Abstract
Objective
The goal of this study was to examine associations between physicians’ clinical assessments, their certainty in these assessments, and the likelihood of a patient-centered recommendation about colorectal cancer (CRC) screening in the elderly.
Methods
Two hundred seventy six primary care physicians in the United States read three vignettes about an 80 year old female patient and answered questions about her life expectancy, their confidence in their life expectancy estimate, the balance of benefits/downsides of CRC screening, their certainty in their benefit/downside assessment, and the best course of action regarding CRC screening. We used logistic regression to determine the relationship between these variables and patient-centered recommendations about CRC screening.
Results
In bivariate analyses, physicians had higher odds of making a patient-centered recommendation about CRC screening when their clinical assessments did not lead to a clear screening recommendation or when they experienced uncertainty in their clinical assessments. However, in a multivariate regression model, only benefit/downside assessment and best course of action remained statistically significant predictors of a patient-centered recommendation.
Conclusions
Our findings demonstrate that when the results of clinical assessments do not lead to obvious screening decisions or when the physician feels uncertain about their clinical assessments, they are more likely to make patient-centered recommendations. Existing uncertainty frameworks do not adequately describe the uncertainty associated with patient-centered recommendations found in this study. Adapting or modifying these frameworks to better reflect the constructs associated with uncertainty and the interactions between uncertainty and the complexity inherent in clinical decisions will facilitate a more complete understanding of how and when physicians choose to include patients in clinical decisions.
Introduction
Medical uncertainty in the primary care setting has been well-documented (1–3) and researchers have developed strategies for categorizing, coping with, reducing, and communicating uncertainty in a variety of medical settings. (e.g. 4–6) However, while physicians and patients can manage and reduce uncertainty through various means, they can rarely – if ever – eliminate it. Not surprisingly, physicians perceive and react to medical uncertainty differently. Because physicians’ reactions to uncertainty may influence their decision making, a critical component of medical care, it is important to understand how physicians perceive and respond to uncertainty. Previous research has documented physicians’ varying reactions to uncertainty. (7) These reactions can, in turn, affect how doctors practice medicine, including how they interpret mammograms (8), their willingness to communicate with patients (9), the costs associated with their care (10), and their willingness to engage in shared decision making (11).
Despite the extent of research about uncertainty, it remains a complex concept. Multiple frameworks have been proposed for understanding and classifying uncertainty which attempt to distinguish among multiple types and sources of uncertainty. (2,5,12) For example, technical uncertainty (12) or ambiguity (2,5) may arise because the information needed simply does not exist – there isn’t sufficient or appropriate research or the information is simply unknown. Conceptual uncertainty (12), complexity (2), or risk (5) stems from the challenges inherent to applying data or guidelines generated at the population level to specific situations for individual patients. This type of uncertainty includes the inability to know which patients will experience a complication or negative outcome from a procedure or treatment, or how to apply treatment guidelines to a specific patient, especially when that patient’s characteristics do not precisely match those in the guidelines. Finally, uncertainty that stems from uncertainty about patients’ preferences, wishes, or goals of care may also be at play, often referred to as personal uncertainty. (2,12) These frameworks are useful when conceptualizing uncertainty because they distinguish among uncertainty from unknown or unknowable data, uncertainty related to the inability to apply risk information to specific individuals, and uncertainty about an individual’s unique characteristics (5),
One area in which clinicians face relatively high levels of uncertainty is in elderly patients with complex medical problems. Clinicians face uncertainty when there is limited evidence directly relevant to this population, such as when the elderly are not included in research or treatment guidelines. Additionally, clinicians may face uncertainty because standard clinical guidelines may not directly apply to an individual within a population, and physicians often individualize certain decisions based on an individual’s health states and life expectancies, rather than on standard clinical guidelines. (13,14) Further, they may face personal uncertainty associated with not knowing patients’ wishes or goals of care.
