Abstract
The phosphatidylinositol-3-kinase (PI3K)-Akt signaling pathway regulates several key cellular functions including protein synthesis, cell growth, glucose metabolism, and inflammation. Many viruses have evolved mechanisms to manipulate this signaling pathway to ensure successful virus replication. The human herpesviruses undergo both latent and lytic infection, but differ in cell tropism, growth kinetics, and disease manifestations. Herpesviruses express multiple proteins that target the PI3K/Akt cell signaling pathway during the course of their life cycle to facilitate viral infection, replication, latency, and reactivation. Rare human genetic disorders with mutations in either the catalytic or regulatory subunit of PI3K that result in constitutive activation of the protein predispose to severe herpesvirus infections as well as to virus-associated malignancies. Inhibiting the PI3K/Akt pathway or its downstream proteins using drugs already approved for other diseases can block herpesvirus lytic infection and may reduce malignancies associated with latent herpesvirus infections.
Keywords: PI3K, Akt, herpesvirus, herpes simplex, varicella-zoster, cytomegalovirus, Epstein-Barr virus, Kaposi's sarcoma associated herpesvirus
Introduction
The phosphatidylinositol-3-kinase (PI3K)-Akt signaling pathway regulates multiple key cellular functions including protein synthesis, cell growth, glucose metabolism, and inflammation. Viruses are obligatory intracellular pathogens and they usurp host functions for viral gene transcription and translation, genome replication, and progeny virion production. Viruses also suppress host cell stress responses induced by accumulation of viral proteins, free DNA ends associated with virus replication, nutrient and energy depletion, or hypoxia. To manipulate the intracellular environment for optimal viral replication, viruses including the human herpesviruses, have evolved multiple ways to hijack cellular signaling pathways that are critical for maintaining normal cellular functions such as mitogen-activated protein kinase (MAPK), NF-κB, JAK/STAT, and PI3K/Akt pathways. There are eight human herpesviruses-herpes simplex virus (HSV)-1 and -2, varicella zoster virus (VZV), cytomegalovirus (CMV), Epstein–Barr virus (EBV), human herpesvirus (HHV)-6 and HHV-7, and Kaposi sarcoma-associated herpesvirus (KSHV, HHV-8). Each of these viruses replicate in the nucleus and have dual life cycles- lytic replication and latent infection. They encode from about 70 (in the case of VZV) to over 200 (in the case of CMV) proteins, and differ in cell tropism, replication kinetics, and disease manifestations. Like other viruses, human herpesviruses exploit the PI3K/Akt pathway to optimize virus entry, replication, latency, reactivation, and modulation of host innate immune responses (reviewed in (Alwine, 2008; Bhatt and Damania, 2012; Buchkovich et al., 2008; Cooray, 2004; Diehl and Schaal, 2013; Dunn and Connor, 2012; Tsalikis et al., 2013; Walsh and Mohr, 2011)). Here we highlight recent findings on the ability of human herpesviruses to modulate the PI3K/Akt signaling pathway, the effects of mutations in PI3K on herpesvirus infections in humans, and potential strategies to inhibit PI3K to treat herpesvirus infections and virus-associated malignancies.
The PI3K/Akt pathway
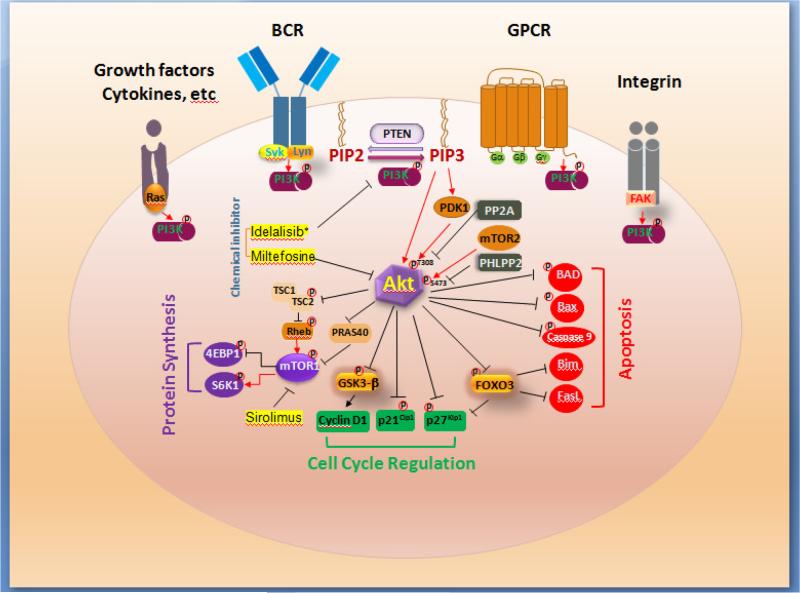
PI3K is activated when extracellular stimuli such as cytokines, growth factors, or viruses bind to cell surface receptors such as G protein coupled receptors (GPCRs), B cell receptors (BCR), or integrins that have tyrosine kinase activity (Fig. 1). This results in translocation of the PI3K complex, which usually consists of a p85 regulatory domain and a p110 catalytic domain, from the cytoplasm to the plasma membrane. Binding of the phosphorylated tyrosine residues on receptors or adapter proteins to the p85 regulatory subunit of PI3K relieves its inhibitory activity on the p110 catalytic domain of PI3K (Cuevas et al., 2001) and allows p110 to phosphorylate membrane-bound phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). This results in recruitment of pleckstrin homology domain containing proteins, including phosphoinositide-dependent protein kinase 1 (PDK1) and the proto-oncoprotein serine/threonine kinase Akt to PIP3 to the plasma membrane. PDK1 phosphorylates Akt at threonine 308, and the mammalian target of rapamycin (mTOR) complex 2 (mTORC2) phosphorylates Akt at serine 473 site to fully activate Akt. PIP3 also binds to its receptor on the endoplasmic reticulum (ER), leading to calcium release from the ER, and activation of calcium signaling which is important for cytoskeletal organization, filopodia formation, and cell-cell fusion.
Fig. 1.
Receptor-mediated activation of PI3K-Akt pathway and signaling. Binding of ligands to cell surface receptors induces activation of PI3K. Activated PI3K converts membrane-bound PIP2 to PIP3. PTEN dephosphorylates PIP3 to form PIP2. PIP3 recruits PDK1 and Akt to the plasma membrane, resulting in Akt phosphorylation by PDK1. Akt can be dephosphorylated by PP2A and PHLPP2. Activated Akt (a) stimulates protein synthesis by phosphorylation of mTOR inhibitor TSC2, leading to mTOR1 activation, and phosphorylation of 4EBP1 (an inhibitor of translation) and S6K1, (b) stimulates cell cycle progression by phosphorylation of cell cycle inhibitors p21Cip1 and p27Kip1 for their degradation, and phosphorylation and inactivation of transcriptional factors GSK-3β and FOXO3, leading to increased cyclin D1 and reduced p27Kip1 expression, and (c) inhibits apoptosis by phosphorylation and inactivation of proapoptotic proteins BAD, Bax, caspase 9, and transcriptional factor FOXO3 to reduce Bim and FasL expression. * indicates that idelalisib blocks PI3Kδ only.
Activated Akt induces phosphorylation of multiple downstream targets. Phosphorylation of cell cycle inhibitors p21Cip1 and p27Kip1 inactivates these proteins and enhances progression of cells from the G1 to S phase of the cell cycle. Phosphorylation and inactivation of glycogen synthase kinase (GSK)-3β by Akt promotes cell growth. Akt phosphorylation of pro-apoptotic proteins BAD, Bim, caspase 9, and phosphorylation of the transcription factor FOXO1results in inactivation of these proteins and inhibits apoptosis. Similarly, increased expression of the anti-apoptotic proteins X-linked inhibitor of apoptosis protein (XIAP), Bcl-xL, Bcl-2, and myeloid cell leukemia 1 (Mcl-1) by Akt enhances cell survival. Phosphorylation and inactivation of the tuberous sclerosis protein 2 (TSC2) results in mTOR1 activation which in turn phosphorylates and inhibits the translational inhibitor eukaryotic translation initiation factor 4E binding protein 1 (4EBP1) and activates S6K1 to stimulate protein synthesis (Hassan et al., 2013; Hemmings and Restuccia, 2012; Vanhaesebroeck et al., 2012).
PI3Ks are divided into three subclasses on the basis of structure, regulation, and lipid substrate specificity. Class I PI3Ks are often involved in the pathogenesis of human cancers and are extensively targeted by viruses (Engelman, 2009; Walsh and Mohr, 2011). Class I PI3Ks are heterodimers consisting of a p110 catalytic subunit (p110α, β, δ, or γ) and a regulatory subunit (p50, p55, p85, or p101). PI3K p110α and p110β are ubiquitously expressed, whereas p110δ and p110γ are primarily found in white blood cells (Okkenhaug, 2013).
PI3K/Akt activity is tightly controlled at multiple steps. PI3K signaling is negatively regulated by several phosphoinositide phosphatases, including the tumor suppressor phosphatase and tensin homolog (PTEN) which dephosphorylates the 3-phosphate from the inositol ring of PIP3 (Stambolic et al., 1998), inositol polyphosphate-4-phosphatase, type II (INPP4B) which dephosphorylates the 4-position phosphate from the inositol ring of PIP2, the PH domain and leucine rich repeat protein phosphatase 2 (PHLPP2) which dephosphorylates Akt at Ser 473 (Brognard et al., 2007), and the protein phosphatase 2 (PP2A) which dephosphorylates Akt at Thr 308 (Andjelkovic et al., 1996; Kuo et al., 2008).
