Abstract
The threat of West Nile virus (WNV) epidemics with increasingly severe neuroinvasive infections demands the development and licensing of effective vaccines. To date, vaccine candidates based on inactivated, live-attenuated, or chimeric virus, and viral DNA and WNV protein subunits have been developed. Some have been approved for veterinary use or are under clinical investigation, yet no vaccine has been licensed for human use. Reaching the milestone of a commercialized human vaccine, however, may largely depend on the economics of vaccine production. Analysis suggests that currently only novel low-cost production technologies would allow vaccination to outcompete the cost of surveillance and clinical treatment. Here, we review progress using plants to address the economic challenges of WNV vaccine production. The advantages of plants as hosts for vaccine production in cost, speed and scalability, especially those of viral vector-based transient expression systems, are discussed. The progress in developing WNV subunit vaccines in plants is reviewed within the context of their expression, characterization, downstream processing, and immunogenicity in animal models. The development of vaccines based on enveloped and non-enveloped virus-like particles is also discussed. These advancements suggest that plants may provide a production platform that offers potent, safe and affordable human vaccines against WNV.
Keywords: Nicotiana benthamiana, Plant biotechnology, Plant-made vaccines, Vaccine, West Nile virus (WNV)
1 Introduction
West Nile virus (WNV) is a mosquito-borne flavivirus in the Flaviviridae family closely related to the Japanese encephalitis (JEV), Kunjin (KUN), St Louis encephalitis, Murray Valley encephalitis, dengue (DENV), yellow fever (YFV), and tick borne encephalitis viruses [1]. WNV has a single-stranded positive sense RNA genome of approximately 11 kilobases, which contains a single open reading frame (ORF) flanked by 5′ and 3′ non-coding regions [1]. The translation of the ORF produces a single polypro-tein, which is processed into three structural proteins (capsid [CP], premembrane [prM], and envelope [E]) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [2]. The translation of NS induces the formation of complex three-dimensional networks of membranes in which the replication of viral RNA occurs [3]. This leads to the production of negative sense RNA copies of the genome, each of which serves as a template for the replication of multiple copies of positive sense genomes. Each nascent genome either serves as a template for additional polyprotein translation or binds multiple copies of CP to form a nucleocapsid [3]. The nucleo-capsid then buds into the lumen of the endoplasmic reticulum (ER), where E and prM proteins are anchored to form the immature virions. Cleavage of the N-terminal peptide of prM by cellular furin during the maturation pathway releases matured virions containing membrane (M) proteins from the cell though exocytosis [4]. As a result, the mature WNV is an enveloped virus of approximately 50 nm in diameter with the nucleocapsid surrounded in a host ER-derived membrane that has been modified by the insertion of E and M proteins [4].
For WNV, five distinct lineages have been described [5]. Lineage 1 includes strains that can cause neuroinvasive diseases in animals and humans, and have a worldwide distribution associated with epidemics in North America, Europe and Middle East [6]. Lineage 2 strains can also cause neuroinvasive infections and have recently spread from southern Africa into southern and central Europe [7]. Lineage 3 and 4 were identified in the Czech Republic and Russia, respectively, with each represented by a single isolate [8]. Lineage 5 strains have only been found in India and have not been documented to cause neuroinvasive infections [8]. WNV infection in humans causes a wide range of clinical manifestations, from mild fevers to fatal neuroinvasive diseases. Up to 80% of infected individuals may display no clinical symptoms or have mild symptoms of fever, headache, body ache, fatigue and skin rash [1]. In North America, approximately 1% of people infected develop severe neuroinvasive encephalitis, meningitis or poliomyelitis with acute flaccid paralysis [1]. The fatality rate of WNV neuroinvasive infections is approximately 10%, which increases dramatically with age and in immunocompromised individuals [1].
In addition to humans, WNV also infect mosquitoes, ticks, birds, and other mammals [1]. Culex mosquitoes are primarily responsible for the transmission of WNV from wild birds – its main reservoir to humans and other mammals, which are dead-end hosts [1]. Migrating birds are primarily responsible for the global transmission of WNV [1]. In addition to mosquitos, cases of WNV infection have also been reported as a result of blood transfusion, organ transplantation, breastfeeding and intra-uterine exposure [9].
Historically, WNV was an Old World disease found mostly in the Eastern Europe, Africa, and the Middle East. However, in 1999, WNV entered the American continent and subsequently spread across the United States (US), Canada, Caribbean, and Latin America, with outbreaks occurring on an annual basis [1]. In the US, the frequency and severity of WNV outbreaks have increased significantly in recent years, with a higher incidence of neuroinvasive infections, marking 2012 as one of the deadliest (286 fatalities) on record [1]. Currently, no vaccine or therapeutic agent has been approved for human use. The global threat of WNV epidemics and the lack of treatment warrant the development of vaccines and production platforms that can bring products to market at low cost.
2 WNV vaccine development and current vaccine candidates
Studies have shown that neutralizing humoral response is critical for protective immunity against WNV and is a potential correlate of vaccine-induced protection [10]. To maximize the induction of protective antibodies, several different types of vaccine candidates against WNV are being developed, including candidates based on inactivated, live-attenuated, or chimeric virus, viral DNA, and WNV protein subunits. While some of these vaccines are available for use in animals and have been evaluated in clinical trials (Table 1), a licensed human vaccine remains elusive.
Table 1.
West Nile virus vaccines licensed for veterinary use or in human clinical trials
| Antigen | Development Stage | Seroconversion rate | Sponsor | References |
|---|---|---|---|---|
| Whole inactivated WNV | Licensed for veterinary use | 100% in horses | Fort Dodge Animal Health | [12] |
| Whole inactivated WNV | Licensed for veterinary use | NR | Boehringer Ingelheim | NR |
| Canarypox expressing WNV prM-E | Licensed for veterinary use | 100% in horses | Merial-Sanofi | [11] |
| YFV17D backbone expressing WNV prM-E | Licensed for veterinary use (Recalled in 2010) | 100% in horses | Intervet | [11] |
| Plasmid DNA expressing WNV prM-E | Licensed for veterinary use (Discontinued) | 100% in horses | Fort Dodge Animal Health | [30] |
| DENV-4 backbone expressing WNV prM-E | Phase I | 75–89% | NIAID | [26] |
| YFV17D backbone expressing WNV prM-E | Phase I | 100% | Sanofi | [22] |
| YFV17D backbone expressing WNV prM-E | Phase II | 95.4–97.3% | Sanofi | [23, 24] |
| Soluble WNV E protein | Phase I | 100% | Hawaii Biotech | [37] |
| Plasmid DNA expressing WNV prM-E | Phase I | 96.6–100% | Vical-NIAID | [31, 79] |
NR: Not reported; NIAID: The National Institute of Allergy and Infectious Diseases.