Colorectal cancer screening decision making in the elderly may be a useful context for examining physicians’ uncertainty and perceptions of this uncertainty because physicians may face one or more of the various types of uncertainty described above. Guidelines for colorectal cancer (CRC) screening endorse individualized decision making for patients ages 75 years and older. (13,15) Variation in health state and life expectancy leads to variation in individuals’ likelihood of benefitting from screening. To provide individualized care about CRC screening, physicians must make several clinical assessments including estimating an individual’s life expectancy, weighing the expected benefits and downsides of CRC screening, and assessing whether screening is in the patient’s best interest. The uncertainty that physicians experience with each of these clinical assessments may differ depending on the clinical context. For healthy patients who have a long life expectancy (traditionally accepted as 10 years or more) (16), evidence indicates that CRC screening is likely beneficial and physicians may find recommending CRC screening a relatively easy decision to make. (17,18) Physicians may also find it straightforward to counsel the sickest patients against screening, given that they are unlikely to benefit from screening. However, for patients with moderate morbidities, it may be challenging to make clinical estimates weighing the benefits and downsides of CRC screening and determine the best course of action. Therefore, depending on the individual patient’s health state, physicians may have varying levels of uncertainty about their clinical assessments.
The purpose of this study was to understand more about the relationship between uncertainty and patient-centered decision making in the context of colorectal cancer screening decisions in the elderly. We define patient-centered recommendation as the physician initiating a discussion of colon cancer screening with the patient and basing their recommendation about colon cancer screening on that discussion. Previous studies (17,19–21) have evaluated physicians’ recommendations for CRC screening in the elderly. This study contributes to that literature by examining the association between physicians’ clinical assessments, their confidence and certainty in these assessments, and the likelihood of a patient-centered recommendation when physicians considered CRC screening in three clinical vignettes of 80 year old women in good, fair and poor health.
Methods
This study is part of a larger study examining physicians’ recommendations for CRC screening in elderly patients. This paper reports physicians’ confidence and uncertainty when making clinical assessments about the potential benefits or downsides of CRC screening in case vignettes presented by survey. The Office of Human Research Ethics at the University of North Carolina at Chapel Hill reviewed and approved this study and exempted it from written informed consent. The funding sources had no role in the study.
Participants
We used the American Medical Association Physician Masterfile to identify potentially eligible primary care physicians from across the US based on their self-designated primary specialty of practice code. We included both family physicians and general internists. We excluded internists who practiced a subspecialty, physicians not currently practicing, and geriatricians. Using these eligibility criteria, the Masterfile vendor Medical Marketing Service, Inc. provided a list of 5000 randomly identified eligible general internists and family physicians, from which we randomly selected 650 participants for study to create a self-weighting sample.
Screening questions at the beginning of the survey determined whether physicians 1.)provided direct patient care involving health maintenance for patients 75 years and older and 2.) practiced family medicine or general internal medicine.
Mailings and Follow-up Contacts
The initial mailings occurred in January and February of 2008 and consisted of a cover letter, a 41-item questionnaire, a pre-addressed stamped return envelope, and a small cash incentive. Follow up mailings were sent to non-responders after 2 and 4 weeks.
After three mailings, we attempted to contact the non-responders via fax and offered a larger incentive of $50 to encourage physicians to complete the questionnaire. Physicians who indicated interest in participating were mailed another questionnaire.
In the event that mail was returned indicating that a physician was no longer at the last known address in the Masterfile, we conducted a web search to find a more recent work address or fax number and sent the questionnaire there.
