Abstract
Background
Parkinson’s disease (PD) is a common neurodegenerative disorder associated with gray matter atrophy. Cortical atrophy patterns may further help distinguish between PD motor subtypes. Comparable differences in subcortical volumes have not been found.
Methods
Twenty-one cognitively intact and treated PD patients, including 12 tremor dominant (TD) subtype, Nine postural instability gait dominant (PIGD) subtype, and 20 matched healthy control subjects underwent 3.0 Tesla high-resolution structural MRI scanning. Subcortical volumetric analysis was performed using FreeSurfer and shape analysis was performed with FIRST to assess for differences between PD patients and controls and between PD subtypes.
Results
No significant differences in subcortical volumes were found between motor PD subtypes, but comparing grouped PD patients with controls revealed a significant increase in hippocampal volume in PD patients (p=0.03). A significant shape difference was detected in the right nucleus accumbens (NAcc) between PD and controls and between motor subtypes. Shape differences were driven by positive deviations in the TD subtype. Correlation analysis revealed a trend between hippocampal volume and decreasing MDS-UPDRS (p=0.06).
Conclusion
While no significant differences in subcortical volumes between PD motor subtypes were found, increased hippocampal volumes were observed in PD patients compared to controls. Right NAcc shape differences in PD patients were driven by changes in the TD subtype. These unexpected findings may be related to the effects of chronic dopaminergic replacement on the mesolimbic pathway. Further studies are needed to replicate and determine the clinical significance of such morphologic changes.
Keywords: Parkinson’s disease, hippocampus, volumetric analysis, dopamine, shape analysis, nucleus accumbens
INTRODUCTION
Parkinson’s disease (PD) is a common neurodegenerative disease pathologically defined by neuronal depletion within the substantia nigra pars compacta (SN) and the presence of Lewy bodies and neurites in diseased neurons. PD patients can be divided into tremor dominant (TD) and postural instability gait dominant (PIGD) subtypes based on their predominant motor symptoms. TD and PIGD patients have been found to differ in their rates of disease progression and in the frequency and severity of non-motor clinical features including cognitive decline and dementia [1].
Although no distinct pathological or imaging finding can distinguish between TD and PIGD, pathological studies have shown that the severity of cortical Lewy body formation and amyloid plaque load is greater in PIGD [2]. Also, using voxel based morphometry (VBM), Rosenberg-Katz et al. recently reported greater cortical gray matter atrophy [3] in PIGD compared with TD patients in regions involving motor, cognitive, limbic and associative function. The authors hypothesized that these differences may play a role in the distinct clinical features of the subtypes.
Using dopamine transporter (DaT) single photon emission computed tomography (SPECT) imaging, TD and PIGD subtypes have also been shown to differ in presynaptic dopamine neuronal function [4] suggesting that differential degrees of disease burden may be present at the subcortical level as well. Only only one volumetric MR study to date has investigated subcortical volumes in TD and PIGD patients. Linder et al. reported finding no significant subcortical volumetric differences between subtypes [5]. This study, however, was conducted on a 1.5 T MRI magnet and involved visual analysis, which may be less sensitive than quantitative imaging methods for detecting small volumetric differences.
In the present study, we utilized a robust, automated volumetric MRI method to determine whether subcortical gray matter volumes differ between TD and PIGD subtypes. To complement our volumetric analysis, we also conducted automated shape analysis, which may be more sensitive in detecting local, sub-structural pathology related morphologic changes than overall volumetric measurement. Based on previous work [1–5], we hypothesized that there would be smaller subcortical gray matter volume in PIGD compared to TD patients.
METHODS
Study participants
Twenty-one patients with idiopathic PD (12 male; mean age ± S.D.: 61.1 ± 7.6 years) and 20 age and gender matched healthy controls (HC; 11 male; mean age ± S.D.: 61.1 ± 8.8 years) were recruited from the University of Colorado Denver Movement Disorders Center. All patients met UK Parkinson Society Brain Bank criteria for clinical PD diagnosis, were Hoehn & Yahr stage I-III, and were taking and responsive to dopaminergic medication. All patients provided written informed consent approved by the Colorado Multiple Institutional Review Board.
Clinical assessments
A Montreal Cognitive Assessment (MoCA) was acquired for all subjects. All patients were evaluated with the revised Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) in the “on” and practical “off” state (dopaminergic medications held for ≥ 12 hours). Subtype assignments were based on previously described methods [6]. Twelve patients were classified as TD and 9 as PIGD (none were indeterminate). Patients were given the Beck Depression Inventory and Neuropsychiatric Inventory to assess for psychiatric comorbidities.
