Abstract
A role for the beta genus HPVs in keratinocyte carcinoma (KC) remains to be established. In this article we examine the potential role of the beta HPVs in cancer revealed by the epidemiology associating these viruses with KC and supported by oncogenic properties of the beta HPV proteins. Unlike the cancer associated alpha genus HPVs, in which transcriptionally active viral genomes are invariably found associated with the cancers, that is not the case for the beta genus HPVs and keratinocyte carcinomas. Thus a role for the beta HPVs in KC would necessarily be in the carcinogenesis initiation and not in the maintenance of the tumor.
Keywords: Papillomavirus, Keratinocyte, Cancer, MAML1, Notch, Epidemiology
Introduction
The papillomaviridae family comprises approximately 200 different human papillomavirus (HPV) types, which are clustered among the five genera alpha, beta, gamma, mu, and nu (www.hpvcenter.se/html/refclones.html). Among the genus alpha HPV types are those that induce benign, non-genital skin warts, the low-risk mucosotropic HPVs that induce genital warts and laryngeal papillomas, and the high-risk mucosotropic HPVs that are associated with cervical cancer, other anogenital cancers and oropharyngeal cancers (Howley, Schiller, and Lowy, 2013).
Genus beta HPV were first identified in flat warts and macular, red, brown, or achromic lesions as well as cutaneous squamous cell carcinomas (SCC) of patients with the rare disease epidermodysplasia verruciformis (EV) and were initially referred to as EV-HPVs. Cutaneous SCC arise in 30% to 60% of EV-patients during the second to fourth decade of life, 10 to 30 years after the onset of benign skin lesions. Cancers are frequently localized in sun-exposed areas of the skin (Orth, 1986). SCC in EV harbor multiple genome copies of specific HPV types, especially HPV5 and HPV8 but also HPV14, HPV20, and a few others (de Oliveira et al., 2004; Dell'Oste et al., 2009; Orth, 1986). Viral transcripts have been described in several of these EV-associated SCCs (reviewed in (Pfister and Ter Schegget, 1997)). In 2009, HPV5 and HPV8 were classified by IARC as “possibly carcinogenic” in EV patients (Bouvard et al., 2009).
The oncogenic potential of some of the beta HPV types (described below) and the consistent finding of certain beta genus HPV types in EV-associated SCC make HPV an attractive etiologic agent for at least some SCCs in individuals who do not have EV (Feltkamp et al., 2008). In the general population, the prevalence of beta HPV-DNA in cutaneous SCC is lower than in EV. In contrast to EV, a diverse spectrum of beta HPV types has been detected and no single types predominate. In those positive tumors, the viral load in tumor biopsies is less than one genome per cell (Weissenborn et al., 2005). In addition, transcriptome sequencing failed to identify papillomavirus expression in any of non-EV associated SCCs indicating that beta HPV is not active in cutaneous SCC (Arron et al., 2011). Thus, in contrast to the alpha genus HPV-associated cancers where transcriptionally active HPV DNA is required for tumor maintenance, the presence of a beta HPV is obviously not mandatory for maintenance of the malignant phenotype in SCCs in the general population. Thus if any of the beta HPVs are associated with SCCs in the general population, their role must be in the initiation of the cancer rather than its maintenance. The prevalence of beta HPV-DNA in precancerous actinic keratosis is higher than in SCCs and higher HPV-DNA loads (up to 50 copies/cell) are compatible with a carcinogenic role of the beta HPVs in early phases of skin cancer development (Weissenborn et al., 2005). Active replication and expression of beta HPVs has been demonstrated in actinic keratosis lesions as well as in the adjacent pathological epithelium of SCCs in renal transplant patients, where beta HPV expression is also associated with increased expression of the cellular proliferation marker MCM7 (Borgogna et al., 2014).
Epidemiology of beta HPV infections
The significance of the association of the beta HPVs and skin cancer is challenged somewhat since these HPV types are ubiquitous and infect the skin of all people as a commensal flora. More than 80% of healthy donors were positive for beta HPV DNA in skin swabs or plucked eyebrow hairs where many different beta HPV types are frequently observed (Antonsson et al., 2000; de Koning et al., 2009). HPV positivity can be demonstrated soon after birth (Antonsson et al., 2003; Weissenborn et al., 2009). The hair follicle is regarded as the natural reservoir for the cutaneous beta HPVs. HPV is present in hair follicles from different body sites such as scalp, eyebrow, arm, trunk, leg, and pubic region (Köhler et al., 2007). The beta HPV type spectrum in eyebrow hair follicles is to a significant degree representative of different body sites suggesting generalized infection of the entire skin. Since eyebrow hairs have therefore served as easily collected marker in many recent epidemiological studies (Plasmeijer et al., 2010).
Case control studies of SCC examining beta HPV-DNA and/or serum antibody have revealed moderate odds ratios in the range of 1.5 to 2.8 with lower 95% confidence interval limits between 1.0 and 1.4 (Bouwes Bavinck et al., 2010; Karagas et al., 2006; Struijk et al., 2003). To assess beta HPV expression serum samples were mostly analyzed for antibodies to the major capsid protein L1. More recently, multiplex serology was used, which allows the simultaneous detection of antibodies against more than 15 beta HPV types. A prospective pilot study based on the prevalence of serum antibodies failed to reveal differences between cases and controls in blood samples taken more than 18 months prior to diagnosis of SCC (Casabonne et al., 2007). This was interpreted to suggest that the antibody response observed in people with SCC is a consequence of tumor formation rather than evidence for previous viral activity and resulted in an IARC working group in 2009 to consider the beta HPVs as not classifiable as to their carcinogenicity in humans (Bouvard et al., 2009). However, this conclusion may not be valid since seroconversion after beta HPV infections has been shown to be extremely slow, occuring years or even decades after initial contact (Michael et al., 2008). Persistent beta HPV infection, as such, may only poorly drive serologic responses without additional “danger” signals such as local inflammation or tumor growth. This could explain that an association with beta HPV seropositivity became only obvious rather late in individuals with prevalent tumors or less than 18 months prior to diagnosis of SCC (Casabonne et al., 2007). Earlier critical viral activities are not excluded by seronegativity. It is highly interesting that beta HPV seropositivity around transplantation implied a hazard ratio of 2.8 to develop KC in organ transplant recipients, who were followed for up to 22 years (Genders et al., 2014).