2.1 Inactivated WNV vaccines
Two inactivated whole WNV vaccines have been approved for veterinary use. The first inactivated vaccine (WN-Innovator) is based on whole NY99, a North American highly virulent WNV strain. It requires two doses and an annual booster shot, and can offer protection from fatal neuroinvasive disease in horses and hamsters [11, 12]. Baboons immunized with the same vaccine also showed strong IgG and IgM responses and exhibited low viremia upon challenge [13]. Similarly, other inactivated virus vaccine candidates have shown protection against lethal WNV challenges in geese and mice, respectively [14]. Recently, a hydrogen peroxide-inactivated KUN virus candidate was shown to protect mice against lethal challenge of NY99 strain [15]. One potential issue of using inactivated virus as vaccines is the generation of viral sequence variants during processing of parent virus stocks. To minimize such risk, a cDNA clone of NY99 was synthesized to produce the RNA viral genome. The inactivated WNV derived from the synthetic genome was shown to elicit strong protection in mice following two doses delivery [16]. While successful in eliciting protective immunity, inactivated whole WNV as human vaccines will face safety concerns and the corresponding regulatory hurdles.
2.2 Live-attenuated WNV vaccines
Live-attenuated WNV vaccines based on naturally attenuated strains or infectious clones have been developed to enhance the induction of immune response to NS. Thus, this strategy may evoke cellular immune responses that contribute to clearance of subsequent virus infection. KUN shares all the neutralizing epitopes and 98% of its amino acid sequence with the WNV NY99, but causes far less severe infections [17]. When KUN is delivered into mice, a strong neutralizing antibody response against NY99 was detected in immunized animals. On a lethal dose challenge with NY99, 80–100% immunized mice were protected [17]. A naturally attenuated lineage 2 strain derived from an infectious clone (WN1415) has also been tested as a live vaccine candidate. The attenuation is due to a set of mutations in the genes of NS; this strain can elicit a robust immune response that protects mice from a lethal NY99 challenge [18]. Other live-attenuated vaccine candidates include strains with mutations at glycosylation sites of the E and NS1 proteins or at specific sites of the E protein that are associated with attenuation of JEV [19, 20]. These strains are highly attenuated in causing neuroinvasive diseases, but still can stimulate neutralizing humoral response that provides protection against WNV challenge [19, 20].
2.3 Live-attenuated WNV chimeric vaccines
The safety profile of other existing attenuated flavivirus vaccines can be exploited to develop chimeric vaccines that carry WNV antigens. For example, the attenuated chimeric vaccine commercialized for veterinary use (ChimerVax-VN01), was developed based on the parent YFV 17D vaccine by replacing the prM and E genes of YFV with those of NY99 [21]. For human application, three mutations in the E protein responsible the attenuation of JEV SA14-14-2 vaccine were introduced to further attenuate the chimeric virus (ChimerVax-VN02) [21]. The safety of this live chimeric vaccine was demonstrated in a Phase I clinical trial with healthy adults of 18–40 years old. Strong and durable (12 months) neutralizing antibodies were detected in all singly inoculated subjects (103 or 105 PFU per dose), and T-cell responses specific to the WNV E protein was also identified in 83–87% of vaccinated individuals [22]. The safety and immunogenicity of this chimeric vaccine was further demonstrated in two Phase II clinical trials in three adult age groups of 18–40, 41–64 and > 65 years old [23, 24]. Another example of chimeric vaccine is constructed by replacing DENV-4 prM and E genes with their equivalent genes of WNV. The WNV/DENV-4 chimeric viruses are highly attenuated, but are highly immunogenic in mice, geese and non-human primates [25]. Two Phase I clinical trials on healthy adults (18–50 years old) have been recently completed and the results indicated that the candidate was well tolerated and immunogenic. Specifically, seroconversion to WNV NY99 was observed in 74% (103 PFU), 75% (104 PFU), and 55% (105 PFU) of subjects after a single dose, and a second 105 PFU dose given six months after the first dose increased the seroconversion rate to 89% [26]. Since this vaccine is attenuated by a dual-strategy mechanism, i.e. chimerization of WNV with a non-neuroinvasive flavivirus, DENV-4, and a 30-nucleotide deletion in the 3′ UTR, this makes reversion to a wild-type WNV or DENV within a vaccinated host very unlikely [25]. However, because this chimeric virus can be transmitted by a known vector mosquito (Aedes albopictus) for both WNV and DENV [27], potential safety issues have to be addressed for its further development.
2.4 Vectored virus and DNA WNV vaccines
Since vectored viruses that are commonly used to express heterologous antigens replicate poorly in mammalian cells, vaccines based on these vectors often have superior safety profiles than live-attenuated vaccines. Importantly, these vectors can induce strong humoral and cell-mediated immune responses due to the robust expression of antigens and the process of abortive replication, which mimics a natural viral infection. For example, a canarypox viral vector that expresses the WNV prM and E proteins has been shown to elicit protective immunity in several animal species and has been approved for veterinary use [11]. A WNV E protein-expressing vesicular stomatitis virus vector also induced cell-mediated responses and protected mice from a lethal challenge of WNV [28]. Other examples include WNV-E expressing lentiviral vectors, which, in a single dose, fully protected mice from a lethal WNV challenge [29].
DNA-based WNV Innovator vaccine encodes genes for the coexpression of the WNV prM and E proteins (prM-E), which facilitates the formation of virus-like particles (VLPs) in host cells, and induce protective immunity in horses, mice and several bird species [30]. A similar DNA vaccine was tested in Phase I clinical trials, demonstrating its ability to induce neutralizing antibodies and CD4+ and/or CD8+ T-cell responses specific to WNV M or E proteins [31]. Other DNA vaccine candidates include constructs that coexpress the domain III (DIII) of the WNV E protein and interleukin-15 (IL-15) to enhance humoral immunity, and that expresses a fusion protein of prM-E with lysosome-associated membrane protein to improve MHC-II presentation and neutralizing antibody response [32]. An interesting variation of this strategy is to express a CP-deleted WNV or KUN subgenome with the expression of CP supplied in trans. This design allows the production of a “single round infectious particles.” The single-cycle pseudoinfectious virions replicate once and express WNV antigens to generate VLPs in host cells, which mimic live viral infection and, therefore, greatly enhance their immunogenicity and protection against WNV challenges in small animal and non-human primate models [33].
2.5 Subunit WNV vaccines
The search for safer vaccines has driven the development of vaccines based on WNV protein subunits. The WNV E protein has been shown to be essential for virus attachment and subsequent entry into host cells, and contains the majority of protective epitopes for neutralizing antibodies [10]. Crystal structure analysis revealed the three domain architecture of the E protein: a central β-barrel domain I (DI), an elongated domain II (DII) containing the fusion loop conserved in all flaviviruses, and a C-terminal DIII with an immunoglobulin-like fold [4]. It was found that antibody response to different domains of the E protein has different properties in neutralization, cross-reactivity, and maturation sensitivity. For example, weakly or non-neutralizing antibodies induced by WNV in humans are typically against the epitopes on the fusion loop of DII [34]. These antibodies are also cross-reactive amongst flaviviruses and can neutralize the partially but not fully matured WNV [35]. In contrast, epitopes for the most potently neutralizing antibodies are localized in DIII [36]. These neutralizing antibodies are WNV and often genotype specific, and can equivalently neutralize immature and fully matured WNV [35].
