Abstract
Background
Historically, recruitment and retention of young women in intervention-based clinical trials has been challenging. In August 2012, enrollment for a clinical trial testing of an investigational human papillomavirus (HPV) therapeutic vaccine called PepCan was opened at our institution. This study was an open-label, single arm, single institution, dose-escalation Phase I clinical trial. Women with recent Papanicolau smear results showing high-grade squamous intraepithelial lesions (HSILs) or cannot rule out HSIL were eligible to enroll. Patients with biopsy-confirmed HSIL were also eligible. Colposopy was performed at the screening visit, and participants became eligible for vaccination when the diagnosis of HSIL was confirmed with biopsy and other inclusion criteria were met.
Purpose
The aim of this study was to identify strategies and factors effective in recruitment and retention of study participants.
Methods
Potential vaccine candidates were recruited through direct advertisement as well as referrals, including through the Arkansas telecolposcopy network. The network is a federally funded program, administered by physicians and advanced practice nurses. The network telemedically links rural health sites and allows physician-guided colposcopy and biopsies to be conducted by advanced practice nurses. A variety of strategies were employed to assure good retention including face-to-face contact with the study coordinator at the time of consent and most of study visits, frequent contact using text messaging, phone calls, and e-mails, and creation of a private Facebook page to improve communication among research staff and study participants. A questionnaire, inquiring about motivation for joining the study, occupation, education, household income, number of children, and number of sexual partners, was administered at the screening visit with the intent of identifying factor(s) associated with recruitment and retention.
Results
Thirty-seven participants were enrolled between September 2012 and March 2014. The largest proportion of participants (46%) was enrolled from the telecolposcopy network. Others were enrolled through outside institutions (43%), in-house referrals (8%), or direct advertisement (3%). Most participants were motivated to join the study to take care of their health issues. Only 2 participants joined the Facebook private page. Of 24 participants who qualified for vaccination, only 1 terminated early due to an unanticipated move.
Limitations
As with most Phase I clinical trials, the number of participants is small.
Conclusions
The availability of a large number of potential participants from the telecolposcopy network increased recruitment to this clinical trial by 85% over other traditional means of recruitment. The telecolposcopy network is not only a means of providing a gynecological service to women who otherwise would forego care, but also a novel and valuable resource in recruiting participants for a clinical trial.
Keywords: Telecolposcopy network, recruitment, human papillomavirus, therapeutic vaccine, Facebook
Introduction
Cervical cancer is the fourth most common cancer among women globally with annual incidence of 528,000 cases and mortality of 266,000 cases (1). Effective means of screening for early detection, such as Papanicolaou (Pap) smears and human papillomavirus (HPV)-deoxyribonucleotide testing, are available. Furthermore, prophylactic vaccines [Gardasil® (Merck & Co., Whitehouse Station, NJ) and Cervarix® (GalaxoSmithKline, Middlesex, United Kingdom)] which prevent cervical cancer by preventing HPV infection, have been approved by the Food and Drug Administration in 2006 and 2009, respectively. However, due to a lack of thorough implementation, cervical cancer remains to be a serious health problem. Only 32% of the targeted group (girls aged 13-17 years) has been vaccinated in the United States (2).
Almost all cases of cervical cancer are caused by HPV. HPV also causes other cancers, including anal, oropharyngeal, penile, vaginal, vulvar, and is estimated to be responsible for 5.2% of the cancer burden worldwide (3, 4). Effective surgical treatments, such as loop electrical excision procedure (LEEP), are available to treat precancerous cervical lesions called high-grade squamous intraepithelial lesion (HSIL, also known as cervical intraepithelial lesion 2/3). However, they result in an unintended side effect of doubling the rate of preterm delivery in future pregnancies (5, 6). Therefore, non-surgical alternatives for treating HSIL are needed. Currently, many therapeutic HPV vaccines are in development (7).