MRI acquisition
3.0 Tesla high- resolution T1-weighted images were obtained using an 8-channel coil on a GE Signa scanner. MRI parameters were as follows: FOV=22, matrix 256 × 256, 140 slices, and slice thickness = 1.2 mm, coronal plane, resulting in voxel resolution of 1×1×1.2 mm3.
Image processing and analysis
Automated segmentation of subcortical gray matter structures and calculation of total intracranial volume (TICV) was conducted using FreeSurfer (v4.5.0) (https://surfer.nmr.mgh.harvard.edu/). We examined the following structures: putamen, caudate nucleus, globus pallidus, hippocampus, amygdala, and nucleus accumbens (NAcc). Segmentation results for each subject were visually reviewed independently by three of the authors (JH, TK and ES) for segmentation errors prior to group analysis. For each structure, shape analysis was performed using FIRST (v5.0.0) (http://fsl.fmrib.ox.ac.uk/fsl/). From the statistical maps generated from this analysis we extracted signed mean scalar values (representing shape change from the average surface) for all the voxels with significant shape differences for each subject.
Statistical analyses
PD groups and subtypes were compared on continuous and dichotomous demographic variables using independent t-tests and chi-square tests, respectively. For each structure, left, right and bilateral gray matter volumes were analyzed for the main effect of PD group and subtype using analysis of covariance (ANCOVA), after adjusting for age and TICV. Statistical analyses were performed using IBM SPSS Statistics (IBM, Armonk, NY, USA). A significant difference between groups was defined as p < 0.05. Effects of PD group and subtype on shape adjusted for age and TICV were tested using Randomise (FSL) using a standard general linear model f-test, 5000 iterations and a family-wise error correction threshold of p < 0.05.
RESULTS
Participants
Subject characteristics are summarized in Table 1. PD patients had mild to moderate disease as demonstrated by a mean Hoehn & Yahr stage of 2.2 ± 0.7, and had no significant cognitive impairment (MoCA: 27.7 ± 1.5), psychiatric symptoms (NPI: 2.6 ± 2.6) or significant depression (BDI: 8.2 ± 5.7). Mean levodopa daily dose (LEDD) was 505 ± 367.
Table 1.
PD Patient and Control Demographics and Clinical Scores
| PD Patients | Subtype differences p-value | Healthy Controls (N=20) | PD vs Healthy Controls p-Value | |||
|---|---|---|---|---|---|---|
| All (N=21) | TD (N=12) | PIGD (N=9) | ||||
| Age (mean ± SD) | 61.1 ± 7.6 | 60.9 ± 8.3 | 60.2 ± 7.4 | 0.88 | 61.1± 8.8 | 0.97 |
| Gendera | 12M: 9 F | 9M: 3F | 3M: 6F | 0.06 | 11M: 9 F | 0.89 |
| MoCA | 27.7 ± 1.5 | 27.8 ± 1.5 | 27.6 ± 1.7 | 0.78 | 28.2 ± 1.7 | 0.30 |
| Education (years) | 16 ± 2.4 | 16.4 ± 1.4 | 15.3 ± 3.3 | 0.32 | 17 ± 1.9 | 0.20 |
| Hoehn & Yahr stage | 2.2 ± 0.7 | 2.0 ± .04 | 2.5 ± 0.9 | 0.08 | - | - |
| Disease duration (years) | 5.5 ± 3.4 | 5.2 ± 2.7 | 5.9 ± 4.3 | 0.63 | - | - |
| Levodopa Equivalent Daily Dose | 505 ± 367 | 559 ± 395 | 463 ± 360 | 0.58 | - | - |
| MDS-UPDRS Total | 53.6 ± 10.7 | 56.9 ± 10.3 | 49.3 ± 11.2 | 0.26 | - | - |
| MDS-UPDRS III–OFF | 31.4 ± 10.0 | 33.4 ± 9.9 | 28.7 ± 9.9 | 0.35 | - | - |
| MDS-UPDRS III–ON | 22.2 ± 9.6 | 23.5 ± 8.4 | 20.6 ± 11.4 | 0.50 | - | - |
| Neuropsychiatric Inventory-Severity | 2.6 ± 2.6 | 2.2 ± 1.8 | 3.1 ± 3.4 | 0.42 | - | - |
| Beck Depression Inventory | 8.2 ± 5.7 | 8.5 ± 5.3 | 7.8 ± 6.4 | 0.78 | - | - |
Abbreviations: PD Parkinson’s Disease; MDS-UPDRS Movement Disorders Society-Unified Parkinson’s Disease Rating Scale.