E protein has been examined as the prime candidate of subunit vaccines against WNV. For example, an insect cell-produced E protein offered protection against WNV challenge in mice, hamsters, chickens, geese and rhesus monkeys; and it was well tolerated and induced a neutralizing antibody response in all immunized human subjects [37]. A recent study showed that E protein can also elicit durable and Th1/Th2 balanced humoral and cellular immune responses against both lineage 1 and 2 WNV when a saponin-based adjuvant is used [38]. E DIII has also been explored as a target for developing WMV subunit vaccines. For example, E. coli and insect cell-produced DIII conferred protection against a lethal WNV challenge in mice [39].
Coexpression of prM and E often leads to the assembly of VLPs that share many immunogenic properties with the native WNV [40]. Insect cell-produced prM-E VLPs were shown to protect mice from lethal WNV challenge and induced sterilizing immunity [41]. A single inoculation of mammalian cell-derived lineage 1 WNV prM-E VLPs also protected mice against a lethal challenge with both lineage 1 and 2 WNV, demonstrating that VLP-based vaccines are more immunogenic than those based-on individual subunit antigens [42].
3 WNV vaccine candidates produced in plants
3.1 Plants as a production system for WNV vaccines
Despite the development of aforementioned vaccine candidates, the eventual approval and commercialization of human vaccines against WNV may largely depend on the economics of vaccine production and implementation of a vaccination program. Studies have shown that a universal WNV vaccination program produced under current vaccine platforms would not be cost effective compared with that of post-exposure treatment [43]. Another analysis indicated that vaccines based on technologies with lower production costs are needed because only they could outcompete the costs associated with surveillance and treatment [44]. Because plants can produce large quantities of recombinant proteins at low cost, plant-based systems may provide solutions to overcome the economic challenge of WNV vaccine production [45]. Plant biomass generation does not require prohibitive capital investment for building fermentation facilities and there is no need to construct duplicate facilities for scale-up operation [46]. As a result, upstream processing in plant-based systems can be operated and scaled-up in a flexible and cost-efficient manner that cannot be easily matched by fermentation-based technologies currently used for vaccine production [47, 48].
Systems based on transgenic plants were first explored to produce subunit vaccines for flavivirus. For example, the JEV E protein accumulated to a low level of 1.1–1.9 μg/mg of total soluble protein in transgenic rice leaves; and E-containing leaf extracts induced an E-specific neutralizing antibody response in mice with similar titers as that induced by an E. coli-produced E antigen [49]. The issue of low vaccine accumulation in early transgenic systems has been overcome by using improved promoters [45, 50]. The development of transient expression systems based on plant viruses provides another alternative platform for vaccine production. These transient expression systems drive high-level accumulation of pharmaceutical protein within one to two weeks of vector delivery [51–58]. The speed and high-yield benefits of the transient system offer the plant-expression system the versatility to quickly produce subunit vaccines against viruses such as WNV that have multiple lineages with unpredictable outbreaks in various parts of the world.
3.2 Plant-produced subunit vaccines against WNV
As DIII of WNV E contains the majority of the neutralizing epitopes that induce strong host antibody responses and/or protective immunity against WNV, we explored the possibility of producing DIII in plants [59, 60]. The coding sequence of DIII was cloned into the expression cassette in a deconstructed viral expression vector and delivered into lettuce (with geminiviral vector) or Nicotiana benthamiana (with TMV-based MagnICON vector) plants through agroinfiltration [61, 62] for accumulation in ER, cytosol and chloroplast. Western blot analysis detected DIII antigen in plant samples that were infiltrated with DIII construct with the expected molecular weight. It appeared that DIII was stable during expression and isolation, as only the full-length DIII was observed [59]. Further ELISA analysis confirmed the expression of DIII in plants and indicated that DIII was produced quickly in plants and reached the highest accumulation level within four days post infiltration (DPI) with an average level of 100 μg/g leaf fresh weight (LFW) [59]. This level of expression is higher than DENV DIII expression with a similar vector system and presents the highest level of accumulation ever reported in plants at that time [59, 63]. Detailed analysis indicated that the expression level of DIII in plant tissue is affected by the particular subcellular compartment where DIII accumulates. For example, if DIII was targeted to the cytosol or chloroplast, the maximal levels of accumulation are below 1.16 μg/g LFW, approximately 86 times less than that in ER [59]. The overall DIII expression levels in plants is lower than that of other vaccine candidates we have produced using the MagnICON vectors [52]. Since leaf necrosis was observed in DIII-construct infiltrated plants, DIII may be toxic to plant cells that may shorten the window of its accumulation, contributing to the lower expression level. Because DIII was produced under standard conditions, its expression could be further enhanced by genetic manipulations of the DIII gene and the plant host [45].
Plant-derived DIII was further examined for its structural and immunological properties. We first tested its ability in binding E16, a MAb that neutralizes WNV potently and protects mice against a lethal infection of WNV in both prophylactic and post-exposure models [64–66]. ELISA showed that plant DIII specially bound to E16. The epitope for E16 consists of four discontinuous regions of DIII, thus, the results demonstrate that plant-produced DIII was folded into a tertiary structure similar to that of the native viral DIII. The immunogenicity of plant DIII was evaluated in mice with four doses of 5 μg or 25 μg DIII injected subcutaneously with alum as adjuvant. WNV E DIII-specific IgG was detected after the first dose in all mice immunized with 25 μg of plant-produced DIII, while the 5 μg dosage induced a response after the third DIII injection (Fig. 1). Results also demonstrated that plant-derived DIII elicited at least equivalent anti-DIII IgG titers as those of E. coli-produced DIII (P > 0.5) [59]. This result is in contrast to the low titers induced by a plant-produced DENV DIII even when the TiterMax Gold was used as adjuvant [63]. Further analysis of IgG subtypes indicated that > 99% of DIII-specific IgG was the IgG1 subtype, suggesting an overwhelmingly Th2-type response [59]. A previous study reported that E. coli-produced DIII with CpG adjuvant induced a Th1-biased response [39]. This inconsistency is not surprising, as studies have shown that flavivirus antigens tend to stimulate a Th2-type response when alum is used as the adjuvant, while CpG is likely to skew the response toward the Th1 type [67]. Flow cytometry analysis of antisera from plant-DIII immunized mice showed that they contain antibodies that can recognize DIII in its native conformation (Fig. 2) and possibly bind to the same protective epitope as E16 [59].