Our group initiated enrollment in 2012 for a single-institution, single-arm, dose-escalation, National Cancer Institute-funded Phase I clinical trial of one such candidate vaccine called PepCan. A variety of strategies were employed to enhance recruitment and retention in this clinical trial. Here, we report a novel approach of enhancing recruitment of young women into a clinical trial through a telemedicine network.
Methods
Clinical trial
PepCan consists of four current good manufacturing practice-grade synthetic peptides covering the amino acid sequences of human papillomavirus type 16 E6 protein and Candin® (Allermed, San Diego, CA), a colorless extract of Candida albicans, as a novel adjuvant (8) because of its ability to promote T-cell proliferation (8, 9), interleukin-12 secretion by Langerhans cells (8, 9), and skin wart (another condition caused by HPV) regression (10). Women with recent Pap smear results with HSIL or cannot rule out HSIL were eligible to enroll. A written informed consent was obtained from each participant. At the screening visit, colposcopy guided biopsy was obtained, and if the diagnosis of HSIL was confirmed, participants were eligible to receive vaccination. Women with previous biopsy results confirming HSIL prior to study enrollment were also eligible to join as long as they were able to receive the first vaccination within 60 days of the biopsy date. The vaccination was administered intradermally in any extremity every three weeks. Three months after the fourth vaccination, LEEP was performed. Six participants were enrolled into each of the four dose levels (i.e., 50, 100, 250, and 500 μg per peptide per injection). The therapeutic dose of Candin (300 μl/injection) was used for all participants (10). All participants received 4 vaccinations except for 1 participant who exited the study early after 2 vaccinations. The main outcome measure was safety. Upon completion of the study, study participants received $250 in compensation for their time. Those who traveled equal to or more than 50 miles one way were also eligible to receive a travel stipend ($40 to $100 per visit, depending on the distance travelled) at each visit.
Recruitment strategy
Direct advertising efforts to attract potential participants included placing advertisements (a local newspaper, a local college-campus newspaper, and a local radio station), posting flyers and making brochures available throughout the University of Arkansas for Medical Science (UAMS) campus (including in primary care clinics) as well as in local health units, physician’s offices, and college heath centers. Information about the study was disseminated at state fairs, various health fairs, and through a university-sponsored texting campaign to pre-registered potential study participants. To encourage referrals, letters along with flyers and brochures were mailed to local health departments and private gynecology clinics informing them about the clinical trial and encouraging practitioners to refer eligible patients. Potential study participants in the UAMS healthcare system were approached in person and/or by mail (letter describing the study and brochure). Information about the study was also available online (www.uams.edu/papresults) and a Facebook page titled UAMS HPV Vaccine Trial. Interviews with local radio stations and television news stations also disseminated information about the study in local communities. In addition, a partnership was developed with the Arkansas telecolposcopy network, allowing us to recruit women who were recently diagnosed with HSIL by biopsy.This network provides colposcopy services to women who have had abnormal Pap smear results from any of the 90 county health units in Arkansas (11). Colposcopy is performed by trained nurse practitioners under the guidance of UAMS gynecologists who communicate through real-time telemedicine video connection.
Retention strategy
Attempts were made for the study coordinator to be present not only at the time of consent, but also at as many study visits as possible in order to develop good rapport. In addition to the study coordinator keeping close contact with the subjects (via e-mails, texting, phone calls) especially around the time of study visits, all subjects who presented to the screening visit received an invitation to join a Facebook private page. Postings by the study staff included study milestones reached, information regarding women’s health, media coverage of the study, etc.
Identifying factor(s) associated with recruitment and retention
Questions about motivation of joining the study, occupation, education, household income, number of children, and number of sexual partners were asked in the Screening Visit Questionnaire. It also included questions about whether they have received HPV prophylactic vaccines and whether they used social media. An Exit Visit Questionnaire or Early Termination Questionnaire was also administered to assess satisfaction level and reasons for early termination if applicable.
