Abstract
The Paramyxoviridae include some of the great and ubiquitous disease-causing viruses of humans and animals. In most paramyxoviruses, two viral membrane glycoproteins, fusion protein (F) and receptor binding protein (HN, H or G) mediate a concerted process of recognition of host cell surface molecules followed by fusion of viral and cellular membranes, resulting in viral nucleocapsid entry into the cytoplasm. The interactions between the F and HN, H or G viral glycoproteins and host molecules are critical in determining host range, virulence and spread of these viruses. Recently, atomic structures, together with biochemical and biophysical studies, have provided major insights into how these two viral glycoproteins successfully interact with host receptors on cellular membranes and initiate the membrane fusion process to gain entry into cells. These studies highlight the conserved core mechanisms of paramyxovirus entry that provide the fundamental basis for rational anti-viral drug design and vaccine development.
Keywords: Membrane fusion, Membrane glycoproteins, Viral envelope proteins, Paramyxovirus entry, Atomic structure of viral glycoproteins, Viral receptors, Fusion protein
Highlights
-
•
New structural and functional insights into paramyxovirus entry mechanisms.
-
•
Current data on paramyxovirus glycoproteins suggest a core conserved entry mechanism.
-
•
Diverse mechanisms preventing premature fusion activation exist in these viruses.
-
•
Precise spacio-temporal interplay between paramyxovirus glycoproteins initiate entry.
Introduction
Paramyxoviruses are a diverse family of viruses, which includes many human and animal pathogens that are of global importance to public health and economy. Highly infectious pathogens like respiratory syncytial virus (RSV), measles virus (MeV), mumps virus (MuV), parainfluenza viruses 1–5 (PIV1–5) and human metapneumovirus (hMPV) contribute significantly to the annual global disease burden in humans, infecting millions of individuals worldwide and leading to a large number of deaths in areas having inadequate health care resources. Many of these viruses are also re-emerging in previously immune populations due to a decrease in vaccination and corresponding breakdown of herd immunity (Gahr et al., 2014, Munoz-Alia et al., 2014, Rubin et al., 2012, Yang et al., 2014). Other paramyxoviruses are more sporadic in their outbreaks and viruses like the zoonotic Nipah virus (NiV) and Hendra virus (HeV) cause deadly localized outbreaks, resulting in high morbidity and mortality in human populations around the world. NiV and HeV are classified as Biosafety Level 4 (BSL-4) select agents. Cases of human-to-human transmission of NiV have become more prevalent in recent outbreaks in Bangladesh generating significant concern in terms of the epidemiology and transmission of these diseases (Daszak et al., 2012, Luby et al., 2009, Luby et al., 2006, Mahalingam et al., 2012). Animal viruses like the Newcastle disease virus (NDV) cause severe and sometimes fatal epidemics in poultry populations, leading to extensive economic losses. Canine distemper virus (CDV) is a fatal, highly contagious disease affecting canines. Many recent host reservoir sampling studies have indicated that a large number of paramyxoviruses remain undiscovered and uncharacterized, with no existing knowledge of the zoonotic potential, spread or host range of these viruses (Drexler et al., 2012, Lamb and Parks, 2013, Marsh et al., 2012). Both well-characterized and yet undiscovered paramyxoviruses highlight the considerable hazard posed by such emerging pathogens in an era of increasing global population, human-wildlife territorial conflicts and international travel.
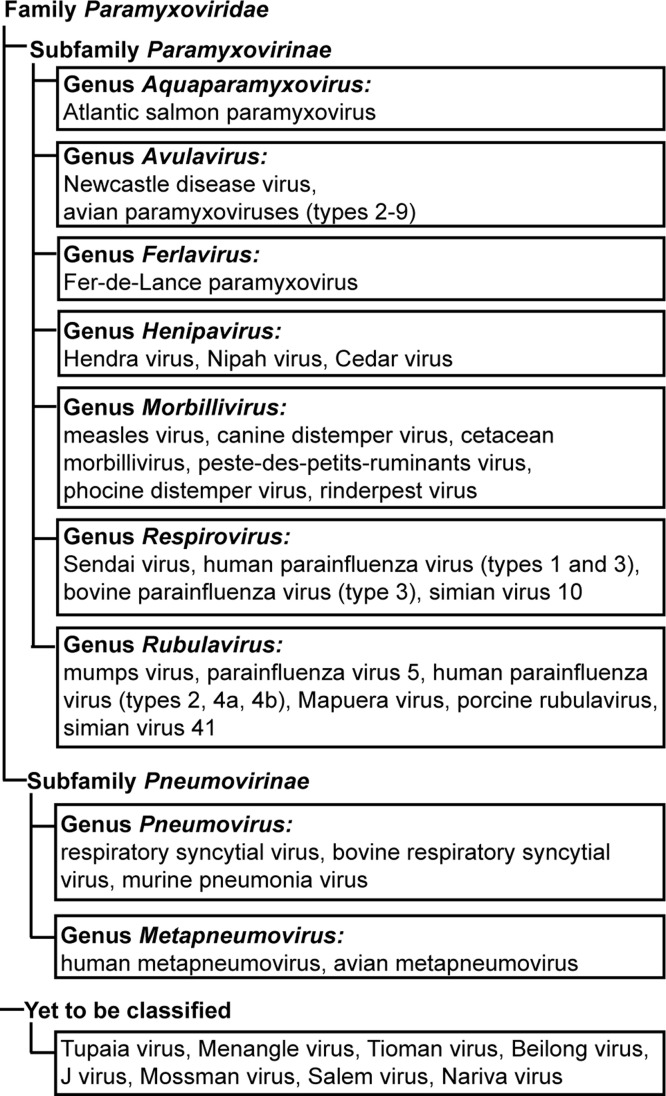
Paramyxoviruses are enveloped viruses harboring a negative-sense RNA genome. Based on sequence homology and protein functions, paramyxoviruses are classified into two sub-families – Pneumovirinae and Paramyxovirinae, with the two sub-families further divided into multiple genera ( Fig. 1). Like most viruses, paramyxoviruses utilize molecules present on cellular membranes, to identify host cells. Attachment via these viral ‘receptors’ leads to fusion of viral and cellular membranes and entry of the viral genome in the form of a nucleocapsid, into the host cell cytoplasm (Lamb and Parks, 2013). To infect host cells, most paramyxoviruses depend on the concerted actions of two major glycoproteins present on the viral membrane, namely the attachment protein (HN, H or G), and the fusion (F) protein (Heminway et al., 1994a, Horvath et al., 1992, Hu et al., 1992, Morrison and Portner, 1991, Yao et al., 1997). The membrane fusion event that mediates viral entry appears to occur at neutral pH on the plasma membrane for most paramyxoviruses. Unlike viruses of the subfamily Paramyxovirinae, in members of the subfamily Pneumovirinae, the F protein was found to be sufficient for viral propagation in cell culture (Biacchesi et al., 2005, Biacchesi et al., 2004, Karron et al., 1997) and the cellular pathway of entry for this subfamily of viruses is yet unclear with membrane fusion at the cell membrane (Srinivasakumar et al., 1991), clathrin-mediated endocytosis (Kolokoltsov et al., 2007, Schowalter et al., 2009, Schowalter et al., 2006) or macropinocytosis (Krzyzaniak et al., 2013), suggested as entry routes for various members of this subfamily. Clathrin-mediated endocytosis (CME) was proposed as an entry pathway for RSV based on interactions with clathrin light chain proteins (Kolokoltsov et al., 2007) and association with cholesterol microdomains and membrane Rho-GTPases, (San-Juan-Vergara et al., 2012). Recently, Krzyzaniak and colleagues suggested macropinocytosis as the initial uptake step of RSV, based on the dependence of RSV infection on Rab5 and other macropinocytosis-associated proteins (Krzyzaniak et al., 2013). Thus Pneumovirinae appear to utilize one or more of these pathways to gain access to the host cell cytoplasm, while Paramyxovirinae primarily utilize the cellular surface entry route.
Fig. 1.
Family Paramyxoviridae. Classification of viruses in the family Paramyxoviridae, showing subfamilies – Paramyxovirinae and Pneumovirinae, along with the various genera and representative examples of each genus.
Gaining access to the cytoplasm: viral membrane fusion proteins
Paramyxovirus glycoproteins F and HN, H or G are important for the initial infection step, as well as subsequent cell–cell spread. The latter mode of transmission has being suggested as the major clinical route of spread within tissues of a living host (Duprex et al., 1999, Ehrengruber et al., 2002, Sattentau, 2008). F and HN, H or G transiently expressed in cells are able to cause cell–cell fusion, potentially creating a transmission route for the viral nucleocapsid between adjacent cells (McChesney et al., 1997). Additionally, a recent report shows a secondary route for cell–cell spread of PIV5 using actin-associated intercellular connections that may bypass membrane fusion requirements between some cells of a tissue (Roberts et al., 2015).
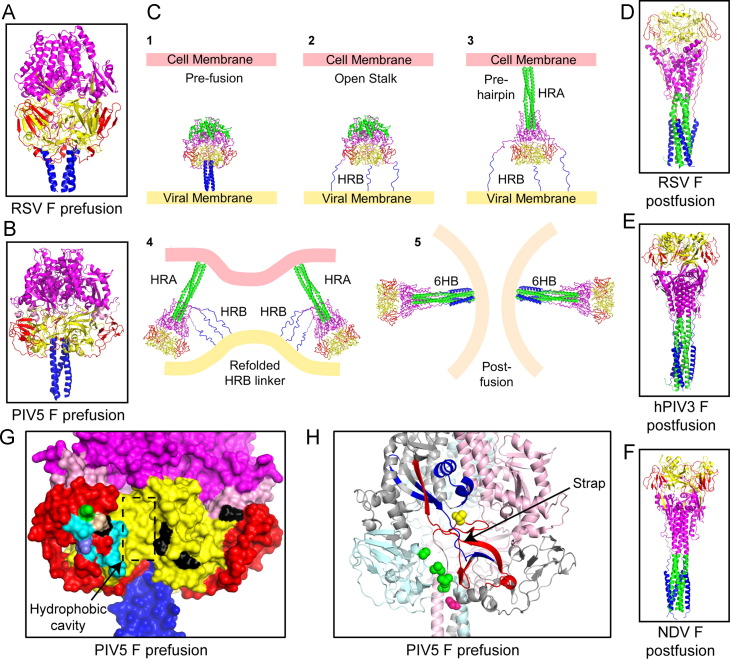
Paramyxovirus F proteins are Class I viral membrane fusion proteins which are structurally and functionally similar to other Class I viral membrane fusion proteins from viruses that include Ebola virus, human immunodeficiency virus (HIV), influenza virus and severe acute respiratory virus-coronavirus SARS-CoV among many others (Bartesaghi et al., 2013, Caffrey et al., 1999, Chan et al., 1997, Julien et al., 2013, Lee et al., 2008a, Li et al., 2005, Malashkevich et al., 1999, McLellan et al., 2013, McLellan et al., 2011, Pancera et al., 2014, Swanson et al., 2010, Varghese and Colman, 1991, Weissenhorn et al., 1998, Wiley and Skehel, 1977, Wiley and Skehel, 1987, Wilson et al., 1981, Yin et al., 2005, Yin et al., 2006, Zhao et al., 2000), reviewed in (Lamb and Jardetzky, 2007). F proteins on synthesis fold into a metastable, prefusion trimer conformation ( Fig. 2A–B). The transition of these metastable, higher energy prefusion trimers to stable, low energy post-fusion trimers drives the process of viral and cellular membrane merger down an energy gradient without requiring ATP hydrolysis, making this transition irreversible in nature (Lamb et al., 2006) (Fig. 2C). Ultimately F proteins are converted to their stable post-fusion trimeric form on completion of membrane merger (Fig. 2D–F). For the Paramyxovirinae subfamily, the attachment proteins are believed to provide the trigger for this refolding process by overcoming an activation energy barrier when they bind a cellular receptor (Heminway et al., 1994a, Horvath et al., 1992, Hu et al., 1992, Morrison and Portner, 1991, Yao et al., 1997). Heat acting as a surrogate can also be used to artificially overcome this thermodynamic barrier and convert prefusion F to its post-fusion form (Ader et al., 2013, Bose et al., 2012, Chan et al., 2012, Connolly et al., 2006).
Fig. 2.
The fusion proteins of paramyxoviruses mediate merger of viral and cellular envelopes through molecular refolding. (A–B) Atomic resolution structures of paramyxovirus F proteins in their prefusion forms, (A) RSV F (PDB ID: 4JHW), B) PIV5 F (PDB ID: 2B9B). The fusion protein domains are colored as follows: domain I, yellow; domain II, red; domain III, purple; fusion peptide, pink; HRB domain, blue. C) Schematic model depicting proposed rearrangements of the activated prefusion F proteins leading to fusion peptide insertion in the target membrane and refolding into a post-fusion form through a series of intermediates, eventually causing membrane merger. (D–F) Atomic resolution structures of paramyxovirus F proteins in their post-fusion forms, (D) RSV F (PDB ID: 3RRT), (E) hPIV3 F (PDB ID: 1ZTM) and (F) NDV F (PDB ID: 3MAW). In addition to the color-coding scheme described above, the HRA domain is colored green for (C–F). (G) Surface representation of the PIV5 F prefusion trimer (PDB ID: 2B9B) showing the potential areas of attachment protein interaction. Positions of mutations in the Ig-like domain and the adjoining hydrophobic cavity and the bordering flexible strap are shown. Various colors mark the residues that are important for interaction of PIV5 F with PIV5 HN (cyan) or MeV F and MeV H (black) (based on sequence alignment) or residues that affect both PIV5 F/HN and CDV F/H interactions (based on sequence alignment) (green) or residues that affect both MeV F/H and PIV5 F/HN interactions (slate) or those that align for all the three F proteins above and disrupt all three pairs of F–HN or F–H interactions (silver). (H) Cartoon depiction of the PIV5 F structure showing the ‘strap’ region composed of beta sheets. The protomers of the F are colored variously. The most dynamic peptides identified by FPOP labeling during the process of F-refolding are marked in red. A region of the strap responsible for transfer of HN specificity between closely related paramyxoviruses is shown in blue. Point mutations that destabilize PIV5 F (green) or MeV F (pink) or CDV F (yellow) are located on this ‘strap’ region or within the adjoining hydrophobic cavity at the junction of two protomers of F.
