it is worth discussing the immune-modulating characteristics of MSCs. Numerous studies have shown that MSCs play a role in both the adaptive and innate immune systems [13; [40; [41; [42]. Based on these immunoregulatory properties, it was hypothesized that MSCs could be useful in the treatment of disorders such as GvHD. While early studies on the use of MSCs for GvHD were promising [43; [44], the results of subsequent clinical trials were less so [45]. Although the therapeutic advantage was less definitive than had been originally hoped, the use of MSCs for GvHD is still being actively investigated, refined and optimized. Interestingly, a few studies have shown that UCB- and placental-derived MSCs may have significantly greater immunosuppressive potential than other sources of MSCs [31; [46; [47; [48]. While large head-to-head clinical trials would be needed to prove that UCB MSCs are in fact superior to other sources of MSCs for the treatment of GvHD, these findings are very encouraging, particularly because, due to the repetitive cycles of blistering and healing, RDEB patients live in a near-constant state of inflammation. As our knowledge of the immune-modulating properties of MSCs improves, we may discover novel indications for the use of MSC-based therapies in the treatment of RDEB and other inflammatory conditions.
3.2 Unrestricted somatic stem cells
An additional population of stromal stem cells that can be found in UCB are known as unrestricted somatic stem cells (USSCs) [49]. Similar to MSCs, USSCs have a greater in vitro expansion potential than BM MSCs [50], express C7, are capable of differentiating into keratinocytes in vitro, and have been shown to promote wound healing after migrating to the skin after both intradermal and tail vein injections in a mouse model [51]. These characteristics certainly make USSCs an interesting candidate for the development of future EB treatments; however, future research is needed to determine what therapeutic advantages, if any, USSCs would have over MSCs.
3.3 Induced pluripotent stem cells
One of the most promising developments in the treatment of EB is the combined use of induced pluripotent stem (iPS) cells and gene editing. Cells isolated from a variety of sources can be reprogrammed to pluripotency using the canonical Yamanaka factors Oct4, Sox2, Klf4, and c-Myc [52]. If correction of the COL7A1 mutation in these cells were followed by directed differentiation, it would provide a patient-specific and renewable source of cells for transplant. Tolar et al. demonstrated the derivation of iPS cells from both fibroblasts and keratinocytes taken from a patient with RDEB [53]. These iPS cells were capable of differentiation into both hematopoietic and non-hematopoietic lineages, and were shown to form skin-like structures in mice. Osborn et al. expanded on this work by using TALEN-based gene editing to correct the COL7A1 mutation in fibroblasts taken from an RDEB patient. These gene-corrected fibroblasts were then used to derive and expand an iPS cell line to a level that could theoretically be used in a human transplant [54]. There are also examples of RDEB patients with spontaneous reversion of the COL7A1 mutation, where self-corrected keratinocytes produce functional C7 in patches of healthy-looking skin. These corrected keratinocytes could be reprogrammed into iPS cells capable of differentiation into epidermal and hematopoietic cell lineages, setting the stage for autologous cellular therapies using this ‘natural’ gene therapy [55]. While the advantage of transplanting hematopoietic cells is their ability to target the systemic manifestations of RDEB, the increased expression of C7 found in epidermal stem cells may make them an equally viable candidate for cellular therapies. Yet another approach, taken by Sebastiano et al., used adeno-associated virus to correct the COL7A1 mutation in patient-derived iPS cells that could then be differentiated into epithelial sheets of keratinocytes [56]. Overall, iPS cell generation and gene editing techniques are improving at a rapid pace and the ability to translate these technologies into clinical therapies is now becoming a reality. In September 2014 the first clinical trial with human iPS cells began, when autologous iPS cells derived from six patients with age-related macular degeneration were differentiated into retinal pigment epithelial cell sheets for surgical implantation in the eye [57]. If this study establishes the safety and feasibility of using iPS cells in humans, trials using gene-corrected iPS cells may not be far behind.