Results
Recruitment
Thirty-seven participants were enrolled between September 2012 and March 2014. The mean age was 31 with a range of 22-49, with 20 (54%) African Americans. Although many inquiries were received through direct advertisement efforts, particularly through the local radio station advertisement, only one participant initially heard about the study through this route (saw a flyer and picked up a brochure). As it was routine to perform HPV testing on patients diagnosed with atypical squamous cells of unknown significance on Pap smears, some women who called may have tested positive for HPV but were not eligible to enroll in the study because they did not have HSIL. The remaining 36 participantsincluded telecolposcopy network referrals (46%), outside referrals (43%), or in-house referrals (8%). Outside referrals were from a local county health unit (n = 10), non-local county health units (n = 4), a local college health clinic (n = 1), and a private clinic (n = 1). Therefore, the availability of the telecolposcopy network increased the number of referrals by 85%.
Subject characteristics
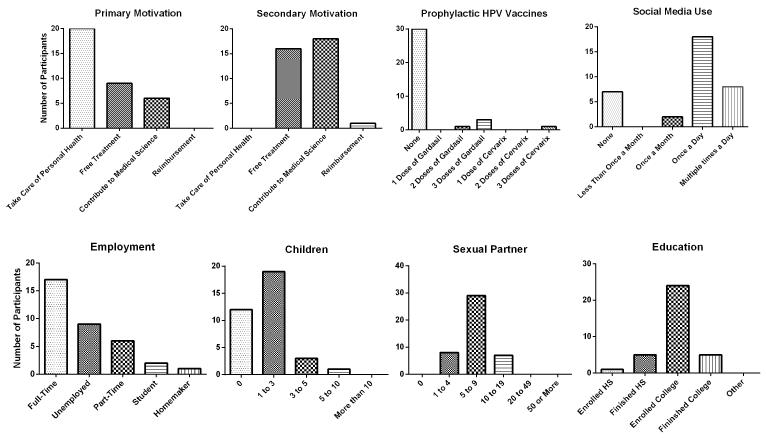
Two participants (one for non-compliance and the other one for developing an unrelated serious illness) were removed from the study prior to their screening visits. Results of the Screening Visit Questionnaire (Fig. 1) showed the primary motivations for participating in the clinical trial to be “taking care of personal health (57%)”, “possible free treatment (26%)”, and “contribution to medical science (17%).” Secondary motivations were identified as “contribution to medical science (51%)”, “possible free treatment (46%)”, and “possible reimbursement (3%).” Overall, the participants appeared to be motivated by taking care of their health issues rather than by financial gains in the form of reimbursement and/or free treatment.
Fig. 1.
A summary of screening visit questionnaire results. Questions about the subjects’ motivations to participate in the study (primary and secondary), administration of HPV prophylactic vaccines, routine use of social media, employment status, number of children, number of life time sexual partners, and the level of education were asked. The participants were mainly motivated to take care of personal healthy, and to contribute to medical science.
Most participants had not received any prophylactic HPV vaccines (86%) as expected as most participants were older than the targeted ages of 13 to 17 when the vaccine became available (2). Eighty percent of the participants were using social media on a regular basis. Occupations varied widely: manager (n = 3), customer service (n = 2), nursing aid (n = 2), retail sales (n = 2), personal/homecare aid (n = 2), cashier (n = 1), school teacher (n = 1), teaching assistant (n = 1), and waitress (n = 1).
Retention
HSILs were confirmed with biopsy in 24 participants, and they proceeded to the vaccination phase of the study. Only 2 participants (who both happened to proceed to the vaccination phase) joined the Facebook private page, and none entered any direct postings. However, 5 (22%) participants had reported to have viewed the site at least once. All but 1 participant, who exited the study due to a move to a distant state, completed the study. Therefore, an unexpected circumstance rather than any identifiable factors contributed to earlytermination. The average number of communications recorded in electronic case reporting forms were 23.5 ± 6.9 (Range 14-37) for the 23 subjects who completed the study, and 10 communications for 1 subject who exited early. Therefore, a traditional means of keeping in contact with study participants such as e-mails, phone calls, and texting appeared to be sufficient for good retention and good overall satisfaction as assessed by the Exit Visit Questionnaire. Eight of 24 (33%) vaccine recipients qualified and received travel stipend for having had to travel equal to or more than 50 miles each way to study visits.