Enhanced PI3K/Akt signaling has been identified in many human cancers including mutation or amplification of the genes encoding catalytic subunits of PI3K p110α and p110δ the gene product of PIK3CA and PI3KCD, respectively) (Angulo et al., 2013; Lucas et al., 2014; Perez-Tenorio et al., 2007; Samuels et al., 2004), loss of function of PTEN (Perez-Tenorio et al., 2007), and/or INPP4B (Balakrishnan and Chaillet, 2013; Bertucci and Mitchell, 2013; Gewinner et al., 2009), or mutation and/or amplification of the proto-oncogenes AKT1 and AKT2 (Ruggeri et al., 1998; Staal, 1987). Therefore, the PI3K/Akt pathway is an important target for drug development for treatment of human malignancies as well as for virus infections.
Herpesviruses modulate the PI3K/Akt pathway
Herpesviruses enhance their replication by modulating the intracellular environment through altering cell signaling pathways to control transcription and translation, regulate cell cycle progression, inhibit apoptosis, evade host defense systems, and alter cellular metabolism. Herpesvirus activation of the PI3K/Akt pathway manipulates many of these activities to favor virus replication or latency. Activation of PI3K/Akt signaling can occur at multiple steps during the virus life cycle including (a) entry and virus glycoprotein binding, (b) release of tegument proteins after virus delivery into the cell, (c) virus replication, and (d) virus latency and reactivation.
Entry of herpesviruses in cells induces activation of PI3K/Akt
Human herpesviruses infect different cell types including epithelial and endothelial cells, macrophages, and lymphocytes. These viruses can enter cells by viral glycoprotein binding to receptors and fusion of viral and cellular membranes either at the cell surface or after endocytosis. The envelope of human herpesviruses contains several glycoproteins, including gB and gH/gL which are shared by all herpesviruses and are essential for mediating membrane fusion. Additional glycoproteins are important for entry of specific viruses such as gD for HSV, gp350 and gp42 for EBV, and UL128, UL130, and UL131A for CMV.
Binding of HSV virions to cellular receptors on the plasma membrane induces changes in cellular gene expression resulting in activation of PI3K/Akt, NF-κB and JAK/STAT signaling (MacLeod and Minson, 2010). Activation of the PI3K/Akt signaling pathway is required for HSV entry into cells. Chemical inhibition of PI3K activity with LY294002 blocked HSV entry and fusion mediated by viral glycoproteins (Tiwari and Shukla, 2010). Inhibition of PI3K with wortmannin blocked trafficking of HSV virions to the periphery of the nucleus (Nicola and Straus, 2004). PI3K inhibition with LY294002 reduced expression of HSV-1 ICP0 and increased the cleavage of caspase-3, caspase-7, and poly ADP-ribose polymerase (PARP), implying that PI3K may reduce apoptosis in HSV-infected cells (Hsu, Wu et al. 2010). HSV infection triggers Akt phosphorylation within minutes after infection (Cheshenko et al., 2013; Hsu et al., 2010; MacLeod and Minson, 2010). Inhibiting Akt expression with siRNA or with miltefosine, which blocks Akt phosphorylation, inhibited virus-induced release of calcium, HSV entry, and plaque formation (Cheshenko et al., 2013). Deletion of HSV glycoprotein D (gD) or gB prevents virus-induced Akt phosphorylation, and Akt interacts directly with gB, but not with gD (Cheshenko et al., 2013) (Table 1).
Table 1.
Herpesvirus proteins that modulate the PI3K/Akt pathway
| Virus Protein | Location in virus, expression kinetics | Cellular target for viral protein | Effect of modulating PI3K/Akt signaling | |
|---|---|---|---|---|
| HSV1 | gB | Envelope, γ | Akt, PILRα | Virus entry |
| Envelope, γ | Nectin-1, HVEM, 3-OS HS | Virus entry | ||
| gH | Envelope, γ | integrins | Virus entry | |
| VP11/12 | Tegument, γ | Lck, p85 subunit of PI3K | ||
| US3 | Tegument, γ | GSK-3, FOX1, TSC2 | Protein translation | |
| LAT | Latency | Latency | ||
| HSV2 | ICP10 | Lytic, β | caspase-8 | Anti-apoptosis |
| VZV | ORF12 | Tegument, γ* | p85 subunit of PI3K | Cell cycle progression |
| ORF47 | Tegument, γ* | |||
| ORF66 | Tegument, γ* | |||
| HCMV | gB | Envelope, γ | integrins | Virus entry |
| IE1 | Lytic, α | Anti-apoptosis | ||
| IE2 | Lytic, α | Anti-apoptosis | ||
| EBV | gp350 | Envelope, γ | CD21 | Virus entry |
| BRLF1 (Rta) | Lytic, α | Reactivation from latency | ||
| LMP1 | Latency | CD40 mimic, TRAFs, p85 subunit of PI3K | Anti-apoptosis, protein translation, transformation | |
| LMP2A | Latency | BCR mimic, Syk, Lyn, Shb | Anti-apoptosis, protein translation | |
| EBNA-2 | Latency | p55α subunit of PI3K | B cell proliferation | |
| KSHV | gB | Lytic, γ* | Integrins | Virus entry |
| K1 | Lytic, β, low level expression in latency | BCR mimic, p85 subunit of PI3K, Syk, Lyn | Anti-apoptosis, protein translation | |
| vGPCR | Lytic, β | GPCR mimic | Anti-apoptosis, protein translation | |
| vIL-6 | Lytic, β, low level expression in latency | IL-6 mimic (binds to gp130) | Anti-apoptosis; Reprogramming of endothelial cells to lymphatic cells | |
| Rta | Lytic, α | Reactivation from latency |
Putative kinetic assignment, inferred from its homolog in HSV
PILRα, paired immunoglobulin-like type 2 receptor-α; HVEM, herpesvirus entry mediator; 3-OS
HS, 3-O-sulfated heparan sulfate
Integrins serve as HSV entry mediators and HSV gH binds to αvβ3 integrin (Parry et al., 2005). The binding of gH to αvβ3 integrin activates Akt and triggers intracellular calcium release (Cheshenko et al., 2007; Cheshenko et al., 2014). Inhibition of integrin αvβ3 expression with siRNA reduced virus entry, calcium release, and plaque formation. HSV deleted for gH binds to cells and activates Akt, but is impaired for calcium signaling and virus entry. Activation of Akt by HSV is followed by integrin signaling, release of intracellular calcium, and phosphorylation of focal adhesion kinase (FAK) which provides a favorable environment for entry of the virus into the cell (Cheshenko et al., 2014). Binding of HSV-1 to cells also activates the epidermal growth factor receptor (EGFR)- PI3K signaling pathway, resulting in phosphorylation of cofilin and polymerization of actin which facilitates virus entry (Zheng et al., 2014).
CMV attachment and receptor binding, like HSV, induces PI3K/Akt signaling. CMV infection triggers PI3K activation in serum-starved human embryonic lung fibroblasts within the first 30 minutes of infection with UV-inactivated CMV. PI3K activation subsides 2 hours after infection and resumes 4 hour after infection (Johnson et al., 2001; McFarlane et al., 2011). While CMV protein synthesis is dispensable for the first phase of PI3K activation, it is necessary for the second phase of activation. Inhibition of PI3K with LY294002 delays CMV entry, and reduces immediate-early and early gene expression, and viral DNA replication. Infection of cells with CMV results in phosphorylation of platelet-derived growth factor receptor (PDGFR)-α which interacts with the p85 subunit of PI3K and activates Akt (Soroceanu et al., 2008). Akt activation may be mediated by CMV gB binding to cells, since overexpression of gB induces phosphorylation of Akt and PDGFR-α (Cobbs et al., 2014). Treatment of cells with CMV neutralizing antibody reduced Akt activation (Andreoni et al., 2002). HCMV activates PI3K/Akt and inhibits apoptosis in monocytes by upregulating Mcl-1 (Chan et al., 2010).
Entry of EBV and KSHV into cells also induces PI3K/Akt signaling. EBV gp350 binding to CD21, the virus receptor on B cells, triggers Akt and GSK-3β activation (Barel et al., 2003). The interaction of KSHV glycoproteins with integrins induces phosphorylation of FAK and subsequently Src, PI3K, and c-Cbl (Chakraborty et al., 2011; Krishnan et al., 2006; Naranatt et al., 2003; Sharma-Walia et al., 2004; Valiya Veettil et al., 2010; Veettil et al., 2006). KSHV gB induces phosphorylation of Akt (Sharma-Walia et al., 2004; Zhang et al., 2005). PI3K is important for induction of Cdc42 Rho and RhoA GTPases and cytoskeletal changes in KSHV-infected cells (Sharma-Walia, Naranatt et al. 2004). PI3K inhibition reduces infectivity and cytoskeletal changes associated with KSHV gB, but does not affect virus binding to cells (Valiya Veettil, Sadagopan et al. 2010 Tiwari and Shukla 2010). KSHV induces phosphorylation of the p85 subunit of PI3K within one minute of infection which return to normal levels 30 minutes after infection (Kerur et al., 2010).
Herpesvirus tegument proteins activate PI3K/Akt
Herpesvirus tegument proteins are located between the viral envelope and capsid and are released into the cell immediately after virus entry. HSV encodes two tegument proteins, VP11/12 and US3 protein kinase, that modulate the PI3K/Akt pathway (Benetti and Roizman, 2006; Eaton et al., 2014; Wagner and Smiley, 2011). VP11/12, the most abundant HSV tegument protein, is phosphorylated by Lck and interacts with the p85 subunit of PI3K. VP11/12 is essential for activation of PI3K/Akt by HSV (Wagner and Smiley 2011). The carboxyl terminal region of VP11/12 contains a PI3K p85 subunit binding domain (YTHM) and two Src family kinase (SFK) motifs (YETV and YEEI) which together are important for activation of Lck and binding to PI3K p85 (Strunk et al., 2013).