Questionnaire
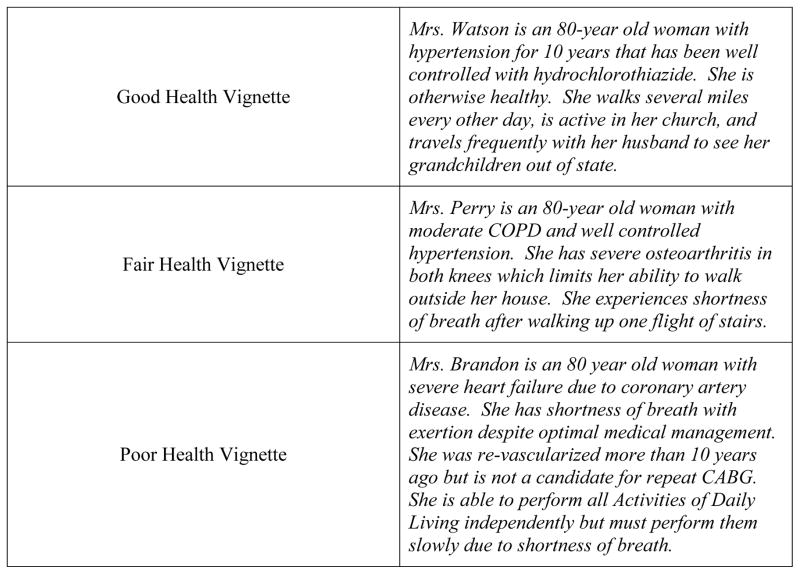
The questionnaire presented three nearly identical vignettes, each followed by an identical set of questions. All vignettes described an 80 year old woman with a negative previous colonoscopy 10 years earlier, but each vignette variably presented clinical data that placed the patient in good, fair, or poor health (Figure 1). All physicians saw all three vignettes. We purposefully chose 80 as the age of interest because the U.S. Preventive Services Task Force recommendations state that CRC screening should be individualized between the ages of 75 and 84. (22) We thus examined decision making within this age group where clinical judgment about the risks and benefits for individuals is expected to drive screening recommendations. The questionnaire was comprised of 11 questions for each vignette, 5 of which form the basis for the analysis presented here, and an additional 8 demographic questions. Except where noted as such, the questions pertaining to the vignettes were Likert-style items with five response categories, the endpoints of which are noted in parentheses after each question described below. The data utilized for this analysis include two types of questions: clinical assessments and physicians’ confidence and certainty in those clinical assessments. The clinical assessment questions include: What is your best estimate of [Patient Name]’s life expectancy? (<2 years, 2–5 years, 6–10 years, and 10+ years); When balancing the potential benefits of colon cancer screening (decrease in colon cancer mortality) against the downsides (risk of perforation from colonoscopy, cost, discomfort, and inconvenience) of screening tests for [Patient’s Name], I believe that: (The benefits clearly outweigh the downsides – The downsides clearly outweigh the benefits); What do you think is the best course of action for [Patient’s Name] regarding colon cancer screening? (I strongly believe that undergoing screening would be in her best interest – I strongly believe that not undergoing screening is in her best interest). The confidence and certainty questions include: How confident are you in the accuracy of your life expectancy estimate for [Patient’s Name]? (Extremely confident – Not at all confident); How certain are you in the way you weighed the potential benefits of colon cancer screening against the downsides of screening tests for [Patient’s Name]? (Extremely certain – Extremely uncertain).
Figure 1.
Clinical Vignettes
Data analysis
We used descriptive statistics to present a summary of the distribution of key variables by vignette. We found no differences between Family Medicine and Internal Medicine physicians’ characteristics or their responses for each of the vignettes and therefore combined the results. We used survey logistic regression techniques in SAS (Cary, NC) to determine the likelihood of physicians making a patient-centered recommendation.
Because we wanted to assess each step of the decision making process, we created six regression models. Five of the models focus on our five independent variables: life expectancy estimate, confidence in life expectancy estimate, benefit/downside assessment, certainty of benefit/downside assessment, and best course of action. We also wanted to explore which of these variables might be most important in influencing a patient-centered recommendation, so the sixth model includes all five components of the decision making process. In all models, we combined results from all three vignettes and controlled for vignette with a dummy variable.
We assessed the risk of multicollinearity by looking at the Spearman correlation between each of the independent predictors used in Models 1 through 5. The benefit/downside and best course of action constructs were the ones most highly correlated with each other, which could account for similarities in their univariate output. However, the relationship between these variables was not strong enough to cause concern with multicollinearity affecting the model estimates. We ran the full model including both the benefit-downsides and the best course of action constructs, and then we removed one or the other and compared the coefficient and standard error estimates and did not observe a substantial change. (23) We also inspected the correlation and covariance matrices of the model and did not find any large off diagonal terms. (24)
Results
Of the 650 questionnaires, 69 physicians were ineligible by the questionnaire’s screening questions and 42 physicians could not be contacted through any available address. We received 276 responses from eligible physicians, with a corrected response rate of 52%.