Recent natural history studies of beta HPV infections have emphasized that the prevalent beta HPVs should be differentiated into types that are transiently detectable and those that persist, with the belief that the beta HPV types that result in persistent infections are more likely to have pathological consequences. Studies of intrafamilial transmission revealed a majority of frequently exchanged beta HPV types and a minority of persisting beta HPV types among individuals (Hsu et al., 2009; Weissenborn et al., 2009). The grouping together of the beta HPV types regardless of whether or not they can cause persistent infections has potentially masked differences among different beta HPV types and has undoubtedly significantly handicapped epidemiologic case control studies. Although more recent epidemiologic studies have tried to focus on the biologically more relevant beta HPV infections, it is still uncertain, which parameters best reflect a biologically relevant beta HPV infection. Different studies have focused on HPV-DNA positivity in the presence of a type specific serological response (Proby et al., 2011), on high viral DNA loads and/or multiple HPV types present (Neale et al., 2013), and/or the presence in the SCC of a patient (Iannacone et al., 2014; Iannacone et al., 2012).
The beta HPV-DNA loads in plucked eyebrow hairs of immunocompetent and immunosuppressed people span 7 orders of magnitude (Weissenborn et al., 2012). Only the beta HPV loads within the highest load decile are close to those observed in EV patients, who are highly predisposed to SCC development (Dell'Oste et al., 2009). The significantly higher proportion of samples from immunosuppressed individuals within the highest load decile correlated with a more than 100-fold increased incidence of cutaneous SCC (Weissenborn et al., 2012). In case control studies, high beta HPV-DNA loads turned out to be associated with cutaneous SCC (Neale et al., 2013). Odds ratios of three were observed for having four or more beta HPV types in the highest load tertile in plucked eyebrow hairs both in immunocompetent Australians (95% CI: 1.11 – 8.09) and in immunosuppressed organ transplant recipients (95% CI: 1.35 – 7.62).
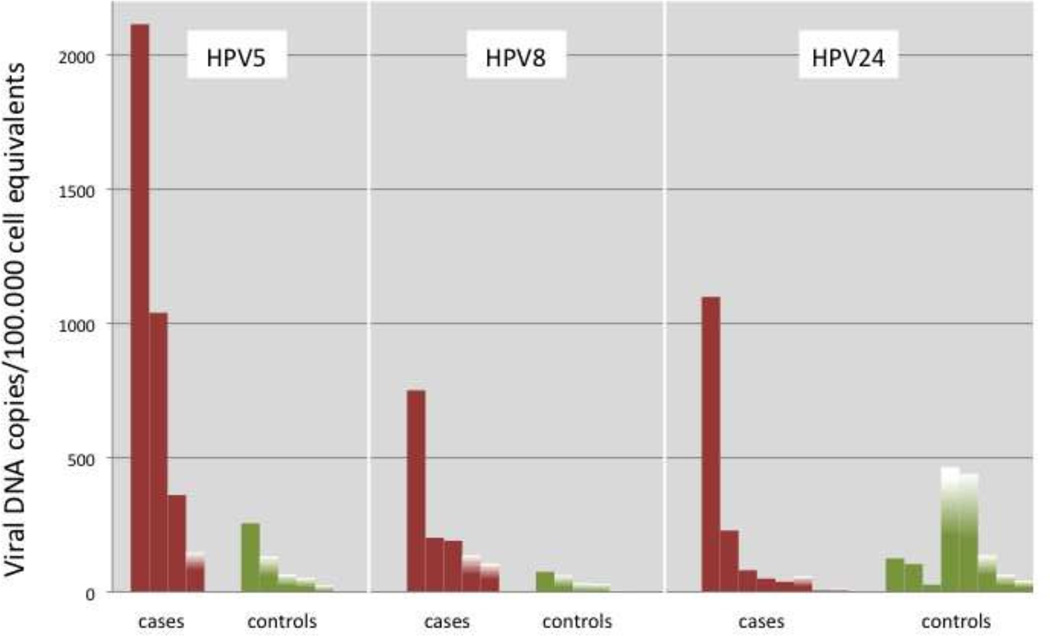
When the association between beta HPV type-specific seropositivity and SCC was stratified by DNA status of the same HPV type in the SCC, significant odds ratios of 3.4 – 3.8 were observed for HPV5, HPV17, and HPV24 (Iannacone et al., 2012). When the association between beta HPV type-specific DNA-positivity in eyebrow hairs and SCC was stratified by DNA status of the same HPV type in the SCC, the DNA prevalence in eyebrow hairs was significantly higher among cases compared to the controls for 11 of 25 genus beta types tested (HPV5, -12, -19, -21, -24 of species beta 1, HPV22, -23, -38, -80 of species beta 2, HPV75 of species beta 3, and HPV92 of species beta 4) (Iannacone et al., 2014). Striking differences were observed in genus beta type-specific DNA loads in plucked eyebrow hairs between controls and SCC cases with DNA of the same HPV type in the tumor (Fig. 1).
Figure 1.
Genus beta type-specific DNA loads in plucked eyebrow hairs of controls and SCC cases who were concordant for a single HPV type in their eyebrow hair and tumor tissue. Controls had a single HPV type in their eyebrow hair that was the same detected in the SCC cases. Fully colored bars show the result of quantitative PCR. Partially colored bars represent quantitative PCR negative but qualitative PCR positive samples with the height giving the threshold of detection of quantitative PCR (Iannacone et al., 2014).
Taken together, recent case control studies have revealed weak beta HPV type-specific associations of seropositivity, DNA prevalence and viral load with SCC. The meaning of the multiplicity of infections and of viral load may provide some suggestion of a dose-response relationship. These observed, although weak associations are consistent with the potential for an etiological role for a subset of the beta HPVs that can establish persistent infections when combined with the growing appreciation for the potential oncogenic activities encoded by the beta HPVs.
Oncogenicity of beta HPVs
The potential oncogenicity of the beta HPVs has been examined using tissue culture transformation and immortalization assays as well as transgenic mice. Several studies have compared various beta HPV types with respect to the ability of their E6 and E7 proteins to transform rodent cells and to immortalize human foreskin keratinocytes (HFKs) (Cornet et al., 2012; Massimi et al., 2008). The oncogenicity of the beta HPVs however is perhaps best demonstrated in transgenic mouse studies. The complete early genome region of HPV8 or the E6 and E7 genes of HPV38 under the control of the human keratin 14 promoter are oncogenic (Schaper et al., 2005; Viarisio et al., 2011). HPV8 mice spontaneously develop papillomas with varying degrees of epidermal dysplasia and SCC develop in 6% of the mice without any additional treatment with physical or chemical carcinogens (Schaper et al., 2005). The HPV38 E6/E7 transgenic mice are highly susceptible to two stage chemical carcinogenesis. Chronic UV irradiation induced actinic keratosis-like lesions and subsequently SCC (Viarisio et al., 2011).