Cleavage by cellular and tissue proteases converts F into an active, pre-triggered form
Paramyxovirus F proteins, like the other Class I fusion proteins are synthesized as a biologically inactive precursor (F0) that has to be cleaved to the biologically active form, F1 and F2, which are linked together by a disulfide bond. Cleavage releases a hydrophobic fusion peptide at the N-terminus of the membrane anchored F1 fragment. The cleaved F1 protein on activation by the attachment protein or heat undergoes a refolding process that results in the fusion peptide being inserted into the target membrane. Subsequent refolding, through a ‘hairpin-like’ intermediate brings together the viral and cellular membranes for merger (Jardetzky and Lamb, 2014, Lamb and Jardetzky, 2007, Lamb et al., 2006) (Fig. 2C). For most paramyxoviruses, the cleavage activation event is believed to occur in the trans Golgi network, through the action of cellular furin-like proteases during F protein transport to the cellular surface (Homma, 1971, Homma and Ohuchi, 1973, Muramatsu and Homma, 1980, Scheid and Choppin, 1974). For Henipaviruses, the F0 protein is recycled from the cell surface by endocytosis into endosomes, where it is cleaved by cathepsin L (Diederich et al., 2005, Pager et al., 2006, Pager and Dutch, 2005). Most paramyxovirus F0 proteins have a single cleavage site, but the RSV F protein is cleaved at two sites, releasing a short, soluble peptide fragment (Gonzâlez-Reyes et al., 2001). Recently, Krzyzaniak and colleagues demonstrated a sequential cleavage of RSV-F with the first cleavage occurring during its transport through the exocytic pathway to the cell surface and a second cleavage, by a furin-like protease, occurring after the virus particle is internalized into endosomes by macropinocytosis (Krzyzaniak et al., 2013). This second cleavage has been implicated to destabilize F and initiate refolding leading to membrane fusion (Gonzâlez-Reyes et al., 2001). Interestingly, a similar hypothesis has very recently been suggested for another Class I fusion protein – the Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) S (spike) protein, perhaps suggesting a convergence of molecular mechanisms of fusion (Burkard et al., 2014, Millet and Whittaker, 2014). Other examples of Class I fusion proteins like SARS-CoV S and Ebola virus GP, are likewise proteolytically activated in the endosomal compartment (Chandran et al., 2005, Simmons et al., 2004). Interestingly, transplanting the two RSV F cleavage sites into Sendai virus (a member of the Paramyxovirinae subfamily) F protein caused the Sendai F protein to lose its dependence on the Sendai HN protein for activation (Rawling et al., 2011, Rawling et al., 2008) presumably because the sequential cleavage of the RSV F cleavage sites destabilized the chimeric F protein. Though the mechanism for F-activation is yet unclear for RSV, taken together, these data suggest that perhaps F proteins from some viruses of the Pneumovirinae subfamily, with their unique sequential cleavage and the minimal requirement for an attachment protein for fusion, might share the molecular mechanisms of F activation more closely with viruses that utilize a single Class I fusion protein for receptor binding and fusion.
Unlike the Pneumovirinae however, for the Paramyxovirinae subfamily of viruses, the single cleavage event of F is not sufficient to trigger F to refold from its prefusion to post-fusion form. A soluble form of the PIV5 F protein when cleaved did not show significant changes in the conformation of its metastable, prefusion form (Welch et al., 2012). In addition, biochemical data shows that cleaved F can be detected on the surface of cells using prefusion F antibodies (Connolly et al., 2009, Connolly et al., 2006). Thus for Paramyxovirinae, the timing and location of membrane fusion is determined by interaction with the attachment protein, when the latter binds to receptors on the host cell.
Atomic structures and biophysical studies of paramyxovirus F proteins provide insights into the refolding process that lead to membrane fusion
The exact steps involved in the refolding event converting F from a prefusion form to a post-fusion form are not yet completely understood, but biochemical and biophysical evidence, together with X-ray crystal structures have started to provide a clearer picture of how cellular and viral membranes are fused by F proteins. Atomic structures of F proteins from various paramyxoviruses – hPIV3, RSV and NDV, have been obtained in their post-fusion forms (McLellan et al., 2011, Swanson et al., 2010, Yin et al., 2005, Zhao et al., 2000) (Fig. 2D–F). In addition, atomic structures of stabilized prefusion forms of PIV5 F and more recently, RSV F have been obtained (Fig. 2A–B) (McLellan et al., 2013, Yin et al., 2006). The human metapneumovirus F (hMPV-F) was co-crystallized with an anti-hMPV-F antibody, and it was found that the hMPV-F structure partially resembled the prefusion form (Wen et al., 2012). All the paramyxovirus F proteins known so far are trimeric in nature. There was a strong structural conservation across F proteins obtained from the various paramyxoviruses. Comparison between prefusion and post-fusion atomic structures of RSV F and comparison between the prefusion structure of PIV5 F and the post-fusion structure of the closely related hPIV3 F show that the prefusion F proteins assume a more rounded shape of the globular heads, while the post-fusion F proteins׳ globular heads are angular in shape. This difference in shape of the F protein heads can also be observed through electron microscopy of purified soluble forms of prefusion and post-fusion F (Connolly et al., 2006). In the F protein structures the head domains are composed of domains I, II and III. Heptad repeat regions HRA and HRB flank these domains. In the prefusion forms of F, HRA domains remain globular with a series of short connected helices and HRB domains form the C-terminal stalk of the trimer, which leads into the transmembrane domain followed by a short cytoplasmic tail (Fig. 2A–B). On cleavage and activation, the HRA regions convert from a set of compact helical structures into an extended 107 Å long helical trimeric coiled-coil domain with hydrophobic fusion peptides at their end. Short peptides targeted to trap F proteins during refolding, suggested that paramyxovirus F proteins at this stage attain an extended intermediate conformation followed by a hairpin structure, during its transition along the energy landscape towards a final stable post-fusion form (Chan et al., 2012, Russell et al., 2001, Russell et al., 2003). The extended intermediate stage is probably where the liberated fusion peptides of the F trimer insert into the host cell membrane (Fig. 2C), and when visualized by electron microscopy, show the F protein bridging two cellular membranes at an average distance of 210 Å (Kim et al., 2011). The HRB domains on the other hand come apart from the central core of the globular head and flip around 196° and ‘zipper up’ with the HRA trimeric coiled-coil to form a stable 6-helix bundle (6HB), which is characteristic of Class I fusion protein structures obtained so far from a variety of viruses (Lamb and Parks, 2007). However, a recent study has suggested that the final step of full zippering up of the heptad repeat domains to form a complete 6HB is not an absolute requirement for membrane fusion. A partially completed 6HB is able to bring the membranes close enough for merger (Brindley et al., 2014). A more detailed insight into the refolding process of F has recently been obtained (Poor et al., 2014). This study utilized oxidative footprinting, followed by high performance liquid chromatography and tandem mass spectrometry (HPLC–MS–MS) to observe the transitions of the refolding process during F-activation by incubation of purified PIV5 F protein at various temperatures. In this study, it was found that the ׳strap׳ peptide was the first region of F to be released (Poor et al., 2014).
Once F is triggered, successful membrane merger requires that the target membrane must be within the range of insertion of the fusion peptide, estimated at a distance of ~210 Å (Kim et al., 2011). The viral membrane attachment proteins HN, H or G, bound to receptors on the host membrane presumably brings cellular and viral membranes within this range such that productive insertion of the F fusion peptide into the target membrane can occur to initiate the fusion process. However, due to the irreversible nature of the F refolding process, this event must be triggered only at the correct time and location when the anchoring and target membranes are physically within the above range. Recent structural and functional studies of various paramyxovirus HN, H and G proteins have yielded significant insights into this precisely choreographed process between the fusion protein and the attachment protein that results in viral entry.
Multifunctional attachment proteins recognize and bind to host cell molecules as receptors
To detect and bind the host cell, most paramyxoviruses have evolved to recognize a variety of cellular surface molecules as viral receptors through their attachment proteins. The Paramyxovirinae subfamily viruses that bear HN as the attachment protein (e.g. parainfluenza viruses 1–5, mumps virus and NDV) bind to sialic acid as receptor. In addition, these HN proteins cleave sialic acid from complex carbohydrate chains on glycoproteins and glycolipids (neuraminidase activity). This enzymatic activity occurs during egress from the cell to prevent the progeny virus from re-associating with the same cell or themselves. The affinities of different HN proteins to their receptors vary according to the virus type (Villar and Barroso, 2006) and receptor binding preferences to different sialic acid end glycans have been extensively studied in paramyxoviruses hPIV1–3 (Song et al., 2011). On the other hand, those viruses bearing H or G as the attachment protein bind protein receptors. MeV H binds to cell surface molecules CD46, CD150/SLAM or Nectin-4, depending upon virus strain and tissue type (Dorig et al., 1993, Manchester et al., 2000, Muhlebach et al., 2011, Naniche et al., 1993, Noyce et al., 2011, Tatsuo et al., 2000, Tatsuo et al., 2001). Receptors bound by the NiV and HeV G proteins include Ephrin B2 and Ephrin B3 (Bonaparte et al., 2005, Negrete et al., 2005, Negrete et al., 2006). Viruses classified under the Pneumovirinae subfamily (e.g. RSV, hMPV) incorporate a G protein different from those found in the Paramyxovirinae subfamily, in terms of size, sequence and domain composition (Doreleijers et al., 1996, Langedijk et al., 1996). The available data suggests that RSV or hMPV G proteins may be dispensable in tissue culture (Biacchesi et al., 2005, Biacchesi et al., 2004, Karron et al., 1997), but the presence of RSV G appears to somewhat enhance cell–cell fusion activity of RSV F in transfected tissue culture monolayers (Heminway et al., 1994b). These results suggest that RSV or hMPV F could itself bind host cell receptors. Molecules including intercellular adhesion molecule-1 (ICAM-1), heparin, annexin II, integrins and heparan have been proposed as receptors for RSV F or hMPV F (Behera et al., 2001, Chang et al., 2012, Krusat and Streckert, 1997, Malhotra et al., 2003). However, the specific role of those molecules in RSV or hMPV entry is not clear as multiple viruses are known to bind to host cells initially through weak interactions with these ubiquitously expressed cell surface molecules. Recently, nucleolin was reported as a receptor for RSV F based on the observation that expression of human nucleolin renders non-permissive Spodoptera frugiperda (Sf9) cells susceptible to infection (Tayyari et al., 2011). However, based on tissue and cell surface distributions, the presence of nucleolin in a large 500 kD multiprotein complex, the lack of membrane anchoring domains of nucleolin, and the fact that nucleolin-RSV F interactions are abolished in the presence of heparin (Holguera et al., 2014, Srivastava and Pollard, 1999), the specific role of nucleolin as a receptor in RSV entry is as yet unclear.
Receptor binding by the attachment proteins initiates activation of the fusion protein
Paramyxovirus attachment proteins are single-pass, type II membrane proteins. X-ray crystal structures and electron micrographs of soluble attachment protein ectodomains from a variety of different paramyxoviruses have indicated that HN/H or G ectodomains consist of a large globular head connected to a stalk. The atomic structures of the HN, H or G globular head domains have been determined for PIV5, NDV, NiV, HeV, MeV and hPIV3 (Bowden et al., 2008a, Bowden et al., 2010, Colf et al., 2007, Crennell et al., 2000, Hashiguchi et al., 2007, Hashiguchi et al., 2011, Lawrence et al., 2004, Santiago et al., 2010, Xu et al., 2008, Yuan et al., 2012, Yuan et al., 2011, Yuan et al., 2005), and were found to be highly conserved in terms of protein secondary structure folds and overall shape. The globular head domain of HN, H or G binds receptor through a typical six-bladed β-propeller fold common to sialidases. In HN proteins, sialic acid is bound through an active site in the center of the β-propeller fold; NiV and HeV G proteins bind Ephrin B2 or B3 through residues located on the top of the G globular head (Bowden et al., 2008a, Xu et al., 2008) and various binding sites were identified on the sides of the MeV H heads that bind CD46, CD150/SLAM or Nectin-4 (Colf et al., 2007, Hashiguchi et al., 2007, Hashiguchi et al., 2011, Mateo et al., 2013, Mateo et al., 2014, Santiago et al., 2010). In NDV HN and hPIV3 HN, evidence has been presented of a second sialic acid binding site, which lacks neuraminidase activity (Bousse et al., 2004, Mahon et al., 2011, Porotto et al., 2012b, Zaitsev et al., 2004). An atomic structure of the globular head domain of NDV HN from a low virulence (lentogenic) strain (Ulster) (Yuan et al., 2012) showed a longer C-terminal extension (Gorman et al., 1990, Nagai et al., 1976, Sakaguchi et al., 1989) that was found to be involved in auto-inhibition of receptor binding by obscuring both the primary and secondary sialic acid receptor binding sites of NDV HN (Yuan et al., 2012). Proteolytic cleavage of this C-terminal extension is required for receptor binding and fusion in these NDV strains (Yuan et al., 2012). However for hPIV3 HN, the second receptor binding site is masked by an N-linked glycan at residue 523, and removal of this N-glycan by mutating the residue at position 523 restores activity of this second site (Mishin et al., 2010) making it difficult to draw conclusive interpretations from results obtained in a study that investigated the biological significance of the second site in hPIV3 HN (Porotto et al., 2006, Porotto et al., 2007).