Figure 1.
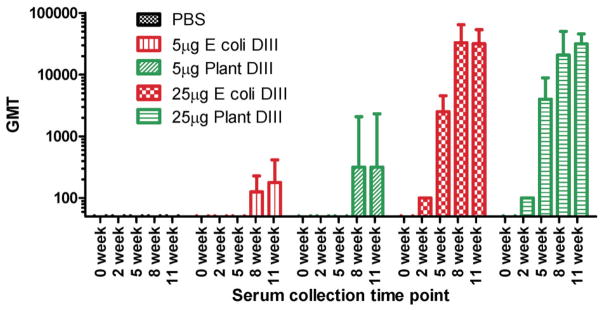
DIII-specific antibody responses in mice upon subcutaneous delivery of plant-derived DIII. BALB/C mice (n = 6 per group) were injected on weeks 0, 3, 6 and 9 with the indicated dosage of antigen. Blood samples were collected on the indicated weeks and serum IgG was measured by ELISA. The y axis shows the geometric means titers (GMT) and the error bars show the 95% level of confidence of the mean [59].
Figure 2.
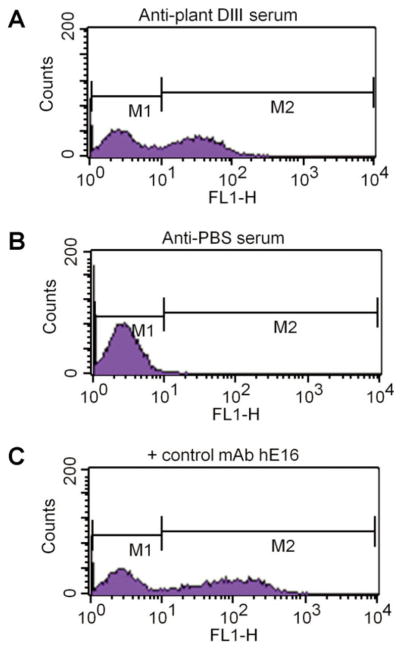
Recognition of antibodies in sera to DIII displayed on yeast cell surface. DIII-displaying yeast cells were incubated with pooled sera collected on week 11 from mice injected with either 25 μg of plant-produced DIII (A) or PBS (B) with hE16 as the positive control MAb (C). Yeast cells were subsequently stained with an Alexa Fluor 488-conjugated goat anti-mouse (A and B) or goat anti-human (C) secondary antibody and processed by flow cytometry [59].
To overcome the relative low level expression of DIII in plants, we examined the accumulation of the WNV E protein. It was shown that including DI and DII of the E protein greatly reduced leaf necrosis and, hence, increased the subunit protein expression level from 100 μg/g LFW to > 600 μg/g LFW (Chen, manuscript in preparation). To avoid the potential adverse effect derived from the plant-specific glycosylation pattern on E protein, we also used a glycol-engineered plant line and yielded E protein with full mammalian glycoforms [66]. Furthermore, our data also showed that plant-derived E protein can be easily purified to homogeneity with a similar procedure as for DIII from plants, and immunization with alum as adjuvant in mice induced robust neutralizing antibody responses specific for both WNV E and DIII, skewing towards Th2 type in both IgG subtypes and cytokine profiles (Chen, manuscript in preparation). These results are consistent with the observation that JEV E protein can be successfully expressed alone, without prM in plants, in contrast to animal cells where prM is necessary for the proper expressing and folding of the JEV E protein [68].
3.3 VLP-based WNV vaccines produced in plants
Since VLPs mimic the architecture of infectious viruses but lack the viral genome, they often elicit more potent cellular and humoral immune responses without adjuvants than other recombinant antigens and present a safer vaccine alternative than attenuated or inactivated viruses [69]. As WNV is a virus surrounded with a lipid membrane, enveloped VLPs are produced when prM and E protein are co-expressed in insect and mammalian cells, which have been shown to induce more potent immune response than E protein alone and are being investigated in clinical trials [40–42] The feasibility of using plants to produce enveloped VLPs as vaccines against WNV was explored. When the NY99 prM-E construct was coexpressed in plants, prM and E protein were both detected at the expected sizes by western blot analysis (Fig. 3). In addition, a positive band corresponding to the size of the processed mature membrane (M) protein was also detected by anti-WNV M-E antibodies with the relative band intensity of prM and M (Fig. 3) comparable to that in the purified WNV virion [70]. This suggests that WNV prM to M processing was similar between plant-derived recombinant antigen and virion protein. Results of sucrose gradient centrifugation confirmed the assembly of VLPs containing both E and prM/M proteins of WNV [69]. The immunogenicity of plant-derived WNV enveloped VLPs are being evaluated in mice.
Figure 3.
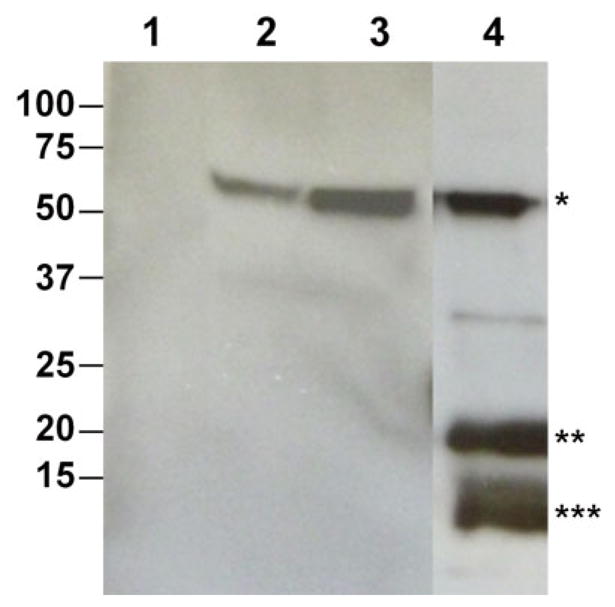
Production of enveloped VLPs based on WNV prM-E protein in N. benthamiana plants. Leaf tissue was infiltrated with the WNV prM-E construct. PrM-E VLPs were extracted from leaves and isolated by PEG precipitation. Samples were separated on 4–12% SDS-PAGE gels and blotted onto PVDF membranes for western blot analysis with an anti-WNV E antibody (Lanes 1–3) or an anti-WNV M-E antibody (Lane 4). Lane1: Sample from buffer-infiltrated leaves, Lane 2: Purified WNV E protein as positive control, Lanes 3–4: Samples from leaves infiltrated with the prM-E construct. *: E protein; **: prM protein; ***: Processed M protein [69].