HPV8 E6 (Marcuzzi et al., 2009) and HPV8 E2 (Pfefferle et al., 2008) are independently able to induce skin cancer but with marked differences in kinetics and histology. The HPV8 E6 mice are a phenocopy of mice expressing the complete early genome region, whereas the HPV8 E2 transgenic mice spontaneously develop infundibular hyperplasia and acanthosis and the SCC show an unusually high proportion of spindle cells. The rate of tumor formation in E2 mice critically depends on E2 mRNA levels. In three established lines the E2 mRNA levels differed in the ratio 6:3:1. The mice with the highest E2 mRNA levels developed tumors in their first year of life, only about 60% of mice with medium mRNA levels developed tumors at the end of their second year of life and only few animals of the line with the lowest mRNA levels developed tumors at an age of more than two years. In the HPV8 transgenic mice, a single UV irradiation or mechanical wounding are highly efficient triggers of papilloma development within three weeks. HPV8 oncogene mRNA is induced about 10-fold one day after UV irradiation (Hufbauer et al., 2010; Marcuzzi et al., 2009). Even about one third of the E2 mice with the lowest mRNA level developed skin lesions within three weeks after UV irradiation and a highly aggressive SCC developed in one case within 13 weeks (Pfefferle et al., 2008). Knocking down HPV8 E6 expression by tattooing gene specific siRNA delayed and partially prevented papilloma development induced by wounding in the course of tattooing (Hufbauer et al., 2010). These studies identify enhanced HPV8 oncogene expression as a necessary prerequisite for tumor development and suggest oncogene expression above a certain threshold can induce SCCs. In certain aspects, the mouse model parallels the natural history of human beta HPV infections. Despite continuous, low level expression of the oncogenic transgene in all skin keratinocytes, it takes months and sometimes more than one year to develop skin tumors without experimental induction. Beta HPV infections in humans are ubiquitous and acquired in early childhood but usually very well controlled, persisting with low viral DNA loads. Extensive sun exposure and impaired control following immunosuppression or late in life due to immunosenescence are likely to induce oncogene expression and to allow transgression of a critical threshold of mRNA levels in line with the increased risk of skin cancer. These transgenic models establish the potential oncogenicity of specific beta HPVs. As potential cancer viruses in humans the role of the beta HPVs must however be at an initiation role since their continued expression would not be required in human SCCs.
Oncogenic characteristics of the beta HPV E6 proteins
The high-risk alpha HPVs encode three oncogenes (E5, E6 and E7) of which E6 and E7 are expressed in the associated cancers. Although the beta HPVs do not encode an E5 gene, the beta E6 and E7 genes are structurally quite similar to their alpha genus counterparts. Recent proteomic studies have highlighted similarities and differences between the alpha and beta genus E6 and E7 proteins.
The E6 proteins have been implicated in the potential carcinogenic activities of the beta HPVs. The skin cancers observed in EV patients mostly occur in sun exposed areas suggesting a synergism between the beta HPV infections and the carcinogenic effects of UV light. An important contribution to SCC development could come from interference of the beta E6 proteins with DNA repair and UV-induced apoptosis (Giampieri and Storey, 2004; Jackson and Storey, 2000; Underbrink et al., 2008), allowing keratinocytes with UV-induced mutations to survive and progress to carcinomas. The E6 proteins of HPV5, -8, -20, -22, -38, -76, -92, and -96 have been reported to target the proapoptotic protein Bak for degradation and thus prevent UV-induced apoptosis (Jackson and Storey, 2000; Underbrink et al., 2008). E6 of HPV5 and HPV8 bind p300 resulting in delayed ATR activation (Muller-Schiffmann, Beckmann, and Steger, 2006; Wallace et al., 2012). The delayed ATR activation leads to delayed accumulation of p53, reduced G1 arrest, the increased likelihood of double strand breaks, and delayed repair of UV-damaged DNA. The beta HPV E6 proteins vary however in their effects on p53 stabilization following DNA damage (White et al., 2014) and several (HPV5, -8 and -38) have been shown to inhibit its stabilization following DNA damage (Wallace, Robinson, and Galloway, 2014). Several of the beta E6 proteins have been shown to induce telomerase activity resulting in life span extension (Bedard et al., 2008; Gabet et al., 2008). These functions would support a potential role for the beta HPVs in the initiation of SCC, but not be required for tumor maintenance.
Systematic proteomic studies of the HPV E6 proteins from both the alpha and beta genera have revealed that the alpha HPV E6 proteins are bound to the ubiquitin ligase E6AP whereas the beta HPV E6 proteins are bound to the transcriptional co-activator mastermind (MAML1) (White et al., 2012a). MAML1 is a transcriptional regulator that is involved in several cell signaling pathways and the result of the E6-MAML interaction is the inhibition of Notch mediated transcription (Brimer et al., 2012; Meyers, Spangle, and Munger, 2013; Tan et al., 2012). Although Notch functions as an oncogene in some T-cell leukemias, Notch acts as a tumor suppressor in squamous epithelial cells (McElhinny, Li, and Wu, 2008; Wu et al., 2000). Notch has been found often to be mutated in head and neck cancers (Agrawal et al., 2011; Stransky et al., 2011) . Notch has a role in keratinocyte differentiation; its activation leads to induction of the cell cycle inhibitor p21 as well as the expression of keratinocyte cellular differentiation markers (Rangarajan et al., 2001; Restivo et al., 2011). In addition to binding MAML1, most of the beta E6 proteins are also found in complex with several MAML1 binding partners including Notch1 and RBPJ (Rozenblatt-Rosen et al., 2012; White et al., 2012a).
The genus-specific interactions of the alpha genus E6s with E6AP and the beta genus E6s with MAML1 respectively involve a very similar alpha helical LXXLL binding motif in each of these cellular proteins (Brimer et al., 2012; Chen et al., 1995; Nuber et al., 1996; Tan et al., 2012). This LXXLL motif is believed to be engaged by a flexible linker region on E6 located between the two globular domains that compose the N- and C-terminal halves of E6s (Nomine et al., 2006).