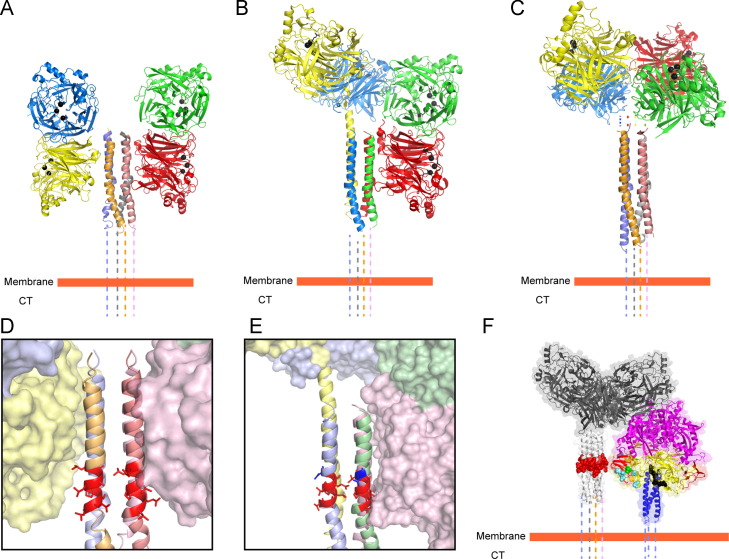
The atomic structures of the attachment proteins together with numerous biochemical and biophysical studies (Bossart et al., 2005, Bowden et al., 2008b, Brindley and Plemper, 2010, Ng et al., 1990, Yuan et al., 2008, Yuan et al., 2005) indicate that all of the HN, H or G proteins show a tetrameric or more precisely, a dimer-of-dimer arrangement of the globular heads, with the stalk domains playing a major stabilizing role in the oligomerization process through covalent or non-covalent associations (Ng et al., 1990, Ng et al., 1989, Parks and Lamb, 1990, Yuan et al., 2008). The two X-ray crystal structures of the stalk domains of paramyxovirus attachment proteins obtained thus far indicate that the stalk domains of NDV HN and PIV5 HN proteins are 4-helix bundles (4HBs), with strong central hydrophobic cores (Bose et al., 2011, Yuan et al., 2011) ( Fig. 3A–C). Based on these structures, subsequent studies have shown that sequences of other paramyxovirus receptor-binding protein stalks can also be modeled as 4HBs (Ader et al., 2012, Maar et al., 2012, Porotto et al., 2012b). Though receptor engagement occurs through the globular head domains of the attachment proteins, an overwhelming amount of biochemical and biophysical evidence suggests that HN, H or G proteins physically interact with the F protein through these stalk domains. This interaction presumably triggers the F protein into carrying out its rearrangements leading to membrane fusion (Bishop et al., 2007, Bose et al., 2014, Bose et al., 2011, Bousse et al., 1994, Corey and Iorio, 2007, Deng et al., 1999, Deng et al., 1995, Ennis et al., 2010, Melanson and Iorio, 2004, Melanson and Iorio, 2006, Paal et al., 2009, Porotto et al., 2003, Stone-Hulslander and Morrison, 1999, Tanabayashi and Compans, 1996).
Fig. 3.
Various observed arrangements of paramyxovirus HN proteins in X-ray crystal atomic structures suggest a molecular mechanism of activation of the fusion protein. (A) NDV HN ‘4 heads down’ arrangement (PDB ID: 3T1E), (B) PIV5 HN ‘2 heads up-2 heads down arrangement (PDB ID: 4JF7) and (C) PIV5 HN receptor binding domains in a ‘4 heads up arrangement’ (PDB ID 1Z4X) placed with respect to the PIV5 HN stalk 4HB domain (PDB ID: 3TSI). (D–E) Variations in interaction surfaces between the globular head domains and the stalk 4HB domains of (D) NDV HN (PDB ID: 3T1E) and (E) PIV5 HN (PDB ID: 4JF7). The respective F-activation domains on the two stalk 4HBs are highlighted in red. The single charged residue that determines specificity between Rubulaviruses is highlighted in blue on the PIV5 HN stalk. (F) Images reconstructed from X-ray crystal structures by aligning the PIV5 HN stalk structure (PDB ID: 3TSI) with the PIV5 HN 4-heads up structure (PDB ID 1Z4X). The 20 ectodomain residues missing from the PIV5-HN stalk structure have been replaced by dotted lines. Cleaved PIV5 F-GCNt (PDB ID: 4GIP) is modeled next to the constructed PIV5 HN ‘4 heads up’ conformation described above to indicate the approximate relative heights of the interacting surfaces of the two glycoproteins.
Interestingly, atomic structures of NDV HN (Fig. 3A) and PIV5 HN (Fig. 3B) (Welch et al., 2013, Yuan et al., 2011) full-length proteins displayed two drastically different arrangements of the globular head domains with respect to the stalk, when compared to the previously observed PIV5 HN dimer-of-dimer arrangement (Fig. 3C) (Yuan et al., 2005). In all these arrangements, the individual monomers and the dimer interface remain constant, while the dimer-of-dimer interface is drastically altered. In a recent study by Hashiguchi and colleagues, the atomic structure of MeV H protein bound to its cellular receptor SLAM, suggested that the measles H dimer-of-dimers could potentially be arranged into two different tetrameric conformations (Hashiguchi et al., 2011). Thus, taking together these data, along with electron micrographs of purified HN proteins (Bose et al., 2012, Yuan et al., 2008), it is evident that paramyxovirus attachment proteins globular head domains are connected to the stalk domains through flexible, unstructured linkers as a result of which, the globular head domains can attain various different positions with respect to the stalk domains.
Unlike the HN, H and G proteins of the Paramyxovirinae subfamily viruses, atomic structures of G proteins of pneumoviruses like RSV or hMPV have not been obtained to date. Biochemical studies of hMPV G and bovine RSV G predict these to be made up of a hydrophobic center between two large mucin-rich domains (Doreleijers et al., 1996, Langedijk et al., 1996), which makes the protein appear physically larger than F and possibly aids in the immune evasion strategies of the virus (Leyrat et al., 2014). Though hMPV G has been associated with binding to cellular glycosaminoglycans (Thammawat et al., 2008), this was found to be a strain specific effect (Adamson et al., 2012), suggesting that Pneumovirinae G proteins are not intimately associated with membrane fusion like the HN, H and G proteins from members of the Paramyxovirinae subfamily.
The fusion protein and the attachment protein physically interact to mediate membrane fusion
For Paramyxovirinae subfamily viruses, the attachment protein and the fusion protein are believed to directly interact with each other, while resident on the same membrane and generally this interaction requires homotypic pairs of fusion and attachment proteins derived from the same virus. A few exceptions have been identified where heterotypic paramyxovirus F–HN interactions are functional in in-vitro assays. For example, hPIV2 HN is able to substitute for hPIV4a HN, and SV41 HN or MuV HN are able to substitute for hPIV2 HN or PIV5 HN in transfected tissue culture cells (Bose et al., 2014, Tsurudome et al., 1998, Tsurudome et al., 1995), suggesting that the F–HN interactions may be conserved among closely related viruses. The exact nature of the F-G/H/HN interaction and how this interaction spatially and temporally couples receptor binding mediated by HN, H or G, and membrane fusion mediated by F has been a topic of considerable debate. Some HN, H or G stalk mutants, that are deficient for fusion, block the attachment protein-fusion protein interaction directly (as assessed by co-immunoprecipitation) (Melanson and Iorio, 2006, Paal et al., 2009, Stone-Hulslander and Morrison, 1999). However, the cellular location, duration and strength of the F–HN, H or G association have been found to differ widely between viruses. Studies of MeV H and CDV H as well as NiV G and HeV G proteins indicate that the strength of the F–G or F–H interaction is inversely related to their fusion activity, suggesting a more intimate association between the two proteins prior to F triggering (Aguilar et al., 2006, Bishop et al., 2008, Plemper et al., 2001, Plemper et al., 2002). On the contrary, certain mutations in the NDV HN stalk showed a direct relationship between fusion activity and F–HN binding, while other mutations in the NDV HN and MeV H stalk domains decrease fusion, but retain F-association (Brindley et al., 2012, Corey and Iorio, 2007, Melanson and Iorio, 2004, Mirza and Iorio, 2013). In Henipaviruses, F–G complexes are also believed to remain associated on cell surfaces and F is released to be endocytosed, cleavage-activated and re-transported to the plasma membrane (Diederich et al., 2005, Pager et al., 2006, Pager and Dutch, 2005). For morbilliviruses, F and H remain associated together in fusion complexes during transport to the cell surface (Ader et al., 2012, Lee et al., 2008b, Paal et al., 2009, Plemper et al., 2001, Plemper et al., 2002), unlike PIV5 and hPIV3 F and HN (Paterson et al., 1997).
Two distinct molecular hypotheses attempt to explain the interaction between the fusion protein and the attachment protein
Based on the above biochemical data, two distinct models have emerged that could explain F-activation at the right place and the right time. The ‘dissociation’ or ‘clamp’ model (Bossart et al., 2013, Plemper et al., 2001, Sergel et al., 1993) proposes that the attachment protein associates with F intracellularly and ‘clamps’ the F protein during transport to the cell surface, preventing F from prematurely converting into the post-fusion form. On binding receptor, the attachment protein releases F allowing the metastable F protein to be destabilized and undergo the refolding process leading to membrane fusion. In the second model, known as the ‘association’ or ‘provocateur’ model (Connolly et al., 2009), the attachment protein, on binding receptor, actively triggers the metastable F protein through destabilization. This destabilization and F-activation can also be brought about by heat acting as a surrogate for HN, H or G, which indicates that the attachment protein interaction with F overcomes an energy barrier of F-activation (Ader et al., 2013, Bose et al., 2012, Chan et al., 2012, Connolly et al., 2009). Based on the differences in the biochemical data of F interactions with HN, H and G protein stalks, it was suggested that for viruses binding protein receptors (with G or H as attachment proteins), the closer F–G or F–H associations indicate an F-stabilization through a ‘clamp’ hypothesis, while for those that bound sialic acid (possessing HN as attachment protein), the more transient F–HN interaction destabilizes F and activates it for fusion (provocateur hypothesis). However, recent data for MeV, CDV, NiV, HeV, as well as PIV5 and hPIV3 are not compatible with the ‘clamp’ hypothesis. Many of the F proteins from the above viruses can be expressed in their prefusion form without the attachment protein needing to stabilize F, suggesting that prefusion F proteins that are involved in F–H, F–G or F–HN interactions do not require the attachment protein ‘clamp’ for stabilization (Ader et al., 2013, Bose et al., 2012, Brindley et al., 2012, Chan et al., 2012, Connolly et al., 2006). Also many of these prefusion F proteins could be converted to the postfusion form by elevated temperature, consistent with the notion that the energy barrier of metastable F proteins could be overcome by external heat, according to the ‘provocateur’ hypothesis. However, the two models can be reconciled if not all F–H or F–G interactions are productive (Jardetzky and Lamb, 2014). Non-productive F–G or F–H associations may occur through alternative interaction regions of F and H/G (Liu et al., 2015, Liu et al., 2013) or through a conformation of the attachment protein that does not allow productive triggering of F (Avila et al., 2014, Brindley et al., 2014a). Such interactions between the attachment and fusion proteins in the ‘pre-receptor binding’ stage may perhaps be necessary for proper folding, cellular transport or increased F–G or F–H concentrations in fusion complexes that can be rapidly triggered (Avila et al., 2014, Brindley et al., 2014a). However, based on the above evidence, such ‘pre-receptor binding’ F–G/H or HN interactions do not appear to be essential to stabilize prefusion F, as proposed by the ‘clamp’ hypothesis.
In recent years, X-ray crystal structures of the fusion and attachment proteins from various paramyxoviruses have significantly aided in identifying the productive interactions between F–HN, F–H or F–G that lead to membrane fusion. In particular, atomic structures of HN stalk domains have helped to provide residue-level information of the potential region of contact between F and the HN protein stalks. The atomic structures of the PIV5 HN and NDV HN stalk domains indicate that the stalk 4HB arrangements have a strong hydrophobic core, with a kink in the central portion of the stalks (Bose et al., 2011, Yuan et al., 2011). Mutagenesis studies along the length of the HN, H or G stalks have suggested that HN, H or G proteins productively interact with the F protein broadly through this central region of the stalk domain (Ader et al., 2012, Apte-Sengupta et al., 2013, Bishop et al., 2007, Bose et al., 2014, Bose et al., 2011, Corey and Iorio, 2007, Deng et al., 1999, Deng et al., 1995, Liu et al., 2013, Melanson and Iorio, 2004, Navaratnarajah et al., 2012, Stone-Hulslander and Morrison, 1999, Wang and Iorio, 1999, Yuan et al., 2011). A large proportion of these residues in the central part of attachment protein stalks are hydrophobic in nature. In closely related HN proteins, the F-interaction region was narrowed down to a highly conserved stretch of 7–8 residues flanking the kink region of the 4HB (Bose et al., 2014) (Fig. 3D–E). Interestingly, a single acidic amino acid immediately adjacent to this region determines the homotypic specificity of F–HN interaction in the rubulavirus subfamily (Bose et al., 2014). A synthetic antibody bound to this ‘F-activation’ region of the PIV5 HN stalk domain, was found to strongly neutralize the fusion promotion activity of PIV5 HN, without affecting the neuraminidase and receptor-binding activities resident in the globular head domain (Welch et al., 2014). Escape mutants generated in residues of the F-activation domain abrogated the neutralizing activity of the above antibody (Welch et al., 2014). In more distantly related paramyxoviruses, the F-activating region, though located close to the central part of the stalk, appears to vary somewhat in its location or is more extensive in nature (Aguilar et al., 2009, Lee et al., 2008b, Liu et al., 2015, Maar et al., 2012, Paal et al., 2009). Insertion of structurally rigid α-helical segments within the MeV H stalk to increase the distance of the F-activating region of the stalk from the anchoring membrane abrogated fusion, while insertion of these α-helical segments above this region did not (Paal et al., 2009), suggesting that this F-interacting region must be present at an optimal height matching with the corresponding H-interaction region of MeV F.