Among the four demographic characteristics available, only specialty was associated with likelihood of responding, with family physicians being more likely to respond than internists (57% vs. 46%; p<0.01). The average age of respondents was 48 years. Seventy-one percent were men, and 74% were non-Hispanic white. On average, respondents reported that 27% of their patients were age 75 or older.
Clinical assessments and physician perceptions for each vignette
Physician responses to the clinical assessment questions varied by vignette (Table 1). For the good health vignette, most physicians: endorsed life expectancies of 6 or more years (93%), felt that the benefits of screening clearly or probably outweighed the downsides (77%), and believed that screening was the best course of action (79%). Conversely, for the poor health vignette, most physicians: endorsed a life expectancy of 5 years or less (99%), reported that the potential downsides of screening outweighed the benefits (85%), and did not believe screening was in the patient’s best interest (77%). The fair health vignette saw the widest distribution of responses. Most physicians endorsed a life expectancy of 2–5 years (68%). A plurality of physicians felt that the downsides of screening probably outweighed the benefits (40%). More than one-third (36%) were unsure of the best course of action. These results demonstrate the internal validity of this study by affirming our expectation that screening decisions typically correlate with patients’ health status.
Table 1.
Physicians’ assessments and uncertainty, by vignette
| Vignette | |||
|---|---|---|---|
| Good Health n(%) |
Fair Health n(%) |
Poor Health n(%) |
|
| Life Expectancy Estimate | |||
| <2 years | 1(0) | 20(7) | 181(66) |
| 2–5 years | 17(6) | 187 (68) | 90(33) |
| 6–10 years | 127(46) | 66(24) | 5(2) |
| >10 years | 131(47) | 3(1) | 0(0) |
| Confidence in Life Expectancy Estimate | |||
| Extremely Confident | 15(5) | 4(1) | 23(8) |
| Very Confident | 113(41) | 102(37) | 136(49) |
| Neither Confident nor Unconfident | 118(43) | 144(52) | 97(35) |
| Not Very Confident | 26(9) | 24(9) | 18(7) |
| Not at all Confident | 4(1) | 2(1) | 1(0) |
| Potential Benefits of Cancer Screening | |||
| Benefits Clearly Outweigh Downsides | 90 (33) | 23 (8) | 7 (3) |
| Benefits Probably Outweigh Downsides | 121 (44) | 55 (20) | 9 (3) |
| Benefits and Downsides Equal | 42 (15) | 64 (23) | 25 (9) |
| Downsides Probably Outweigh Benefits | 22 (8) | 111 (40) | 108 (39) |
| Downsides Clearly Outweigh Benefits | 1 (0) | 23 (8) | 126 (46) |
| Certainty of Benefits | |||
| Extremely Certain | 32 (12) | 15 (5) | 29 (11) |
| Very Certain | 151 (55) | 128 (47) | 142 (52) |
| Neither Certain nor Uncertain | 85 (31) | 117 (43) | 80 (29) |
| Very Uncertain | 7 (3) | 12 (4) | 18 (7) |
| Extremely Uncertain | 1 (0) | 3 (1) | 6 (2) |
| Best Course of Action | |||
| Strongly Believe Screening is in Patient’s Best Interest | 70 (25) | 21 (8) | 4 (1) |
| Screening is Probably in Patient’s Best Interest | 148 (54) | 55 (20) | 16 (6) |
| Unsure | 42 (15) | 100 (36) | 44 (16) |
| Believe Screening is Probably NOT in Patient’s Best Interest | 15 (5) | 79 (29) | 109 (40) |
| Strongly Believe Screening is NOT in Patient’s Best Interest | 0 (0) | 20 (7) | 102 (37) |
In response to questions about confidence and uncertainty, few physicians clearly endorsed options indicating low confidence or high uncertainty for any of the vignettes. However, a significant proportion in each vignette endorsed the neutral option, being neither confident nor unconfident in their life expectancy estimate (35–52%) and neither certain nor uncertain about their benefit/downside assessment (29%–43%). (Table 1)
Associations with patient-centered recommendations for CRC screening