Aside from MAML1 and other Notch associated components, no other cellular proteins complex with all of the beta E6 proteins (White et al., 2012a). A number of other cellular proteins have been shown to bind the beta E6 proteins, but in each case only a subset of E6s (White and Howley, 2013). For instance, several studies have shown that E6 from HPVs 5, 8, 20 and 25 (all from genus beta, species 1) bind to the acetyltransferases CBP and p300 (Howie et al., 2011; Rozenblatt-Rosen et al., 2012; White et al., 2012a). E6 from HPV38 (genus beta, species 2) binds p300 much weaker than E6 from species 1 HPV types (Howie et al., 2011; Muench et al., 2010). In addition, several of the beta E6s (HPV38 and 92) bind and stabilize p53 (White et al., 2012a), however in response to DNA damage, HPV 38 is reported to attenuate p53 signaling (Wallace, Robinson, and Galloway, 2014). Human keratinocyte cell lines expressing various E6 proteins have been profiled for their transcriptional responses to DNA damage and the beta E6 proteins vary in their ability to block such responses (White et al., 2014). Finally, it HPV5 E6 has been to bind SMAD3 and repress TGFβ signaling (Mendoza et al., 2006). These observed differences in biological responses combined with the differences among E6 cellular binding partners indicate that the beta HPVs are not homogeneous with regards to their biologies or the pathologies with which they are associated.
Oncogenic characteristics of the beta HPV E7 proteins
Results from systematic proteomic studies of the HPV E7 proteins revealed patterns of cellular binding proteins that were quite different from those seen with the E6 proteins. Common binding partners were seen for E7 proteins of both the alpha and beta genera suggesting that functions are conserved among the various E7 proteins. The beta E7 proteins, like the alpha E7 proteins, all bind pRB1. The alpha and beta E7 proteins all contain an LXCXE motif responsible for binding to pRB1 and the related ‘pocket proteins’ pRBL1 (p107) and pRBL2 (p130) (White et al., 2012b). In addition to the pRB family of pocket proteins, the pRB E2F partners were observed binding to many of the E7 proteins. There have also been a number of specific studies of the beta HPV E7 proteins with pRB. For instance, HPV38 E7 has been shown to bind pRB with a similar efficiency as HPV16 E7 and promote pRB destabilization (Caldeira et al., 2003). HPV8 E7 binds to pRB only weakly but is nevertheless able to inactivate pRB to deregulate G1/S transition control (Akgül et al., 2007). It is interesting to note in this context that UV-induced oncogene expression in HPV8 transgenic mice was associated with an upregulation of the oncogenic miRNA 106a and a downregulation of its known cellular target pRB (Hufbauer et al., 2011).
In addition to pRB all the alpha and beta HPV E7 proteins bind UBR4, also known as p600 (White et al., 2012b). UBR4 was initially shown to bind E7s from HPV16, 6b, and 11 as well as from the bovine papillomavirus (BPV1) (DeMasi et al., 2005; Huh et al., 2005). UBR4 contains a UBR box, a motif that is common to proteins involved in the N-end rule-mediated degradation of proteins (Tasaki et al., 2005; Tasaki et al., 2009), and it is suspected that it is a functional E3 ubiquitin ligase that functions in an N-end rule pathway. The conservation of the UBR4 interaction with E7s across several PV genera, suggests this interaction is likely to mediate important PV-related functions that have not yet been discovered. In addition, all E7’s complex with KCMF1, another putative E3 ubiquitin ligase that is a binding partner of UBR4 (White et al., 2012b).
Another HPV E7 cellular binding partner shared across the alpha and beta genera is PTPN14, a non-receptor tyrosine phosphatase (White et al., 2012b). PTPN14 has been implicated in density-dependent cell growth (Wang et al., 2012). PTPN14 binds YES1, a regulator of Hippo signaling, and PTPN14 negatively regulates YES1 when cells are at high density. Nothing is known yet about the consequences of E7 binding to PTPN14 and role of this binding in PV biology, but E7/PTPN14 binding suggests the possibility that E7 may affect the Hippo pathway.
Concerning the potential oncogenic role of the beta E7s, it is noteworthy that HPV8 E7 upregulates the expression of the invasion-promoting MT1-matrix metalloproteinase (Akgül et al., 2005; Smola-Hess et al., 2005) and causes invasion of keratinocytes into the dermis of organotypic keratinocyte cultures (Akgül et al., 2005). E7 of HPV5, -8, and -20 increases the number of stem cell-like keratinocytes, defined by high expression of surface markers CD44 and EpCAM, with increased clonogenicity and strongly positive in tumor sphere assays (Hufbauer et al., 2013).
Oncogenic characteristics of the beta HPV E2 proteins
As noted above, the most compelling evidence for the oncogenicity of E2 derives from transgenic mouse experiments in which HPV8 E2 is expressed from the human keratin 14 promoter (Pfefferle et al., 2008). The E2 gene is highly conserved among all papillomaviruses and has key roles in the regulation of virus transcription, viral DNA replication and in plasmid maintenance in dividing cells (Howley, Schiller, and Lowy, 2013). The C-terminal portion of E2 is conserved and consists of a dimerization and sequence specific DNA-binding domain that recognizes cognate sites within the non-coding region upstream of the E6 and E7 genes and adjacent to the origin of DNA replication. The N-terminal portion of E2 is also conserved and binds the viral E1 helicase protein to help recruit it to the origin to initiate viral DNA replication. The conserved N-terminus also binds a number of cellular proteins that are involved in mediating E2’s functions. Proteomic studies have defined many binding partners for the alpha and beta genus E2 proteins that are involved in transcription, cell cycle control, RNA processing and apoptosis, among other processes (Muller et al., 2012). The oncogenicity of HPV8 E2 is not yet well understood but could involve the transcriptional regulation of a cellular gene or genes or it could be the consequence of binding to a specific cellular protein or complex.
Summary: A Role for the Beta HPVs in Keratinocyte Carcinoma
The consideration of an etiologic role for the beta HPVs in KCs has been framed largely by the knowledge of the mechanisms by which the alpha genus HPVs cause cancer in which the expression of the viral oncoproteins are required for the maintenance in addition to the initiation of the cancers. Clearly that is not the case for the KCs in which viral transcription is not detected and viral genome load in the cancers is significantly less than one copy per cell. Given the ubiquitous nature of the beta HPVs, epidemiologic studies have proven difficult. The increase of KCs in immunosuppressed patients is highly suggestive of a viral etiology to the cancers (Wieland, Kreuter, and Pfister, 2014). However, a role for these viruses in KCs must be at the early stages of cancer formation; the viruses must participate in cancer initiation.
