Abstract
Background/Aims
Suicide is a major public health concern, yet there are very few randomized clinical trials that have been conducted to reduce suicidal ideation in patients at risk for suicide. We describe the rationale and refinements of such a trial that is designed to assess the effect of a hypnotic medication on suicidal ideation in adult outpatients currently experiencing suicidal ideation.
Methods
“Reducing Suicidal Ideation Through Insomnia Treatment (REST-IT)” is a multi-site randomized clinical trial that includes 3 recruiting sites and one data management site. This 4-year study is in its second year of recruitment. The purpose of the study is to compare hypnotic medication versus placebo as an add-on treatment to a selective serotonin reuptake inhibitor as a means of reducing suicidal ideation in depressed adult outpatients with insomnia and suicidal ideation. The safety features of the study follow the 2001 NIH guidelines for studies that include patients at risk of suicide.
Results
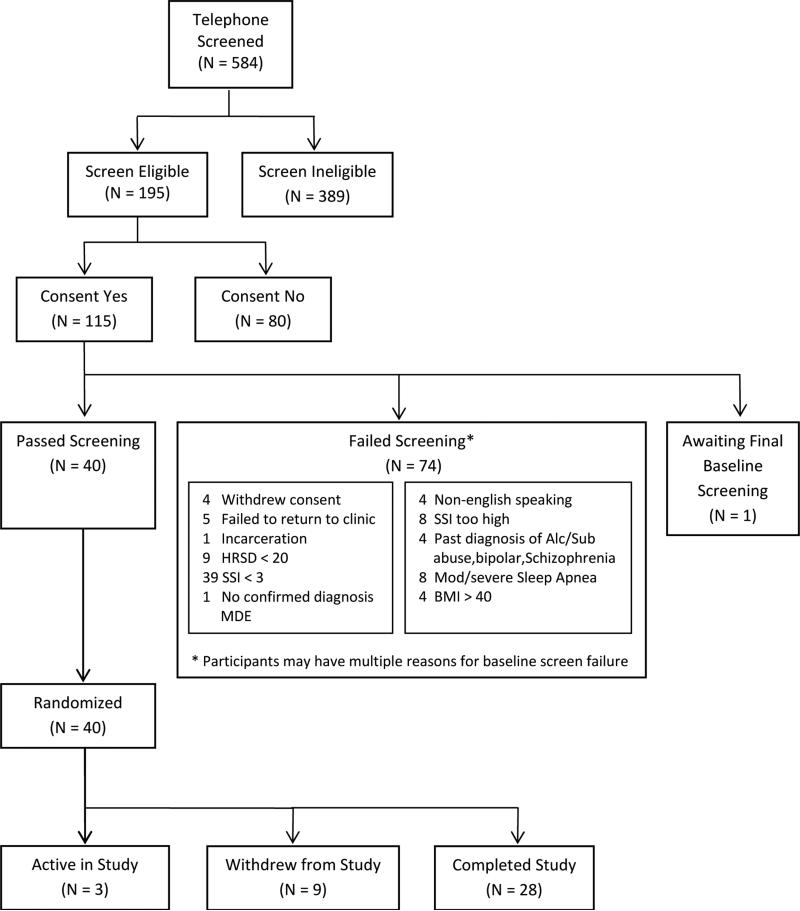
Five hundred and eighty-four potential participants have undergone telephone screening; 67% of these failed the phone screen, most often due to an absence of expressed suicidal ideation (26% of the telephone screen fails). One hundred and twelve persons appeared for a face-to-face baseline assessment, and 40 of these had completed a taper of their ineffective psychotropic medications before the baseline assessments. Sixty-four% of those who completed baseline assessments failed to proceed to randomization, most commonly because of no clinically significant suicidal ideation (51% of those excluded at baseline). One participant was offered and accepted voluntary psychiatric hospitalization in lieu of study participation. Thus far, 40 participants have been randomized into the study, 88.7% of scheduled visits have been attended, with 93.8% adherence for the SSRI and 91.6% adherence for the randomized hypnotic versus placebo. None of the randomized participants have required hospitalization or had a suicide attempt.
Conclusions
By carefully considering the inclusion and exclusion criteria and other safety features, the safe conduct of randomized clinical trials in suicidal adult patients is possible, including the inclusion of participants who have undergone a prescribed tapering of psychotropic medications prior to baseline assessment.