Regions of the fusion protein involved in attachment protein interactions offer insights into the F-triggering process
A number of regions on various paramyxovirus F proteins have been found to potentially interact with the HN, H or G proteins. Many of these regions harbor hydrophobic residues and are surface exposed, suggesting a protein–protein interaction interface with the hydrophobic F-interacting region on the central part of the attachment protein stalks, discussed above (Apte-Sengupta et al., 2012, Avila et al., 2014, Bose et al., 2013, Lee et al., 2007) (Fig. 3F). A number of such residues identified on the PIV5 F protein and the MeV F protein appear to be localized near an immunoglobulin-like (Ig-like domain) fold of domain II and also within an adjacent hydrophobic cavity (Apte-Sengupta et al., 2012, Bose et al., 2013) (Fig. 2G). This cavity is created by residues from two adjacent protomers of F (Apte-Sengupta et al., 2012, Avila et al., 2014, Bose et al., 2013), that juxtapose next to two long β-strands forming a ‘strap’ that connects the HRA domains of the F protein head (Fig. 2H). Mutations of residues in these three adjacent regions – the Ig-like domain, the hydrophobic cavity and the ‘strap’ regions of different paramyxovirus F proteins have many effects including, transfer of HN specificity among different F proteins (Tsurudome et al., 2011, Tsurudome et al., 2013), complete abrogation of F-activation or destabilization of the F protein, making F less reliant on the attachment protein trigger (Avila et al., 2014, Bose et al., 2013, Plattet et al., 2009). Through a more recent dynamic view of PIV5 F refolding it was observed that only the outer loop of the Ig like domain shows conformational mobility, while the rest of the Ig-like domain remains unchanged. In contrast, the ‘strap’ regions underwent some of the largest conformational changes as F protein converted from its prefusion to its post-fusion form (Poor et al., 2014). Based on these data an attractive hypothesis for F triggering could be that HN, H or G interactions through the structurally well defined Ig-like domain could dock the attachment protein stalk into the adjacent hydrophobic cavity, which in turn destabilizes the F protein by initiating structural rearrangements in the adjoining ‘strap’ regions and ultimately culminating in the sequential cascade of refolding events that convert F into its postfusion form. Further studies are required to tease out more of the details as to how HN interaction with F leads to F destabilization and eventual membrane merger (Fig. 2G–H).
Molecular cooperation between the different domains of the attachment protein ensures timing of F-activation and membrane fusion
In silico models of MeV F and H proteins (Lee et al., 2008b, Paal et al., 2009) suggested that prior to fusion activation, the MeV prefusion F and H protein heads are positioned at different levels to each other (‘staggered heads’ arrangement), where the H protein head rises above that of the F protein. However, conflicting results from electron micrograph studies of virions suggested that the glycoprotein spikes appear to be of the same height (“parallel-head” model) (Jain et al., 2008). Recently obtained atomic structures of HN proteins of PIV5 (Welch et al., 2013, Yuan et al., 2005) and NDV (Yuan et al., 2011) and electron microscopy data of purified proteins (Bose et al., 2012, Yuan et al., 2008) have suggested that the attachment protein globular heads can attain various different conformational arrangements. Of these, a ‘four heads down’ structure observed for NDV HN has the globular head dimers folded back and making contacts with the 4HB stalk domains (Yuan et al., 2011) thus lowering the height of HN. This, or a similar arrangement could represent the “parallel head” model observed by EM on virions. On the other hand, the ‘four heads up’ arrangement of PIV5 HN globular heads forming a dimer-of-dimer interface (Yuan et al., 2005) represents a taller form of the HN protein as proposed by the ‘staggered head’ model. Interestingly, PIV5 HN was also crystallized in a third arrangement, which showed a shifted dimer-of dimer interface and appeared to represent a hybrid between the two HN arrangements described above (2 heads up-2 heads down arrangement) (Welch et al., 2013), with one dimer making contacts with the 4HB stalk domain and the other maintaining the arrangement seen in the 4-heads up structure. As there was very little change in conformation of HN, H and G monomers when they bind receptor (Bowden et al., 2008a, Colf et al., 2007, Lawrence et al., 2004, Santiago et al., 2010, Xu et al., 2008, Yuan et al., 2005), these data suggested that the rearrangements of the attachment protein globular head domains might have a role in translating the receptor binding event into F-activation through the stalk domains. Notably however, except for PIV5 HN (Yuan et al., 2005), all the other receptor-ligand complexes studies were carried out with soluble attachment protein globular heads.
A possible model of how the F-activation signal could be linked to receptor binding proposed the globular heads of HN, H or G on binding their specific receptors, transmit a series of specific conformational changes through the linkers down into the stalk domain. The stalk domain in turn undergoes a series of conformational changes that mediate a productive F-interaction leading to F-activation (Plemper et al., 2011, Porotto et al., 2011, Porotto et al., 2012a, Porotto et al., 2012c). However, this model has been difficult to rationalize in the light of some recent results. Firstly, measles H can be re-targeted to bind alternative molecules on cells by inserting 6-His tags or other protein-binding domains into the globular head (Allen et al., 2006, Navaratnarajah et al., 2011, Paraskevakou et al., 2007), suggesting that the specificity of the receptor-binding interaction, though critical for determining the virus host range, is not critical in transmitting specific conformational changes to the stalk. Also measles H binds multiple receptors (e.g. SLAM, Nectin 4 or CD46) through partially overlapping but very different binding surfaces in the globular head (Colf et al., 2007, Hashiguchi et al., 2007, Hashiguchi et al., 2011, Mateo et al., 2013, Mateo et al., 2014, Santiago et al., 2010), but they eventually channel all these inputs into a single output – F-activation and membrane fusion. Secondly, many different functionally active chimeric proteins have been generated by appending the globular head of one attachment protein to the stalk of another (Bose et al., 2012, Deng et al., 1995, Farzan et al., 2011, Porotto et al., 2011, Porotto et al., 2012b, Talekar et al., 2013, Wang et al., 2004). Importantly, in all of these chimeric proteins, the F proteins that can be successfully activated are always homotypic to the stalk domain of the chimeric attachment protein. Thus, if a specific receptor-binding signal propagated down the flexible linkers into the stalk these chimeric proteins should have failed to maintain the conformational crosstalk between the head and the stalk. Thirdly, as there is very little change in the globular heads on receptor binding, the rearrangement generated would not be substantial enough to propagate down into the 4HB stalk through the highly flexible linker domains connecting the head and the stalk domains. Fourthly, it was observed that a headless stalk of PIV5 HN protein could successfully activate PIV5 F (Bose et al., 2012), suggesting that productive F-activation is possible even in complete absence of the head domains. In addition, this PIV5 HN headless stalk domain could activate F more efficiently at sub-optimal temperatures when compared to the full-length HN protein and also activate a hypofusogenic mutant of PIV5 F (P22L) at significantly higher levels compared to full length HN (Bose et al., 2012), suggesting that the HN globular heads are associated with an energy requirement for F activation. Subsequently through the work of different groups, fusion activation by headless stalks has now been extended to other paramyxoviruses of different genera – MeV H, NiV G, MuV HN and NDV HN (Bose et al., 2014, Brindley et al., 2013, Liu et al., 2013) – demonstrating that the receptor binding event itself is not specific in the process of F protein activation. Nonetheless, if receptor-binding to the HN, H or G globular head domains are not directly responsible for productive F-triggering, then what role do they play in mediating the membrane fusion process and entry of paramyxoviruses?
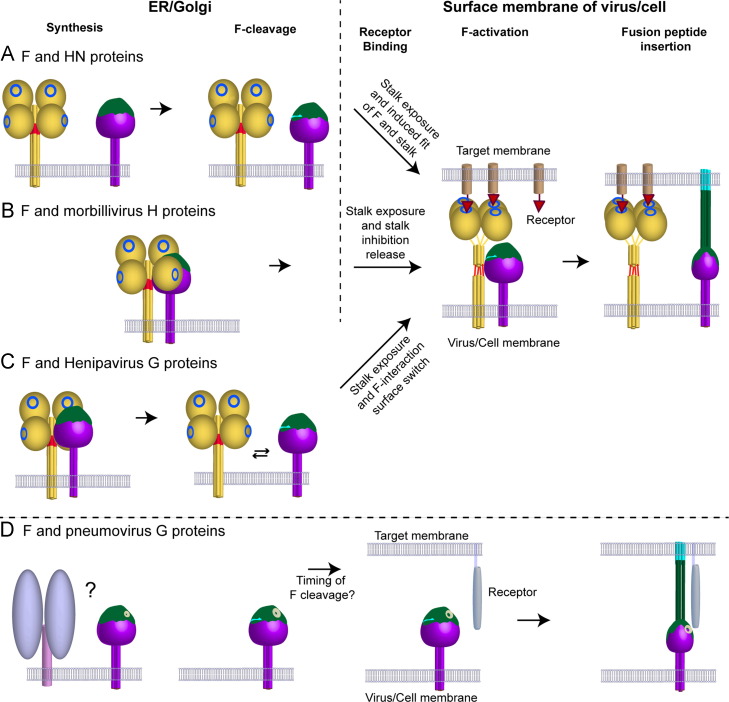
Taking together the above structural and functional data, a new model for F activation has recently been proposed (Bose et al., 2012) ( Fig. 4). The ‘stalk exposure’ model suggests that prior to binding receptor, the globular heads of HN, H or G proteins physically restrict the access of the F protein to the F-activating region residing on the HN, H or G stalk domains. This could be possible if the attachment protein heads attain an arrangement with respect to the stalk 4HB, similar to the ‘4 heads down’ arrangement observed for NDV HN (Yuan et al., 2011). Binding of receptor results in a molecular rearrangement of the dimer-of-dimer globular heads of attachment proteins, causing them to attain a conformation close to that observed in the PIV5 HN 4-heads up atomic structure (Yuan et al., 2005) or the PIV5 HN 2 heads up -2 heads down atomic structure (Welch et al., 2013). These conformations expose the F-activating regions of the HN, H or G stalk domains, allowing the F protein to interact with this region of the HN, H or G stalk and be activated to undergo refolding and subsequent membrane fusion (Bose et al., 2014, Bose et al., 2012). Interestingly, it was observed that the F-activating function of the HN protein could be maintained even after the PIV5 HN molecule was locked into its 2 heads up- 2 heads down conformation using engineered disulfide bonds (Welch et al., 2013) and that binding of only a single dimer of measles H to its receptor was sufficient to trigger F (Brindley et al., 2012). The ‘stalk exposure’ model suggests that the globular heads of HN, H or G are involved in the critical role of ‘regulatory domains’, restricting access of F to interact with the ‘activating domains’ present in the stalk. The restriction is only lifted when the head domains bind receptor. The ‘stalk exposure’ model accounts for the fact that a diverse variety of natural host cell receptors or artificial molecules can be recognized through different interfaces and multiple active sites of the HN, H or G proteins as inputs, all of which can then be converted into a common output that is, F-activation.
Fig. 4.
(A–C) Models of receptor-dependent fusion activation for paramyxoviruses of the Paramyxovirinae subfamily. Structural and functional data indicate that the HN, H or G attachment proteins (yellow) of this subfamily are structurally related and have a globular, C-terminal receptor-binding domain (head) that can interact with various types of host receptors, through receptor binding-sites in the globular heads (blue rings). Putative regions of F-interaction on HN, H or G proteins are indicated on the stalk domains in red. The F proteins (purple) fold into a functional, prefusion state in the presence or absence of the attachment proteins, but without requiring the attachment protein as a stabilizing ‘clamp’. The F proteins are cleaved by proteases to release a hydrophobic fusion peptide (light blue). The HRA domain of F, which refolds into elongated helices post triggering is shown in dark green. (A) F and HN proteins are transported individually to the cell surface and biochemical data suggests that the F–HN interaction is transient. Receptor binding by HN heads results in stalk exposure and possibly a ‘induced fit’ mechanism between the F protein head and the exposed F-activating region on the HN stalk that triggers F to undergo refolding. (B) In morbilliviruses, where F and H are intimately associated in fusion complexes during cellular transport, it has been proposed that the H globular head domains maintain the stalk domains in a ‘pre-triggering’ conformation, possibly through H head-stalk contacts. Stalk exposure, coupled with release of the stalk domains from the globular heads, allows the F protein to mediate productive interaction with the F-activating regions of the H stalk. (C) In Henipaviruses, the initial F–G interaction may be mediated through F interacting with the globular head domains of G or the C-terminal upper portion of the G stalk, which prevents premature F activation during cellular transport within F–G complexes. Stalk exposure and a switch of binding interfaces, allows F to undergo productive interaction with the exposed F-activating domains in the G stalk and be triggered to undergo refolding. (D) For Pneumovirinae, the mechanism of F triggering is yet unclear and it is likely that the distinct G protein does not play a role in this process. The F protein is believed to bind specifically to cellular receptors through binding sites in the F globular head (white ring) and the timing of F-cleavage by both cellular and extracellular proteases is believed to play a role in the triggering process for some of the Pneumovirinae.
Diverse inhibitory mechanisms found in different paramyxoviruses appear to regulate access of the fusion protein to the attachment protein stalk prior to ‘stalk exposure’
In Henipavirus and morbilliviruses, preformed F–G or F–H complexes are transported to the cell surface respectively before incorporation into viral particles, while for those paramyxoviruses with HN as the receptor binding protein F–HN interactions occur primarily at the cell surface. Preformed F–G or F–H complexes likely increase the efficiency of productive F interactions but increase the risk of premature F-triggering within these complexes. Separate transport of F and HN protein possibly keeps these two glycoproteins from interacting prior to HN binding receptor. Recent studies in Henipaviruses and morbilliviruses have suggested that in addition to stalk exposure, mechanisms involving the globular heads or the upper parts of the stalk may prevent F from interacting prematurely with the F-activating domain of the H and G stalks, (Avila et al., 2014, Brindley et al., 2014a, Liu et al., 2015, Liu et al., 2013).
In many mutagenesis studies involving HN proteins, the globular head-proximal upper part of the stalks of these HN proteins were shown to tolerate mutations and addition of carbohydrate moieties within the 4HB, without affecting the F-activation process (Bishop et al., 2007, Bose et al., 2011, Corey and Iorio, 2007, Melanson and Iorio, 2004, Stone-Hulslander and Morrison, 1999, Wang and Iorio, 1999). On the other hand, many lines of evidence have shown that the central F-activating region of almost all the stalk domains of HN, H or G require some structural rearrangements to interact with and activate F (Ader et al., 2012, Apte-Sengupta et al., 2013, Bose et al., 2014, Brindley et al., 2012, Navaratnarajah et al., 2012). This requirement of a ‘primed’ stalk domain conformation that can successfully interact with and activate F is also evident in the headless stalks. However, while MeV headless stalks required an active stabilization through tags to keep them functional (Brindley et al., 2013), the NDV HN headless stalk domains were inhibited in their ability to activate F by engineering disulfide bonds within the 4 HB, similar to that observed in full length NDV HN (Bose et al., 2014). Thus for F–HN protein interactions it has been proposed that the F proteins might interact with the central part of the HN stalks through an ‘induced fit’ mechanism, where the conformational changes in the stalk are passive and do not directly depend on the presence of the globular head domains (Bose et al., 2014) (Fig. 4A).