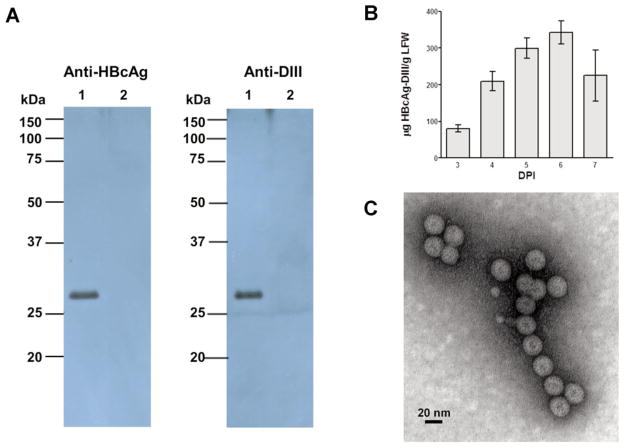
For many viruses, VLPs assembled from CPs have also been shown to trigger strong protective immune responses at very low doses even in the absence of adjuvants [71]. Like native non-enveloped viruses, their quasi-crystalline surface with arrays of repetitive epitopes is the prime target for B-cell recognition and can efficiently crosslink epitope-specific immunoglobulins (Ig) on B cells inducing strong B-cell responses [72]. The particulate nature and high-density presentation of CP on their surface make VLPs an attractive carrier for displaying foreign epitopes. The immunogenicity of displayed heterologous antigen is enhanced through multiple potential mechanisms as it is anchored in the VLP and presented in a high-density repetitive array, thereby, enhancing immune cell uptake and stimulation. To develop a CP-based VLP vaccine against WNV, we first fused the coding sequence of DIII of WNV E to the 3′ end of hepatitis B core antigen (HBcAg) gene, aiming to create an HBcAg-DIII chimeric VLP that displays the DIII epitopes on its surface. Expression of this construct in N. benthamiana rendered robust production of the HBcAg-DIII fusion antigen at the expected molecular size (~27 kDa) in plant leaves, as verified by western blot analysis with both anti-HBcAg and anti-WNV DIII antibodies (Fig. 4A). Further analysis revealed that high-level (~ 350 μg/g LFW) accumulation of this fusion protein was achieved within 6 DPI through transient expression (Fig. 4B). Analyses with sucrose gradient centrifugation and electron microscopy confirmed the assembly of the chimeric VLPs (Fig. 4C). Competitive ELISA indicated that HBcAg-DIII effectively competed with soluble DIII in binding to an anti-DIII MAb E16, confirming that DIII was displayed on the surface of the chimeric VLPs [58]. Furthermore, immunization of mice with a single dose (25 μg) of these chimeric VLPs induced strong DIII-specific B and T-cell responses that are superior to that of the non-fused DIII antigen. We also explored the expression of HBcAg-DIII with MagnICON vectors. This led to even higher levels accumulation (> 1,000 μg/g LFW) of HBcAg-DIII VLPs that have similar structural and immunological properties as those obtained by geminiviral vectors.
Figure 4.
Plant-derived chimeric HBcAg-WNV DIII VLPs. (A) Western blot analysis. Chimeric VLPs were extracted from HBcAg-WNV DIII construct-infiltrated N. benthamiana leaves, purified and separated on 10% SDS-PAGE gels. Proteins were transferred onto PVDF membranes which were subsequently incubated with an anti-HBcAg antibody or an anti-WNV DIII antibody. Lane1: Proteins extracted from HBcAg-DIII construct-infiltrated leaves, Lane 2: Equivalent proteins from un-infiltrated leaves. (B) Temporal expression pattern of HBcAg-WNV DIII. Leaf proteins were extracted from infiltrated leaves 3 to 7 DPI and analyzed with a sandwich ELISA that detects HBcAg. Mean ± standard error (SEM) of samples from three independent infiltration experiments are presented. (C) Electron microscopy of chimeric HBcAg-WNV DIII VLPs. HBcAg-DIII chimeric VLPs were purified from infiltrated leaves, stained with 0.2% aqueous uranyl acetate, and analyzed by transmission electron microscopy [58, 69].
3.4 Downstream processing of plant-derived vaccines against WNV
The lack of scalable downstream processing procedures, the uncertainty of regulatory compliance for production processes, and the lack of demonstration to date of plant-derived vaccines that meet the required safety standards of regulatory agencies are some of the major challenges to the commercialization of plant-made vaccines [45, 46, 73]. To overcome these challenges, we have developed a novel processing scheme for recovering VLPs from plant tissue and through it, successfully demonstrated the feasibility of operating the upstream and downstream production processes under the US Food and Drug Administration (FDA) current Good Manufacture Practice (cGMP) regulations, producing high quality VLPs that meet all preset release specifications in identity, purity, potency and safety [74]. Such a first precedent of producing vaccine candidates under FDA regulations in an academic setting is an important step towards the commercialization of plant-derived vaccines. This scalable downstream process also allowed us to extract and purify HBcAg-DIII VLPs to homogeneity [58]. It not only effectively separated the chimeric VLPs from other leaf components, but also preserved the structural integrity of the fusion particle to yield assembled VLPs with consistent size (Fig. 4C). Similarly, a robust downstream processing procedure for recovering and purifying prM-E enveloped VLPs from N. benthamiana has also been established. It consists of leaf homogenization, clarification of extract by centrifugation, and purification by a series of chromatographic steps including ion-exchange and affinity chromatography similar to that for processing VLP vaccines against influenza [75]. Collectively, these results demonstrate the robustness of the plant transient expression system and the availability of scalable downstream schemes, which will facilitate the broad application of plants as hosts for the development and production of vaccines against WNV.
4 Conclusions
The expanding epidemics of WNV around the world demand the development of effective vaccines and production platforms that can quickly transfer the vaccine candidates into the clinical setting at low cost. The results reviewed here demonstrate that plants provide a viable alternative system for the production of subunit vaccines against WNV that can potentially meet these needs. Specifically, the expression of major human WNV vaccine candidates that are being tested in clinical trials based on the E protein, its DIII fragment, or prM-E VLPs have all been successfully demonstrated in plants. Chimeric VLPs that display DIII on its surface have also been produced. Transient expression based on deconstructed viral vectors has allowed the high level accumulation of these vaccine candidates. Furthermore, a simple, scalable and cGMP compliant downstream processing scheme has also been developed to effectively recover and purify these vaccine candidates from plants. The potency for some of these vaccine candidates has been demonstrated in mice, which is at least equivalent to subunit-based candidates produced by other production systems. With the demonstrated unmatchable flexibility and cost-efficiency in the upstream processing of plant-based systems [48], these results indicate that plants can produce WNV vaccines with comparable potency as other production platforms but with much lower cost. Remaining challenges for WNV vaccine development include the need to address safety and efficacy concerns for the “at risk” populations of elderly and immune-comprised individuals and the potential risk of ADE. Plants may play an important role in overcoming these challenges. For example, the recent development of glycoengineered plants would facilitate the understanding of carbohydrate moiety’s function in inducing ADE by antibodies [65, 76], which would guide future vaccine design. A lingering skepticism of plant-based manufacturing systems has been the absence of approved human products in the US [77]. This barrier has finally been overcome by the FDA approval of a plant-produced glucocerebrosidase for treating Gaucher disease [69]. In a remarkable unprecedented and exciting development, an experimental cocktail of three plant-made MAbs was recently used to treat several Ebola patients, showing promising results [78]. We speculate that plant-based systems will offer a more favorable cost/benefit ratio for WNV vaccination programs and encourage the eventual licensure and commercial production of human vaccines against WNV.