Discussion
For clinical trials investigating the utility of putative HPV therapeutic vaccines, patients with biopsy-confirmed HSILs are a group often selected to be vaccinated. Although HSIL is not an unusual condition, recruiting a large number of such participants is challenging, necessitating opening of multiple study sites. For example, the Phase II/III study of DNA-based HPV therapeutic vaccine, ZYC101a, opened 26 sites to vaccinate 251 participants (9.7 participants per site), and the Phase II study of vaccinia virus-based HPV therapeutic vaccine (RO5217790) opened 66 sites to vaccinate 206 participants [3.1 participants per site, (12)]. Had we relied on in-house referrals only, we would only have been able to enroll a few participants as well. Therefore, we engaged in a wide array of strategies to enhance enrollment.
Direct advertisement was woefully ineffective, resulting in only 1 participant being enrolled. The vast majority of those who showed interest in joining the study as a result of direct advertising did not meet enrollment criteria, likely due to having been told to have HPV without being diagnosed with HSIL. Having access to patients from a wide geographical region through the telecolposcopy network and outside referrals from many different locations resulted in our successful recruitment results.
With the intent of enhancing subject retention, we created a private Facebook page to enhance communication between study staff and participants. However, this strategy was not embraced by the participants as only 2 subjects joined the private group, and neither created any direct postings. However, one of them did contact the Principal investigator through Facebook to ask a question. As the time period in which the participants were enrolled in the clinical trial was relatively short (6 to 7 months), communication in the form of e-mails, texting, and phone calls between the study coordinator and the participants appeared to have been sufficient for the excellent retention rate of this clinical trial.
Some studies have reported using Facebook as a successful recruitment tool; some of them were survey studies for which there were no geographical limitations (13-15). Arcia reported using Facebook advertisement to recruit nulliparous women in the first 20 weeks of pregnancy for an online survey to ask about their childbirth preferences (13). A little more than 1,000 women were consented to participate, and 344 of them met the eligibility criteria for the study. Fenner and colleagues used Facebook to advertise a health survey study for women ages 16 to 25 (14). A total of 551 young women responded to the advertisement, 426 agreed to participate, and 278 eventually completed the survey. Zaid et al. attempted to recruit a more restrictive subject population (i.e., patients with neuroendocrine tumors of the cervix to conduct a cross-sectional epidemiologic and quality of life survey) through Facebook (15). The support group of patients with this tumor was identified on Facebook, and the group members were asked to participate in the survey. Fifty seven women responded and completed the survey. Therefore, Facebook appears to be an effective tool to recruit participants into survey-based studies.
Social networking sites have also been utilized to enroll specific groups of participants into interventional studies (16-21). Devising methods to reach populations such as immigrants with language and cultural barriers for tobacco and alcohol use screening (16) and HIV-negative men who have unprotected anal intercourse with men (19) have been reported. On a similar note, Young et al. reported successfully using social media to recruit peer leaders for HIV prevention and general health interventions (21). Furthermore, this group demonstrated that delivering information about HIV and general health through Facebook groups by peer leaders was successful in increasing home-based HIV testing among at-risk populations (20). Martinez et al. have reported successfully developing a social media recruitment protocol which resulted in being able to meet their goal of recruiting 14 couples into an HIV interventional adaptation study for Latino gay couples in 1 month (18). Enhanced recruitment of young women ages 18 to 35 into nutritional research studies through the use of social media has also been reported (17).
A few studies, including ours, have tried to use Facebook to promote retention. We created a Facebook private page to enhance communication among study participants, investigators, and staff. However, this approach was ineffective with only 2 participants joining the group. In a study aiming to promote literacy and social development through pre-school program for at-risk families in which subjects were expected to participate for several years, Facebook was used to locate and re-establish communication (22). They were able to find 19 subjects who would otherwise been “lost to follow-up” resulting in improved retention. Therefore, Facebook maybe more useful in locating and communicating with subjects rather than in establishing rapport through exchanging postings.