Placental cells could also potentially play a role in these developing iPS cell therapies. It has been well documented that iPS cells can be produced from cord blood cells [58; [59; [60]. In addition to the ease with which iPS cells can be generated from cord blood, they have been generated from cords stored as long as 23 years [61], making the vast stores of banked cord blood cells available for treatments. For hematopoietic cell transplants, the neonatal nature of cord blood cells makes them immunologically immature and therefore safer to use for partially HLA-matched transplants without a corresponding increase in GvHD [62]. Although obtaining an adequate dose of hematopoietic stem cells (HSCs) for an adult patient from a single cord blood unit is challenging, ex vivo expansion of cord blood cells is possible [63; [64]. An additional advantage is that they are obtained from neonates, so they may have not had time to accumulate as many nuclear and somatic mutations as aged cells [65; [66]. A recent study by Tomassetti et al. found that 65% of the differences in cancer risk among different tissue types could be explained by the rate at which the stem cells in those particular tissues divide [67]. As HSCs are highly proliferative, undergoing 1011 divisions during a lifetime, the use of neonatal HSCs obtained from cord blood for HCT is further supported. Combining cord blood HCTs with the synergistic action of AMD3100 and tacrolimus, which has been found to augment cutaneous wound healing by mobilizing endogenous HSCs, could be the key to effectively resolving the skin lesions of patients with RDEB [68; [69].
Generating iPS cells from cord blood could offer an epigenetic advantage as well. It has been shown that after reprogramming to pluripotency, low passage iPS cells retain epigenetic marks based on the cell type they were derived from. This bias can be substantial, as Kim et al. found when iPS cells derived from keratinocytes were compared to those derived from cord blood. This study found that iPS cells derived from keratinocytes have 9.4 times the potential to form keratinocytes, while iPS cells derived from cord blood have increased potential to form cells of myeloid lineage [70]. In addition to the retention of DNA methylation profiles and chromatin marks, the miRNA network unique to the starting cell type remains intact for low passage iPS cells [71]. Thus far, attempts to derive fully engraftable HSCs from human iPS cells have failed, possibly due to the complex microenvironment necessary for the development of functional HSCs in vivo [72]. Differentiating low passage iPS cells derived from cord blood could take advantage of this epigenetic and miRNA bias and, in combination with the co-culture method developed by Suzuki et al., has the potential to improve the yield of CD34+ HSCs that can repopulate the BM after transplant [72].
3.4 Amniotic membrane grafting
Despite the progress made in systemic therapies, the treatment of individual wounds still remains a major aspect of the care of RDEB patients. Amniotic membrane grafts are a type of biological dressing that has previously been used to successfully treat leg ulcers and burns. A recent retrospective chart review by Lo et al. evaluated the use of amniotic grafting to treat chronic wounds in two patients with RDEB. Based on a qualitative wound score, a significant clinical response was noted in four of eight treated wounds, with complete healing in one [73].
3.5 Cord blood platelet gel
Similarly, autologous PB platelet-rich gel has also previously been shown to be both safe and effective at treating cutaneous wounds [74]. However, recent research has shown that cord blood platelet gels (CBPGs) may be better suited to the treatment of skin wounds. In addition to being more easily obtained than autologous PB platelet gels, CBPGs have a lower risk of infection and contain a higher concentration of the growth factors believed to be responsible for the regenerative effect of platelet gels. These growth factors include platelet-derived growth factor, transforming growth factor, fibroblast growth factor, and vascular endothelial growth factor [75]. In a recent case report, severe chronic wounds in three RDEB patients were treated with CBPGs for three weeks. Wounds treated with CBPGs healed faster than untreated control wounds and relapses were not observed in the four weeks following treatment [76].
While the results of both amniotic membrane transplants and CBPGs are encouraging, additional prospective studies are needed before any definitive conclusions about their effectiveness can be made [77]. Further, it is important to consider the potential impact of these treatments within the context of the pathophysiology of RDEB. Even if future studies clearly demonstrate that these therapies are superior to current wound care strategies in EB (which they likely are), without C7 to provide stability at the DEJ, new wounds will inevitably arise. As discussed in a recent editorial, the true potential of these treatments may lie in combination with other therapies currently being developed [5]. For example, the growth factors secreted by CBPGs could potentially be used to enhance the homing and engraftment potential of both local and systemic cellular therapies.