For morbilliviruses, the F-interaction region on the H stalk domain appears to be more extensive compared to those of HN proteins (Apte-Sengupta et al., 2013, Lee et al., 2008b, Navaratnarajah et al., 2014, Paal et al., 2009), but overlaps with residues co-linear to those of the HN stalk implicated for F triggering. The MeV H stalk also contains a structurally stabilized region in the C-terminal part of the stalk that extends beyond that of HN stalks (Navaratnarajah et al., 2014, Navaratnarajah et al., 2012, Paal et al., 2009). This extensive contact region may provide clues to how F-H interactions are mediated within the fusion complexes and how F is prevented from triggering prematurely. A recent study has suggested that the MeV H globular heads actively inhibit the structural rearrangements required by the H stalk to trigger F (Brindley et al., 2015). On receptor binding, the H globular heads undergo a conformational change, exposing the F-activating domain and releasing the inhibition on the stalk to allow conformational changes in the stalk that trigger F (Avila et al., 2014, Brindley et al., 2014a). These studies also demonstrated that during cellular transport of the associated F–H glycoprotein complexes, F cleavage was found to weaken the F–H interaction, following which F is primed for activation and dissociates from the F–H complex. However, it is yet unclear whether the F–H stalk contacts remain constant during this process or switch from less productive to more productive interactions within the extensive F-interacting domain of H. It may be possible for MeV F to form an initial association with the H stalk while the globular heads of H are folded down in a ‘4-heads down-like’ conformation. On F cleavage, this interaction is weakened and as soon as at least one H dimer binds receptor (Brindley et al., 2012) and the stalk is exposed, F could now switch to a more productive interaction with the stalk. However, importantly this switch should not be able to occur by stalk exposure alone, as cleavage of F as well as rearrangements of the stalk appear to be important for productive F interaction. An initial MeV F interaction with the MeV H stalk could be possible considering that ׳heads down׳ orientations of the globular head monomers with respect to the 4HB stalks of closely related NDV HN and PIV5 HN expose variable regions of the stalk domain (compare Fig. 3D and E). This, and the various observed MeV globular head arrangements in the atomic structure (Hashiguchi et al., 2011) suggest that the extent of F accessibility to the stalk in the ‘4 heads down’-like conformation may vary significantly for morbillivirus H proteins. Though the exact details of how morbillivirus F–H complexes initially prevent premature F activation require to be worked out in detail, biochemical and modeling studies of morbilliviruses (Brindley et al., 2014a, Navaratnarajah et al., 2014, Navaratnarajah et al., 2012) suggest that the core paramyxovirus fusion mechanism is conserved according to the ‘stalk exposure’ model, where the HN, H or G head domains act as regulators and the stalks act as activating domains (Bose et al., 2012).
Liu and colleagues recently proposed a 3-step process of Henipavirus F activation, which suggests that, a bidentate G–F interaction of the F protein head with the G protein upper stalk or head prevents F from interacting with the G stalk F-activating region prematurely (Liu et al., 2013). On G binding receptor, globular head rearrangement and stalk exposure allows the F interaction to switch to a more productive association with the central part of the stalk, leading to F-triggering and membrane fusion. Though atomic structures of full-length Henipavirus G proteins have not yet been obtained, given that Henipavirus G proteins possess a unique, highly structured, disulfide-stabilized region in the upper stalk (Aguilar et al., 2009, Maar et al., 2012), which was found to be important for fusion promotion; the initial pre-receptor-bound G-F complex interaction could possibly occur through this region (Fig. 4C) (Liu et al., 2013). A very recent study by this group has identified a stretch of amino acids at the membrane-distal portion of the NiV G stalk that are important for G–F interactions, receptor induced conformational changes and F-triggering (Liu et al., 2015). Similar to the F–H interactions, it is possible for F–G interactions to occur through the upper C-terminal part of the attachment protein stalk even when all four heads are in a ‘down’ conformation as the shape of the G heads and angle of contact with the stalk most likely varies among paramyxoviruses (compare Fig. 3D and E).
Thus for paramyxoviruses that utilize two glycoproteins to mediate cell entry, it is becoming increasingly clear that the core molecular mechanism of membrane fusion is highly conserved. The attachment protein globular heads act as regulatory domains, which on the binding of host receptors determine the correct spatio-temporal juncture at which the fusion protein must refold in order to productively carry out membrane fusion. To prevent premature F-activation, paramyxoviruses have evolved various different mechanisms that either separate the glycoprotein pairs physically during transport through the exocytic pathway to prevent F–HN interaction or abrogate F-triggering through various intramolecular and intermolecular exchanges between these glycoproteins. For some Pneumovirinae, the spatio-temporal nature of F protein cleavage appears to play a role in activation of F, but significant details in the mechanism of activation for membrane fusion in this subfamily of paramyxoviruses remain unknown (Fig. 4D).
Acknowledgments
Research in the authors׳ laboratories was supported in part by National Institutes of Health Research Grants R01-AI-23173 (to R.A.L.), and R01-GM-61050 (to T.S.J.). R.A.L. is an Investigator of the Howard Hughes Medical Institute
Contributor Information
Sayantan Bose, Email: sayantan_bose@hms.harvard.edu.
Robert A. Lamb, Email: ralamb@northwestern.edu.
References
- Adamson P., Thammawat S., Muchondo G., Sadlon T., Gordon D. Diversity in glycosaminoglycan binding amongst hMPV G protein lineages. Viruses. 2012;4:3785–3803. doi: 10.3390/v4123785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ader N., Brindley M., Avila M., Orvell C., Horvat B., Hiltensperger G., Schneider-Schaulies J., Vandevelde M., Zurbriggen A., Plemper R.K., Plattet P. Mechanism for active membrane fusion triggering by morbillivirus attachment protein. J. Virol. 2013;87:314–326. doi: 10.1128/JVI.01826-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ader N., Brindley M.A., Avila M., Origgi F.C., Langedijk J., Orvell C., Vandevelde M., Zurbriggen A., Plemper R.K., Plattet P. Structural rearrangements of the central region of the morbillivirus attachment protein stalk domain trigger F protein refolding for membrane fusion. J. Biol. Chem. 2012;287:16324–16334. doi: 10.1074/jbc.M112.342493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar H.C., Ataman Z.A., Aspericueta V., Fang A.Q., Stroud M., Negrete O.A., Kammerer R.A., Lee B. A novel receptor-induced activation site in the Nipah virus attachment glycoprotein (G) involved in triggering the fusion glycoprotein (F) J. Biol. Chem. 2009;284:1628–1635. doi: 10.1074/jbc.M807469200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar H.C., Matreyek K.A., Filone C.M., Hashimi S.T., Levroney E.L., Negrete O.A., Betrolotti-Ciarlet A., Choi D.Y., McHardy I., Fulcher J.A., Su S.V., Wolf M.C., Kohatsu L., Baum L.G., Lee B. N-glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J. Virol. 2006;80:4878–4889. doi: 10.1128/JVI.80.10.4878-4889.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C., Vongpunsawad S., Nakamura T., James C.D., Schroeder M., Cattaneo R., Giannini C., Krempski J., Peng K.W., Goble J.M., Uhm J.H., Russell S.J., Galanis E. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66:11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- Apte-Sengupta S., Navaratnarajah C.K., Cattaneo R. Hydrophobic and charged residues in the central segment of the measles virus hemagglutinin stalk mediate transmission of the fusion-triggering signal. J. Virol. 2013;87:10401–10404. doi: 10.1128/JVI.01547-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte-Sengupta S., Negi S., Leonard V.H., Oezguen N., Navaratnarajah C.K., Braun W., Cattaneo R. Base of the measles virus fusion trimer head receives the signal that triggers membrane fusion. J. Biol. Chem. 2012;287:33026–33035. doi: 10.1074/jbc.M112.373308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila M., Alves L., Khosravi M., Ader-Ebert N., Origgi F., Schneider-Schaulies J., Zurbriggen A., Plemper R.K., Plattet P. Molecular determinants defining the triggering range of prefusion F complexes of canine distemper virus. J. Virol. 2014;88:2951–2966. doi: 10.1128/JVI.03123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartesaghi A., Merk A., Borgnia M.J., Milne J.L., Subramaniam S. Prefusion structure of trimeric HIV-1 envelope glycoprotein determined by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2013;20:1352–1357. doi: 10.1038/nsmb.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera A.K., Matsuse H., Kumar M., Kong X., Lockey R.F., Mohapatra S.S. Blocking intercellular adhesion molecule-1 on human epithelial cells decreases respiratory syncytial virus infection. Biochem. Biophys. Res. Commun. 2001;280:188–195. doi: 10.1006/bbrc.2000.4093. [DOI] [PubMed] [Google Scholar]
- Biacchesi S., Pham Q.N., Skiadopoulos M.H., Murphy B.R., Collins P.L., Buchholz U.J. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J. Virol. 2005;79:12608–12613. doi: 10.1128/JVI.79.19.12608-12613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biacchesi S., Skiadopoulos M.H., Yang L., Lamirande E.W., Tran K.C., Murphy B.R., Collins P.L., Buchholz U.J. Recombinant human metapneumovirus lacking the small hydrophobic SH and/or attachment G glycoprotein: deletion of G yields a promising vaccine candidate. J. Virol. 2004;78:12877–12887. doi: 10.1128/JVI.78.23.12877-12887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop K.A., Hickey A.C., Khetawat D., Patch J.R., Bossart K.N., Zhu Z., Wang L.F., Dimitrov D.S., Broder C.C. Residues in the stalk domain of the Hendra virus G glycoprotein modulate conformational changes associated with receptor binding. J. Virol. 2008;82:11398–11409. doi: 10.1128/JVI.02654-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop K.A., Stantchev T.S., Hickey A.C., Khetawat D., Bossart K.N., Krasnoperov V., Gill P., Feng Y.R., Wang L., Eaton B.T., Wang L.F., Broder C.C. Identification of Hendra virus G glycoprotein residues that are critical for receptor binding. J. Virol. 2007;81:5893–5901. doi: 10.1128/JVI.02022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaparte M.I., Dimitrov A.S., Bossart K.N., Crameri G., Mungall B.A., Bishop K.A., Choudhry V., Dimitrov D.S., Wang L.F., Eaton B.T., Broder C.C. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc. Natl. Acad. Sci. USA. 2005;102:10652–10657. doi: 10.1073/pnas.0504887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Heath C.M., Shah P.A., M. A., Jardetzky T.S., Lamb R.A. Mutations in the paramyxovirus 5 fusion protein reveal domains importnant for fusion triggering and metastability. J. Virol. 2013;87:13520–13531. doi: 10.1128/JVI.02123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Song A.S., Jardetzky T.S., Lamb R.A. Fusion activation through attachment protein stalk domains indicates a conserved core mechamism of paramyxovirus entry into cells. J. Virol. 2014;88:3925–3941. doi: 10.1128/JVI.03741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Welch B.D., Kors C.A., Yuan P., Jardetzky T.S., Lamb R.A. Structure and mutagenesis of the parainfluenza virus 5 hemagglutinin-neuraminidase stalk domain reveals a four-helix bundle and the role of the stalk in fusion promotion. J. Virol. 2011;85:12855–12866. doi: 10.1128/JVI.06350-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Zokarkar A., Welch B.D., Leser G.P., Jardetzky T.S., Lamb R.A. Fusion activation by a headless parainfluenza virus 5 hemagglutinin-neuraminidase stalk suggests a modular mechanism for triggering. Proc. Natl. Acad. Sci. USA. 2012;109:E2625–E2634. doi: 10.1073/pnas.1213813109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart K.N., Crameri G., Dimitrov A.S., Mungall B.A., Feng Y.R., Patch J.R., Choudhary A., Wang L.F., Eaton B.T., Broder C.C. Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble G glycoprotein of Hendra virus. J. Virol. 2005;79:6690–6702. doi: 10.1128/JVI.79.11.6690-6702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart K.N., Fusco D.L., Broder C.C. Paramyxovirus entry. Adv. Exp. Med. Biol. 2013;790:95–127. doi: 10.1007/978-1-4614-7651-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousse T., Takimoto T., Gorman W.L., Takahashi T., Portner A. Regions on the hemagglutinin-neuraminidase proteins of human parainfluenza virus type-1 and Sendai virus important for membrane fusion. Virology. 1994;204:506–514. doi: 10.1006/viro.1994.1564. [DOI] [PubMed] [Google Scholar]
- Bousse T.L., Taylor G., Krishnamurthy S., Portner A., Samal S.K., Takimoto T. Biological significance of the second receptor binding site of Newcastle disease virus hemagglutinin-neuraminidase protein. J. Virol. 2004;78:13351–13355. doi: 10.1128/JVI.78.23.13351-13355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden T.A., Aricescu A.R., Gilbert R.J., Grimes J.M., Jones E.Y., Stuart D.I. Structural basis of Nipah and Hendra virus attachment to their cell-surface receptor ephrin-B2. Nat. Struct. Biol. 2008;15:567–572. doi: 10.1038/nsmb.1435. [DOI] [PubMed] [Google Scholar]