HSV US3, one of two HSV encoded protein kinases, does not share sequence homology with Akt or activate Akt directly, but serves as an functional homolog of Akt and phosphorylates several Akt substrates including GSK-3β, FOXO1, TSC2 (Chuluunbaatar et al., 2010). Phosphorylation of TSC2 by US3 at the same sites as those phosphorylated by Akt results in activation of mTORC1, which enhances protein translation and HSV replication. Phosphorylation of GSK3β by US3 inactivates GSK3β and promotes stable microtubule formation and virus spread (Naghavi et al., 2013). Infection of cells with an HSV US3 null mutant results in constitutive Akt activation. Deletion of US3 results in increased phosphorylation of VP11/12 by SFKs and by the HSV UL13 protein kinase (Eaton et al., 2014). Thus, US3 inhibits phosphorylation of VP11/12 by SFKs and UL13 resulting in inhibition of VP11/12 signaling and Akt activation. While Akt is activated throughout the entire replicative cycle during infection of cells with an HSV US3 null mutant, Akt is only activated at early time points after infection with wild-type HSV (Benetti and Roizman, 2006).
VZV infection activates Akt and inhibition of PI3K or Akt reduces VZV replication (Rahaus et al., 2007; Sen et al., 2014). Infection with VZV results in phosphorylation of downstream targets of Akt including mTOR, FOXO1, 4EBP1, and S6K1. Expression of VZV protein kinases ORF47 and ORF66 increases Akt activation; conversely VZV deleted for ORF47 and ORF66 results in reduced phosphorylation of Akt and GSK-3β. The VZV ORF12 tegument protein associates with the p85 subunit of PI3K and activates Akt at both threonine 308 and serine 473 (Liu and Cohen, 2013). Activation of Akt by ORF12 protein is important for cell cycle progression in VZV-infected cells, since inhibition of Akt activity reduces the differences observed in cell cycle progression with wild-type and ORF12 deleted VZV. The role of CMV, EBV, and KSHV tegument proteins in activating the PI3K/Akt pathway has not been reported.
Activation of the PI3K/Akt pathway by herpesvirus proteins expressed during virus replication
While entry of herpesviruses into cells and subsequent release of tegument proteins can transiently activate the PI3K/Akt pathway, viral protein synthesis is required to sustain activation of PI3K/Akt. HSV 2 UL39 encodes the large subunit of HSV ribonucleotide reductase (ICP10) which contains an amino terminal serine–threonine protein kinase domain (ICP10PK). ICP10PK functions as a constitutively activated growth-factor receptor that activates PI3K/Akt and Ras/ERK pathways (Laing et al., 2008; Laing et al., 2010; Smith, 2005). While ICP10PK mediated PI3K activation was initially believed to responsible for preventing apoptosis in HSV-2 infected cells (Laing et al., 2008; Laing et al., 2010; Perkins et al., 2002a; Perkins et al., 2002b), more recent studies indicate that ICP10PK protects cells from apoptosis by binding to caspase-8 and disrupting its interaction with FADD, which is independent of activation of PI3K (Dufour et al., 2011). These observations are supported by the fact that the HSV-2 homolog of UL39 in HSV-1 (ICP6) does not have similar serine-threonine kinase activity and does not activate Akt, but also binds caspase-8 and blocks apoptosis (Chung et al., 1989; Dufour et al., 2011).
While activation of Akt by CMV can be detected 96 hours after infection, long term activation requires the expression of CMV proteins during viral replication. Expression of CMV major immediate-early protein 1 (IEP72) or 2 (IEP86) activates PI3K and Akt and inhibits apoptosis (Cobbs et al., 2008; Yu and Alwine, 2002). PI3K activity is required for upregulation of the anti-apoptotic protein c-FLIP by CMV IEP86 (Chiou et al., 2006).
EBV encodes two immediate-early proteins BRLF1 and BZLF1 which are essential for lytic replication and reactivation from latency. Overexpression of BRLF1, but not BZLF1, in normal human fibroblasts activates PI3K/Akt signaling (Darr et al., 2001). Activation of PI3K/Akt signaling is required for BRLF1 activation of the BZLF1 and BMRF1 early promoters, but not the SM early promoter, in epithelial cells.
KSHV G-protein-coupled receptor (vGPCR), transmembrane protein K1, and viral IL-6 (vIL-6) all activate PI3K/Akt. KSHV vGPCR activates multiple signaling pathways including ERK, p38, NF-AT, and PI3K (Bais et al., 1998; Cannon and Cesarman, 2004; Montaner et al., 2001; Pati et al., 2003; Smit et al., 2002). Expression of KSHV vGPCR results in translocation of Akt to the plasma membrane and increased levels of bcl-2 mRNA and protein which inhibits apoptosis (Abboud et al., 2013). Activation of PI3K/Akt by vGPCR results in inactivation of GSK-3β and activation of NF-AT which is a transcription factor that mediates expression of inflammatory cytokines (Bais et al., 1998; Cannon and Cesarman, 2004; Montaner et al., 2001; Pati et al., 2003; Smit et al., 2002). PI3K/Akt activation by GPCR also results in phosphorylation of TSC-2, mTOR, 4EPB1, and S6K1 to enhance translation and cell proliferation (Sodhi, Chaisuparat et al. 2006). Constitutive activation of Akt by vGPCR has an essential role in KSHV sarcomagenesis (Sodhi et al., 2004). KSHV K1, which is a functional mimic for BCR signaling, has a carboxyl terminal ITAM motif and recruits Lyn, Syk, and the p85 subunit of PI3K to constitutively activate PI3K/Akt (Prakash et al., 2005; Tomlinson and Damania, 2004; Xue et al., 2014). KSHV K1 activation of Akt is associated with phosphorylation and inhibition of FOX01, GSK3β, and BAD which are important for inhibition of apoptosis, and phosphorylation of mTOR which may increase translation and endothelial cell transformation (Wang et al., 2006). K1 also inhibits expression of PTEN, which inhibits the activity of PI3K. KSHV vIL-6 binds to its receptor gp130 to activate PI3K/Akt and the JAK2/STAT3 pathway which contributes to reprogramming of endothelial cells to lymphatic cells (Morris et al., 2008; Morris et al., 2012). Expression of the KSHV immediate-early protein Rta, which is required for reactivation from latency, also activates Akt (Li et al., 2012).
Activation of PI3K during herpesviruses latency and reactivation
Herpesviruses establish latency in different cell types with limited or no expression of viral proteins. The PI3K/Akt pathway is activated in cells latently infected with human herpesviruses, and is important for both latency and reactivation. Additional signaling pathways are also important for EBV and KSHV that infect B cells to allow latent infection in these proliferating cells as well as to inhibit apoptosis.
HSV latently infected neurons express no viral proteins, but do express the latency-associated transcript (LAT) which is important for virus reactivation. Mouse neuroblastoma cells stably expressing LAT have higher levels of phosphorylated and total Akt and are more resistant to apoptosis after serum starvation compared with cells not expressing LAT (Li et al., 2010). Maintenance of HSV-1 latency requires persistent PI3K activation which is established by binding of nerve growth factor to the TrkA receptor tyrosine kinase (RTK) (Camarena et al., 2010). The p110α subunit of PI3K is essential to activate PDK1 and maintain HSV-1 latency; treatment of latently infected neurons with inhibitors of PI3K results in HSV-1 reactivation.
Primary B cells latently infected and transformed with EBV express EBV nuclear antigens (EBNAs) and latent membrane proteins (LMPs) and have activated PI3K/Akt (Wlodarski et al., 2005). LMP1 is a functional homolog of constitutive CD40 signaling, and LMP2A is a mimic for constitutive BCR signaling; these viral proteins activate multiple cell signaling pathways required to initiate and maintain B cell transformation and virus latency. LMP1 is a transmembrane protein with intracellular carboxyl terminal activating regions CTAR1 and CTAR2. The CTAR domains associate with TNF receptor–associated factors (TRAFs) to activate multiple signaling pathways including PI3K/Akt, NF-κ B, MAPK, JNK, AP1, and JAK/STAT that regulate cell growth and transformation (Brinkmann and Schulz, 2006; Eliopoulos and Young, 2001; Lam and Sugden, 2003; Mainou et al., 2007; Soni et al., 2007). The CTAR1 domain of LMP1 associates with the p85 subunit of PI3K to activate PI3K (Dawson et al., 2003) and contributes to transformation of rodent fibroblasts and growth of EBV-positive nasopharyngeal carcinoma cells in soft agar (Mainou et al., 2005; Shair et al., 2008). Similarly, survival and growth of LMP-1 transgenic B lymphocytes and lymphoma cells requires activation of Akt signaling (Shair et al., 2007). LMP1 activation of PI3K/Akt results in inactivation of FOXO3α, reduction of expression of DNA damage-binding protein 1 (DDB1), and repression of the DNA repair response which may increase genomic instability and the risk of transformation (Chen et al., 2008). Activation of PI3K/Akt by LMP1 is required for interleukin (IL)-10 production and phosphorylation of GSK-3β and S6K1 (Lambert and Martinez 2007).
LMP1 activation of Akt has an important role in preventing apoptosis. Activation of PI3K/Akt by LMP1 inhibits apoptosis mediated by TRAIL and increases expression of the anti-apoptotic c-FLIP protein (Li et al., 2011). Akt inhibits translocation of the pro-apoptotic protein Bax from the cytoplasm to the mitochondria; Bax localization in the mitochondria results in cytochrome release and apoptosis (Tsuruta et al., 2002). LMP1 activation of Akt/PI3K and FOXO3 induces expression of miR-21 (Yang et al., 2013) and upregulates Mcl-1 both of which reduce apoptosis (Kim et al., 2012).