Our regression results include both physicians’ clinical assessments and their perceptions of those assessments (Table 2). We look first at the relationships between physicians’ clinical assessments and the likelihood that the physician would discuss the decision with the patient (Models 1, 3, and 5). Regression analysis of these associations demonstrated statistically significant relationships (Table 2). As shown in Model 1, the odds of a patient-centered recommendation were higher when the patient’s life expectancy was 2–5 years [OR 2.70; 95% CI 1.58, 4.61] or 6–10 years [OR 2.46; 95% CI 1.20, 5.08], compared to when the life expectancy was shorter (<2 years) or longer (10+ years). The odds of the physician discussing the decision with the patient were about 14 times higher [OR 13.98; 95% CI 6.76, 28.94] when physicians felt that the benefits and downsides of screening were about equal, compared to when they felt the benefits clearly outweighed the downsides (Model 3). The results were similar when we assessed responses to the best course of action question: The odds of discussing the decision with the patient were about 11 times higher [OR 10.98; 95% CI 5.28, 22.74] when the physician was ‘Not sure whether screening is in best interest,’ compared to when they ‘Strongly believe screening is in best interest’ (Model 5). These results support our hypothesis that physicians seek greater patient involvement in the screening decision when their clinical assessments do not directly correspond with screening guidelines for or against screening.
Table 2.
Relationships between Physicians’ Clinical Assessments, Perceptions of their Assessments, and Patient-Centered Screening Recommendations
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) |
p value |
Odds Ratio (95% CI) |
p value |
Odds Ratio (95% CI) |
p value |
Odds Ratio (95% CI) |
p value |
Odds Ratio (95% CI) |
p value |
Odds Ratio (95% CI) |
p value |
|
| Variable | ||||||||||||
| Life Expectancy | 0.0012 | 0.2293 | ||||||||||
| <2 years | 1 [Ref Group] | 1 [Ref Group] | ||||||||||
| 2–5 years | 2.70 (1.58, 4.61) | 1.81 (0.97, 3.39) | ||||||||||
| 6–10 years | 2.46 (1.20, 5.08) | 1.55 (0.66, 3.62) | ||||||||||
| 10+ years | 1.66 (0.72, 3.83) | 1.37 (0.49, 3.79) | ||||||||||
| Confidence in life expectancy estimate | 0.0005 | 0.2147 | ||||||||||
| Extremely confident | 1 [Ref Group] | 1 [Ref Group] | ||||||||||
| Very confident | 2.81 (1.27, 6.20) | 1.55 (0.49, 4.92) | ||||||||||
| Neither confident nor unconfident | 3.94 (1.80, 8.61) | 1.30 (0.42, 4.00) | ||||||||||
| Not very confident | 5.93 (2.31, 15.22) | 2.08 (0.52, 8.42) | ||||||||||
| Not at all confident | 11.49 (1.23, 107.18) | 7.02 (1.04, 47.23) | ||||||||||
| Benefit/Downside Assessment | <0.0001 | 0.0006 | ||||||||||
| Benefits clearly outweigh downsides | 1 [Ref Group] | 1 [Ref Group] | ||||||||||
| Benefits probably outweigh downsides | 5.07 (2.71, 9.49) | 2.71 (1.17, 6.29) | ||||||||||
| Benefits and downsides are about equal | 13.98 (6.76, 28.94) | 6.75 (2.52, 18.08) | ||||||||||
| Downsides probably outweigh benefits | 3.49 (1.66, 7.35) | 3.37 (1.14, 9.93) | ||||||||||
| Downsides clearly outweigh benefits | 0.51 (0.19, 1.39) | 1.88 (0.50, 7.07) | ||||||||||
| Certainty of Benefit/Downside Assessment | <0.0001 | 0.6946 | ||||||||||
| Extremely certain | 1 [Ref Group] | 1 [Ref Group] | ||||||||||
| Very certain | 3.91 (1.56, 9.84) | 1.32 (0.45, 3.91) | ||||||||||
| Neither certain nor uncertain | 9.27 (3.56, 24.13) | 1.56 (0.48, 5.12) | ||||||||||
| Very uncertain | 6.91 (2.04, 23.45) | 1.26 (0.28, 5.66) | ||||||||||
| Extremely uncertain | 2.55 (0.29, 22.84) | 0.62 (0.11, 3.49) | ||||||||||
| Best Course of Action | <0.0001 | <0.0001 | ||||||||||
| Strongly believe screening is in best interest | 1 [Ref Group] | 1 [Ref Group] | ||||||||||
| Believe that screening is probably in best interest | 5.03 (2.65, 9.53) | 2.25 (1.02, 4.93) | ||||||||||
| Not sure whether screening is in best interest | 10.96 (5.28, 22.74) | 2.98 (1.14, 7.82) | ||||||||||
| Believe NOT screening is probably in best interest | 2.08 (0.94, 4.55) | 0.77 (0.26, 2.32) | ||||||||||
| Strongly believe NOT screening is in best interest | 0.23 (0.23, 1.00) | 0.17 (0.03, 0.96) | ||||||||||