5 – Conclusions
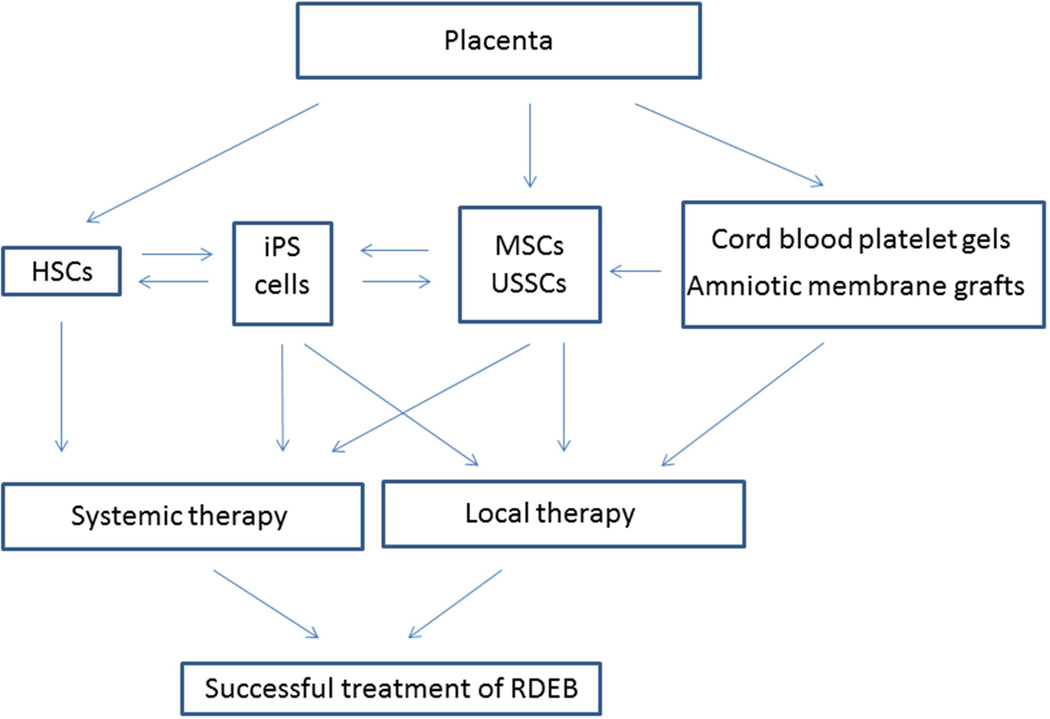
Over the past decade, significant advances have been made in the treatment of EB. Patients who were once offered nothing beyond palliative measures are now being offered hope based on the early success of HCT. Despite this progress, many obstacles still remain and, as is often the case with complex diseases, the optimal treatment of RDEB will ultimately rely on the combined effects of multiple therapies. Recent advances in placenta-based therapies are promising, both as a way to improve upon existing treatments and as novel therapies (Figure 2). Furthermore, while the focus of this review has been on the application of placenta-based therapies for the treatment of RDEB, it is important to remember that there are many genetic skin disorders, some of which, like RDEB, are both debilitating and currently without an effective cure. It is therefore imperative that, as we continue to advance our collective knowledge through the treatment of RDEB, we make an effort to apply what we have learned to the treatment of additional diseases.
Figure 2.
Placenta-based therapies for the treatment of recessive dystrophic epidermolysis bullosa.
Acknowledgements
We apologize to the authors whose work could not be quoted because of space constraints and thank Nancy Griggs Morgan for editorial assistance. JT is supported in part by R01 AR063070, R01 AR059947, DOD W81XWH, EB Research Partnership, EB Medical Research Foundation, DEBRA, and the Sohana Research Fund.
Abbreviations
- BM
bone marrow
- C7
type VII collagen
- CBPG
cord blood platelet gel
- DEJ
dermal-epidermal junction
- EB
epidermolysis bullosa
- GvHD
graft versus host disease
- HCT
hematopoietic cell transplantation
- HLA
human leukocyte antigen
- HSC
hematopoietic stem cell
- IL
interleukin
- iPS
induced pluripotent stem
- MAC
myeloablative conditioning
- MSC
mesenchymal stromal/stem cell
- PB
peripheral blood
- RDEB
recessive dystrophic epidermolysis bullosa
- RIC
reduced intensity conditioning
- UCB
umbilical cord blood
- USSC
unrestricted somatic stem cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interest – Authors declare no conflicts of interest.