EBV LMP2A is a transmembrane protein expressed during latency. LMP2A mimics BCR signaling and is important for EBV latency and virus-induced oncogenesis (Fotheringham et al., 2012; Scholle et al., 2000; Swart et al., 2000). LMP2A associates with Syk and Lyn tyrosine kinases and with scaffold protein Shb to activate Ras, PI3K and Akt (Fukuda and Longnecker, 2007; Matskova et al., 2007; Swart et al., 2000). The ITAM motif of LMP2A is required for activation of Akt (Morrison and Raab-Traub, 2005). Activation of Akt increases the level of XIAP (Hatton et al., 2011) and BclxL (Portis and Longnecker, 2004) which increase survival of EBV-infected B cells. LMP2A activation of Akt in nasopharyngeal carcinoma cells results in activation of mTOR and phosphorylation of 4EBP1 which enhances translation of cellular proteins (Moody et al., 2005). LMP2A activation of Akt also increases survival of epithelial cells (Scholle, Bendt et al. 2000) and inhibits apoptosis mediated by TGF-β in epithelial and Burkitt lymphoma cells (Fukuda, 2004). Activation of PI3K/Akt by LMP2A is important for phosphorylation of FOXO1 and GSK-3β, and for translocation of β-catenin to the nucleus of epithelial cells which may inhibit their differentiation (Morrison et al., 2003). EBV latent EBNA-2 may also contribute to P13K/Akt activation by induction of the p55α regulatory subunit of PI3K (Spender et al., 2006).
Other mechanisms can lead to activation of Akt in EBV-infected cells. EBV encodes microRNA miR-BART7-3p that targets PTEN, enhances activation of Akt, and increases cell migration of nasopharyngeal carcinoma cells (Cai et al., 2014). Hypermethylation of the promoter of INPP4B, a phosphatase that inhibits PI3K/Akt signaling, enhances the PI3K/Akt pathway in EBV-positive nasopharyngeal carcinoma cells (Yuen et al., 2014).
PI3K/Akt has an important role in EBV reactivation from latency. Inhibition of PI3K reduces EBV reactivation induced by BCR signaling in EBV-positive Burkitt lymphoma cell lines (Iwakiri and Takada 2004, Goswami, Gershburg et al. 2012). Similarly, blocking PI3K impairs TGF-β-induced reactivation of EBV in Burkitt lymphoma cells (Oussaief et al., 2009) and methotrexate-induced EBV reactivation in lymphoblastoid cell lines (Feng et al., 2004).
Activation of Akt/PI3K by KSHV is important for survival of latently infected primary effusion lymphoma cells (Uddin et al., 2005) and monocytes (Gonnella et al., 2013), and for tubule formation in endothelial cells (Wang and Damania, 2008). Akt activation contributes to increased expression of XIAP, phosphorylation of FOXO1 and GSK-3β, prevention of cytochrome c release from mitochondria, and inhibition of cleavage of caspase-3, caspase-9, and PARP in KSHV-positive primary effusion lymphoma cells (Uddin et al., 2005).
The KSHV latency associated proteins include LANA (ORF73), v-cyclin (ORF72), v-FLIP (ORF71), Kaposins, LANA2, vIRF3, and K10.5. Inhibition of PI3K activity results in increased cytoplasmic localization of LANA2 which may reduce the ability of LANA2 to block apoptosis (Munoz-Fontela et al., 2005). KSHV v-FLIP induces secretion of cytokines which activate Akt (Sharma-Walia et al., 2012). Inhibition of Akt activity inhibits KSHV reactivation from latently infected primary effusion lymphoma cells induced by treatment with a p53 activator or a Cdk1 inhibitor, but enhances KSHV reactivation induced by treatment with phorbol ester (TPA) (Li et al., 2012; Peng et al., 2010). Inhibition of PI3K reduces KSHV reactivation induced by BCR signaling in KSHV-positive Burkitt lymphoma cell lines, and this effect is associated with reduced expression of KSHV Rta (Kati et al., 2013).
Thus the PI3K/Akt pathway is critical for maintaining HSV, EBV, and KSHV latent infection; inhibition of PI3K/Akt reduces EBV and KSHV reactivation induced by BCR signaling, but enhances reactivation of HSV and of KSHV induced by treatment with other stimuli.
PI3K and immunodeficiencies
Recently two immunodeficiencies have been reported in association with mutations in the p110 catalytic or p85 regulatory subunits of PI3K. Two groups have reported patients with severe herpesvirus infections who have heterozygous gain-of-function mutations in PIK3CD, which encodes PI3Kδ (Angulo et al., 2013; Lucas et al., 2014a). These patients presented with fatal varicella-zoster virus pneumonia, persistent CMV viremia, CMV lymphadenitis, persistent EBV viremia, EBV-positive B cell lymphomas, or other infections including severe otitis, sinusitis, pneumonia, and bacterial meningitis. They also had prominent lymphadenopathy, nodular lymphoid hyperplasia, increased levels of IgM, and impaired responses to vaccination. In each patient a dominant gain-of-function mutation in one allele of PIK3CD resulted in constitutive activation and phosphorylation of Akt and increased activation of mTOR. The patients had reduced CD4 T cells, reduced naïve T cells, reduced memory B cells, increased effector memory T cells, increased senescent CD8 effector T cells, and enhanced activation-induced T cell death. Treatment of one patient with sirolimus reduced lymphoid hyperplasia, and increased naïve T cells (Lucas et al., 2014a), while hematopoietic stem cell transplant was curative in another patient (Angulo et al., 2013). The disease is referred to as APDS (activated PI3K-delta syndrome (Angulo et al., 2013)) or PASLI (p110delta-activating mutation causing senescent T cells, lymphadenopathy, and immunodeficiency (Lucas et al., 2014). Additional patients have been described with EBV-negative lymphomas and with infections due to pathogens other than herpesviruses (Crank et al., 2014; Hartman et al., 2014; Kracker et al., 2014).
Patients with heterozygous gain-of-function mutations in PIK3R1, which encodes the p85 regulatory subunit of PI3K, were reported with an immunodeficiency syndrome with recurrent bacterial upper and lower respiratory tract infections (Deau et al., 2014; Lucas et al 2014b). One patient had persistent asymptomatic CMV and EBV viremia as well as gastroenteritis due to enterovirus. Like patients with mutations in PIK3CD, patients with mutations in PIK3R1 also have increased phosphorylation of Akt, increased mTOR signaling, increased IgM, reduced naïve T cells, reduced memory B cell function, increased senescent CD8 T cells, and enhanced activation-induced T cell death. The disease is referred to as APDS2 (activated PI3Kdelta syndrome 2).
PI3K/Akt pathway in herpesvirus associated malignancies
EBV and KSHV are oncogenic viruses associated with B cell and epithelial cell malignancies. PI3K/Akt is activated in EBV-positive post-transplant lymphoproliferative disease, Hodgkin lymphoma, nasopharyngeal carcinoma, and gastric carcinoma (Alsayed et al., 2008; Chen, 2012). Similarly, the PI3K/Akt pathway is activated in Kaposi's sarcoma and primary effusion lymphoma (Bhatt et al., 2010; Sodhi et al., 2004; Uddin et al., 2005). Therefore, inhibition of the PI3K/Akt pathway is a potential target for the treatment of EBV and KSHV associated malignancies.
Targeting the PI3K/Akt pathway to inhibit virus replication and virus-associated malignancies
While a number of small molecule inhibitors are available to block PI3K/Akt activity in vitro and many of these have been shown to inhibit herpesvirus replication, most of these drugs are not licensed for use in humans. Recently miltefosine, which blocks Akt phosphorylation, has been approved for use in leishmaniasis. Pre-treatment of cultured cells with miltefosine, followed by HSV-2 infection, inhibited virus plaque formation (Cheshenko, Trepanier et al. 2013). Further studies showed the miltefosine blocked virus entry into epithelial cells, calcium release, and reactivation of virus from explanted ganglia to epithelial cells. Miltefosine inhibited replication of acyclovir-sensitive and acyclovir resistant HSV-2 strains. Several PI3K inhibitors are currently in trials for treatment of malignancies (Bauer et al., 2014; Blachly and Baiocchi, 2014; Tasian et al., 2014). Recently, a small molecule inhibitor of PI3Kδ (idelalisib) was approved for treatment of patients with relapsed chronic lymphocytic leukemia and follicular B cell lymphoma (Furman et al., 2014; Gopal et al., 2014). Thus, Akt or PI3K inhibitors might be used for treatment of anti-viral resistant herpesvirus infections in the future.
The PI3K/Akt pathway results in activation of mTOR. While several mTOR inhibitors are in clinical trials for cancer therapy (Bauer et al., 2014; Blachly and Baiocchi, 2014; Tasian et al., 2014), at present only sirolimus (also known as rapamycin), everolimus, and temserolimus are approved for use in humans. These drugs are immunosuppressive and used in transplant recipients and for treating selected malignancies. Sirolimus inhibits the growth of KSHV primary effusion lymphoma cells in vitro (Sin et al., 2007). Replacement of cyclosporine with sirolimus therapy in 15 kidney transplant recipients with Kaposi's sarcoma resulted in the resolution of Kaposi's sarcoma skin lesions (Stallone et al., 2005). Substitution of calcineurin inhibitors with sirolimus in 14 patients with post-transplant Kaposi's sarcoma resulted in complete remissions in two patients and partial responses in 8 patients (Lebbe et al., 2006). Similarly, a pooled analysis from several transplant centers showed that substitution of immunosuppressive regimens in 12 renal transplant recipients with regimens containing either sirolimus or everolimus resulted in resolution of Kaposi's sarcoma lesions in 11 patients (Campistol and Schena, 2007).