Next we look at the relationship between physicians’ perceptions of their clinical assessments and the likelihood that the patient would discuss the decision with the patient (Models 2 and 4). Among physicians’ perceptions of their clinical assessments, less confidence in their life expectancy estimate was associated with higher odds of a patient-centered recommendation (Model 2). A physician who reported not being at all confident had 11.5 times higher odds of discussing the decision with the patient than a physician who was extremely confident [OR 11.49; 95% CI 1.23, 107.18]. Regarding certainty about the benefits/downsides of screening (Model 4), the odds of a physician discussing the decision with the patient were considerably higher when the physician was ‘neither certain nor uncertain’ [OR 9.27, 95% CI 3.56, 24.13] or ‘very uncertain’ [OR 6.91, 95% CI 2.04, 23.45], compared to when they were ‘extremely certain.’ These results support our hypothesis that less confidence and greater uncertainty are associated with a greater likelihood of a patient-centered recommendation.
In the final model (Model 6), which includes all of the dependent variables – clinical assessments as well as physicians’ perceptions – two of the clinical assessments (benefit/downside assessment and best course of action) remained statistically significant, while life expectancy estimates, confidence in life expectancy estimate, and certainty of benefit/downside assessment were no longer statistically significant. The implications of these results are discussed below.
Discussion
As expected, physicians’ clinical assessments varied by the health status of the patient in each vignette. Most physicians reported that the benefits outweighed the harms and that screening was the best course of action for the good health vignette. Conversely, for the poor health vignette, most physicians reported that the harms outweighed the benefits and that screening was not in the patient’s best interest. For the fair health vignette, physicians were more than twice as likely to report being unsure about the best course of action (36%) than in to the best and worst health vignettes (15% and16%, respectively). When we explored associations with patient-centered recommendations – that is, seeking patient input before making a screening recommendation – we found that physicians have greater odds of making a patient-centered recommendation about CRC screening in elderly patients when their clinical assessments of life expectancy, the benefits and downsides of screening, and the best course of action do not lend themselves to a clear screening recommendation. They also have greater odds of a patient-centered recommendation when they are less confident about their assessments of the patient’s life expectancy or experience greater uncertainty in their assessment of the risks and benefits of screening. However, when both clinical assessments and physicians’ confidence and certainty in those assessments are taken together, as in the full regression model (Model 6), only two clinical assessments – benefit/downside assessment and best course of action – remain statistically significant. These results suggest that clinicians’ subjective uncertainty in their clinical assessments may be a key driver in making patient centered recommendations. Interestingly, we found that few physicians clearly endorsed or acknowledged uncertainty in response to direct questioning (the confidence and certainty questions). However, a larger proportion indirectly implied uncertainty by indicating that they were unsure about the best course of action.
Shared or individualized decision making has been promoted for use when the best course of action is a close call (25) or when the best course of action depends upon how individual patients value specific potential outcomes. (3) Our findings support this idea by demonstrating that physicians are more likely to consult with the patient before making a recommendation when they are uncertain or when the results of their clinical assessments do not suggest an obvious screening decision. These results confirm the findings of our previous research (18) and demonstrate that uncertainty is correlated with patient-centered recommendations in a national survey of physicians. The current findings also extend our previous research because we explored which steps in the clinical assessment are associated with a patient-centered recommendation..