A number of studies have established the potential oncogenicity of the viruses and their individual genes in transgenic mice as well as in tissue culture models. Furthermore detailed studies of the viral E6 and E7 genes provide mechanistic insights as to how the beta HPVs might participate in initiating carcinogenic processes. The increased risk of KCs in sun exposed areas in patients with EV and in immuno-suppressed patients suggests a synergism between DNA damage from UV light and a mechanistic contribution from the beta HPVs. Several studies with specific beta HPV E6s have shown that they can block DNA damage pathways and inhibit apoptosis in mechanisms that appear to involve E6 binding p300 and /or CBP. The beta E6 proteins also target MAML1, a necessary component of Notch signaling, to inhibit Notch signaling in keratinocytes. Notch plays a role in keratinocyte differentiation and functions as a tumor suppressor gene in epithelial cells. Therefore, inhibiting Notch signaling in keratinocytes can inhibit differentiation and perhaps contribute to the continued proliferation of the HPV infected cell. Through targeting p300 as well as MAML1 and Notch signaling, E6 could function in cancer initiation by allowing a beta HPV infected cell to accumulate the genomic mutations necessary to progress to cancer. The beta HPV E7s also have oncogenic activities. Its binding partners are similar to those of the alpha genus HPVs, and include the pRB family of pocket proteins that are well established as tumor suppressor genes. They also include binding proteins that have been less well studied with regard to their roles in papillomavirus host cell interactions, including UBR4 and PTPN14 (White et al., 2012b).
A major unanswered question assuming the beta HPVs indeed do have an etiologic role in the initiation of KC is why the presence of the viral genome and its expression are not detectable in the cancers. Is there active selection against the beta HPVs? If so, is this selection immunological, or is continued expression of the virus toxic to the cell? One trivial explanation would be that the beta HPV DNA is lost because it its expression is not required for maintenance of the cancer and there is therefore no selection for its persistence. In contrast to the alpha HPV-positive anogenital cancers integration of beta HPV DNA into the cellular genome appears to occur extremely rarely in EV-KC. In one EV-patient HPV5 DNA was not yet integrated in the primary cancer but only in a metastasis (Yabe et al., 1989). Therefore if the major contribution of the beta HPVs to KC is at the initiation stage and viral functions are not required for cancer maintenance since the malignant growth of cancer cells is driven by the accumulated DNA mutations, there would be no selective pressure to maintain the viral DNA.
The beta HPVs have been associated with skin cancers, but an etiological role remains to be proven.
A role for the beta HPVs in non-melanoma skin cancer would be in initiation, not maintenance.
The beta E2, E6 and E7 genes may each have oncogenic functions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J, Zhang N, El-Naggar AK, Jasser SA, Weinstein JN, Trevino L, Drummond JA, Muzny DM, Wu Y, Wood LD, Hruban RH, Westra WH, Koch WM, Califano JA, Gibbs RA, Sidransky D, Vogelstein B, Velculescu VE, Papadopoulos N, Wheeler DA, Kinzler KW, Myers JN. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgül B, Garcia-Escudero R, Ghali L, Pfister HJ, Fuchs PG, Navsaria H, Storey A. The E7 protein of cutaneous human papillomavirus type 8 causes invasion of human keratinocytes into the dermis in organotypic cultures of skin. Cancer Res. 2005;65(6):2216–2223. doi: 10.1158/0008-5472.CAN-04-1952. [DOI] [PubMed] [Google Scholar]
- Akgül B, Ghali L, Davies D, Pfister H, Leigh IM, Storey A. HPV8 early genes modulate differentiation and cell cycle of primary human adult keratinocytes. Exp Dermatol. 2007;16(7):590–599. doi: 10.1111/j.1600-0625.2007.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol. 2000;74(24):11636–11641. doi: 10.1128/jvi.74.24.11636-11641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson A, Karanfilovska S, Lindqvist PG, Hansson BG. General acquisition of human papillomavirus infections of skin occurs in early infancy. J Clin Microbiol. 2003;41(6):2509–2514. doi: 10.1128/JCM.41.6.2509-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arron ST, Ruby JG, Dybbro E, Ganem D, Derisi JL. Transcriptome sequencing demonstrates that human papillomavirus is not active in cutaneous squamous cell carcinoma. J Invest Dermatol. 2011;131(8):1745–1753. doi: 10.1038/jid.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard KM, Underbrink MP, Howie HL, Galloway DA. The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes. J Virol. 2008;82:3894–3902. doi: 10.1128/JVI.01818-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgogna C, Lanfredini S, Peretti A, De Andrea M, Zavattaro E, Colombo E, Quaglia M, Boldorini R, Miglio U, Doorbar J, Bavinck JN, Quint KD, de Koning MN, Landolfo S, Gariglio M. Improved detection reveals active beta-papillomavirus infection in skin lesions from kidney transplant recipients. Mod Pathol. 2014;27(8):1101–1115. doi: 10.1038/modpathol.2013.240. [DOI] [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V Group, W. H. O. I. A. f. R. o. C. M. W. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- Bouwes Bavinck JN, Neale RE, Abeni D, Euvrard S, Green AC, Harwood CA, de Koning MN, Naldi L, Nindl I, Pawlita M, Pfister H, Proby CM, Quint WG, ter Schegget J, Waterboer T, Weissenborn S, Feltkamp MC. Multicenter study of the association between betapapillomavirus infection and cutaneous squamous cell carcinoma. Cancer Res. 2010;70(23):9777–9786. doi: 10.1158/0008-5472.CAN-10-0352. [DOI] [PubMed] [Google Scholar]