Keywords: randomized clinical trial, suicide, depression, insomnia, outpatient, fluoxetine, zolpidem
In the last two decades, the world rates of mortality due to infectious disease and malnutrition have declined while the rates of suicide have not improved.1 In the USA, mortality rates of 8 of the 10 most common causes of death showed a reduction between 2011 and 2012, while only one of the top-10 causes of death showed a statistically significant increase – suicide.2 Thus, US and global progress against suicide has been stagnant. This stagnation has many causes including stigma, fear, and lack of research into targeted treatment of risk factors for suicide. To date, most research into suicide prevention has focused on improved surveillance for risk factors and population-based interventions designed to reduce psychosocial risk factors. In contrast, randomized clinical trials which recruit actively suicidal outpatients are uncommon.3 There are large-scale clinical trials of interventions in persons at increased risk of suicide (such as persons with prior suicide attempts), but these studies did not specifically recruit patients who were suicidal at the time of enrollment.4-6
Pharmaceutical companies cannot be counted upon to conduct such research, as studies associated with suicide attempts or suicide deaths would potentially tarnish the development of new drugs or the market reputation of existing pharmaceuticals. As a result, the research methods of pharmaceutical companies’ development of new psychotropic medications routinely exclude patients deemed to be at risk for suicide. Thus, there is little definitive information regarding treatment choices for persons who are actively suicidal. This systematic exclusion of suicidal patients from clinical trials creates an ethical problem that is little different from the situation a few decades ago when women and minorities were marginalized in recruitment of studies for cardiovascular disease, which ultimately led to the call for broadly representative recruitment so that all genders, ages, races, and ethnicities could benefit from research discoveries. The exclusion of suicidal patients in industry-supported clinical trials can be aggravated by university institutional review boards which are understandably risk-avoidant and not desiring to approve studies that may have serious adverse events that could reflect badly upon the university. Recently, ethicists have commented upon this dilemma and called upon institutional review boards to accept the possibility of heightened serious adverse events as an acceptable cost to rectify the injustice done by ignoring the needs of high-risk patients (such as suicidal patients) to have access to evidence-based treatments which have been proven in clinical trials.7
Investigators in the field of suicidology have highlighted the need for suicide-focused clinical trials, saying “nowhere is the lack of proven therapeutic methods greater than in the prevention of suicidal behavior.”8 Suicide experts within the National Institute for Mental Health (NIH) have paved the way for ethical conduct of clinical trials in suicidal patients with the publication of specific guidelines that are intended to promote the ethical recruitment and the safety of suicidal participants.9
So, the groundwork has been set for the design of clinical trials in suicidal patients, but where to start? We begin with the realization that the field is not sufficiently developed to accept suicide death as the proper endpoint for a primary aim, for both ethical and practical reasons. One practical reason includes the fact that suicide death is sufficiently uncommon that only exceptionally large RCTs would show an effect of an intervention. So, smaller studies are logical first steps, and more commonly observed events, such as changes in suicidal ideation, become the proxy for suicide, as suicidal ideation is widely accepted as a prelude to suicidal behavior and suicide death.10 Suicidal ideation, then, becomes the primary endpoint. Hereafter, the term ‘suicide’ will be used as the summary term for suicidal ideation, suicidal behavior and suicide death.