References
- 1.Fine JD, Bruckner-Tuderman L, Eady RA, Bauer EA, Bauer JW, Has C, Heagerty A, Hintner H, Hovnanian A, Jonkman MF, Leigh I, Marinkovich MP, Martinez AE, McGrath JA, Mellerio JE, Moss C, Murrell DF, Shimizu H, Uitto J, Woodley D, Zambruno G. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. Journal of the American Academy of Dermatology. 2014;70(6):1103–1126. doi: 10.1016/j.jaad.2014.01.903. [DOI] [PubMed] [Google Scholar]
- 2.Bruckner-Tuderman L. Dystrophic epidermolysis bullosa: pathogenesis and clinical features. Dermatologic clinics. 2010;28(1):107–114. doi: 10.1016/j.det.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CK, Wang SP, Lee JY, McGrath JA. Treatment of hereditary epidermolysis bullosa: updates and future prospects. Am J Clin Dermatol. 2014;15(1):1–6. doi: 10.1007/s40257-013-0059-z. [DOI] [PubMed] [Google Scholar]
- 4.Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT, Chen M, Riddle MJ, Osborn MJ, Lund T, Dolan M, Blazar BR, Tolar J. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. The New England journal of medicine. 2010;363(7):629–639. doi: 10.1056/NEJMoa0910501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolar J, Wagner JE. A biologic Velcro patch. The New England journal of medicine. 2015;372(4):382–384. doi: 10.1056/NEJMcibr1414709. [DOI] [PubMed] [Google Scholar]
- 6.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, Cooke MP. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shenoy S, Boelens JJ. Advances in unrelated and alternative donor hematopoietic cell transplantation for nonmalignant disorders. Current opinion in pediatrics. 2015;27(1):9–17. doi: 10.1097/MOP.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oran B, Shpall E. Umbilical cord blood transplantation: a maturing technology. Hematology Am Soc Hematol Educ Program. 2012;2012:215–222. doi: 10.1182/asheducation-2012.1.215. [DOI] [PubMed] [Google Scholar]
- 9.Shahrokhi S, Menaa F, Alimoghaddam K, McGuckin C, Ebtekar M. Insights and hopes in umbilical cord blood stem cell transplantations. J Biomed Biotechnol. 2012;2012:572821. doi: 10.1155/2012/572821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orchard PJ, Blazar BR, Wagner J, Charnas L, Krivit W, Tolar J. Hematopoietic cell therapy for metabolic disease. The Journal of pediatrics. 2007;151(4):340–346. doi: 10.1016/j.jpeds.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 12.Chino T, Tamai K, Yamazaki T, Otsuru S, Kikuchi Y, Nimura K, Endo M, Nagai M, Uitto J, Kitajima Y, Kaneda Y. Bone marrow cell transfer into fetal circulation can ameliorate genetic skin diseases by providing fibroblasts to the skin and inducing immune tolerance. The American journal of pathology. 2008;173(3):803–814. doi: 10.2353/ajpath.2008.070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolar J, Ishida-Yamamoto A, Riddle M, McElmurry RT, Osborn M, Xia L, Lund T, Slattery C, Uitto J, Christiano AM, Wagner JE, Blazar BR. Amelioration of epidermolysis bullosa by transfer of wild-type bone marrow cells. Blood. 2009;113(5):1167–1174. doi: 10.1182/blood-2008-06-161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita Y, Abe R, Inokuma D, Sasaki M, Hoshina D, Natsuga K, Nishie W, McMillan JR, Nakamura H, Shimizu T, Akiyama M, Sawamura D, Shimizu H. Bone marrow transplantation restores epidermal basement membrane protein expression and rescues epidermolysis bullosa model mice. Proc Natl Acad Sci U S A. 2010;107(32):14345–14350. doi: 10.1073/pnas.1000044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolar J, Blazar BR, Wagner JE. Concise review: Transplantation of human hematopoietic cells for extracellular matrix protein deficiency in epidermolysis bullosa. Stem Cells. 2011;29(6):900–906. doi: 10.1002/stem.647. [DOI] [PubMed] [Google Scholar]
- 16.Murata H, Janin A, Leboeuf C, Soulier J, Gluckman E, Meignin V, Socie G. Donor-derived cells and human graft-versus-host disease of the skin. Blood. 2007;109(6):2663–2665. doi: 10.1182/blood-2006-07-033902. [DOI] [PubMed] [Google Scholar]