- Brimer N, Lyons C, Wallberg AE, Vande Pol SB. Cutaneous papillomavirus E6 oncoproteins associate with MAML1 to repress transactivation and NOTCH signaling. Oncogene. 2012;31:4639–4646. doi: 10.1038/onc.2011.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira S, Zehbe I, Accardi R, Malanchi I, Dong W, Giarre M, de Villiers EM, Filotico R, Boukamp P, Tommasino M. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J Virol. 2003;77(3):2195–2206. doi: 10.1128/JVI.77.3.2195-2206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casabonne D, Michael KM, Waterboer T, Pawlita M, Forslund O, Burk RD, Travis RC, Key TJ, Newton R. A prospective pilot study of antibodies against human papillomaviruses and cutaneous squamous cell carcinoma nested in the Oxford component of the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121(8):1862–1868. doi: 10.1002/ijc.22885. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Reid CE, Band V, Androphy EJ. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science. 1995;269:529–531. doi: 10.1126/science.7624774. [DOI] [PubMed] [Google Scholar]
- Cornet I, Bouvard V, Campo MS, Thomas M, Banks L, Gissmann L, Lamartine J, Sylla BS, Accardi R, Tommasino M. Comparative analysis of transforming properties of E6 and E7 from different Beta human papillomavirus types. J Virol. 2012;86:2366–2370. doi: 10.1128/JVI.06579-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning MN, Weissenborn SJ, Abeni D, Bouwes Bavinck JN, Euvrard S, Green AC, Harwood CA, Naldi L, Neale R, Nindl I, Proby CM, Quint WG, Sampogna F, ter Schegget J, Struijk L, Wieland U, Pfister HJ, Feltkamp MC group, E.-H.-U.-C. Prevalence and associated factors of betapapillomavirus infections in individuals without cutaneous squamous cell carcinoma. J Gen Virol. 2009;90(Pt 7):1611–1621. doi: 10.1099/vir.0.010017-0. [DOI] [PubMed] [Google Scholar]
- de Oliveira WR, He Q, Rady PL, Hughes TK, Neto CF, Rivitti EA, Tyring SK. HPV typing in Brazilian patients with epidermodysplasia verruciformis: high prevalence of EV-HPV 25. J Cutan Med Surg. 2004;8(2):110–115. doi: 10.1007/s10227-003-0100-6. [DOI] [PubMed] [Google Scholar]
- Dell'Oste V, Azzimonti B, De Andrea M, Mondini M, Zavattaro E, Leigheb G, Weissenborn SJ, Pfister H, Michael KM, Waterboer T, Pawlita M, Amantea A, Landolfo S, Gariglio M. High beta-HPV DNA loads and strong seroreactivity are present in epidermodysplasia verruciformis. J Invest Dermatol. 2009;129(4):1026–1034. doi: 10.1038/jid.2008.317. [DOI] [PubMed] [Google Scholar]
- DeMasi J, Huh KW, Nakatani Y, Munger K, Howley PM. Bovine papillomavirus E7 transformation function correlates with cellular p600 protein binding. Proc Natl Acad Sci U S A. 2005;102(32):11486–11491. doi: 10.1073/pnas.0505322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltkamp MC, de Koning MN, Bavinck JN, Ter Schegget J. Betapapillomaviruses: innocent bystanders or causes of skin cancer. J Clin Virol. 2008;43(4):353–360. doi: 10.1016/j.jcv.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Gabet AS, Accardi R, Bellopede A, Popp S, Boukamp P, Sylla BS, Londono-Vallejo JA, Tommasino M. Impairment of the telomere/telomerase system and genomic instability are associated with keratinocyte immortalization induced by the skin human papillomavirus type 38. FASEB J. 2008;22(2):622–632. doi: 10.1096/fj.07-8389com. [DOI] [PubMed] [Google Scholar]
- Genders RE, Mazlom H, Michel A, Plasmeijer EI, Quint KD, Pawlita M, van der Meijden E, Waterboer T, de Fijter H, Claas FH, Wolterbeek R, Feltkamp MC, Bouwes Bavinck JN. The Presence of Betapapillomavirus Antibodies around Transplantation Predicts the Development of Keratinocyte Carcinoma in Organ Transplant Recipients: A Cohort Study. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.456. [DOI] [PubMed] [Google Scholar]
- Giampieri S, Storey A. Repair of UV-induced thymine dimers is compromised in cells expressing the E6 protein from human papillomaviruses types 5 and 18. Br J Cancer. 2004;90(11):2203–2209. doi: 10.1038/sj.bjc.6601829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie HL, Koop JI, Weese J, Robinson K, Wipf G, Kim L, Galloway DA. Beta-HPV 5 and 8 E6 promote p300 degradation by blocking AKT/p300 association. PLoS Pathog. 2011;7(8):e1002211. doi: 10.1371/journal.ppat.1002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley PM, Schiller JT, Lowy DR. Papillomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia: Lippincott, Williams and Wilkins; 2013. pp. 1662–1703. [Google Scholar]
- Hsu JY, Chen AC, Keleher A, McMillan NA, Antonsson A. Shared and persistent asymptomatic cutaneous human papillomavirus infections in healthy skin. J Med Virol. 2009;81(8):1444–1449. doi: 10.1002/jmv.21529. [DOI] [PubMed] [Google Scholar]
- Hufbauer M, Biddle A, Borgogna C, Gariglio M, Doorbar J, Storey A, Pfister H, Mackenzie I, Akgul B. Expression of betapapillomavirus oncogenes increases the number of keratinocytes with stem cell-like properties. J Virol. 2013;87(22):12158–12165. doi: 10.1128/JVI.01510-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufbauer M, Lazic D, Akgul B, Brandsma JL, Pfister H, Weissenborn SJ. Enhanced human papillomavirus type 8 oncogene expression levels are crucial for skin tumorigenesis in transgenic mice. Virology. 2010;403(2):128–136. doi: 10.1016/j.virol.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Hufbauer M, Lazic D, Reinartz M, Akgul B, Pfister H, Weissenborn SJ. Skin tumor formation in human papillomavirus 8 transgenic mice is associated with a deregulation of oncogenic miRNAs and their tumor suppressive targets. J Dermatol Sci. 2011;64(1):7–15. doi: 10.1016/j.jdermsci.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci U S A. 2005;102(32):11492–11497. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannacone MR, Gheit T, Pfister H, Giuliano AR, Messina JL, Fenske NA, Cherpelis BS, Sondak VK, Roetzheim RG, Silling S, Pawlita M, Tommasino M, Rollison DE. Case-control study of genus-beta human papillomaviruses in plucked eyebrow hairs and cutaneous squamous cell carcinoma. Int J Cancer. 2014;134(9):2231–2244. doi: 10.1002/ijc.28552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannacone MR, Gheit T, Waterboer T, Giuliano AR, Messina JL, Fenske NA, Cherpelis BS, Sondak VK, Roetzheim RG, Michael KM, Tommasino M, Pawlita M, Rollison DE. Case-control study of cutaneous human papillomaviruses in squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1303–1313. doi: 10.1158/1055-9965.EPI-12-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Storey A. E6 proteins from diverse cutaneous HPV types inhibit apoptosis in response to UV damage. Oncogene. 2000;19:592–598. doi: 10.1038/sj.onc.1203339. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Nelson HH, Sehr P, Waterboer T, Stukel TA, Andrew A, Green AC, Bavinck JN, Perry A, Spencer S, Rees JR, Mott LA, Pawlita M. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98(6):389–395. doi: 10.1093/jnci/djj092. [DOI] [PubMed] [Google Scholar]