Gender comparison performed using Chi-squared test.
There were no significant differences in age, gender, MoCA scores or level of education between PD subtypes, or between PD patients and controls. There were trends toward higher Hoehn & Yahr stage (p = 0.08) and female gender (p = 0.06) in PIGD compared with TD patients. Motor severity, as measured by MDS-UPDRS III, was not significantly different between the two subtypes.
Subcortical volumes
Volumetric results are displayed in Table 2. While mean subcortical volumes were smaller in the PIGD subtype compared with the TD subtype for all structures except the hippocampus, no statistically significant differences were observed. Post hoc analysis comparing the subcortical volumes of the PD and healthy control groups did reveal a significant difference in total hippocampal volumes with PD patients showing larger volumes compared to healthy controls (p=0.03, Figure 1). Both right and left hippocampal volumes independently demonstrated a trend toward larger volumes in PD patients (p=0.07 and p=0.06, respectively). No other statistically significant differences in volumes between PD patients and controls were found. Post hoc analysis also revealed a trend between increasing total hippocampal volume and lower MDS-UPDRS motor scores (p=0.06 uncorrected).
Table 2.
Subcortical gray matter volumes
| PD Patients | Healthy Controls (N=20) | TD vs PIGD comparison p Value | PD vs HC comparison p Value | |||
|---|---|---|---|---|---|---|
| TD (N=12) | PIGD (N=9) | All (N=21) | ||||
| Putamen | 10178.8 | 9800.7 | 10016.8 | 10164.9 | 0.87 | 0.36 |
| Right | 4940.8 | 4691.0 | 4833.8 | 4926.6 | 0.71 | 0.35 |
| Left | 5238.0 | 5109.7 | 5183.0 | 5238.3 | 0.92 | 0.41 |
| Caudate nucleus | 6892.4 | 6502.7 | 6725.4 | 6987.6 | 0.50 | 0.12 |
| Right | 3438.4 | 3281.3 | 3371.1 | 3513.4 | 0.64 | 0.10 |
| Left | 3454.0 | 3221.3 | 3354.3 | 3474.2 | 0.39 | 0.16 |
| Globus pallidus | 3199.7 | 3080.2 | 3148.5 | 2995.3 | 0.91 | 0.35 |
| Right | 1529.3 | 1466.4 | 1502.3 | 1447.0 | 0.72 | 0.49 |
| Left | 1670.4 | 1613.8 | 1646.1 | 1548.3 | 0.94 | 0.34 |
| Hippocampus | 8327.1 | 8381.4 | 8345.3 | 7835.6 | 0.35 | 0.03 |
| Right | 4247.7 | 4178.2 | 4212.8 | 3954.2 | 0.94 | 0.07 |
| Left | 4079.4 | 4203.2 | 4132.5 | 3881.4 | 0.15 | 0.05 |
| Amygdala | 3218.8 | 3079.6 | 3159.1 | 3056.5 | 0.77 | 0.62 |
| Right | 1704.4 | 1554.3 | 1640.1 | 1560.5 | 0.33 | 0.45 |
| Left | 1514.3 | 1525.2 | 1519.0 | 1496.1 | 0.60 | 0.94 |
| Nucleus accumbens | 1270.0 | 1153.2 | 1220.0 | 1154.2 | 0.21 | 0.32 |
| Right | 636.3 | 560.0 | 603.6 | 593.8 | 0.19 | 0.93 |
| Left | 633.8 | 593.2 | 616.4 | 560.4 | 0.58 | 0.13 |
Volumes uncorrected mm3.
Figure 1.
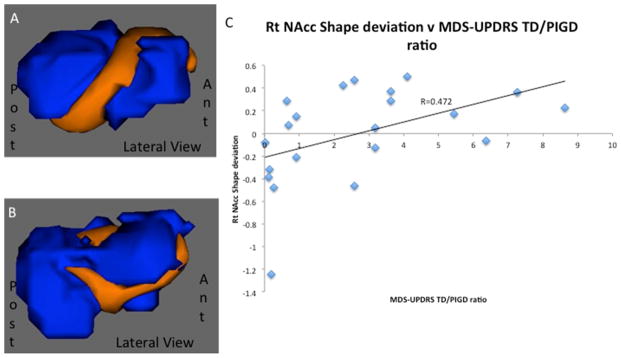
Results of RNAcc shape analysis. A, B) visual representation of effects of group and subtype, respectively; statistically significantly differing vertices are depicted in orange. C) Graph demonstrating significant correlation between degree of shape difference and MDS-UPDRS TD/PIGD ratio.