Telemedicine is an innovative means of extending health care for hard to reach populations utilizing the latest technologies. To our knowledge, only one other study has reported enhanced recruitment into clinical trials through a telemedicine network. Switzer et al. utilized telestroke network to enhance recruitment into studies examining the utility of Factor Seven and minocycline for stroke outcomes (23). Nineteen of 28 participants (68%) enrolled in these trials were referred from the telestroke network. However, the need of transporting the participants to a distant location to initiate the drugs was a hurdle. For our study, potential participants were only able to enroll if they had the time and the means to commute to the study site as well. The availability of travel stipend appears to have been helpful as 33% of subjects received it for having had to travel equal to or more than 50 miles each way. Nevertheless, the availability of telecolposcopy network was a valuable asset in increasing the pool of participants by 85%. In short, our experience conducting this clinical trial has shown that a telemedicine network can enhance recruitment by enabling the investigators to reach potential subjects from wide geographical areas, and that traditional means of engaging subjects through e-mails, texting, and phone calls were sufficient for good retention.
Acknowledgement
The authors would like to thank Lannie Byrd and Lauren Farabough for their assistance in setting up sites for this clinical trial in Facebook and the university webpage. We would like to thank all health unit and clinic staff who referred potential subjects to our study especially Ann Lyp..
Funding This work was supported by grants from the National Institutes of Health (R01 CA143130 and UL1TR000039) and from the Health Resources and Services Administration’s Office for the Advancement of Telemedicine (1 H2AIT16616 and 6 H2ARH24765-02-01).
Grant Support: Grants from the National Institutes of Health (R01 CA143130 and UL1TR000039) and from the Health Resources and Services Administration’s Office for the Advancement of Telemedicine (1 H2AIT16616 and 6 H2ARH24765-02-01)
Footnotes
Conflict of Interest Statement Mayumi Nakagawa is an inventor named in a US patent #8,652,482 titled “HPV-E6 Protein T Cell Epitopes and Uses Thereof” issued on 2/18/2014. She is also named in these patent applications: (1) HPV-E6 Protein T Cell Epitopes and Uses Thereof; Non-Provisional US Patent Application, Continuation In Part of 12/286,822 filed on 8/9/2011, (2) Human Papilloma Virus Therapeutic Vaccine;International Application: (PCT/US14/60198) filed on 10/11/2014. Other authors have no conflicts of interest to declare.
References
- 1.GLOBOCAN 2012 CANCER FACT SHEET. Cedex; France: 2012. Cancer IAfRo. [Google Scholar]
- 2.Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013 Feb 6;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tota JE, Chevarie-Davis M, Richardson LA, Devries M, Franco EL. Epidemiology and burden of HPV infection and related diseases: implications for prevention strategies. Prev Med. 2011 Oct;53(Suppl 1):S12–21. doi: 10.1016/j.ypmed.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010 Apr;46(4 Suppl):S20–6. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Bruinsma FJ, Quinn MA. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta-analysis. BJOG. 2011 Aug;118(9):1031–41. doi: 10.1111/j.1471-0528.2011.02944.x. [DOI] [PubMed] [Google Scholar]
- 6.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013 Apr;121(4):829–46. doi: 10.1097/AOG.0b013e3182883a34. [DOI] [PubMed] [Google Scholar]
- 7.Ma B, Xu Y, Hung CF, Wu TC. HPV and Therapeutic Vaccines: Where are We in 2010? Current Cancer Therapy Reviews. [Review] 2010;6:81–103. [Google Scholar]
- 8.Wang X, Coleman HN, Nagarajan U, Spencer HJ, Nakagawa M. Candida skin test reagent as a novel adjuvant for a human papillomavirus peptide-based therapeutic vaccine. Vaccine. 2013 Dec 2;31(49):5806–13. doi: 10.1016/j.vaccine.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa M, Coleman HN, Wang X, Daniels J, Sikes J, Nagarajan UM. IL-12 secretion by Langerhans cells stimulated with Candida skin test reagent is mediated by dectin-1 in some healthy individuals. Cytokine. 2014 Feb;65(2):202–9. doi: 10.1016/j.cyto.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KH, Horn TD, Pharis J, Kincannon J, Jones R, O’Bryan K, et al. Phase 1 clinical trial of intralesional injection of Candida antigen for the treatment of warts. Arch Dermatol. 2010 Dec;146(12):1431–3. doi: 10.1001/archdermatol.2010.350. [DOI] [PubMed] [Google Scholar]
- 11.Hitt WC, Low G, Bird TM, Ott R. Telemedical cervical cancer screening to bridge medicaid service care gap for rural women. Telemed J E Health. 2013 May;19(5):403–8. doi: 10.1089/tmj.2012.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ClinicalTrials.gov. 2014.