Sirolimus also inhibits EBV-positive B cell lymphomas in a xenogenic mouse model and in EBV LMP2/Myc transgenic mice (Cen and Longnecker, 2011; Nepomuceno et al., 2003). In three patients with EBV-associated post-transplant lymphoma, replacement of calcineurin inhibitors and mycophenolate mofetil or azathioprine with sirolimus resulted in complete resolution of B cell lymphomas in two patients and a temporary remission of a T cell lymphoma in one patient (Boratynska and Smolska, 2008). A pooled analysis of 19 renal transplant recipients with post-transplant lymphoproliferative disease from multiple European transplant centers showed that substitution of calcineurin inhibitors with sirolimus or everolimus, along with rituximab therapy in six patients and chemotherapy in six patients, resulted in complete remission of disease in 15 patients (Pascual, 2007). While sirolimus has a partially inhibitory effect on EBV-positive B cell lymphoma lines and resistance to sirolimus is associated with high levels of phosphorylated Akt, the addition of a PI3Kδ inhibitor to sirolimus enhanced the ability of the latter to kill the cells (Furukawa et al., 2013). Similarly, an experimental drug that inhibits both PI3K and mTOR was more effective than either PI3K or mTOR inhibitors to inhibit the growth of KSHV-positive primary effusion lymphoma cells in vitro and in a xenograft tumor model (Bhatt, Bhende et al. 2010
Conclusions
Human herpesviruses express multiple proteins during the immediate-early, early, and late phases of the virus replication cycle and during latency that activate the PI3K/Akt pathway. Activation of PI3K/Akt by viral glycoproteins such as gB in HSV, CMV, and KSHV, HSV gD, and EBV gp350, as well as tegument proteins of HSV and VZV present during herpesvirus entry is important for preparing the cell for virus infection to optimize virus replication. Similarly, activation of PI3K/Akt by immediate-early proteins such as CMV IE1 and IE2, and Rta in EBV and KSHV are important for the initial stages of virus infection. Herpesvirus proteins expressed in the early phase of virus replication, such as KSHV vIL-6 and vGPCR, also contribute to PI3/Akt activation. PI3K/Akt is also critical for maintaining latent herpesvirus infection and this pathway is activated by HSV LAT and the EBV latency proteins LMP1, LMP2, and EBNA-2. PI3K-Akt signaling is required for optimizing protein synthesis, cell growth, transformation, and inhibiting apoptosis. Constitutive activation of PI3K due to mutations in the cellular genes PIK3CD or PIK3R1 result in severe herpesvirus infections due to impaired cellular immunity.PI3K/Akt is activated in several EBV and KSHV associated B cell and epithelial cell malignancies. Recently, several drugs that block Akt or PI3K have been licensed for treatment of malignancies or for a parasitic infection. These include miltefosine, which blocks Akt phosphorylation and inhibits HSV in vitro and idelalisib, which blocks PI3Kδ. These drugs, and others currently under development, might be used to treat human herpesvirus infections or virus-associated malignancies. Inhibitors of mTOR, such as sirolimus, everolimus, and temserolimus, which block signaling downstream of PI3K/Akt might also have a role in treating herpesvirus infections. Thus, further studies and development of inhibitors of the PI3K/Akt pathway may lead to novel therapies for both acute herpesvirus infections and for virus-associated malignancies.
Highlights.
Human herpesvirus replication and latency proteins activate the PI3K/Akt pathway.
PI3K/Akt is important for protein synthesis, transformation, and blocking apoptosis.
Constitutive activation of PI3K is associated with severe herpesvirus infections.
PI3K/Akt is activated in several EBV and KSHV associated malignancies.
Drugs that block PI3K/Akt might be used to treat herpesvirus infections or cancers.
Acknowledgments
This work was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abboud ER, Shelby BD, Angelova M, Nelson AB, Ferris M, McFerrin HE, Morris CA, Sullivan DE. Kaposi sarcoma-associated herpesvirus g protein-coupled receptor enhances endothelial cell survival in part by upregulation of bcl-2. The Ochsner journal. 2013;13:66–75. [PMC free article] [PubMed] [Google Scholar]
- Alsayed Y, Leleu X, Leontovich A, Oton AB, Melhem M, George D, Ghobrial IM. Proteomics analysis in post-transplant lymphoproliferative disorders. European journal of haematology. 2008;81:298–303. doi: 10.1111/j.1600-0609.2008.01106.x. [DOI] [PubMed] [Google Scholar]
- Alwine JC. Modulation of host cell stress responses by human cytomegalovirus. Current topics in microbiology and immunology. 2008;325:263–279. doi: 10.1007/978-3-540-77349-8_15. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci U S A. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoni KA, Wang X, Huang SM, Huang ES. Human cytomegalovirus hyperimmune globulin not only neutralizes HCMV infectivity, but also inhibits HCMV-induced intracellular NF-kappaB, Sp1, and PI3-K signaling pathways. Journal of medical virology. 2002;67:33–40. doi: 10.1002/jmv.2189. [DOI] [PubMed] [Google Scholar]
- Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, Blake-Palmer K, Perisic O, Smyth D, Maes M, Fiddler C, Juss J, Cilliers D, Markelj G, Chandra A, Farmer G, Kielkowska A, Clark J, Kracker S, Debre M, Picard C, Pellier I, Jabado N, Morris JA, Barcenas-Morales G, Fischer A, Stephens L, Hawkins P, Barrett JC, Abinun M, Clatworthy M, Durandy A, Doffinger R, Chilvers ER, Cant AJ, Kumararatne D, Okkenhaug K, Williams RL, Condliffe A, Nejentsev S. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- Balakrishnan A, Chaillet JR. Role of the inositol polyphosphate-4-phosphatase type II Inpp4b in the generation of ovarian teratomas. Developmental biology. 2013;373:118–129. doi: 10.1016/j.ydbio.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barel M, Balbo M, Le Romancer M, Frade R. Activation of Epstein-Barr virus/C3d receptor (gp140, CR2, CD21) on human cell surface triggers pp60src and Akt-GSK3 activities upstream and downstream to PI 3-kinase, respectively. Eur J Immunol. 2003;33:2557–2566. doi: 10.1002/eji.200324059. [DOI] [PubMed] [Google Scholar]
- Bauer TM, Patel MR, Infante JR. Targeting PI3 kinase in cancer. Pharmacology & therapeutics. 2014 doi: 10.1016/j.pharmthera.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Benetti L, Roizman B. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J Virol. 2006;80:3341–3348. doi: 10.1128/JVI.80.7.3341-3348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertucci MC, Mitchell CA. Phosphoinositide 3-kinase and INPP4B in human breast cancer. Annals of the New York Academy of Sciences. 2013;1280:1–5. doi: 10.1111/nyas.12036. [DOI] [PubMed] [Google Scholar]
- Bhatt AP, Bhende PM, Sin SH, Roy D, Dittmer DP, Damania B. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood. 2010;115:4455–4463. doi: 10.1182/blood-2009-10-251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt AP, Damania B. AKTivation of PI3K/AKT/mTOR signaling pathway by KSHV. Frontiers in immunology. 2012;3:401. doi: 10.3389/fimmu.2012.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachly JS, Baiocchi RA. Targeting PI3-kinase (PI3K), AKT and mTOR axis in lymphoma. British journal of haematology. 2014;167:19–32. doi: 10.1111/bjh.13065. [DOI] [PubMed] [Google Scholar]
- Boratynska M, Smolska D. Inhibition of mTOR by sirolimus induces remission of post-transplant lymphoproliferative disorders. Transplant international : official journal of the European Society for Organ Transplantation. 2008;21:605–608. doi: 10.1111/j.1432-2277.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Brinkmann MM, Schulz TF. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J Gen Virol. 2006;87:1047–1074. doi: 10.1099/vir.0.81598-0. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Molecular cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol. 2008;6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai LM, Lyu XM, Luo WR, Cui XF, Ye YF, Yuan CC, Peng QX, Wu DH, Liu TF, Wang E, Marincola FM, Yao KT, Fang WY, Cai HB, Li X. EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene. 2014 doi: 10.1038/onc.2014.341. [DOI] [PubMed] [Google Scholar]
- Camarena V, Kobayashi M, Kim JY, Roehm P, Perez R, Gardner J, Wilson AC, Mohr I, Chao MV. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons. Cell host & microbe. 2010;8:320–330. doi: 10.1016/j.chom.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campistol JM, Schena FP. Kaposi's sarcoma in renal transplant recipients--the impact of proliferation signal inhibitors. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22(Suppl 1):i17–22. doi: 10.1093/ndt/gfm089. [DOI] [PubMed] [Google Scholar]
- Cannon ML, Cesarman E. The KSHV G protein-coupled receptor signals via multiple pathways to induce transcription factor activation in primary effusion lymphoma cells. Oncogene. 2004;23:514–523. doi: 10.1038/sj.onc.1207021. [DOI] [PubMed] [Google Scholar]