- Bowden T.A., Crispin M., Harvey D.J., Aricescu A.R., Grimes J.M., Jones E.Y., Stuart D.I. Crystal structure and carbohydrate analysis of Nipah virus attachment glycoprotein: a template for antiviral and vaccine design. J. Virol. 2008;82:11628–11636. doi: 10.1128/JVI.01344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden T.A., Crispin M., Harvey D.J., Jones E.Y., Stuart D.I. Dimeric architecture of the Hendra virus attachment glycoprotein: evidence for a conserved mode of assembly. J. Virol. 2010;84:6208–6217. doi: 10.1128/JVI.00317-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley M.A., Chaudhury S., Plemper R.K. Measles virus glycoprotein complexes preassemble intracellularly and relax during transport to the cell surface in preparation for fusion. J. Virol. 2015;89:1230–1241. doi: 10.1128/JVI.02754-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley M.A., Plattet P., Plemper R.K. Efficient replication of a paramyxovirus independent of full zippering of the fusion protein six-helix bundle domain. Proc. Natl. Acad. Sci. USA. 2014;111:E3795–E3804. doi: 10.1073/pnas.1403609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley M.A., Plemper R.K. Blue native PAGE and biomolecular complementation reveal a tetrameric or higher-order oligomer organization of the physiological measles virus attachment protein H. J. Virol. 2013;84:12174–12184. doi: 10.1128/JVI.01222-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley M.A., Suter R., Schestak I., Kiss G., Wright E.R., Plemper R.K. A stabilized headless measles virus attachment protein stalk efficiently triggers membrane fusion. J. Virol. 2013;87:11693–11703. doi: 10.1128/JVI.01945-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley M.A., Takeda M., Plattet P., Plemper R.K. Triggering the measles virus membrane fusion machinery. Proc. Natl. Acad. Sci. USA. 2012;109:E3018–E3027. doi: 10.1073/pnas.1210925109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J., Bosch B.J., de Haan C.A. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10:e1004502. doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M., Kaufman J., Stahl S., Wingfield P., Gronenborn A.M., Clore G.M. Monomer-trimer equilibrium of the ectodomain of SIV gp41: insight into the mechanism of peptide inhibition of HIV infection. Protein Sci. 1999;8:1904–1907. doi: 10.1110/ps.8.9.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D.C., Fass D., Berger J.M., Kim P.S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Chan Y.P., Lu M., Dutta S., Yan L., Barr J., Flora M., Feng Y.R., Xu K., Nikolov D.B., Wang L.F., Skiniotis G., Broder C.C. Biochemical, conformational and immunogenic analysis of soluble trimeric forms of Henipavirus fusion glycoproteins. J. Virol. 2012;86:11457–11471. doi: 10.1128/JVI.01318-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran K., Sullivan N.J., Felbor U., Whelan S.P., Cunningham J.M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A., Masante C., Buchholz U.J., Dutch R.E. Human metapneumovirus (HMPV) binding and infection are mediated by interactions between the HMPV fusion protein and heparan sulfate. J. Virol. 2012;86:3230–3243. doi: 10.1128/JVI.06706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colf L.A., Juo Z.S., Garcia K.C. Structure of the measles virus hemagglutinin. Nat. Struct. Mol. Biol. 2007;14:1227–1228. doi: 10.1038/nsmb1342. [DOI] [PubMed] [Google Scholar]
- Connolly S.A., Leser G.P., Jardetzky T.S., Lamb R.A. Bimolecular complementation of paramyxovirus fusion and hemagglutinin-neuraminidase proteins enhances fusion: implications for the mechanism of fusion triggering. J. Virol. 2009;83:10857–10868. doi: 10.1128/JVI.01191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly S.A., Leser G.P., Yin H.S., Jardetzky T.S., Lamb R.A. Refolding of a paramyxovirus F protein from prefusion to postfusion conformations observed by liposome binding and electron microscopy. Proc. Natl. Acad. Sci. USA. 2006;103:17903–17908. doi: 10.1073/pnas.0608678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey E.A., Iorio R.M. Mutations in the stalk of the measles virus hemagglutinin protein decrease fusion but do not interfere with virus-specific interaction with the homologous fusion protein. J. Virol. 2007;81:9900–9910. doi: 10.1128/JVI.00909-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crennell S., Takimoto T., Portner A., Taylor G. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat. Struct. Biol. 2000;7:1068–1074. doi: 10.1038/81002. [DOI] [PubMed] [Google Scholar]
- Daszak P., Zambrana-Torrelio C., Bogich T.L., Fernandez M., Epstein J.H., Murray K.A., Hamilton H. Interdisciplinary approaches to understanding disease emergence: the past, present, and future drivers of Nipah virus emergence. Proc. Natl. Acad. Sci. USA. 2012;110:3681–3688. doi: 10.1073/pnas.1201243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R., Wang Z., Mahon P.J., Marinello M., Mirza A., Iorio R.M. Mutations in the Newcastle disease virus hemagglutinin-neuraminidase protein that interfere with its ability to interact with the homologous F protein in the promotion of fusion. Virology. 1999;253:43–54. doi: 10.1006/viro.1998.9501. [DOI] [PubMed] [Google Scholar]
- Deng R., Wang Z., Mirza A.M., Iorio R.M. Localization of a domain on the paramyxovirus attachment protein required for the promotion of cellular fusion by its homologous fusion protein spike. Virology. 1995;209:457–469. doi: 10.1006/viro.1995.1278. [DOI] [PubMed] [Google Scholar]
- Diederich S., Moll M., Klenk H.D., Maisner A. The Nipah virus fusion protein is cleaved within the endosomal compartment. J. Biol. Chem. 2005;280:29899–29903. doi: 10.1074/jbc.M504598200. [DOI] [PubMed] [Google Scholar]
- Doreleijers J.F., Langedijk J.P., Hard K., Boelens R., Rullmann J.A., Schaaper W.M., van Oirschot J.T., Kaptein R. Solution structure of the immunodominant region of protein G of bovine respiratory syncytial virus. Biochemistry. 1996;35:14684–14688. doi: 10.1021/bi9621627. [DOI] [PubMed] [Google Scholar]
- Dorig R.E., Marcil A., Chopra A., Richardson C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Muller M.A., Maganga G.D., Vallo P., Binger T., Gloza-Rausch F., Rasche A., Yordanov S., Seebens A., Oppong S., Adu Sarkodie Y., Pongombo C., Lukashev A.N., Schmidt-Chanasit J., Stocker A., Carneiro A.J., Erbar S., Maisner A., Fronhoffs F., Buettner R., Kalko E.K., Kruppa T., Franke C.R., Kallies R., Yandoko E.R., Herrler G., Reusken C., Hassanin A., Kruger D.H., Matthee S., Ulrich R.G., Leroy E.M., Drosten C. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprex W.P., McQuaid S., Hangartner L., Billeter M.A., Rima B.K. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J. Virol. 1999;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber M.U., Ehler E., Billeter M.A., Naim H.Y. Measles virus spreads in rat hippocampal neurons by cell-to-cell contact and in a polarized fashion. J. Virol. 2002;76:5720–5728. doi: 10.1128/JVI.76.11.5720-5728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M.K., Hu C., Naik S.K., Hallak L.K., Peng K.W., Russell S.J., Dingli D. Mutations in the stalk region of the measles virus hemagglutinin inhibit syncytium formation but not virus entry. J. Virol. 2010;84:10913–10917. doi: 10.1128/JVI.00789-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan S.F., Palermo L.M., Yokoyama C.C., Orefice G., Fornabaio M., Sarkar A., Kellogg G.E., Greengard O., Porotto M., Moscona A. Premature activation of the paramyxovirus fusion protein before target cell attachment with corruption of the viral fusion machinery. J. Biol. Chem. 2011;286:37945–37954. doi: 10.1074/jbc.M111.256248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr P., DeVries A.S., Wallace G., Miller C., Kenyon C., Sweet K., Martin K., White K., Bagstad E., Hooker C., Krawczynski G., Boxrud D., Liu G., Stinchfield P., LeBlanc J., Hickman C., Bahta L., Barskey A., Lynfield R. An outbreak of measles in an undervaccinated community. Pediatrics. 2014;134:e220–e228. doi: 10.1542/peds.2013-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzâlez-Reyes L., Ruiz-Argèuello M.B., Garcâia-Barreno B., Calder L., Lâopez J.A., Albar J.P., Skehel J.J., Wiley D.C., Melero J.A. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc. Natl. Acad. Sci. USA. 2001;98:9859–9864. doi: 10.1073/pnas.151098198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman W.L., Gill D.S., Scroggs R.A., Portner A. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and Sendai virus have high structure-function similarity with limited antigenic cross-reactivity. Virology. 1990;175:211–221. doi: 10.1016/0042-6822(90)90201-2. [DOI] [PubMed] [Google Scholar]
- Hashiguchi T., Kajikawa M., Maita N., Takeda M., Kuroki K., Sasaki K., Kohda D., Yanagi Y., Maenaka K. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc. Natl. Acad. Sci. USA. 2007;104:19535–19540. doi: 10.1073/pnas.0707830104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi T., Ose T., Kubota M., Maita N., Kamishikiryo J., Maenaka K., Yanagi Y. Structure of the measles virus hemagglutinin bound to its cellular receptor SLAM. Nat. Struct. Biol. 2011;18:135–141. doi: 10.1038/nsmb.1969. [DOI] [PubMed] [Google Scholar]
- Heminway B.R., Yu Y., Galinski M.S. Paramyxovirus mediated cell fusion requires co-expression of both the fusion and hemagglutinin-neuraminidase glycoproteins. Virus Res. 1994;31:1–16. doi: 10.1016/0168-1702(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Heminway B.R., Yu Y., Tanaka Y., Perrine K.G., Gustafson E., Bernstein J.M., Galinski M.S. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology. 1994;200:801–805. doi: 10.1006/viro.1994.1245. [DOI] [PubMed] [Google Scholar]
- Holguera J., Villar E., Munoz-Barroso I. Identification of cellular proteins that interact with Newcastle disease virus and human respiratory syncytial virus by a two-dimensional virus overlay protein binding assay (VOPBA) Virus Res. 2014;191:138–142. doi: 10.1016/j.virusres.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. I. Restoration of the infectivity for L cells by direct action of trypsin on L cell-borne Sendai virus. J. Virol. 1971;8:619–629. doi: 10.1128/jvi.8.5.619-629.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ohuchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. III. Structural differences of Sendai viruses grown in eggs and tissue culture cells. J. Virol. 1973;12:1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C.M., Paterson R.G., Shaughnessy M.A., Wood R., Lamb R.A. Biological activity of paramyxovirus fusion proteins: factors influencing formation of syncytia. J. Virol. 1992;66:4564–4569. doi: 10.1128/jvi.66.7.4564-4569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Ray R., Compans R.W. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J. Virol. 1992;66:1528–1534. doi: 10.1128/jvi.66.3.1528-1534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., McGinnes L.W., Morrison T.G. Overexpression of thiol/disulfide isomerases enhances membrane fusion directed by the Newcastle disease virus fusion protein. J. Virol. 2008;82:12039–12048. doi: 10.1128/JVI.01406-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T.S., Lamb R.A. Activation of paramyxovirus membrane fusion and virus entry. Curr. Opin. Virol. 2014;5:24–33. doi: 10.1016/j.coviro.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J.P., Cupo A., Sok D., Stanfield R.L., Lyumkis D., Deller M.C., Klasse P.J., Burton D.R., Sanders R.W., Moore J.P., Ward A.B., Wilson I.A. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron R.A., Buonagurio D.A., Georgiu A.F., Whitehead S.S., Adamus J.E., Clements-Mann M.L., Harris D.O., Randolph V.B., Udem S.A., Murphy B.R., Sidhu M.S. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.H., Donald J.E., Grigoryan G., Leser G.P., Fadeev A.Y., Lamb R.A., DeGrado W.F. Capture and imaging of a prehairpin fusion intermediate of the paramyxovirus PIV5. Proc. Natl. Acad. Sci. USA. 2011;108:20992–20997. doi: 10.1073/pnas.1116034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolokoltsov A.A., Deniger D., Fleming E.H., Roberts N.J., Jr., Karpilow J.M., Davey R.A. Small interfering RNA profiling reveals key role of clathrin-mediated endocytosis and early endosome formation for infection by respiratory syncytial virus. J. Virol. 2007;81:7786–7800. doi: 10.1128/JVI.02780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusat T., Streckert H.J. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch. Virol. 1997;142:1247–1254. doi: 10.1007/s007050050156. [DOI] [PubMed] [Google Scholar]
- Krzyzaniak M.A., Zumstein M.T., Gerez J.A., Picotti P., Helenius A. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathog. 2013;9:e1003309. doi: 10.1371/journal.ppat.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R.A., Jardetzky T.S. Structural basis of viral invasion: lessons from paramyxovirus F. Curr. Opin. Struct. Biol. 2007;17:427–436. doi: 10.1016/j.sbi.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R.A., Parks G.D. Paramyxoviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. fifth edition. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1449–1496. [Google Scholar]
- Lamb R.A., Parks G.D. Paramyxoviridae: The viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. sixth edition. Wolters Kluwer/Lippincott, Williams and Wilkins; Philadelphia: 2013. pp. 957–995. [Google Scholar]
- Lamb R.A., Paterson R.G., Jardetzky T.S. Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology. 2006;344:30–37. doi: 10.1016/j.virol.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langedijk J.P., Schaaper W.M., Meloen R.H., van Oirschot J.T. Proposed three-dimensional model for the attachment protein G of respiratory syncytial virus. J. Gen. Virol. 1996;77(Pt 6):1249–1257. doi: 10.1099/0022-1317-77-6-1249. [DOI] [PubMed] [Google Scholar]