- Köhler A, Forschner T, Meyer T, Ulrich C, Gottschling M, Stockfleth E, Nindl I. Multifocal distribution of cutaneous human papillomavirus types in hairs from different skin areas. Br J Dermatol. 2007;156(5):1078–1080. doi: 10.1111/j.1365-2133.2007.07809.x. [DOI] [PubMed] [Google Scholar]
- Marcuzzi GP, Hufbauer M, Kasper HU, Weissenborn SJ, Smola S, Pfister H. Spontaneous tumour development in human papillomavirus type 8 E6 transgenic mice and rapid induction by UV-light exposure and wounding. J Gen Virol. 2009;90(Pt 12):2855–2864. doi: 10.1099/vir.0.012872-0. [DOI] [PubMed] [Google Scholar]
- Massimi P, Thomas M, Bouvard V, Ruberto I, Campo MS, Tommasino M, Banks L. Comparative transforming potential of different human papillomaviruses associated with non-melanoma skin cancer. Virology. 2008;371(2):374–379. doi: 10.1016/j.virol.2007.10.015. [DOI] [PubMed] [Google Scholar]
- McElhinny AS, Li JL, Wu L. Mastermind-like transcriptional co-activators: emerging roles in regulating cross talk among multiple signaling pathways. Oncogene. 2008;27(38):5138–5147. doi: 10.1038/onc.2008.228. [DOI] [PubMed] [Google Scholar]
- Mendoza JA, Jacob Y, Cassonnet P, Favre M. Human papillomavirus type 5 E6 oncoprotein represses the transforming growth factor beta signaling pathway by binding to SMAD3. J Virol. 2006;80(24):12420–12424. doi: 10.1128/JVI.02576-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JM, Spangle JM, Munger K. The human papillomavirus type 8 E6 protein interferes with NOTCH activation during keratinocyte differentiation. J Virol. 2013;87(8):4762–4767. doi: 10.1128/JVI.02527-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael KM, Waterboer T, Sehr P, Rother A, Reidel U, Boeing H, Bravo IG, Schlehofer J, Gartner BC, Pawlita M. Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog. 2008;4(6):e1000091. doi: 10.1371/journal.ppat.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench P, Probst S, Schuetz J, Leiprecht N, Busch M, Wesselborg S, Stubenrauch F, Iftner T. Cutaneous papillomavirus E6 proteins must interact with p300 and block p53-mediated apoptosis for cellular immortalization and tumorigenesis. Cancer Res. 2010;70(17):6913–6924. doi: 10.1158/0008-5472.CAN-10-1307. [DOI] [PubMed] [Google Scholar]
- Muller M, Jacob Y, Jones L, Weiss A, Brino L, Chantier T, Lotteau V, Favre M, Demeret C. Large scale genotype comparison of human papillomavirus E2-host interaction networks provides new insights for e2 molecular functions. PLoS Pathog. 2012;8(6):e1002761. doi: 10.1371/journal.ppat.1002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Schiffmann A, Beckmann J, Steger G. The E6 protein of the cutaneous human papillomavirus type 8 can stimulate the viral early and late promoters by distinct mechanisms. J Virol. 2006;80(17):8718–8728. doi: 10.1128/JVI.00250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale RE, Weissenborn S, Abeni D, Bavinck JN, Euvrard S, Feltkamp MC, Green AC, Harwood C, de Koning M, Naldi L, Nindl I, Pawlita M, Proby C, Quint WG, Waterboer T, Wieland U, Pfister H. Human papillomavirus load in eyebrow hair follicles and risk of cutaneous squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22(4):719–727. doi: 10.1158/1055-9965.EPI-12-0917-T. [DOI] [PubMed] [Google Scholar]
- Nomine Y, Masson M, Charbonnier S, Zanier K, Ristriani T, Deryckere F, Sibler AP, Desplancq D, Atkinson RA, Weiss E, Orfanoudakis G, Kieffer B, Trave G. Structural and functional analysis of E6 oncoprotein: insights in the molecular pathways of human papillomavirus-mediated pathogenesis. Mol Cell. 2006;21(5):665–678. doi: 10.1016/j.molcel.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Nuber U, Schwarz S, Kaiser P, Schneider R, Scheffner M. Cloning of human ubiquitin-conjugating enzymes UbcH6 and UbcH7 (E2-F1) and characterization of their interaction with E6-AP and RSP5. J. Biol. Chem. 1996;271(5):2795–2800. doi: 10.1074/jbc.271.5.2795. [DOI] [PubMed] [Google Scholar]
- Orth G. Epidermodysplasia verruciformis: A model for understanding the oncogenicity of human papillomaviruses. Ciba Found. Symp. 1986;120:157–174. doi: 10.1002/9780470513309.ch11. [DOI] [PubMed] [Google Scholar]
- Pfefferle R, Marcuzzi GP, Akgul B, Kasper HU, Schulze F, Haase I, Wickenhauser C, Pfister H. The human papillomavirus type 8 E2 protein induces skin tumors in transgenic mice. J Invest Dermatol. 2008;128(9):2310–2315. doi: 10.1038/jid.2008.73. [DOI] [PubMed] [Google Scholar]
- Pfister H, Ter Schegget J. Role of HPV in cutaneous premalignant and malignant tumors. Clin Dermatol. 1997;15(3):335–347. doi: 10.1016/s0738-081x(96)00162-9. [DOI] [PubMed] [Google Scholar]
- Plasmeijer EI, Neale RE, O'Rourke P, Mallitt KA, de Koning MN, Quint W, Buettner PG, Pawlita M, Waterboer T, Green AC, Feltkamp MC. Lack of association between the presence and persistence of betapapillomavirus DNA in eyebrow hairs and betapapillomavirus L1 antibodies in serum. J Gen Virol. 2010;91(Pt 8):2073–2079. doi: 10.1099/vir.0.019976-0. [DOI] [PubMed] [Google Scholar]