- Cen O, Longnecker R. Rapamycin reverses splenomegaly and inhibits tumor development in a transgenic model of Epstein-Barr virus-related Burkitt's lymphoma. Molecular cancer therapeutics. 2011;10:679–686. doi: 10.1158/1535-7163.MCT-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, ValiyaVeettil M, Sadagopan S, Paudel N, Chandran B. c-Cbl-mediated selective virus-receptor translocations into lipid rafts regulate productive Kaposi's sarcoma-associated herpesvirus infection in endothelial cells. J Virol. 2011;85:12410–12430. doi: 10.1128/JVI.05953-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Bentz GL, Smith MS, Parmater A, Yurochko AD. PI3K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J Immunol. 2010;184:3213–3222. doi: 10.4049/jimmunol.0903025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Roles of the PI3K/Akt pathway in Epstein-Barr virus-induced cancers and therapeutic implications. World journal of virology. 2012;1:154–161. doi: 10.5501/wjv.v1.i6.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YR, Liu MT, Chang YT, Wu CC, Hu CY, Chen JY. Epstein-Barr virus latent membrane protein 1 represses DNA repair through the PI3K/Akt/FOXO3a pathway in human epithelial cells. J Virol. 2008;82:8124–8137. doi: 10.1128/JVI.00430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N, Liu W, Satlin LM, Herold BC. Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol Biol Cell. 2007;18:3119–3130. doi: 10.1091/mbc.E07-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N, Trepanier JB, Gonzalez PA, Eugenin EA, Jacobs WR, Jr., Herold BC. Herpes simplex virus type 2 glycoprotein H interacts with integrin alphavbeta3 to facilitate viral entry and calcium signaling in human genital tract epithelial cells. J Virol. 2014;88:10026–10038. doi: 10.1128/JVI.00725-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N, Trepanier JB, Stefanidou M, Buckley N, Gonzalez P, Jacobs W, Herold BC. HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:2584–2599. doi: 10.1096/fj.12-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SH, Yang YP, Lin JC, Hsu CH, Jhang HC, Yang YT, Lee CH, Ho LL, Hsu WM, Ku HH, Chen SJ, Chen SS, Chang MD, Wu CW, Juan LJ. The immediate early 2 protein of human cytomegalovirus (HCMV) mediates the apoptotic control in HCMV retinitis through up-regulation of the cellular FLICE-inhibitory protein expression. J Immunol. 2006;177:6199–6206. doi: 10.4049/jimmunol.177.9.6199. [DOI] [PubMed] [Google Scholar]
- Chuluunbaatar U, Roller R, Feldman ME, Brown S, Shokat KM, Mohr I. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev. 2010;24:2627–2639. doi: 10.1101/gad.1978310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung TD, Wymer JP, Smith CC, Kulka M, Aurelian L. Protein kinase activity associated with the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10). J Virol. 1989;63:3389–3398. doi: 10.1128/jvi.63.8.3389-3398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs C, Khan S, Matlaf L, McAllister S, Zider A, Yount G, Rahlin K, Harkins L, Bezrookove V, Singer E, Soroceanu L. HCMV glycoprotein B is expressed in primary glioblastomas and enhances growth and invasiveness via PDGFR-alpha activation. Oncotarget. 2014;5:1091–1100. doi: 10.18632/oncotarget.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs CS, Soroceanu L, Denham S, Zhang W, Kraus MH. Modulation of oncogenic phenotype in human glioma cells by cytomegalovirus IE1-mediated mitogenicity. Cancer research. 2008;68:724–730. doi: 10.1158/0008-5472.CAN-07-2291. [DOI] [PubMed] [Google Scholar]
- Cooray S. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- Crank MC, Grossman JK, Moir S, Pittaluga S, Buckner CM, Kardava L, Agharahimi A, Meuwissen H, Stoddard J, Niemela J, Kuehn H, Rosenzweig SD. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. Journal of clinical immunology. 2014;34:272–276. doi: 10.1007/s10875-014-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas BD, Lu Y, Mao M, Zhang J, LaPushin R, Siminovitch K, Mills GB. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:27455–27461. doi: 10.1074/jbc.M100556200. [DOI] [PubMed] [Google Scholar]
- Darr CD, Mauser A, Kenney S. Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation. J Virol. 2001;75:6135–6142. doi: 10.1128/JVI.75.13.6135-6142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson CW, Tramountanis G, Eliopoulos AG, Young LS. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem. 2003;278:3694–3704. doi: 10.1074/jbc.M209840200. [DOI] [PubMed] [Google Scholar]
- Deau MC, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, Cavazzana M, Picard C, Durandy A, Fischer A, Kracker S. A human immunodeficiency caused by mutations in the PIK3R1 gene. The Journal of clinical investigation. 2014;124:3923–3928. doi: 10.1172/JCI75746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl N, Schaal H. Make yourself at home: viral hijacking of the PI3K/Akt signaling pathway. Viruses. 2013;5:3192–3212. doi: 10.3390/v5123192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour F, Sasseville AM, Chabaud S, Massie B, Siegel RM, Langelier Y. The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFalpha- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis. 2011;16:256–271. doi: 10.1007/s10495-010-0560-2. [DOI] [PubMed] [Google Scholar]
- Dunn EF, Connor JH. HijAkt: The PI3K/Akt pathway in virus replication and pathogenesis. Progress in molecular biology and translational science. 2012;106:223–250. doi: 10.1016/B978-0-12-396456-4.00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton HE, Saffran HA, Wu FW, Quach K, Smiley JR. Herpes simplex virus protein kinases US3 and UL13 modulate VP11/12 phosphorylation, virion packaging, and phosphatidylinositol 3-kinase/Akt signaling activity. J Virol. 2014;88:7379–7388. doi: 10.1128/JVI.00712-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos AG, Young LS. LMP1 structure and signal transduction. Seminars in cancer biology. 2001;11:435–444. doi: 10.1006/scbi.2001.0410. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Feng WH, Cohen JI, Fischer S, Li L, Sneller M, Goldbach-Mansky R, Raab-Traub N, Delecluse HJ, Kenney SC. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. Journal of the National Cancer Institute. 2004;96:1691–1702. doi: 10.1093/jnci/djh313. [DOI] [PubMed] [Google Scholar]
- Fotheringham JA, Coalson NE, Raab-Traub N. Epstein-Barr virus latent membrane protein-2A induces ITAM/Syk- and Akt-dependent epithelial migration through alphav-integrin membrane translocation. J Virol. 2012;86:10308–10320. doi: 10.1128/JVI.00853-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Longnecker R. Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway. J Virol. 2007;81:9299–9306. doi: 10.1128/JVI.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, Ghia P, Eradat H, Ervin T, Lamanna N, Coiffier B, Pettitt AR, Ma S, Stilgenbauer S, Cramer P, Aiello M, Johnson DM, Miller LL, Li D, Jahn TM, Dansey RD, Hallek M, O'Brien SM. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Wei L, Krams SM, Esquivel CO, Martinez OM. PI3Kdelta inhibition augments the efficacy of rapamycin in suppressing proliferation of Epstein-Barr virus (EBV)+ B cell lymphomas. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:2035–2043. doi: 10.1111/ajt.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, Barretina J, Lin WM, Rameh L, Salmena L, Pandolfi PP, Cantley LC. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnella R, Santarelli R, Farina A, Granato M, D'Orazi G, Faggioni A, Cirone M. Kaposi sarcoma associated herpesvirus (KSHV) induces AKT hyperphosphorylation, bortezomib-resistance and GLUT-1 plasma membrane exposure in THP-1 monocytic cell line. Journal of experimental & clinical cancer research : CR. 2013;32:79. doi: 10.1186/1756-9966-32-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, Blum KA, Goy AH, Davies AJ, Zinzani PL, Dreyling M, Johnson D, Miller LL, Holes L, Li D, Dansey RD, Godfrey WR, Salles GA. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. The New England journal of medicine. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman HN, Niemela J, Hintermeyer MK, Garofalo M, Stoddard J, Verbsky JW, Rosenzweig SD, Routes JM. Gain of Function Mutations of PIK3CD as a Cause of Primary Sclerosing Cholangitis. Journal of clinical immunology. 2014 doi: 10.1007/s10875-014-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan B, Akcakanat A, Holder AM, Meric-Bernstam F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surgical oncology clinics of North America. 2013;22:641–664. doi: 10.1016/j.soc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton O, Phillips LK, Vaysberg M, Hurwich J, Krams SM, Martinez OM. Syk activation of phosphatidylinositol 3-kinase/Akt prevents HtrA2-dependent loss of X-linked inhibitor of apoptosis protein (XIAP) to promote survival of Epstein-Barr virus+ (EBV+) B cell lymphomas. J Biol Chem. 2011;286:37368–37378. doi: 10.1074/jbc.M111.255125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harbor perspectives in biology. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MJ, Wu CY, Chiang HH, Lai YL, Hung SL. PI3K/Akt signaling mediated apoptosis blockage and viral gene expression in oral epithelial cells during herpes simplex virus infection. Virus Res. 2010;153:36–43. doi: 10.1016/j.virusres.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wang X, Ma XL, Huong SM, Huang ES. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J Virol. 2001;75:6022–6032. doi: 10.1128/JVI.75.13.6022-6032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kati S, Tsao EH, Gunther T, Weidner-Glunde M, Rothamel T, Grundhoff A, Kellam P, Schulz TF. Activation of the B cell antigen receptor triggers reactivation of latent Kaposi's sarcoma-associated herpesvirus in B cells. J Virol. 2013;87:8004–8016. doi: 10.1128/JVI.00506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerur N, Veettil MV, Sharma-Walia N, Sadagopan S, Bottero V, Paul AG, Chandran B. Characterization of entry and infection of monocytic THP-1 cells by Kaposi's sarcoma associated herpesvirus (KSHV): role of heparan sulfate, DC-SIGN, integrins and signaling. Virology. 2010;406:103–116. doi: 10.1016/j.virol.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim WS, Park C. Epstein-Barr virus latent membrane protein-1 protects B-cell lymphoma from rituximab-induced apoptosis through miR-155-mediated Akt activation and up-regulation of Mcl-1. Leukemia & lymphoma. 2012;53:1586–1591. doi: 10.3109/10428194.2012.659736. [DOI] [PubMed] [Google Scholar]