Acknowledgments
We appreciate the contributions by the members of the Chen laboratory for the results described. We also thank J. Caspermeyer for the critical reading of the manuscript. Research performed by our laboratory in this review was supported in part by NIH-NIAID grants U01 AI075549 and R33AI101329 to Q. Chen.
Abbreviations
- ADE
antibody-dependent enhancement
- BSL
biosafety level
- cGMP
Good Manufacture Practice
- CP
capsid protein
- CPMV
cowpea mosaic virus
- DENV
Dengue virus
- DIII
domain III of the envelope protein
- DPI
days post infiltration
- E
envelope protein
- ELISA
enzyme-linked immunosorbent assay
- ER
endoplasmic reticulum
- FDA
US Food and Drug Administration
- HBcAg
hepatitis B core antigen
- JEV
Japanese encephalitis
- KUN
Kunjin virus
- LFW
leaf fresh weight
- M
membrane
- MAb
monoclonal antibody
- NVCP
Norwalk virus capsid protein
- ORF
open reading frame
- prM
premembrane
- TMV
tobacco mosaic virus
- VLP
virus-like particle
- WNV
West Nile virus
- YFV
Yellow fever virus
Biography
 Dr. Qiang Chen is a Professor in the Biodesign Institute and School of Life Sciences at Arizona State University. Dr. Chen’s lab focuses on developing novel protein biologics and optimizing their expression and assembly in plants, e.g. plant-produced MAbs against Ebola virus formulated in Zmapp as therapeutics. Dr. Chen spent more than ten years in the biotechnology and pharmaceutical industry directing research in vaccine and therapeutic protein development in both plant and mammalian cell culture systems. Prior to joining ASU, Dr. Chen was the Associate Director of division of protein chemistry at Cardinal Health and a Sr. Scientist at Monsanto.
Dr. Qiang Chen is a Professor in the Biodesign Institute and School of Life Sciences at Arizona State University. Dr. Chen’s lab focuses on developing novel protein biologics and optimizing their expression and assembly in plants, e.g. plant-produced MAbs against Ebola virus formulated in Zmapp as therapeutics. Dr. Chen spent more than ten years in the biotechnology and pharmaceutical industry directing research in vaccine and therapeutic protein development in both plant and mammalian cell culture systems. Prior to joining ASU, Dr. Chen was the Associate Director of division of protein chemistry at Cardinal Health and a Sr. Scientist at Monsanto.
Footnotes
The author declares no commercial or financial conflict of interest.
References
- 1.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. Jama. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- 3.Chambers TJ, Hahn CS, Galler R, Rice CM. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 4.Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 5.May FJ, Davis CT, Tesh RB, Barrett AD. Phylogeography of West Nile virus: from the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J Virol. 2011;85:2964–2974. doi: 10.1128/JVI.01963-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanciotti RS, Ebel GD, Deubel V, Kerst AJ, et al. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298:96–105. doi: 10.1006/viro.2002.1449. [DOI] [PubMed] [Google Scholar]
- 7.Rudolf I, Bakonyi T, Sebesta O, Mendel J, et al. West Nile virus lineage 2 isolated from Culex modestus mosquitoes in the Czech Republic, 2013: expansion of the European WNV endemic area to the North? Euro Surveill. 2014;19:2–5. doi: 10.2807/1560-7917.es2014.19.31.20867. [DOI] [PubMed] [Google Scholar]
- 8.Bakonyi T, Hubalek Z, Rudolf I, Nowotny N. Novel flavivirus or new lineage of West Nile virus, central Europe. Emerg Infect Dis. 2005;11:225–231. doi: 10.3201/eid1102.041028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinckley AF, O’Leary DR, Hayes EB. Transmission of West Nile virus through human breast milk seems to be rare. Pediatrics. 2007;119:e666–671. doi: 10.1542/peds.2006-2107. [DOI] [PubMed] [Google Scholar]
- 10.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 11.Seino KK, Long MT, Gibbs EP, Bowen RA, et al. Comparative efficacies of three commercially available vaccines against West Nile Virus (WNV) in a short-duration challenge trial involving an equine WNV encephalitis model. Clin Vaccine Immunol. 2007;14:1465–1471. doi: 10.1128/CVI.00249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng T, Hathaway D, Jennings N, Champ D, et al. Equine vaccine for West Nile virus. Dev Biol (Basel) 2003;114:221–227. [PubMed] [Google Scholar]
- 13.Wolf RF, Papin JF, Hines-Boykin R, Chavez-Suarez M, et al. Baboon model for West Nile virus infection and vaccine evaluation. Virology. 2006;355:44–51. doi: 10.1016/j.virol.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Samina I, Khinich Y, Simanov M, Malkinson M. An inactivated West Nile virus vaccine for domestic geese-efficacy study and a summary of 4 years of field application. Vaccine. 2005;23:4955–4958. doi: 10.1016/j.vaccine.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 15.Pinto AK. A Hydrogen Peroxide-Inactivated Virus Vaccine Elicits Humoral and Cellular Immunity and Protects against Lethal West Nile Virus Infection in Aged Mice. J Virol. 2013;87:1926. doi: 10.1128/JVI.02903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orlinger KK, Holzer GW, Schwaiger J, Mayrhofer J, et al. An inactivated West Nile Virus vaccine derived from a chemically synthesized cDNA system. Vaccine. 2010;28:3318–3324. doi: 10.1016/j.vaccine.2010.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall RA, Nisbet DJ, Pham KB, Pyke AT, et al. DNA vaccine coding for the full-length infectious Kunjin virus RNA protects mice against the New York strain of West Nile virus. Proc Natl Acad Sci USA. 2003;100:10460–10464. doi: 10.1073/pnas.1834270100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamshchikov G, Borisevich V, Seregin A, Chaporgina E, et al. An attenuated West Nile prototype virus is highly immunogenic and protects against the deadly NY99 strain: a candidate for live WN vaccine development. Virology. 2004;330:304–312. doi: 10.1016/j.virol.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Yu L, Robert Putnak J, Pletnev AG, Markoff L. Attenuated West Nile viruses bearing 3′SL and envelope gene substitution mutations. Vaccine. 2008;26:5981–5988. doi: 10.1016/j.vaccine.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 20.Whiteman MC, Li L, Wicker JA, Kinney RM, et al. Development and characterization of non-glycosylated E and NS1 mutant viruses as a potential candidate vaccine for West Nile virus. Vaccine. 2010;28:1075–1083. doi: 10.1016/j.vaccine.2009.10.112. [DOI] [PubMed] [Google Scholar]