- Lawrence M.C., Borg N.A., Streltsov V.A., Pilling P.A., Epa V.C., Varghese J.N., McKimm-Breschkin J.L., Colman P.M. Structure of the haemagglutinin-neuraminidase from human parainfluenza virus type III. J. Mol. Biol. 2004;335:1343–1357. doi: 10.1016/j.jmb.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Lee J.E., Fusco M.L., Hessell A.J., Oswald W.B., Burton D.R., Saphire E.O. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 2008;454:177–182. doi: 10.1038/nature07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Prussia A., Paal T., White L.K., Snyder J.P., Plemper R.K. Functional interaction between paramyxovirus fusion and attachment proteins. J. Biol. Chem. 2008;283:16561–16572. doi: 10.1074/jbc.M801018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Prussia A., Snyder J.P., Plemper R.K. Reversible inhibition of the fusion activity of measles virus F protein by an engineered intersubunit disulfide bridge. J. Virol. 2007;81:8821–8826. doi: 10.1128/JVI.00754-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyrat C., Paesen G.C., Charleston J., Renner M., Grimes J.M. Structural insights into the human metapneumovirus glycoprotein ectodomain. J. Virol. 2014;88:11611–11616. doi: 10.1128/JVI.01726-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Liu Q., Bradel-Tretheway B., Monreal A.I., Saludes J.P., Lu X., Nicola A.V., Aguilar H.C. Nipah virus attachment glycoprotein stalk C-terminal region links receptor binding to fusion triggering. J. Virol. 2015;89:1838–1850. doi: 10.1128/JVI.02277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Stone J.A., Bradel-Tretheway B., Dabundo J., Benavides Montano J.A., Santos-Montanez J., Biering S.B., Nicola A.V., Iorio R.M., Lu X., Aguilar H.C. Unraveling a three-step spatiotemporal mechanism of triggering of receptor-induced Nipah virus fusion and cell entry. PLoS Pathog. 2013;9:e1003770. doi: 10.1371/journal.ppat.1003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby S.P., Gurley E.S., Hossain M.J. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 2009;49:1743–1748. doi: 10.1086/647951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby S.P., Rahman M., Hossain M.J., Blum L.S., Husain M.M., Gurley E., Khan R., Ahmed B.N., Rahman S., Nahar N., Kenah E., Comer J.A., Ksiazek T.G. Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 2006;12:1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maar D., Harmon B., Chu D., Schulz B., Aguilar H.C., Lee B., Negrete O.A. Cysteines in the stalk of the Nipah virus G glycoprotein are located in a distinct subdomain critical for fusion activation. J. Virol. 2012;86:6632–6642. doi: 10.1128/JVI.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S., Herrero L.J., Playford E.G., Spann K., Herring B., Rolph M.S., Middleton D., McCall B., Field H., Wang L.F. Hendra virus: an emerging paramyxovirus in Australia. Lancet Infect. Dis. 2012;12:799–807. doi: 10.1016/S1473-3099(12)70158-5. [DOI] [PubMed] [Google Scholar]
- Mahon P.J., Mirza A.M., Iorio R.M. Role of the two sialic acid binding sites on the newcastle disease virus HN protein in triggering the interaction with the F protein required for the promotion of fusion. J. Virol. 2011;85:12079–12082. doi: 10.1128/JVI.05679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malashkevich V.N., Schneider B.J., McNally M.L., Milhollen M.A., Pang J.X., Kim P.S. Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9-A resolution. Proc. Natl. Acad. Sci. USA. 1999;96:2662–2667. doi: 10.1073/pnas.96.6.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R., Ward M., Bright H., Priest R., Foster M.R., Hurle M., Blair E., Bird M. Isolation and characterisation of potential respiratory syncytial virus receptor(s) on epithelial cells. Microbes Infect. 2003;5:123–133. doi: 10.1016/s1286-4579(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Manchester M., Eto D.S., Valsamakis A., Liton P.B., Fernandez-Munoz R., Rota P.A., Bellini W.J., Forthal D.N., Oldstone M.B. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 2000;74:3967–3974. doi: 10.1128/jvi.74.9.3967-3974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh G.A., de Jong C., Barr J.A., Tachedjian M., Smith C., Middleton D., Yu M., Todd S., Foord A.J., Haring V., Payne J., Robinson R., Broz I., Crameri G., Field H.E., Wang L.F. Cedar virus: a novel henipavirus isolated from Australian bats. PLoS Pathog. 2012;8:e1002836. doi: 10.1371/journal.ppat.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo M., Navaratnarajah C.K., Syed S., Cattaneo R. The measles virus hemagglutinin beta-propeller head beta4-beta5 hydrophobic groove governs functional interactions with nectin-4 and CD46 but not those with the signaling lymphocytic activation molecule. J. Virol. 2013;87:9208–9216. doi: 10.1128/JVI.01210-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo M., Navaratnarajah C.K., Willenbring R.C., Maroun J., Iankov I., Lopez M., Sinn P.L., Cattaneo R. Different roles of the three loops forming the adhesive interface of nectin-4 in measles virus binding and cell entry, nectin-4 homodimerization, and heterodimerization with nectin-1. J. Virol. 2014;88:14161–14171. doi: 10.1128/JVI.02379-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChesney M.B., Miller C.J., Rota P.A., Zhu Y.D., Antipa L., Lerche N.W., Ahmed R., Bellini W.J. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology. 1997;233:74–84. doi: 10.1006/viro.1997.8576. [DOI] [PubMed] [Google Scholar]
- McLellan J.S., Chen M., Leung S., Graepel K.W., Du X., Yang Y., Zhou T., Baxa U., Yasuda E., Beaumont T., Kumar A., Modjarrad K., Zheng Z., Zhao M., Xia N., Kwong P.D., Graham B.S. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan J.S., Yang Y., Graham B.S., Kwong P.D. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J. Virol. 2011;85:7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson V.R., Iorio R.M. Amino acid substitutions in the F-specific domain in the stalk of the Newcastle disease virus HN protein modulate fusion and interfere with its interaction with the F protein. J. Virol. 2004;78:13053–13061. doi: 10.1128/JVI.78.23.13053-13061.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson V.R., Iorio R.M. Addition of N-glycans in the stalk of the Newcastle disease virus HN protein blocks its interaction with the F protein and prevents fusion. J. Virol. 2006;80:623–633. doi: 10.1128/JVI.80.2.623-633.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza A.M., Iorio R.M. A mutation in the stalk of the NDV HN protein prevents triggering of the F protein despite allowing efficient HN-F complex formation. J. Virol. 2013;87:8813–8815. doi: 10.1128/JVI.01066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishin V.P., Watanabe M., Taylor G., Devincenzo J., Bose M., Portner A., Alymova I.V. N-linked glycan at residue 523 of human parainfluenza virus type 3 hemagglutinin-neuraminidase masks a second receptor-binding site. J. Virol. 2010;84:3094–3100. doi: 10.1128/JVI.02331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T., Portner A. Structure, function, and intracellular processing of the glycoproteins of Paramyxoviridae. In: Kingsbury D.W., editor. The Paramyxoviruses. Plenum Press; New York: 1991. pp. 347–382. [Google Scholar]
- Muhlebach M.D., Mateo M., Sinn P.L., Prufer S., Uhlig K.M., Leonard V.H., Navaratnarajah C.K., Frenzke M., Wong X.X., Sawatsky B., Ramachandran S., McCray P.B., Jr., Cichutek K., von Messling V., Lopez M., Cattaneo R. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Alia M.A., Fernandez-Munoz R., Casasnovas J.M., Porras-Mansilla R., Serrano-Pardo A., Pagan I., Ordobas M., Ramirez R., Celma M.L. Measles virus genetic evolution throughout an imported epidemic outbreak in a highly vaccinated population. Virus Res. 2014;196C:122–127. doi: 10.1016/j.virusres.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. V. An activating enzyme for Sendai virus in the chorioallantoic fluid of the embryonated chicken egg. Microbiol. Immunol. 1980;24:113–122. doi: 10.1111/j.1348-0421.1980.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H.-D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976;72:494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T.F., Rossi B., Rabourdidn-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnarajah C.K., Kumar S., Generous A., Apte-Sengupta S., Mateo M., Cattaneo R. The measles virus hemagglutinin stalk: structures and functions of the central fusion activation and membrane-proximal segments. J. Virol. 2014;88:6158–6167. doi: 10.1128/JVI.02846-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnarajah C.K., Negi S., Braun W., Cattaneo R. Membrane fusion triggering: three modules with different structure and function in the upper half of the measles virus attachment protein stalk. J. Biol. Chem. 2012;287:38543–38551. doi: 10.1074/jbc.M112.410563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnarajah C.K., Oezguen N., Rupp L., Kay L., Leonard V.H., Braun W., Cattaneo R. The heads of the measles virus attachment protein move to transmit the fusion-triggering signal. Nat. Struct. Mol. Biol. 2011;18:128–134. doi: 10.1038/nsmb.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete O.A., Levroney E.L., Aguilar H.C., Bertolotti-Ciarlet A., Nazarian R., Tajyar S., Lee B. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature. 2005;436:401–405. doi: 10.1038/nature03838. [DOI] [PubMed] [Google Scholar]
- Negrete O.A., Wolf M.C., Aguilar H.C., Enterlein S., Wang W., Muhlberger E., Su S.V., Bertolotti-Ciarlet A., Flick R., Lee B. Two key residues in ephrinB3 are critical for its use as an alternative receptor for Nipah virus. PLoS Pathog. 2006;2:e7. doi: 10.1371/journal.ppat.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D.T., Hiebert S.W., Lamb R.A. Different roles of individual N-linked oligosaccharide chains in folding, assembly, and transport of the simian virus 5 hemagglutinin-neuraminidase. Mol. Cell. Biol. 1990;10:1989–2001. doi: 10.1128/mcb.10.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D.T., Randall R.E., Lamb R.A. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J. Cell Biol. 1989;109:3273–3289. doi: 10.1083/jcb.109.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce R.S., Bondre D.G., Ha M.N., Lin L.T., Sisson G., Tsao M.S., Richardson C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7:e1002240. doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paal T., Brindley M.A., St, Clair C., Prussia A., Gaus D., Krumm S.A., Snyder J.P., Plemper R.K. Probing the spatial organization of measles virus fusion complexes. J. Virol. 2009;83:10480–10493. doi: 10.1128/JVI.01195-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pager C.T., Craft W.W., Jr., Patch J., Dutch R.E. A mature and fusogenic form of the Nipah virus fusion protein requires proteolytic processing by cathepsin L. Virology. 2006;346:251–257. doi: 10.1016/j.virol.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pager C.T., Dutch R.E. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J. Virol. 2005;79:12714–12720. doi: 10.1128/JVI.79.20.12714-12720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M., Zhou T., Druz A., Georgiev I.S., Soto C., Gorman J., Huang J., Acharya P., Chuang G.Y., Ofek G., Stewart-Jones G.B., Stuckey J., Bailer R.T., Joyce M.G., Louder M.K., Tumba N., Yang Y., Zhang B., Cohen M.S., Haynes B.F., Mascola J.R., Morris L., Munro J.B., Blanchard S.C., Mothes W., Connors M., Kwong P.D. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevakou G., Allen C., Nakamura T., Zollman P., James C.D., Peng K.W., Schroeder M., Russell S.J., Galanis E. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol. Ther. 2007;15:677–686. doi: 10.1038/sj.mt.6300105. [DOI] [PubMed] [Google Scholar]
- Parks G.D., Lamb R.A. Folding and oligomerization properties of a soluble and secreted form of the paramyxovirus hemagglutinin-neuraminidase glycoprotein. Virology. 1990;178:498–508. doi: 10.1016/0042-6822(90)90347-t. [DOI] [PubMed] [Google Scholar]
- Paterson R.G., Johnson M.L., Lamb R.A. Paramyxovirus fusion (F) protein and hemagglutinin-neuraminidase (HN) protein interactions: intracellular retention of F and HN does not affect transport of the homotypic HN or F protein. Virology. 1997;237:1–9. doi: 10.1006/viro.1997.8759. [DOI] [PubMed] [Google Scholar]
- Plattet P., Langedijk J.P., Zipperle L., Vandevelde M., Orvell C., Zurbriggen A. Conserved leucine residue in the head region of morbillivirus fusion protein regulates the large conformational change during fusion activity. Biochemistry. 2009;48:9112–9121. doi: 10.1021/bi9008566. [DOI] [PubMed] [Google Scholar]
- Plemper R.K., Brindley M.A., Iorio R.M. Structural and mechanistic studies of measles virus illuminate paramyxovirus entry. PLoS Pathog. 2011;7:e1002058. doi: 10.1371/journal.ppat.1002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemper R.K., Hammond A.L., Cattaneo R. Measles virus envelope glycoproteins hetero-oligomerize in the endoplasmic reticulum. J. Biol. Chem. 2001;276:44239–44246. doi: 10.1074/jbc.M105967200. [DOI] [PubMed] [Google Scholar]
- Plemper R.K., Hammond A.L., Gerlier D., Fielding A.K., Cattaneo R. Strength of envelope protein interaction modulates cytopathicity of measles virus. J. Virol. 2002;76:5051–5061. doi: 10.1128/JVI.76.10.5051-5061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poor T.A., Jones L.M., Sood A., Leser G.P., Plasencia M.D., Rempel D.L., Jardetzky T.S., Woods R.J., Gross M.L., Lamb R.A. Probing the paramyxovirus fusion (F) protein-refolding event from pre- to postfusion by oxidative footprinting. Proc. Natl. Acad. Sci. USA. 2014;111:E2596–E2605. doi: 10.1073/pnas.1408983111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M., Devito I., Palmer S.G., Jurgens E.M., Yee J.L., Yokoyama C.C., Pessi A., Moscona A. Spring-loaded model revisited: paramyxovirus fusion requires engagement of a receptor binding protein beyond initial triggering of the fusion protein. J. Virol. 2011;85:12867–12880. doi: 10.1128/JVI.05873-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M., Fornabaio M., Greengard O., Murrell M.T., Kellogg G.E., Moscona A. Paramyxovirus receptor-binding molecules: engagement of one site on the hemagglutinin-neuraminidase protein modulates activity at the second site. J. Virol. 2006;80:1204–1213. doi: 10.1128/JVI.80.3.1204-1213.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M., Fornabaio M., Kellogg G.E., Moscona A. A second receptor binding site on human parainfluenza virus type 3 hemagglutinin-neuraminidase contributes to activation of the fusion mechanism. J. Virol. 2007;81:3216–3228. doi: 10.1128/JVI.02617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M., Murrell M., Greengard O., Moscona A. Triggering of human parainfluenza virus 3 fusion protein (F) by the hemagglutinin-neuraminidase (HN) protein: an HN mutation diminishes the rate of F activation and fusion. J. Virol. 2003;77:3647–3654. doi: 10.1128/JVI.77.6.3647-3654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M., Palmer S.G., Palermo L.M., Moscona A. Mechanism of fusion triggering by human parainfluenza virus type III: communication between viral glycoproteins during entry. J. Biol. Chem. 2012;287:778–793. doi: 10.1074/jbc.M111.298059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M., Salah Z., DeVito I., Talekar A., Palmer S.G., Xu R., Wilson I.A., Moscona A. The second receptor binding site of the globular head of the Newcastle disease virus hemagglutinin-neuraminidase activates the stalk of multiple paramyxovirus receptor binding proteins to trigger fusion. J. Virol. 