- 13.Arcia A. Facebook Advertisements for Inexpensive Participant Recruitment Among Women in Early Pregnancy. Health Educ Behav. 2013 Sep 30;41(3):237–41. doi: 10.1177/1090198113504414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenner Y, Garland SM, Moore EE, Jayasinghe Y, Fletcher A, Tabrizi SN, et al. Web-based recruiting for health research using a social networking site: an exploratory study. J Med Internet Res. 2012;14(1):e20. doi: 10.2196/jmir.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaid T, Burzawa J, Basen-Engquist K, Bodurka DC, Ramondetta LM, Brown J, et al. Use of social media to conduct a cross-sectional epidemiologic and quality of life survey of patients with neuroendocrine carcinoma of the cervix: a feasibility study. Gynecol Oncol. 2014 Jan;132(1):149–53. doi: 10.1016/j.ygyno.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlini BH, Safioti L, Rue TC, Miles L. Using Internet to Recruit Immigrants with Language and Culture Barriers for Tobacco and Alcohol Use Screening: A Study Among Brazilians. J Immigr Minor Health. 2014 Feb 23; doi: 10.1007/s10903-013-9934-1. [DOI] [PubMed] [Google Scholar]
- 17.Leonard A, Hutchesson M, Patterson A, Chalmers K, Collins C. Recruitment and retention of young women into nutrition research studies: practical considerations. Trials. 2014;15:23. doi: 10.1186/1745-6215-15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez O, Wu E, Shultz AZ, Capote J, Lopez Rios J, Sandfort T, et al. Still a hard-to-reach population? Using social media to recruit Latino gay couples for an HIV intervention adaptation study. J Med Internet Res. 2014;16(4):e113. doi: 10.2196/jmir.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vial AC, Starks TJ, Parsons JT. Finding and recruiting the highest risk HIV-negative men who have sex with men. AIDS Educ Prev. 2014 Feb;26(1):56–67. doi: 10.1521/aeap.2014.26.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young SD, Cumberland WG, Lee SJ, Jaganath D, Szekeres G, Coates T. Social networking technologies as an emerging tool for HIV prevention: a cluster randomized trial. Ann Intern Med. 2013 Sep 3;159(5):318–24. doi: 10.7326/0003-4819-159-5-201309030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young SD, Zhao M, Teiu K, Kwok J, Gill H, Gill N. A social-media based HIV prevention intervention using peer leaders. J Consum Health Internet. 2013 Oct 1;17(4):353–61. doi: 10.1080/15398285.2013.833445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mychasiuk R, Benzies K. Facebook: an effective tool for participant retention in longitudinal research. Child Care Health Dev. 2012 Sep;38(5):753–6. doi: 10.1111/j.1365-2214.2011.01326.x. [DOI] [PubMed] [Google Scholar]
- 23.Switzer JA, Hall CE, Close B, Nichols FT, Gross H, Bruno A, et al. A telestroke network enhances recruitment into acute stroke clinical trials. Stroke. 2010 Mar;41(3):566–9. doi: 10.1161/STROKEAHA.109.566844. [DOI] [PubMed] [Google Scholar]