- 17.Khan FM, Sy S, Louie P, Smith M, Chernos J, Berka N, Sinclair GD, Lewis V, Russell JA, Storek J. Nasal epithelial cells of donor origin after allogeneic hematopoietic cell transplantation are generated at a faster rate in the first 3 months compared with later posttransplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(12):1658–1664. doi: 10.1016/j.bbmt.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Tamai K, Yamazaki T, Chino T, Ishii M, Otsuru S, Kikuchi Y, Iinuma S, Saga K, Nimura K, Shimbo T, Umegaki N, Katayama I, Miyazaki J, Takeda J, McGrath JA, Uitto J, Kaneda Y. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci U S A. 2011;108(16):6609–6614. doi: 10.1073/pnas.1016753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iinuma S, Aikawa E, Tamai K, Fujita R, Kikuchi Y, Chino T, Kikuta J, McGrath JA, Uitto J, Ishii M, Iizuka H, Kaneda Y. Transplanted Bone Marrow-Derived Circulating PDGFRalpha+ Cells Restore Type VII Collagen in Recessive Dystrophic Epidermolysis Bullosa Mouse Skin Graft. J Immunol. 2015 doi: 10.4049/jimmunol.1400914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- 22.Leeb C, Jurga M, McGuckin C, Moriggl R, Kenner L. Promising new sources for pluripotent stem cells. Stem Cell Rev. 2010;6(1):15–26. doi: 10.1007/s12015-009-9102-0. [DOI] [PubMed] [Google Scholar]
- 23.Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3(4):248–269. [PMC free article] [PubMed] [Google Scholar]
- 24.Zarrabi M, Mousavi SH, Abroun S, Sadeghi B. Potential uses for cord blood mesenchymal stem cells. Cell J. 2014;15(4):274–281. [PMC free article] [PubMed] [Google Scholar]
- 25.Bieback K, Kern S, Kluter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22(4):625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 26.Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ, Han ZC. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–1026. [PubMed] [Google Scholar]
- 27.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 28.Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013;2013:916136. doi: 10.1155/2013/916136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22(5):812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006;326(3):725–736. doi: 10.1007/s00441-006-0270-9. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 33.Alexeev V, Donahue A, Uitto J, Igoucheva O. Analysis of chemotactic molecules in bone marrow-derived mesenchymal stem cells and the skin: Ccl27-Ccr10 axis as a basis for targeting to cutaneous tissues. Cytotherapy. 2013;15(2):171 e171–184 e171. doi: 10.1016/j.jcyt.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conget P, Rodriguez F, Kramer S, Allers C, Simon V, Palisson F, Gonzalez S, Yubero MJ. Replenishment of type VII collagen and re-epithelialization of chronically ulcerated skin after intradermal administration of allogeneic mesenchymal stromal cells in two patients with recessive dystrophic epidermolysis bullosa. Cytotherapy. 2010;12(3):429–431. doi: 10.3109/14653241003587637. [DOI] [PubMed] [Google Scholar]
- 35.Luo G, Cheng W, He W, Wang X, Tan J, Fitzgerald M, Li X, Wu J. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen. 2010;18(5):506–513. doi: 10.1111/j.1524-475X.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim YS, Ahn Y, Kwon JS, Cho YK, Jeong MH, Cho JG, Park JC, Kang JC. Priming of mesenchymal stem cells with oxytocin enhances the cardiac repair in ischemia/reperfusion injury. Cells Tissues Organs. 2012;195(5):428–442. doi: 10.1159/000329234. [DOI] [PubMed] [Google Scholar]
- 37.Flynn A, Barry F, O'Brien T. UC blood-derived mesenchymal stromal cells: an overview. Cytotherapy. 2007;9(8):717–726. doi: 10.1080/14653240701584578. [DOI] [PubMed] [Google Scholar]
- 38.Panepucci RA, Siufi JL, Silva WA, Jr, Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT, Zago MA. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22(7):1263–1278. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 39.Miranda HC, Herai RH, Thome CH, Gomes GG, Panepucci RA, Orellana MD, Covas DT, Muotri AR, Greene LJ, Faca VM. A quantitative proteomic and transcriptomic comparison of human mesenchymal stem cells from bone marrow and umbilical cord vein. Proteomics. 2012;12(17):2607–2617. doi: 10.1002/pmic.201200111. [DOI] [PubMed] [Google Scholar]
- 40.Uccelli A, Mancardi G, Chiesa S. Is there a role for mesenchymal stem cells in autoimmune diseases? Autoimmunity. 2008;41(8):592–595. doi: 10.1080/08916930802200166. [DOI] [PubMed] [Google Scholar]