In this study, we developed questions related to the decision making process for a specific clinical decision - cancer screening in the elderly. While we designed our questions to capture discrete steps in the clinical decision making process and examine the relationship between uncertainty and involving patients in the decision making process, there may be significant overlap in these constructs. We found that benefit/downside and best course of action were correlated, and though the models were stable, these constructs may indeed be conceptually similar. Our findings suggest that one of the challenges in examining drivers of patient centered care may be isolating discrete clinical steps as clinical decision making is such a complex cognitive task.
Consequently, existing frameworks of uncertainty may need to be extended to account for such complexity. For example, assessing subjective uncertainty about providers’ clinical assessments may be an important aspect of clinical decision making in some situations. Further, current frameworks may not adequately account for overlap between the types of uncertainty physicians experience as they make clinical decisions. For example, when physicians balance the benefits and downsides, they may be grappling with several types of uncertainty simultaneously. Our results suggest that one strategy clinicians use to address this uncertainty is to solicit information about the patient’s preferences and goals of care. This may be an effort to decrease one type of uncertainty - personal uncertainty. Extending existing frameworks of uncertainty to capture the interactions between types of uncertainty and cognitive complexity inherent in clinical decisions will facilitate a fuller understanding of how and when physicians choose to include patients in clinical decisions.
There are several limitations to our study. The physicians in this study responded to hypothetical clinical vignettes, which may not accurately capture real world decision making. In addition, the vignettes all discussed an 80 year old female patient and CRC screening, so the results may not generalize to men, the non-elderly, screening for other diseases, or for treatment decisions. CRC decision making may differ from decision making on other topics, most notably because of the long time between the decision and the potential for benefit – typically at least 5 years. Further, this research is exploratory. As the vignettes were always presented in the same order, there may have been an ordering effect. Due to some small cell sizes, the confidence intervals for some regression results are quite large, indicating a lack of precision. To confirm our findings, we have run similar models with collapsed variables and found roughly the same results (models not shown).
Conclusion
Our findings demonstrate that when the results of clinical assessments do not lead to obvious screening decisions or when the physician feels uncertain about their clinical assessments, they are more likely to make patient-centered recommendations. Importantly, the uncertainty associated with patient-centered recommendations in this study is not adequately described by existing uncertainty frameworks. Adapting or modifying these frameworks to better reflect the constructs associated with uncertainty and the interactions between uncertainty and the complexity inherent in clinical decisions will facilitate a more complete understanding of how and when physicians choose to include patients in clinical decisions.
Footnotes
Financial support for this study was provided in part for Dr. Lewis by a grant from the National Cancer Institute (K07 CA104428); for Dr. Pignone by a grant from National Cancer Institute (K05 CA129166); and for Dr. Esserman by a grant from the NIH CTSA (UL1TR000142; UL1TR000083). The funding agreements ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
An earlier version of this work was presented at the 2013 Annual Meeting of the Society of General Internal Medicine, in Denver, CO.
The authors report no conflicts of interest related to this study.