- Proby CM, Harwood CA, Neale RE, Green AC, Euvrard S, Naldi L, Tessari G, Feltkamp MC, de Koning MN, Quint WG, Waterboer T, Pawlita M, Weissenborn S, Wieland U, Pfister H, Stockfleth E, Nindl I, Abeni D, Schegget JT, Bouwes Bavinck JN. A case-control study of betapapillomavirus infection and cutaneous squamous cell carcinoma in organ transplant recipients. Am J Transplant. 2011;11(7):1498–1508. doi: 10.1111/j.1600-6143.2011.03589.x. [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, Miele L, Aguet M, Radtke F, Dotto GP. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20(13):3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo G, Nguyen BC, Dziunycz P, Ristorcelli E, Ryan RJ, Ozuysal OY, Di Piazza M, Radtke F, Dixon MJ, Hofbauer GF, Lefort K, Dotto GP. IRF6 is a mediator of Notch pro-differentiation and tumour suppressive function in keratinocytes. EMBO J. 2011;30(22):4571–4585. doi: 10.1038/emboj.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Deo RC, Padi M, Adelmant G, Calderwood MA, Rolland T, Grace M, Dricot A, Askenazi M, Tavares M, Pevzner SJ, Abderazzaq F, Byrdsong D, Carvunis AR, Chen AA, Cheng J, Correll M, Duarte M, Fan C, Feltkamp MC, Ficarro SB, Franchi R, Garg BK, Gulbahce N, Hao T, Holthaus AM, James R, Korkhin A, Litovchick L, Mar JC, Pak TR, Rabello S, Rubio R, Shen Y, Singh S, Spangle JM, Tasan M, Wanamaker S, Webber JT, Roecklein-Canfield J, Johannsen E, Barabasi AL, Beroukhim R, Kieff E, Cusick ME, Hill DE, Munger K, Marto JA, Quackenbush J, Roth FP, DeCaprio JA, Vidal M. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature. 2012;487(7408):491–495. doi: 10.1038/nature11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper ID, Marcuzzi GP, Weissenborn SJ, Kasper HU, Dries V, Smyth N, Fuchs P, Pfister H. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 2005;65(4):1394–1400. doi: 10.1158/0008-5472.CAN-04-3263. [DOI] [PubMed] [Google Scholar]
- Smola-Hess S, Pahne J, Mauch C, Zigrino P, Smola H, Pfister HJ. Expression of membrane type 1 matrix metalloproteinase in papillomavirus-positive cells: role of the human papillomavirus (HPV) 16 and HPV8 E7 gene products. J Gen Virol. 2005;86(Pt 5):1291–1296. doi: 10.1099/vir.0.80551-0. [DOI] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortes ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareno C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, Grandis JR. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struijk L, Bouwes Bavinck JN, Wanningen P, van der Meijden E, Westendorp RG, Ter Schegget J, Feltkamp MC. Presence of human papillomavirus DNA in plucked eyebrow hairs is associated with a history of cutaneous squamous cell carcinoma. J Invest Dermatol. 2003;121(6):1531–1535. doi: 10.1046/j.1523-1747.2003.12632.x. [DOI] [PubMed] [Google Scholar]
- Tan MJ, White EA, Sowa ME, Harper JW, Aster JC, Howley PM. Cutaneous beta-human papillomavirus E6 proteins bind Mastermind-like coactivators and repress Notch signaling. Proc Natl Acad Sci U S A. 2012;109(23):E1473–E1480. doi: 10.1073/pnas.1205991109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki T, Mulder LC, Iwamatsu A, Lee MJ, Davydov IV, Varshavsky A, Muesing M, Kwon YT. A family of mammalian E3 ubiquitin ligases that contain the UBR box motif and recognize N-degrons. Mol Cell Biol. 2005;25(16):7120–7136. doi: 10.1128/MCB.25.16.7120-7136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki T, Zakrzewska A, Dudgeon DD, Jiang Y, Lazo JS, Kwon YT. The substrate recognition domains of the N-end rule pathway. J Biol Chem. 2009;284(3):1884–1895. doi: 10.1074/jbc.M803641200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol. 2008;82:10408–10417. doi: 10.1128/JVI.00902-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viarisio D, Mueller-Decker K, Kloz U, Aengeneyndt B, Kopp-Schneider A, Grone HJ, Gheit T, Flechtenmacher C, Gissmann L, Tommasino M. E6 and E7 from beta HPV38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog. 2011;7(7):e1002125. doi: 10.1371/journal.ppat.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace NA, Robinson K, Galloway DA. Beta human papillomavirus E6 expression inhibits stabilization of p53 and increases tolerance of genomic instability. J Virol. 2014;88(11):6112–6127. doi: 10.1128/JVI.03808-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace NA, Robinson K, Howie HL, Galloway DA. HPV 5 and 8 E6 abrogate ATR activity resulting in increased persistence of UVB induced DNA damage. PLoS Pathog. 2012;8(7):e1002807. doi: 10.1371/journal.ppat.1002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, Park JI, Chen J. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26(17):1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn S, Neale RE, Waterboer T, Abeni D, Bavinck JN, Green AC, Harwood CA, Euvrard S, Feltkamp MC, de Koning MN, Naldi L, Quint WG, Tessari G, Proby CM, Wieland U, Pfister H group, E.- H.-U.-C. Beta-papillomavirus DNA loads in hair follicles of immunocompetent people and organ transplant recipients. Med Microbiol Immunol. 2012;201(2):117–125. doi: 10.1007/s00430-011-0212-3. [DOI] [PubMed] [Google Scholar]
- Weissenborn SJ, De Koning MN, Wieland U, Quint WG, Pfister HJ. Intrafamilial transmission and family-specific spectra of cutaneous betapapillomaviruses. J Virol. 2009;83(2):811–816. doi: 10.1128/JVI.01338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, Majewski S, Pfister H, Wieland U. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol. 2005;125(1):93–97. doi: 10.1111/j.0022-202X.2005.23733.x. [DOI] [PubMed] [Google Scholar]
- White EA, Howley PM. Proteomic approaches to the study of papillomavirus-host interactions. Virology. 2013;435(1):57–69. doi: 10.1016/j.virol.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EA, Kramer RE, Tan MJ, Hayes SD, Harper JW, Howley PM. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J Virol. 2012a;86(24) doi: 10.1128/JVI.02172-12. 13174-131786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EA, Sowa ME, Tan MJ, Jeudy S, Hayes SD, Santha S, Munger K, Harper JW, Howley PM. Systematic identification of interactions between host cell proteins and E7 oncoproteins from diverse human papillomaviruses. Proc Natl Acad Sci U S A. 2012b;109(5):E260–E267. doi: 10.1073/pnas.1116776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EA, Walther J, Javanbakht H, Howley PM. Genus beta human papillomavirus E6 proteins vary in their effects on the transactivation of p53 target genes. J Virol. 2014;88(15):8201–8212. doi: 10.1128/JVI.01197-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland U, Kreuter A, Pfister H. Human papillomavirus and immunosuppression. Curr Probl Dermatol. 2014;45:154–165. doi: 10.1159/000357907. [DOI] [PubMed] [Google Scholar]
- Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet. 2000;26(4):484–489. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- Yabe Y, Tanimura Y, Sakai A, Hitsumoto T, Nohara N. Molecular characteristics and physical state of human papillomavirus DNA change with progressing malignancy: studies in a patient with epidermodysplasia verruciformis. Int J Cancer. 1989;43(6):1022–1028. doi: 10.1002/ijc.2910430611. [DOI] [PubMed] [Google Scholar]