- 41.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 42.Mougiakakos D, Machaczka M, Jitschin R, Klimkowska M, Entesarian M, Bryceson YT, Henter JI, Sander B, Le Blanc K. Treatment of familial hemophagocytic lymphohistiocytosis with third-party mesenchymal stromal cells. Stem Cells Dev. 2012;21(17):3147–3151. doi: 10.1089/scd.2012.0214. [DOI] [PubMed] [Google Scholar]
- 43.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 44.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 45.Kaipe H, Erkers T, Sadeghi B, Ringden O. Stromal cells-are they really useful for GVHD? Bone marrow transplantation. 2014;49(6):737–743. doi: 10.1038/bmt.2013.237. [DOI] [PubMed] [Google Scholar]
- 46.Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24(11):2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 47.Roelen DL, van der Mast BJ, in't Anker PS, Kleijburg C, Eikmans M, van Beelen E, de Groot-Swings GM, Fibbe WE, Kanhai HH, Scherjon SA, Claas FH. Differential immunomodulatory effects of fetal versus maternal multipotent stromal cells. Hum Immunol. 2009;70(1):16–23. doi: 10.1016/j.humimm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Karlsson H, Erkers T, Nava S, Ruhm S, Westgren M, Ringden O. Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol. 2012;167(3):543–555. doi: 10.1111/j.1365-2249.2011.04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Muller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200(2):123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demerdash Z, El Baz H, Mahmoud F, Mohamed S, Maher K, Gaafar T, Shawky S, Hassan M, Abdelhady D, Taha T. Enhancing ex vivo expansion of cord blood-derived unrestricted somatic stem cells for clinical applications. Cell Prolif. 2013;46(6):628–636. doi: 10.1111/cpr.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao Y, Itoh M, Yang A, Zhu H, Roberts S, Highet AM, Latshaw S, Mitchell K, van de Ven C, Christiano A, Cairo MS. Human cord blood-derived unrestricted somatic stem cells promote wound healing and have therapeutic potential for patients with recessive dystrophic epidermolysis bullosa. Cell Transplant. 2014;23(3):303–317. doi: 10.3727/096368913X663569. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Tolar J, Xia L, Riddle MJ, Lees CJ, Eide CR, McElmurry RT, Titeux M, Osborn MJ, Lund TC, Hovnanian A, Wagner JE, Blazar BR. Induced pluripotent stem cells from individuals with recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2011;131(4):848–856. doi: 10.1038/jid.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osborn MJ, Starker CG, McElroy AN, Webber BR, Riddle MJ, Xia L, DeFeo AP, Gabriel R, Schmidt M, von Kalle C, Carlson DF, Maeder ML, Joung JK, Wagner JE, Voytas DF, Blazar BR, Tolar J. TALEN-based gene correction for epidermolysis bullosa. Mol Ther. 2013;21(6):1151–1159. doi: 10.1038/mt.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tolar J, McGrath JA, Xia L, Riddle MJ, Lees CJ, Eide C, Keene DR, Liu L, Osborn MJ, Lund TC, Blazar BR, Wagner JE. Patient-specific naturally gene-reverted induced pluripotent stem cells in recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2014;134(5):1246–1254. doi: 10.1038/jid.2013.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sebastiano V, Zhen HH, Derafshi BH, Bashkirova E, Melo SP, Wang P, Leung TL, Siprashvili Z, Tichy A, Li J, Ameen M, Hawkins J, Lee S, Li L, Schwertschkow A, Bauer G, Lisowski L, Kay MA, Kim SK, Lane AT, Wernig M, Oro AE. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6(264):264ra163. doi: 10.1126/scitranslmed.3009540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World's First Induced Pluripotent Stem Cells Clinical Study on Humans Launches in Japan [News] 2014 Available from http://stemcellstm.alphamedpress.org/site/misc/News159.xhtml. [Google Scholar]
- 58.Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31(3):458–466. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- 59.Zaehres H, Kogler G, Arauzo-Bravo MJ, Bleidissel M, Santourlidis S, Weinhold S, Greber B, Kim JB, Buchheiser A, Liedtke S, Eilken HM, Graffmann N, Zhao X, Meyer J, Reinhardt P, Burr B, Waclawczyk S, Ortmeier C, Uhrberg M, Scholer HR, Cantz T, Wernet P. Induction of pluripotency in human cord blood unrestricted somatic stem cells. Exp Hematol. 2010;38(9):809–818. doi: 10.1016/j.exphem.2010.05.009. 818 e801–802. [DOI] [PubMed] [Google Scholar]