Shape analysis results are shown in Figure 1. A significant difference in the right NAcc shape was found between the PD group and controls (p=0.005) and between PD subtypes (p=0.02), resulting from local positive (outward) surface deviations in the PD group and TD subtype, respectively. When a post hoc pair-wise effect of subtype analysis was performed comparing the right NAcc shape in TD and PIGD separately with controls, only the TD group differed significantly from the control group (p=0.004, Tukey’s corrected). Post hoc analysis also revealed that the magnitude of shape deviation was significantly correlated with the MDS-UPDRS TD/PIGD ratio (p=0.03).
DISCUSSION
In this study we investigated subcortical volumes in TD and PIGD patients and found no significant differences between these motor subtypes. Our data are consistent with earlier work [5], which similarly found no statistically significant volumetric differences in subcortical gray matter structures between TD and PIGD. Our findings help strengthen the literature in that this finding has been verified using data acquired at a higher field strength MRI magnet and processed with automated segmentation methodology. This suggests that volumetric differences that can distinguish TD from PIGD could be limited to the cortex, which is in line with prior studies suggesting that differences in cortical pathology between the subtypes may account for their varying clinical manifestations and courses [2,3]. It is possible that subcortical volume differences exist, however are so small that more statistical power would be needed to detect such a difference.
Interestingly, when our grouped PD patients were compared with healthy controls, we found a significant increase of hippocampal volumes. Previous studies investigating hippocampal volumes in non-demented PD patients have yielded inconsistent results. Some studies have shown hippocampal atrophy in PD patients but others have found no differences; these are reviewed in a paper by Calabresi, et. al. [7]. One of the larger volumetric studies of PD patients to date, however, reported slightly increased hippocampal volumes in 72 PD patients compared with 46 healthy controls, although the difference was not statistically significant [8].
Shape analysis of the subcortical volumes has begun to provide insights into the morphological changes that are associated with PD [9,10]. Our shape analysis revealed significant differences in shape of the right NAcc between the PD group and controls as well as between PD subtypes and post hoc analysis revealed that the initial group difference was driven by positive changes within the TD subtype. Mean right NAcc volumes were slightly larger in the TD subtype compared to both PIGD patients and controls, however these differences were not significant. Given that NAcc pathology has been linked to akinesia as well as non-motor cognitive and psychiatric symptoms [11], which are known to have a greater burden on PIGD patients, it is possible that positive remodeling in TD patients may be somewhat protective against these clinical changes. Furthermore, the magnitude of right NAcc shape difference correlated with the MDS-UPDRS TD/PIGD ratio (p=0.03), suggesting this metric may be a morphologic marker for motor subtype.
The presence of a strong dopaminergic influence on the mesolimbic pathway, which includes both the hippocampus and the NAcc, has been established in physiologic and pathologic settings [7] and offers a possible biological explanation for the morphologic changes in our PD patients, all of whom were taking dopaminergic medications. In early PD, the dopaminergic deficit predominantly affects the dorsal striatum and spares the ventral striatum and the ventral tegmental area [7]. While dopaminergic therapy may initially restore function to dopamine-dependent pathways in the dorsal striatum, it may also potentiate activity in the ventral-tegmental-area hippocampal loop, subjecting the hippocampus and NAcc to supra-physiologic levels of dopaminergic stimulation [12]. Furthermore, a distinguishing feature of the hippocampus among subcortical structures is that it is a site of continued neurogenesis in the adult human and findings from recent mindfulness-based interventions further support that there is preserved plasticity in the hippocampus even in older individuals with PD [13]. As dopaminergic stimulation has been shown to promote hippocampal neurogenesis in rats [14], it is possible a similar process may underlie our finding of increased hippocampal sizes in PD patients treated with dopaminergic medications.