References
- 1.Eddy DM. Variations in physician practice: The role of uncertainty. Health Aff. 1984;3:74–89. doi: 10.1377/hlthaff.3.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Han PK, Klein WM, Arora NK. Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. 2011;31:828–38. doi: 10.1177/0272989X11393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Politi MC, Lewis CL, Frosch DL. Supporting shared decisions when clinical evidence in low. Med Care Res Rev. 2013;70:113S–128S. doi: 10.1177/1077558712458456. [DOI] [PubMed] [Google Scholar]
- 4.Mishel MH. Uncertainty in illness. Image J Nurs Sch. 1988;20:225–32. doi: 10.1111/j.1547-5069.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 5.Politi MC, Han PKJ, Cole NF. Communicating the uncertainty of harms and benefits of medical interventions. Med Decis Making. 2007;27:681–95. doi: 10.1177/0272989X07307270. [DOI] [PubMed] [Google Scholar]
- 6.Politi MC, Street RL. The importance of communication in collaborative decision making: facilitating shared mind and the management of uncertainty. J Eval Clin Pract. 2011;17:579–84. doi: 10.1111/j.1365-2753.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- 7.Gerrity MS, DeVellis RF, Earp JA. Physicians’ reactions to uncertainty in patient care: A new measure and insights. Med Care. 1990;28:728–36. doi: 10.1097/00005650-199008000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Carney PA, Yi JP, Abraham LA, Miglioretti DL, Aiello EJ, Gerrity MS, Reisch L, Berns EA, Sickles EA, Elmore JG. Reactions to uncertainty and the accuracy of diagnostic mammography. J Gen Intern Med. 2007;22:234–41. doi: 10.1007/s11606-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon GH, Joos SK, Byrne J. Physician expressions of uncertainty during patient encounters. Patient Educ Couns. 2007;40:59–65. doi: 10.1016/s0738-3991(99)00069-5. [DOI] [PubMed] [Google Scholar]
- 10.Allison JJ, Kiefe CI, Cook EF, Gerrity MS, Orav EJ, Centor R. The association of physician attitudes about uncertainty and risk taking with resource use in a Medicare HMO. Med Decis Making. 1998;18:320–9. doi: 10.1177/0272989X9801800310. [DOI] [PubMed] [Google Scholar]
- 11.Politi MC, Légaré F. Physicians’ reactions to uncertainty in the context of shared decision making. Patient Educ Couns. 2010;80:155–7. doi: 10.1016/j.pec.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Beresford EB. Uncertainty and the shaping of medical decisions. Hastings Cent Rep. 1991;21:6–11. [PubMed] [Google Scholar]
- 13.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. J Amer Med Assoc. 2011;285:2750–6. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 14.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. J Amer Med Assoc. 2005;294:716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 15.Gross CP, Soulos PR, Ross JS, Cramer LD, Guerrero C, Tinetti ME, Braithwaite RS. Assessing the Impact of Screening Colonoscopy on Mortality in the Medicare Population. J Gen Intern Med. 2011;26:1441–9. doi: 10.1007/s11606-011-1816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Cornell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colon cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. Brit Med J. 2013;346:e8441. doi: 10.1136/bmj.e8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis CL, Esserman D, DeLeon C, Pignone MP, Pathman DE, Golin C. Physician decision making for colorectal cancer screening in the elderly. J Gen Intern Med. 2013;28:1202–7. doi: 10.1007/s11606-013-2393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis CL, Moore CG, Golin CE, Griffith J, Tytell-Brenner A, Pignone MP. Resident physicians’ life expectancy estimates and colon cancer screening recommendations in elderly patients. Med Decis Making. 2008;28:254–61. doi: 10.1177/0272989X07311756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper GS, Fortinsky RH, Hapke R, Landefeld CS. Primary care physician recommendations for colorectal cancer screening. Patient and practitioner factors. Arch Intern Med. 1997;157:1946–50. [PubMed] [Google Scholar]
- 20.Haggstrom DA, Klabunde CN, Smith JL, Yuan G. Variation in Primary Care Physicians’ Colorectal Cancer Screening Recommendations by Patient Age and Comorbidity. J Gen Intern Med. 2013;28:18–24. doi: 10.1007/s11606-012-2093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahi CJ, van Ryn M, Juliar B, Stuart JS, Imperiale TF. Provider recommendations for colorectal cancer screening in elderly veterans. J Gen Intern Med. 2009;24:1263–8. doi: 10.1007/s11606-009-1110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 23.Stoltzfus JC. Logistic regression: A brief primer. Academic Emergency Medicine. 2011;18:1099–1104. doi: 10.1111/j.1553-2712.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 24.SAS Staff. Usage note 32471: Testing assumptions in logit, probit, Poisson, and other generalized linear models. SAS; [Accessed November 15, 2014]. http://support.sas.com/kb/32/471.html. [Google Scholar]
- 25.Sheridan SL, Harris RP, Woolf SH. Shared decision making about screening and chemoprevention. U.S. Preventive Services Task Force; Shared Decision-Making Workgroup of the U.S. Preventive Services Task Force. [DOI] [PubMed] [Google Scholar]