- 60.Adegani FJ, Langroudi L, Arefian E, Shafiee A, Dinarvand P, Soleimani M. A comparison of pluripotency and differentiation status of four mesenchymal adult stem cells. Mol Biol Rep. 2013;40(5):3693–3703. doi: 10.1007/s11033-012-2445-7. [DOI] [PubMed] [Google Scholar]
- 61.Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ, Mallett C, Ye Z, Witting S, Cornetta K, Cheng L, Yoder MC. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117(18):4773–4777. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J, Gu Q, Hao J, Bai D, Liu L, Zhao X, Liu Z, Wang L, Zhou Q. Generation of induced pluripotent stem cells with high efficiency from human umbilical cord blood mononuclear cells. Genomics Proteomics Bioinformatics. 2013;11(5):304–311. doi: 10.1016/j.gpb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delaney C, Ratajczak MZ, Laughlin MJ. Strategies to enhance umbilical cord blood stem cell engraftment in adult patients. Expert Rev Hematol. 2010;3(3):273–283. doi: 10.1586/ehm.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schuller CE, Jankowski K, Mackenzie KL. Telomere length of cord blood-derived CD34(+) progenitors predicts erythroid proliferative potential. Leukemia. 2007;21(5):983–991. doi: 10.1038/sj.leu.2404631. [DOI] [PubMed] [Google Scholar]
- 65.Arnheim N, Cortopassi G. Deleterious mitochondrial DNA mutations accumulate in aging human tissues. Mutat Res. 1992;275(3–6):157–167. doi: 10.1016/0921-8734(92)90020-p. [DOI] [PubMed] [Google Scholar]
- 66.Ono T, Uehara Y, Saito Y, Ikehata H. Mutation theory of aging, assessed in transgenic mice and knockout mice. Mech Ageing Dev. 2002;123(12):1543–1552. doi: 10.1016/s0047-6374(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 67.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347(6217):78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tolar J, McGrath JA. Augmentation of cutaneous wound healing by pharmacologic mobilization of endogenous bone marrow stem cells. The Journal of investigative dermatology. 2014;134(9):2312–2314. doi: 10.1038/jid.2014.209. [DOI] [PubMed] [Google Scholar]
- 69.Lin Q, Wesson RN, Maeda H, Wang Y, Cui Z, Liu JO, Cameron AM, Gao B, Montgomery RA, Williams GM, Sun Z. Pharmacological mobilization of endogenous stem cells significantly promotes skin regeneration after full-thickness excision: the synergistic activity of AMD3100 and tacrolimus. J Invest Dermatol. 2014;134(9):2458–2468. doi: 10.1038/jid.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Huo H, Loh YH, Aryee MJ, Lensch MW, Li H, Collins JJ, Feinberg AP, Daley GQ. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29(12):1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vitaloni M, Pulecio J, Bilic J, Kuebler B, Laricchia-Robbio L, Izpisua Belmonte JC. MicroRNAs contribute to induced pluripotent stem cell somatic donor memory. J Biol Chem. 2014;289(4):2084–2098. doi: 10.1074/jbc.M113.538702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki N, Yamazaki S, Yamaguchi T, Okabe M, Masaki H, Takaki S, Otsu M, Nakauchi H. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther. 2013;21(7):1424–1431. doi: 10.1038/mt.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lo V, Lara-Corrales I, Stuparich A, Pope E. Amniotic membrane grafting in patients with epidermolysis bullosa with chronic wounds. J Am Acad Dermatol. 2010;62(6):1038–1044. doi: 10.1016/j.jaad.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 74.Driver VR, Hanft J, Fylling CP, Beriou JM Autologel Diabetic Foot Ulcer Study G. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52(6):68–70. 72, 74 passim. [PubMed] [Google Scholar]
- 75.Parazzi V, Lazzari L, Rebulla P. Platelet gel from cord blood: a novel tool for tissue engineering. Platelets. 2010;21(7):549–554. doi: 10.3109/09537104.2010.514626. [DOI] [PubMed] [Google Scholar]
- 76.Tadini G, Guez S, Pezzani L, Marconi M, Greppi N, Manzoni F, Rebulla P, Esposito S. Preliminary evaluation of cord blood platelet gel for the treatment of skin lesions in children with dystrophic epidermolysis bullosa. Blood Transfus. 2014:1–6. doi: 10.2450/2014.0160-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tolar J, Tolar M. A living band-aid for epidermolysis bullosa. Blood Transfus. 2015;13:1–2. doi: 10.2450/2014.0289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]