- 21.Dayan GH, Pugachev K, Bevilacqua J, Lang J, Monath TP. Preclinical and Clinical Development of a YFV 17 D-Based Chimeric Vaccine against West Nile Virus. Viruses-Basel. 2013;5:3048–3070. doi: 10.3390/v5123048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monath TP, Liu J, Kanesa-Thasan N, Myers GA, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci USA. 2006 doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biedenbender R, Bevilacqua J, Gregg AM, Watson M, Dayan G. Phase II, randomized, double-blind, placebo-controlled, multi-center study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J Infect Dis. 2011;203:75–84. doi: 10.1093/infdis/jiq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dayan GH, Bevilacqua J, Coleman D, Buldo A, Risi G. Phase II, dose ranging study of the safety and immunogenicity of single dose West Nile vaccine in healthy adults >/= 50 years of age. Vaccine. 2012;30:6656–6664. doi: 10.1016/j.vaccine.2012.08.063. [DOI] [PubMed] [Google Scholar]
- 25.Pletnev AG, Swayne DE, Speicher J, Rumyantsev AA, Murphy BR. Chimeric West Nile/dengue virus vaccine candidate: pre-clinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine. 2006;24:6392–6404. doi: 10.1016/j.vaccine.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Durbin AP, Wright PF, Cox A, Kagucia W, et al. The live attenuated chimeric vaccine rWN/DEN4Delta30 is well-tolerated and immunogenic in healthy flavivirus-naive adult volunteers. Vaccine. 2013;31:5772–5777. doi: 10.1016/j.vaccine.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanley KA, Goddard LB, Gilmore LE, Scott TW, et al. Infectivity of West Nile/dengue chimeric viruses for West Nile and dengue mosquito vectors. Vector Borne Zoonotic Dis. 2005;5:1–10. doi: 10.1089/vbz.2005.5.1. [DOI] [PubMed] [Google Scholar]
- 28.Iyer AV, Pahar B, Boudreaux MJ, Wakamatsu N, et al. Recombinant vesicular stomatitis virus-based west Nile vaccine elicits strong humoral and cellular immune responses and protects mice against lethal challenge with the virulent west Nile virus strain LSU-AR01. Vaccine. 2009;27:893–903. doi: 10.1016/j.vaccine.2008.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coutant F, Frenkiel MP, Despres P, Charneau P. Protective antiviral immunity conferred by a nonintegrative lentiviral vector-based vaccine. PLoS One. 2008;3:e3973. doi: 10.1371/journal.pone.0003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis BS, Chang G-JJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML. West Nile Virus Recombinant DNA Vaccine Protects Mouse and Horse from Virus Challenge and Expresses In Vitro a Noninfectious Recombinant Antigen That Can Be Used in Enzyme-Linked Immunosorbent Assays. J Virol. 2001;75:4040–4047. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledgerwood JE, Pierson TC, Hubka SA, Desai N, et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203:1396–1404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramanathan MP, Kutzler MA, Kuo YC, Yan J, et al. Coimmunization with an optimized IL15 plasmid adjuvant enhances humoral immunity via stimulating B cells induced by genetically engineered DNA vaccines expressing consensus JEV and WNV E DIII. Vaccine. 2009;27:4370–4380. doi: 10.1016/j.vaccine.2009.01.137. [DOI] [PubMed] [Google Scholar]
- 33.Chang DC, Liu WJ, Anraku I, Clark DC, et al. Single-round infectious particles enhance immunogenicity of a DNA vaccine against West Nile virus. Nature biotechnology. 2008;26:571–577. doi: 10.1038/nbt1400. [DOI] [PubMed] [Google Scholar]
- 34.Vogt MR, Moesker B, Goudsmit J, Jongeneelen M, et al. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J Virol. 2009;83:6494–6507. doi: 10.1128/JVI.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson S, Jost CA, Xu Q, Ess J, et al. Maturation of West Nile Virus Modulates Sensitivity to Antibody-Mediated Neutralization. PLoS Pathog. 2008;4:e1000060. doi: 10.1371/journal.ppat.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nybakken G, Oliphant T, Johnson S, Burke S, et al. Structural basis for neutralization of a therapeutic antibody against West Nile virus. Nature. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coller BA, Pai V, Weeks-Levy C, Ogata S. Recombinant subunit West Nile virus vaccine for protection of human subjects. US20120141520. United States patent application No. 2012:A1.
- 38.Magnusson SE. Matrix-M adjuvanted envelope protein vaccine protects against lethal lineage 1 and 2 West Nile virus infection in mice. Vaccine. 2014;32:800. doi: 10.1016/j.vaccine.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 39.Chu JHJ, Chiang CCS, Ng ML. Immunization of Flavivirus West Nile Recombinant Envelope Domain III Protein Induced Specific Immune Response and Protection against West Nile Virus Infection. J Immunol. 2007;178:2699–2705. doi: 10.4049/jimmunol.178.5.2699. [DOI] [PubMed] [Google Scholar]
- 40.Ohtaki N, Takahashi H, Kaneko K, Gomi Y, et al. Immunogenicity and efficacy of two types of West Nile virus-like particles different in size and maturation as a second-generation vaccine candidate. Vaccine. 2010;28:6588–6596. doi: 10.1016/j.vaccine.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 41.Qiao M, Ashok M, Bernard KA, Palacios G, et al. Induction of sterilizing immunity against West Nile Virus (WNV), by immunization with WNV-like particles produced in insect cells. J Infect Dis. 2004;190:2104–2108. doi: 10.1086/425933. [DOI] [PubMed] [Google Scholar]
- 42.Merino-Ramos T, Blázquez AB, Escribano-Romero E, Cañas-Arranz R, et al. Protection of a Single Dose West Nile Virus Recombinant Subviral Particle Vaccine against Lineage 1 or 2 Strains and Analysis of the Cross-Reactivity with Usutu Virus. PLoS ONE. 2014;9:e108056. doi: 10.1371/journal.pone.0108056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zohrabian A, Hayes EB, Petersen LR. Cost-effectiveness of West Nile virus vaccination. Emerg Infect Dis. 2006;12:375–380. doi: 10.3201/eid1203.050782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martina BE, Koraka P, Osterhaus AD. West Nile Virus: is a vaccine needed? Curr Opin Investig Drugs. 2010;11:139–146. [PubMed] [Google Scholar]
- 45.Chen Q. Expression and manufacture of pharmaceutical proteins in genetically engineered horticultural plants. In: Mou B, Scorza R, editors. Transgenic Horticultural Crops: Challenges and Opportunities – Essays by Experts. Taylor & Francis; Boca Raton: 2011. pp. 83–124. [Google Scholar]
- 46.Chen Q. Expression and Purification of Pharmaceutical Proteins in Plants. Biological Engineering. 2008;1:291–321. [Google Scholar]