2012;86:5730–5741. doi: 10.1128/JVI.06793-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M., Salah Z.W., Gui L., Devito I., Jurgens E.M., Lu H., Yokoyama C.C., Palermo L.M., Lee K., Moscona A. Regulation of paramyxovirus fusion activation: the hemagglutinin-neuraminidase protein stabilizes the fusion protein in a pre-triggered state. J. Virol. 2012;86:12838–12848. doi: 10.1128/JVI.01965-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawling J., Cano O., Garcin D., Kolakofsky D., Melero J.A. Recombinant Sendai viruses expressing fusion proteins with two furin cleavage sites mimic the syncytial and receptor-independent infection properties of respiratory syncytial virus. J. Virol. 2011;85:2771–2780. doi: 10.1128/JVI.02065-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawling J., Garcia-Barreno B., Melero J.A. Insertion of the two cleavage sites of the respiratory syncytial virus fusion protein in Sendai virus fusion protein leads to enhanced cell-cell fusion and a decreased dependency on the HN attachment protein for activity. J. Virol. 2008;82:5986–5998. doi: 10.1128/JVI.00078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K.L., Manicassamy B., Lamb R.A. Influenza A virus uses intercellular connections to spread to neighboring cells. J. Virol. 2015;89:1537–1549. doi: 10.1128/JVI.03306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin S.A., Link M.A., Sauder C.J., Zhang C., Ngo L., Rima B.K., Duprex W.P. Recent mumps outbreaks in vaccinated populations: no evidence of immune escape. J. Virol. 2012;86:615–620. doi: 10.1128/JVI.06125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C.J., Jardetzky T.S., Lamb R.A. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 2001;20:4024–4034. doi: 10.1093/emboj/20.15.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C.J., Kantor K.L., Jardetzky T.S., Lamb R.A. A dual-functional paramyxovirus F protein regulatory switch segment: activation and membrane fusion. J. Cell Biol. 2003;163:363–374. doi: 10.1083/jcb.200305130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T., Toyoda T., Gotoh B., Inocencio N.M., Kuma K., Miyata T., Nagai Y. Newcastle disease virus evolution. I. Multiple lineages defined by sequence variability of the hemagglutinin-neuraminidase gene. Virology. 1989;169:260–272. doi: 10.1016/0042-6822(89)90151-7. [DOI] [PubMed] [Google Scholar]
- San-Juan-Vergara H., Sampayo-Escobar V., Reyes N., Cha B., Pacheco-Lugo L., Wong T., Peeples M.E., Collins P.L., Castano M.E., Mohapatra S.S. Cholesterol-rich microdomains as docking platforms for respiratory syncytial virus in normal human bronchial epithelial cells. J. Virol. 2012;86:1832–1843. doi: 10.1128/JVI.06274-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C., Celma M.L., Stehle T., Casasnovas J.M. Structure of the measles virus hemagglutinin bound to the CD46 receptor. Nat. Struct. Mol. Biol. 2010;17:124–129. doi: 10.1038/nsmb.1726. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P.W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974;57:475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schowalter R.M., Chang A., Robach J.G., Buchholz U.J., Dutch R.E. Low-pH triggering of human metapneumovirus fusion: essential residues and importance in entry. J. Virol. 2009;83:1511–1522. doi: 10.1128/JVI.01381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schowalter R.M., Smith S.E., Dutch R.E. Characterization of human metapneumovirus F protein-promoted membrane fusion: critical roles for proteolytic processing and low pH. J. Virol. 2006;80:10931–10941. doi: 10.1128/JVI.01287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergel T., McGinnes L.W., Peeples M.E., Morrison T.G. The attachment function of the Newcastle disease virus hemagglutinin-neuraminidase protein can be separated from fusion promotion by mutation. Virology. 1993;193:717–726. doi: 10.1006/viro.1993.1180. [DOI] [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. USA. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Yu H., Chen X., Lasanajak Y., Tappert M.M., Air G.M., Tiwari V.K., Cao H., Chokhawala H.A., Zheng H., Cummings R.D., Smith D.F. A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J. Biol. Chem. 2011;286:31610–31622. doi: 10.1074/jbc.M111.274217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasakumar N., Ogra P.L., Flanagan T.D. Characteristics of fusion of respiratory syncytial virus with HEp-2 cells as measured by R18 fluorescence dequenching assay. J. Virol. 1991;65:4063–4069. doi: 10.1128/jvi.65.8.4063-4069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M., Pollard H.B. Molecular dissection of nucleolin׳s role in growth and cell proliferation: new insights. FASEB J. 1999;13:1911–1922. [PubMed] [Google Scholar]
- Stone-Hulslander J., Morrison T.G. Mutational analysis of heptad repeats in the membrane-proximal region of Newcastle disease virus HN protein. J. Virol. 1999;73:3630–3637. doi: 10.1128/jvi.73.5.3630-3637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K., Wen X., Leser G.P., Paterson R.G., Lamb R.A., Jardetzky T.S. Structure of the Newcastle disease virus F protein in the post-fusion conformation. Virology. 2010;402:372–379. doi: 10.1016/j.virol.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talekar A., Moscona A., Porotto M. Measles virus fusion machinery activated by sialic acid binding globular domain. J. Virol. 2013;87:13619–13627. doi: 10.1128/JVI.02256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabayashi K., Compans R.W. Functional interaction of paramyxovirus glycoproteins: identification of a domain in Sendai virus HN which promotes cell fusion. J. Virol. 1996;70:6112–6118. doi: 10.1128/jvi.70.9.6112-6118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuo H., Ono N., Tanaka K., Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- Tatsuo H., Ono N., Yanagi Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 2001;75:5842–5850. doi: 10.1128/JVI.75.13.5842-5850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayyari F., Marchant D., Moraes T.J., Duan W., Mastrangelo P., Hegele R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011;17:1132–1135. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- Thammawat S., Sadlon T.A., Hallsworth P.G., Gordon D.L. Role of cellular glycosaminoglycans and charged regions of viral G protein in human metapneumovirus infection. J. Virol. 2008;82:11767–11774. doi: 10.1128/JVI.01208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurudome M., Ito M., Nishio M., Kawano M., Okamoto K., Kusagawa S., Komada H., Ito Y. Identification of regions on the fusion protein of human parainfluenza virus type 2 which are required for haemagglutinin-neuraminidase proteins to promote cell fusion. J. Gen. Virol. 1998;79:279–289. doi: 10.1099/0022-1317-79-2-279. [DOI] [PubMed] [Google Scholar]
- Tsurudome M., Ito M., Nishio M., Nakahashi M., Kawano M., Komada H., Nosaka T., Ito Y. Identification of domains on the fusion (F) protein trimer that influence the hemagglutinin-neuraminidase specificity of the f protein in mediating cell-cell fusion. J. Virol. 2011;85:3153–3161. doi: 10.1128/JVI.01666-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurudome M., Kawano M., Yuasa T., Tabata N., Nishimo M., Komada H., Ito Y. Identification of regions on the hemagglutinin-neuraminidase protein of human parainfluenza virus type 2 important for promoting cell fusion. Virology. 1995;213:190–203. doi: 10.1006/viro.1995.1559. [DOI] [PubMed] [Google Scholar]
- Tsurudome M., Nakahashi M., Matsushima Y., Ito M., Nishio M., Kawano M., Komada H., Nosaka T. Full conversion of the hemagglutinin-neuraminidase specificity of the parainfluenza virus 5 fusion protein by replacement of 21 amino acids in its head region with those of the simian virus 41 fusion protein. J. Virol. 2013;87:8342–8350. doi: 10.1128/JVI.03549-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese J.N., Colman P.M. Three-dimensional structure of the neuraminidase of influenza virus A/Tokyo/3/67 at 2.2 Å resolution. J. Mol. Biol. 1991;221:473–486. doi: 10.1016/0022-2836(91)80068-6. [DOI] [PubMed] [Google Scholar]
- Villar E., Barroso I.M. Role of sialic acid-containing molecules in paramyxovirus entry into the host cell: a minireview. Glycoconj. J. 2006;23:5–17. doi: 10.1007/s10719-006-5433-0. [DOI] [PubMed] [Google Scholar]
- Wang Z., Iorio R.M. Amino acid substitutions in a conserved region in the stalk of the Newcastle disease virus HN glycoprotein spike impair its neuraminidase activity in the globular domain. J. Gen. Virol. 1999;80:749–753. doi: 10.1099/0022-1317-80-3-749. [DOI] [PubMed] [Google Scholar]
- Wang Z., Mirza A.M., Li J., Mahon P.J., Iorio R.M. An oligosaccharide at the C-terminus of the F-specific domain in the stalk of the human parainfluenza virus 3 hemagglutinin-neuraminidase modulates fusion. Virus Res. 2004;99:177–185. doi: 10.1016/j.virusres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W., Calder L.J., Wharton S.A., Skehel J.J., Wiley D.C. The central structural feature of the membrane fusion protein subunit from the Ebola virus glycoprotein is a long triple-stranded coiled coil. Proc. Natl. Acad. Sci. USA. 1998;95:6032–6036. doi: 10.1073/pnas.95.11.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch B.D., Liu Y., Kors C.A., Leser G.P., Jardetzky T.S., Lamb R.A. Structure of the cleavage-activated prefusion form of the parainfluenza virus 5 fusion protein. Proc. Nat. Acad. Sci. USA. 2012;109:16672–16677. doi: 10.1073/pnas.1213802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch B.D., Paduch M., Leser G.P., Bergman Z., Kors C.A., Paterson R.G., Jardetzky T.S., Kossiakoff A.A., Lamb R.A. Probing the functions of the paramyxovirus glycoproteins F and HN with a panel of synthetic antibodies. J. Virol. 2014;88:11713–11725. doi: 10.1128/JVI.01707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch B.D., Yuan P., Bose S., Kors C.A., Lamb R.A., Jardetzky T.S. Structure of the parainfluenza virus 5 (PIV5) hemagglutinin-neuraminidase (HN) ectodomain. PLoS Pathog. 2013;9:e1003534. doi: 10.1371/journal.ppat.1003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Krause J.C., Leser G.P., Cox R.G., Lamb R.A., Williams J.V., Crowe J.E., Jr., Jardetzky T.S. Structure of the human metapneumovirus fusion protein with neutralizing antibody identifies a pneumovirus antigenic site. Nat. Struct. Mol. Biol. 2012;19:461–463. doi: 10.1038/nsmb.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D.C., Skehel J.J. Crystallization and x-ray diffraction studies on the haemagglutinin glycoprotein from the membrane of influenza virus. J. Mol. Biol. 1977;112:343–347. doi: 10.1016/s0022-2836(77)80149-6. [DOI] [PubMed] [Google Scholar]
- Wiley D.C., Skehel J.J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wilson I.A., Skehel J.J., Wiley D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature. 1981;289:366–375. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Xu K., Rajashankar K.R., Chan Y.P., Himanen J.P., Broder C.C., Nikolov D.B. Host cell recognition by the Henipaviruses: crystal structures of the Nipah G attachment glycoprotein and its complex with ephrin-B3. Proc. Natl. Acad. Sci. USA. 2008;105:9953–9958. doi: 10.1073/pnas.0804797105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T.U., Kim J.W., Eom H.E., Oh H.K., Kim E.S., Kang H.J., Nam J.G., Kim K.S., Kim S.S., Lee C.K., Park Y.J., Park O. Resurgence of measles in a country of elimination: interim assessment and current control measures in the Republic of Korea in early 2014. Int. J. Infect. Dis. 2014;33C:1–14. doi: 10.1016/j.ijid.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Yao Q., Hu X., Compans R.W. Association of the parainfluenza virus fusion and hemagglutinin-neuraminidase glycoproteins on cell surfaces. J. Virol. 1997;71:650–656. doi: 10.1128/jvi.71.1.650-656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.-S., Paterson R.G., Wen X., Lamb R.A., Jardetzky T.S. Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc. Natl. Acad. Sci. USA. 2005;102:9288–9293. doi: 10.1073/pnas.0503989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.S., Wen X., Paterson R.G., Lamb R.A., Jardetzky T.S. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature. 2006;439:38–44. doi: 10.1038/nature04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Leser G.P., Demeler B., Lamb R.A., Jardetzky T.S. Domain architecture and oligomerization properties of the paramyxovirus PIV5 hemagglutinin-neuraminidase (HN) protein. Virology. 2008;378:282–291. doi: 10.1016/j.virol.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Paterson R.G., Leser G.P., Lamb R.A., Jardetzky T.S. Structure of the Ulster strain Newcastle disease virus hemagglutinin-neuraminidase reveals auto-inhibitory interactions associated with low virulence. PLoS Pathog. 2012;8:e1002855. doi: 10.1371/journal.ppat.1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Swanson K.A., Leser G.P., Paterson R.G., Lamb R.A., Jardetzky T.S. Structure of the Newcastle disease virus hemagglutinin-neuraminidase (HN) ectodomain reveals a four-helix bundle stalk. Proc. Natl. Acad. Sci. USA. 2011;108:14920–14925. doi: 10.1073/pnas.1111691108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Thompson T., Wurzburg B.A., Paterson R.G., Lamb R.A., Jardetzky T.S. Structural studies of the parainfluenza virus 5 hemagglutinin-neuraminidase tetramer in complex with its receptor, sialyllactose. Structure. 2005;13:1–13. doi: 10.1016/j.str.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Zaitsev V., Von Itzsteine M., Groves D., Kiefel M., Takimoto T., Portner A., Taylor G. Second sialic acid binding site in Newcastle disease virus hemagglutinin-neuraminidase: implications in fusion. J. Virol. 2004;78:3733–3741. doi: 10.1128/JVI.78.7.3733-3741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Singh M., Malashkevich V.N., Kim P.S. Structural characterization of the human respiratory syncytial virus fusion protein core. Proc. Natl. Acad. Sci. USA. 2000;97:14172–14177. doi: 10.1073/pnas.260499197. [DOI] [PMC free article] [PubMed] [Google Scholar]