Potential dopaminergic influence on hippocampal volume might help explain the findings in a study by Lee et al [15] of smaller hippocampal volumes in PD patients at the time of diagnosis, before dopaminergic therapy had been initiated, compared to controls. This potential confound may also help explain the inconsistency of previous studies. In a recent longitudinal study of hippocampal volumes in PD, low hippocampal volume in PD was suggested to be predictive of the progression of cognitive impairment [16]. Our finding of enlarged hippocampal volumes in our selected cognitively intact PD cohort could therefore stem from selection bias for patients who have protective mechanisms against cognitive decline. Furthermore, correlation analysis revealed a trend between hippocampal volume and lower MDS-UPDRS scores (p=0.06). Continued investigation into the effects of dopaminergic therapy on mesolimbic morphology in cognitively intact PD patients is needed, and whether such effects are associated with any clinically meaningful changes.
In conclusion, we found no significant volumetric differences in the subcortical gray matter structures between TD and PIGD subtypes of PD patients. An unexpected finding was the presence of larger hippocampal volumes in our cognitively intact PD patients compared with healthy controls. Shape analysis further revealed evidence of remodeling of the right NAcc in PD patients that was driven by changes in the TD subtype, which suggests this finding may have utility as an imaging biomarker for subtype. These novel findings may be related to the use of improved automated methods or due to biologic changes, possibly related to dopaminergic stimulation affecting the mesolimbic pathways. Further studies are needed to test this possibility and to determine the clinical significance of such morphologic changes.
Highlights.
Cognitively intact Parkinson’s disease patients showed larger hippocampal volumes than controls
Shape changes in the right nucleus accumbens in the tremor dominant PD patients may be an imaging biomarker for PD subtype
Morphologic changes in the hippocampus and nucleus accumbens may be related to effects of dopaminergic replacement on the mesolimbic pathway
Further studies are needed to determine clinical significance of volumetric changes
Acknowledgments
Funding sources: Supported by NIH/NCATS Colorado CTSI Grant Number KL2 TR001080.
We would like to thank our patients and control subjects for their generous participation in this project.
Footnotes
Financial disclosures and Conflicts of interest: None
Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burn DJ, et al. Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2006;77:585–589. doi: 10.1136/jnnp.2005.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selikhova M, et al. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132:2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg-Katz K, et al. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology. 2013;80:1476–1484. doi: 10.1212/WNL.0b013e31828cfaa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eggers C, Kahraman D, Fink GR, Schmidt M, Timmermann L. Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT Single photon emission computed tomography. Mov Disord. 2011;26:416–423. doi: 10.1002/mds.23468. [DOI] [PubMed] [Google Scholar]
- 5.Linder J, et al. Degenerative changes were common in brain magnetic resonance imaging in patients with newly diagnosed Parkinson’s disease in a population-based cohort. J Neurol. 2009;256:1671–1680. doi: 10.1007/s00415-009-5177-4. [DOI] [PubMed] [Google Scholar]
- 6.Stebbins GT, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov Disord. 2013;28:668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi P, Castrioto A, Di Filippo M, Picconi B. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson’s disease. Lancet Neurol. 2013;12:811–821. doi: 10.1016/S1474-4422(13)70118-2. [DOI] [PubMed] [Google Scholar]
- 8.Messina D, et al. Patterns of brain atrophy in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Parkinsonism Relat Disord. 2011;17:172–176. doi: 10.1016/j.parkreldis.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Nemmi F, Sabatini U, Rascol O, Péran P. Parkinson’s disease and local atrophy in subcortical nuclei: insight from shape analysis. Neurobiol Aging. 2015;36:424–433. doi: 10.1016/j.neurobiolaging.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Menke RAL, et al. Comprehensive morphometry of subcortical grey matter structures in early-stage Parkinson’s disease. Hum Brain Mapp. 2014;35:1681–1690. doi: 10.1002/hbm.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mavridis IN. Mavridis’ atrophy begins in early stage Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:1323. doi: 10.1016/j.parkreldis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Swainson R, et al. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 13.Pickut BA, et al. Mindfulness based intervention in Parkinson’s disease leads to structural brain changes on MRI: A randomized controlled longitudinal trial. Clin Neurol Neurosurg. 2013;115:2419–2425. doi: 10.1016/j.clineuro.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Takamura N, et al. The effect of dopamine on adult hippocampal neurogenesis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:116–124. doi: 10.1016/j.pnpbp.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Lee HM, et al. Subcortical grey matter changes in untreated, early stage Parkinson’s disease without dementia. Parkinsonism Relat Disord. 2014;20:622–626. doi: 10.1016/j.parkreldis.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Kandiah N, et al. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:1203–1208. doi: 10.1016/j.parkreldis.2014.08.024. [DOI] [PubMed] [Google Scholar]