- Kracker S, Curtis J, Ibrahim MA, Sediva A, Salisbury J, Campr V, Debre M, Edgar JD, Imai K, Picard C, Casanova JL, Fischer A, Nejentsev S, Durandy A. Occurrence of B-cell lymphomas in patients with activated phosphoinositide 3-kinase delta syndrome. The Journal of allergy and clinical immunology. 2014;134:233–236. doi: 10.1016/j.jaci.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HH, Sharma-Walia N, Streblow DN, Naranatt PP, Chandran B. Focal adhesion kinase is critical for entry of Kaposi's sarcoma-associated herpesvirus into target cells. J Virol. 2006;80:1167–1180. doi: 10.1128/JVI.80.3.1167-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- Laing JM, Golembewski EK, Wales SQ, Liu J, Jafri MS, Yarowsky PJ, Aurelian L. Growth-compromised HSV-2 vector Delta RR protects from N-methyl-D-aspartate-induced neuronal degeneration through redundant activation of the MEK/ERK and PI3-K/Akt survival pathways, either one of which overrides apoptotic cascades. Journal of neuroscience research. 2008;86:378–391. doi: 10.1002/jnr.21486. [DOI] [PubMed] [Google Scholar]
- Laing JM, Smith CC, Aurelian L. Multi-targeted neuroprotection by the HSV-2 gene ICP10PK includes robust bystander activity through PI3-K/Akt and/or MEK/ERK-dependent neuronal release of vascular endothelial growth factor and fractalkine. J Neurochem. 2010;112:662–676. doi: 10.1111/j.1471-4159.2009.06475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam N, Sugden B. CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal. 2003;15:9–16. doi: 10.1016/s0898-6568(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Lebbe C, Euvrard S, Barrou B, Pouteil-Noble C, Garnier JL, Glotz D, Legendre C, Frances C. Sirolimus conversion for patients with posttransplant Kaposi's sarcoma. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:2164–2168. doi: 10.1111/j.1600-6143.2006.01412.x. [DOI] [PubMed] [Google Scholar]
- Li S, Carpenter D, Hsiang C, Wechsler SL, Jones C. Herpes simplex virus type 1 latency-associated transcript inhibits apoptosis and promotes neurite sprouting in neuroblastoma cells following serum starvation by maintaining protein kinase B (AKT) levels. J Gen Virol. 2010;91:858–866. doi: 10.1099/vir.0.015719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SS, Yang S, Wang S, Yang XM, Tang QL, Wang SH. Latent membrane protein 1 mediates the resistance of nasopharyngeal carcinoma cells to TRAIL-induced apoptosis by activation of the PI3K/Akt signaling pathway. Oncology reports. 2011;26:1573–1579. doi: 10.3892/or.2011.1423. [DOI] [PubMed] [Google Scholar]
- Li X, Chen S, Sun R. Cdk1 inhibition induces mutually inhibitory apoptosis and reactivation of Kaposi's sarcoma-associated herpesvirus. J Virol. 2012;86:6668–6676. doi: 10.1128/JVI.06240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cohen JI. Varicella-zoster virus ORF12 protein activates the phosphatidylinositol 3-kinase/Akt pathway to regulate cell cycle progression. J Virol. 2013;87:1842–1848. doi: 10.1128/JVI.02395-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M, Stoddard J, Ouyang W, Frucht DM, Rao VK, Atkinson TP, Agharahimi A, Hussey AA, Folio LR, Olivier KN, Fleisher TA, Pittaluga S, Holland SM, Cohen JI, Oliveira JB, Tangye SG, Schwartzberg PL, Lenardo MJ, Uzel G. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nature immunology. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CL, Zhang Y, Venida A, Wang Y, Hughes J, McElwee J, Butrick M, Matthews H, Price S, Biancalana M, Wang X, Richards M, Pozos T, Barlan I, Ozen A, Rao VK, Su HC, Lenardo MJ. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. The Journal of experimental medicine. 2014;211:2537–2547. doi: 10.1084/jem.20141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod IJ, Minson T. Binding of herpes simplex virus type-1 virions leads to the induction of intracellular signalling in the absence of virus entry. PLoS One. 2010;5:e9560. doi: 10.1371/journal.pone.0009560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainou BA, Everly DN, Jr., Raab-Traub N. Epstein-Barr virus latent membrane protein 1 CTAR1 mediates rodent and human fibroblast transformation through activation of PI3K. Oncogene. 2005;24:6917–6924. doi: 10.1038/sj.onc.1208846. [DOI] [PubMed] [Google Scholar]
- Mainou BA, Everly DN, Jr., Raab-Traub N. Unique signaling properties of CTAR1 in LMP1-mediated transformation. J Virol. 2007;81:9680–9692. doi: 10.1128/JVI.01001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matskova LV, Helmstetter C, Ingham RJ, Gish G, Lindholm CK, Ernberg I, Pawson T, Winberg G. The Shb signalling scaffold binds to and regulates constitutive signals from the Epstein-Barr virus LMP2A membrane protein. Oncogene. 2007;26:4908–4917. doi: 10.1038/sj.onc.1210298. [DOI] [PubMed] [Google Scholar]
- McFarlane S, Nicholl MJ, Sutherland JS, Preston CM. Interaction of the human cytomegalovirus particle with the host cell induces hypoxia-inducible factor 1 alpha. Virology. 2011;414:83–90. doi: 10.1016/j.virol.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer research. 2001;61:2641–2648. [PubMed] [Google Scholar]
- Moody CA, Scott RS, Amirghahari N, Nathan CO, Young LS, Dawson CW, Sixbey JW. Modulation of the cell growth regulator mTOR by Epstein-Barr virus-encoded LMP2A. J Virol. 2005;79:5499–5506. doi: 10.1128/JVI.79.9.5499-5506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris VA, Punjabi AS, Lagunoff M. Activation of Akt through gp130 receptor signaling is required for Kaposi's sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells. J Virol. 2008;82:8771–8779. doi: 10.1128/JVI.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris VA, Punjabi AS, Wells RC, Wittkopp CJ, Vart R, Lagunoff M. The KSHV viral IL-6 homolog is sufficient to induce blood to lymphatic endothelial cell differentiation. Virology. 2012;428:112–120. doi: 10.1016/j.virol.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JA, Klingelhutz AJ, Raab-Traub N. Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J Virol. 2003;77:12276–12284. doi: 10.1128/JVI.77.22.12276-12284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JA, Raab-Traub N. Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of {beta}-catenin signaling. J Virol. 2005;79:2375–2382. doi: 10.1128/JVI.79.4.2375-2382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Fontela C, Collado M, Rodriguez E, Garcia MA, Alvarez-Barrientos A, Arroyo J, Nombela C, Rivas C. Identification of a nuclear export signal in the KSHV latent protein LANA2 mediating its export from the nucleus. Experimental cell research. 2005;311:96–105. doi: 10.1016/j.yexcr.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Naghavi MH, Gundersen GG, Walsh D. Plus-end tracking proteins, CLASPs, and a viral Akt mimic regulate herpesvirus-induced stable microtubule formation and virus spread. Proc Natl Acad Sci U S A. 2013;110:18268–18273. doi: 10.1073/pnas.1310760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J Virol. 2003;77:1524–1539. doi: 10.1128/JVI.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer research. 2003;63:4472–4480. [PubMed] [Google Scholar]
- Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78:7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annual review of immunology. 2013;31:675–704. doi: 10.1146/annurev-immunol-032712-095946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oussaief L, Hippocrate A, Ramirez V, Rampanou A, Zhang W, Meyers D, Cole P, Khelifa R, Joab I. Phosphatidylinositol 3-kinase/Akt pathway targets acetylation of Smad3 through Smad3/CREB-binding protein interaction: contribution to transforming growth factor beta1-induced Epstein-Barr virus reactivation. J Biol Chem. 2009;284:23912–23924. doi: 10.1074/jbc.M109.036483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry C, Bell S, Minson T, Browne H. Herpes simplex virus type 1 glycoprotein H binds to alphavbeta3 integrins. J Gen Virol. 2005;86:7–10. doi: 10.1099/vir.0.80567-0. [DOI] [PubMed] [Google Scholar]
- Pascual J. Post-transplant lymphoproliferative disorder--the potential of proliferation signal inhibitors. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22(Suppl 1):i27–35. doi: 10.1093/ndt/gfm088. [DOI] [PubMed] [Google Scholar]
- Pati S, Foulke JS, Jr., Barabitskaya O, Kim J, Nair BC, Hone D, Smart J, Feldman RA, Reitz M. Human herpesvirus 8-encoded vGPCR activates nuclear factor of activated T cells and collaborates with human immunodeficiency virus type 1 Tat. J Virol. 2003;77:5759–5773. doi: 10.1128/JVI.77.10.5759-5773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Wu TT, Tchieu JH, Feng J, Brown HJ, Feng J, Li X, Qi J, Deng H, Vivanco I, Mellinghoff IK, Jamieson C, Sun R. Inhibition of the phosphatidylinositol 3-kinase-Akt pathway enhances gamma-2 herpesvirus lytic replication and facilitates reactivation from latency. J Gen Virol. 2010;91:463–469. doi: 10.1099/vir.0.015073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjold B, Rutqvist LE, Skoog L, Stal O. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- Perkins D, Pereira EF, Gober M, Yarowsky PJ, Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J Virol. 2002a;76:1435–1449. doi: 10.1128/JVI.76.3.1435-1449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D, Yu Y, Bambrick LL, Yarowsky PJ, Aurelian L. Expression of herpes simplex virus type 2 protein ICP10 PK rescues neurons from apoptosis due to serum deprivation or genetic defects. Experimental neurology. 2002b;174:118–122. doi: 10.1006/exnr.2001.7849. [DOI] [PubMed] [Google Scholar]