- 47.Chen Q, Zhang C, Santi L. Plant-Made Biologics. Biomed Res Int. 2014;2014:3. doi: 10.1155/2014/418064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuse D, Tu T, McDonald K. Manufacturing Economics of Plant-Made Biologics: Case Studies in Therapeutic and Industrial Enzymes. Biomed Res Int. 2014;2014:10. doi: 10.1155/2014/256135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Deng H, Zhang X, Xiao H, et al. Generation and immunogenicity of Japanese encephalitis virus envelope protein expressed in transgenic rice. Biochem Biophys Res Commun. 2009;380:292–297. doi: 10.1016/j.bbrc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q, Mason H, Mor T, Cardineau GA, et al. Subunit vaccines produced using plant biotechnology. In: Levine MM, editor. New Generation Vaccines. Informa Healthcare USA, Inc; New York: 2009. pp. 306–315. [Google Scholar]
- 51.Lico C, Chen Q, Santi L. Viral vectors for production of recombinant proteins in plants. J Cell Physiol. 2008;216:366–377. doi: 10.1002/jcp.21423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santi L, Batchelor L, Huang Z, Hjelm B, et al. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine. 2008;26:1846–1854. doi: 10.1016/j.vaccine.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phoolcharoen W, Bhoo SH, Lai H, Ma J, et al. Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant Biotechnology Journal. 2011;9:807–816. doi: 10.1111/j.1467-7652.2011.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phoolcharoen W, Dye JM, Kilbourne J, Piensook K, et al. A non-replicating subunit vaccine protects mice against lethal Ebola virus challenge. Proc Natl Acad Sci USA. 2011;108:20695–20700. doi: 10.1073/pnas.1117715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai H, He J, Engle M, Diamond MS, Chen Q. Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnology Journal. 2012;10:95–104. doi: 10.1111/j.1467-7652.2011.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Z, Chen Q, Hjelm B, Arntzen C, Mason H. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol Bioeng. 2009;103:706–714. doi: 10.1002/bit.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Z, Phoolcharoen W, Lai H, Piensook K, et al. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol Bioeng. 2010;106:9–17. doi: 10.1002/bit.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Q, He J, Phoolcharoen W, Mason HS. Geminiviral vectors based on bean yellow dwarf virus for production of vaccine antigens and monoclonal antibodies in plants. Hum Vaccin. 2011;7:331–338. doi: 10.4161/hv.7.3.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He J, Peng L, Lai H, Hurtado J, et al. A Plant-Produced Antigen Elicits Potent Immune Responses against West Nile Virus in Mice. Biomed Res Int. 2014;2014:10. doi: 10.1155/2014/952865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He J, Lai H, Brock C, Chen Q. A Novel System for Rapid and Cost-Effective Production of Detection and Diagnostic Reagents of West Nile Virus in Plants. Journal of Biomedicine and Biotechnology. 2012;2012:1–10. doi: 10.1155/2012/106783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leuzinger K, Dent M, Hurtado J, Stahnke J, et al. Efficient Agroinfiltration of Plants for High-level Transient Expression of Recombinant Proteins. Journal of Visualized Experiments. 2013 doi: 10.3791/50521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Q, Lai H. Gene delivery into plant cells for recombinant protein production. Biomed Res Int. 2014;2014:10. doi: 10.1155/2015/932161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saejung W, Fujiyama K, Takasaki T, Ito M, et al. Production of dengue 2 envelope domain III in plant using TMV-based vector system. Vaccine. 2007;25:6646–6654. doi: 10.1016/j.vaccine.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 64.Lai H, Engle M, Fuchs A, Keller T, et al. Monoclonal antibody produced in plants efficiently treats West Nile virus infection in mice. Proc Natl Acad Sci USA. 2010;107:2419–2424. doi: 10.1073/pnas.0914503107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He J, Lai H, Engle M, Gorlatov S, et al. Generation and Analysis of Novel Plant-Derived Antibody-Based Therapeutic Molecules against West Nile Virus. PLoS ONE. 2014;9:e93541. doi: 10.1371/journal.pone.0093541. doi:93510.91371/journal.pone.0093541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai H, He J, Hurtado J, Stahnke J, et al. Structural and functional characterization of an anti-West Nile virus monoclonal antibody and its single-chain variant produced in glycoengineered plants. Plant Biotechnology Journal. 2014;12:1098–1107. doi: 10.1111/pbi.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Demento SL, Bonafe N, Cui W, Kaech SM, et al. TLR9-Targeted Biodegradable Nanoparticles as Immunization Vectors Protect against West Nile Encephalitis. The Journal of Immunology. 2010;185:2989–2997. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Appaiahgari MB, Abdin MZ, Bansal KC, Vrati S. Expression of Japanese encephalitis virus envelope protein in transgenic tobacco plants. J Virol Methods. 2009;162:22–29. doi: 10.1016/j.jviromet.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Chen Q, Lai H. Plant-derived virus-like particles as vaccines. Human Vaccines & Immunotherapeutics. 2013;9:26–49. doi: 10.4161/hv.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis BS, Chang GJ, Cropp B, Roehrig JT, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75:4040–4047. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 72.Fehr T, Skrastina D, Pumpens P, Zinkernagel RM. T cell-independent type I antibody response against B cell epitopes expressed repetitively on recombinant virus particles. Proc Natl Acad Sci USA. 1998;95:9477–9481. doi: 10.1073/pnas.95.16.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faye L, Gomord V. Success stories in molecular farming – a brief overview. Plant Biotechnology Journal. 2010;8:525–528. doi: 10.1111/j.1467-7652.2010.00521.x. [DOI] [PubMed] [Google Scholar]
- 74.Lai H, Chen Q. Bioprocessing of plant-derived virus-like particles of Norwalk virus capsid protein under current Good Manufacture Practice regulations. Plant Cell Reports. 2012;31:573–584. doi: 10.1007/s00299-011-1196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Landry N, Ward BJ, Trepanier S, Montomoli E, et al. Preclini-cal and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS One. 2010;5:e15559. doi: 10.1371/journal.pone.0015559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castilho A, Steinkellner H. Glyco-engineering in plants to produce human-like N-glycan structures. Biotechnology Journal. 2012;7:1088–1098. doi: 10.1002/biot.201200032. [DOI] [PubMed] [Google Scholar]
- 77.Chen Q. Turning a new leaf. European Biopharm Rev. 2011;2:64–68. [Google Scholar]
- 78.Check Hayden E, Reardon S. Should experimental drugs be used in the Ebola outbreak? Nature (London) 2014 http://dx.doi.org/10.1038/nature.2014.15698.
- 79.Martin JE, Pierson TC, Hubka S, Rucker S, et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196:1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]