- Portis T, Longnecker R. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene. 2004;23:8619–8628. doi: 10.1038/sj.onc.1207905. [DOI] [PubMed] [Google Scholar]
- Prakash O, Swamy OR, Peng X, Tang ZY, Li L, Larson JE, Cohen JC, Gill J, Farr G, Wang S, Samaniego F. Activation of Src kinase Lyn by the Kaposi sarcoma-associated herpesvirus K1 protein: implications for lymphomagenesis. Blood. 2005;105:3987–3994. doi: 10.1182/blood-2004-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaus M, Desloges N, Wolff MH. Varicella-zoster virus requires a functional PI3K/Akt/GSK-3alpha/beta signaling cascade for efficient replication. Cell Signal. 2007;19:312–320. doi: 10.1016/j.cellsig.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Molecular carcinogenesis. 1998;21:81–86. [PubMed] [Google Scholar]
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74:10681–10689. doi: 10.1128/jvi.74.22.10681-10689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N, Mukherjee G, Sen A, Bendall SC, Sung P, Nolan GP, Arvin AM. Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell reports. 2014;8:633–645. doi: 10.1016/j.celrep.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shair KH, Bendt KM, Edwards RH, Bedford EC, Nielsen JN, Raab-Traub N. EBV latent membrane protein 1 activates Akt, NFkappaB, and Stat3 in B cell lymphomas. PLoS Pathog. 2007;3:e166. doi: 10.1371/journal.ppat.0030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shair KH, Schnegg CI, Raab-Traub N. EBV latent membrane protein 1 effects on plakoglobin, cell growth, and migration. Cancer research. 2008;68:6997–7005. doi: 10.1158/0008-5472.CAN-08-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78:4207–4223. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Patel K, Chandran K, Marginean A, Bottero V, Kerur N, Paul AG. COX-2/PGE2: molecular ambassadors of Kaposi's sarcoma-associated herpes virus oncoprotein-v-FLIP. Oncogenesis. 2012;1:e5. doi: 10.1038/oncsis.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin SH, Roy D, Wang L, Staudt MR, Fakhari FD, Patel DD, Henry D, Harrington WJ, Jr., Damania BA, Dittmer DP. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood. 2007;109:2165–2173. doi: 10.1182/blood-2006-06-028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit MJ, Verzijl D, Casarosa P, Navis M, Timmerman H, Leurs R. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via G(i) and phospholipase C-dependent signaling pathways. J Virol. 2002;76:1744–1752. doi: 10.1128/JVI.76.4.1744-1752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC. The herpes simplex virus type 2 protein ICP10PK: a master of versatility. Frontiers in bioscience : a journal and virtual library. 2005;10:2820–2831. doi: 10.2741/1738. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Patel V, Gomez-Roman JJ, Li Y, Sausville EA, Sawai ET, Gutkind JS. Akt plays a central role in sarcomagenesis induced by Kaposi's sarcoma herpesvirus-encoded G protein-coupled receptor. Proc Natl Acad Sci U S A. 2004;101:4821–4826. doi: 10.1073/pnas.0400835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni V, Cahir-McFarland E, Kieff E. LMP1 TRAFficking activates growth and survival pathways. Advances in experimental medicine and biology. 2007;597:173–187. doi: 10.1007/978-0-387-70630-6_14. [DOI] [PubMed] [Google Scholar]
- Soroceanu L, Akhavan A, Cobbs CS. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature. 2008;455:391–395. doi: 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- Spender LC, Lucchesi W, Bodelon G, Bilancio A, Karstegl CE, Asano T, Dittrich-Breiholz O, Kracht M, Vanhaesebroeck B, Farrell PJ. Cell target genes of Epstein-Barr virus transcription factor EBNA-2: induction of the p55alpha regulatory subunit of PI3-kinase and its role in survival of EREB2.5 cells. J Gen Virol. 2006;87:2859–2867. doi: 10.1099/vir.0.82128-0. [DOI] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. The New England journal of medicine. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Strunk U, Saffran HA, Wu FW, Smiley JR. Role of herpes simplex virus VP11/12 tyrosine-based motifs in binding and activation of the Src family kinase Lck and recruitment of p85, Grb2, and Shc. J Virol. 2013;87:11276–11286. doi: 10.1128/JVI.01702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart R, Ruf IK, Sample J, Longnecker R. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-Kinase/Akt pathway. J Virol. 2000;74:10838–10845. doi: 10.1128/jvi.74.22.10838-10845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasian SK, Teachey DT, Rheingold SR. Targeting the PI3K/mTOR Pathway in Pediatric Hematologic Malignancies. Frontiers in oncology. 2014;4:108. doi: 10.3389/fonc.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CC, Damania B. The K1 protein of Kaposi's sarcoma-associated herpesvirus activates the Akt signaling pathway. J Virol. 2004;78:1918–1927. doi: 10.1128/JVI.78.4.1918-1927.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalikis J, Croitoru DO, Philpott DJ, Girardin SE. Nutrient sensing and metabolic stress pathways in innate immunity. Cell Microbiol. 2013;15:1632–1641. doi: 10.1111/cmi.12165. [DOI] [PubMed] [Google Scholar]
- Tsuruta F, Masuyama N, Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002;277:14040–14047. doi: 10.1074/jbc.M108975200. [DOI] [PubMed] [Google Scholar]
- Uddin S, Hussain AR, Al-Hussein KA, Manogaran PS, Wickrema A, Gutierrez MI, Bhatia KG. Inhibition of phosphatidylinositol 3′-kinase/AKT signaling promotes apoptosis of primary effusion lymphoma cells. Clin Cancer Res. 2005;11:3102–3108. doi: 10.1158/1078-0432.CCR-04-1857. [DOI] [PubMed] [Google Scholar]
- Valiya Veettil M, Sadagopan S, Kerur N, Chakraborty S, Chandran B. Interaction of c-Cbl with myosin IIA regulates Bleb associated macropinocytosis of Kaposi's sarcoma-associated herpesvirus. PLoS Pathog. 2010;6:e1001238. doi: 10.1371/journal.ppat.1001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J Virol. 2006;80:11432–11446. doi: 10.1128/JVI.01342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Smiley JR. Herpes simplex virus requires VP11/12 to activate Src family kinasephosphoinositide 3-kinase-Akt signaling. J Virol. 2011;85:2803–2812. doi: 10.1128/JVI.01877-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D, Mohr I. Viral subversion of the host protein synthesis machinery. Nat Rev Microbiol. 2011;9:860–875. doi: 10.1038/nrmicro2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Damania B. Kaposi's sarcoma-associated herpesvirus confers a survival advantage to endothelial cells. Cancer research. 2008;68:4640–4648. doi: 10.1158/0008-5472.CAN-07-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dittmer DP, Tomlinson CC, Fakhari FD, Damania B. Immortalization of primary endothelial cells by the K1 protein of Kaposi's sarcoma-associated herpesvirus. Cancer research. 2006;66:3658–3666. doi: 10.1158/0008-5472.CAN-05-3680. [DOI] [PubMed] [Google Scholar]
- Wlodarski P, Kasprzycka M, Liu X, Marzec M, Robertson ES, Slupianek A, Wasik MA. Activation of mammalian target of rapamycin in transformed B lymphocytes is nutrient dependent but independent of Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase, insulin growth factor-I, and serum. Cancer research. 2005;65:7800–7808. doi: 10.1158/0008-5472.CAN-04-4180. [DOI] [PubMed] [Google Scholar]
- Xue M, Yao S, Hu M, Li W, Hao T, Zhou F, Zhu X, Lu H, Qin D, Yan Q, Zhu J, Gao SJ, Lu C. HIV-1 Nef and KSHV oncogene K1 synergistically promote angiogenesis by inducing cellular miR-718 to regulate the PTEN/AKT/mTOR signaling pathway. Nucleic acids research. 2014;42:9862–9879. doi: 10.1093/nar/gku583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GD, Huang TJ, Peng LX, Yang CF, Liu RY, Huang HB, Chu QQ, Yang HJ, Huang JL, Zhu ZY, Qian CN, Huang BJ. Epstein-Barr Virus_Encoded LMP1 upregulates microRNA-21 to promote the resistance of nasopharyngeal carcinoma cells to cisplatin-induced Apoptosis by suppressing PDCD4 and Fas-L. PLoS One. 2013;8:e78355. doi: 10.1371/journal.pone.0078355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Alwine JC. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3'-OH kinase pathway and the cellular kinase Akt. J Virol. 2002;76:3731–3738. doi: 10.1128/JVI.76.8.3731-3738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen JW, Chung GT, Lun SW, Cheung CC, To KF, Lo KW. Epigenetic inactivation of inositol polyphosphate 4-phosphatase B (INPP4B), a regulator of PI3K/AKT signaling pathway in EBV-associated nasopharyngeal carcinoma. PLoS One. 2014;9:e105163. doi: 10.1371/journal.pone.0105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang JF, Chandran B, Persaud K, Pytowski B, Fingeroth J, Groopman JE. Kaposi's sarcoma-associated herpesvirus activation of vascular endothelial growth factor receptor 3 alters endothelial function and enhances infection. J Biol Chem. 2005;280:26216–26224. doi: 10.1074/jbc.M411392200. [DOI] [PubMed] [Google Scholar]
- Zheng K, Xiang Y, Wang X, Wang Q, Zhong M, Wang S, Wang X, Fan J, Kitazato K, Wang Y. Epidermal growth factor receptor-PI3K signaling controls cofilin activity to facilitate herpes simplex virus 1 entry into neuronal cells. mBio. 2014;5:e00958–00913. doi: 10.1128/mBio.00958-13. [DOI] [PMC free article] [PubMed] [Google Scholar]