Selecting an intervention for a suicide-clinical trial starts with a review of the known risk factors for suicide. Unmodifiable risk factors for suicide death in adults include male gender, advancing age, and Caucasian ethnicity. Well-established modifiable risk factors include Major Depressive Disorder, active substance abuse, social isolation, widowhood, and feelings of hopelessness.10-12 The most recent item to be added to the list of modifiable risk factors is sleep disturbance, especially insomnia and nightmares.13-15 Insomnia is a frequent symptom within the Major Depressive Disorder syndrome, affecting up to 60% of adult outpatients with Major Depressive Disorder and 90% of inpatients.16,17 The cause-and-effect relationship of insomnia and Major Depressive Disorder is complex, as insomnia may either precede, follow, or be coincident with the onset of an episode of Major Depressive Disorder.18,19
More than 40 studies in adults have shown that insomnia stands as an independent risk factor for suicide,15 thus raising the possibility that treatment of insomnia may mitigate suicidal risk. The mechanism by which insomnia confers risk for suicide is not completely understood, but possible mediation paths include insomnia's tendency to promote despair, its pernicious influence upon decision making, and its impact on the serotonergic function in the brain.15
Treatment of depression with antidepressant medication is not always a sufficient means to relieve insomnia, as (1) antidepressants work relatively slowly, over weeks,20 and (2) insomnia is the symptom of depression least likely to resolve with what otherwise would be considered a successful course of antidepressant medication therapy.21 For all of these reasons, specific, targeted treatment of insomnia is a justifiable approach in reducing the risk of suicide. The two widely accepted approaches to treating insomnia include FDA-approved hypnotic medications22 and cognitive behavioral therapy for insomnia.23 While both treatments are effective, hypnotics work faster but then may lose effectiveness when they are withdrawn, while cognitive behavior therapy has a slower onset of action, but may have persistent benefit after formal conclusion of therapy. Recently, we have embarked upon an NIH-supported clinical trial of the hypnotic medication zolpidem versus placebo for outpatients with insomnia and suicidal ideation within the context of Major Depressive Disorder. Since there were so few prior clinical trials in this topic to which to refer as models for study design,24 the authors relied upon their own early experiences and NIH safety guidelines in the initial study design, with subsequent modifications as needed.
The remainder of this paper describes our experience in refining the methods of our clinical trials in suicidal adult participants.
Methods
Overview
The clinical trial “Reducing Suicidal Ideation Through Insomnia Treatment (REST-IT)” enrolls adult outpatients 18-65 years old who met criteria for Major Depressive Disorder, complicated by insomnia and suicidal ideation. Qualified participants are prescribed an open label selective serotonin reuptake inhibitor (such as fluoxetine 20 mg, sertraline 50 mg, paroxetine 20, or citalopram 20 daily), with an option to increase the dose after 4 weeks if antidepressant response is inadequate. Participants also receive a daily bedtime dose of either generic zolpidem controlled release 6.25 mg or placebo in a 1:1 blinded randomization. The dose of zolpidem/placebo may be increased to 12.5 mg any time during the study after the first week if the initial dose produces inadequate relief of insomnia and is also free of side effects such as morning hangover. The placebo was custom ordered from the University of Georgia College of Pharmacy to resemble the generic form of zolpidem controlled release. The period of randomized treatment is 8 weeks.
All study medications are managed by the clinical research pharmacy at the Georgia Regents Medical Center and shipped to recruiting sites as necessary. Data are entered at each site into a centrally managed shared web-based database. At the time of randomization, the website randomization facility assigns a kit number to the participant which corresponds to a treatment assignment known only to the central pharmacy. The pharmacy is automatically notified of the kit number upon randomization and provides the appropriate treatment to the recruitment site. Wake Forest School of Medicine is the Data Management site, while The Medical College of Georgia, Duke University School of Medicine, and the University of Wisconsin are the recruiting sites. Each recruiting site has access to an inpatient psychiatric ward, thus satisfying the 2001 NIH guidelines for studies which include patients at risk of suicide to “consider and establish criteria for hospitalization, where the hospitalization will take place and the procedures within the hospital that provide additional safety”.9 The institutional review board at each site approved the conduct of the study, and each participant provided written, informed consent to participate.
The safety features of the study were designed to address the NIH publication “Issues to consider in intervention research with persons at high risk for suicidality”,9 summarized in Table 1 .
Framing the sample
The tension in defining the sample frame comes from selecting a group of patients with ‘clinically relevant’ suicidal ideation, without including patients with such intense suicidal ideation (including a plan with imminent intent for suicide) that randomization could not be ethically justified, or as would require hospitalization. The 2001 NIH guidelines state: “provide specific inclusion criteria and their measurement with regards to suicidality”.9 Our primary endpoint is the intensity of suicidal ideation as measured with the Scale for Suicide Ideation, which is a 19-item self-report instrument, with each item scored 0-2, for a maximum total score of 38. A score of > 3 has been reported to confer increased risk for eventual suicide death.10 We originally proposed to include patients with SSI > 0, but this was judged by the study section at NIH to include patients with such low levels of suicidal ideation (i.e., scores of ‘1’ or ‘2’) as to be not clinically relevant. As a result, the inclusion criterion was revised upward to a Scale for Suicide Ideation score > 3.
It was equally important to operationalize the exclusion criterion of ‘too suicidal’ to be safely included. The need for an operating rule was made more urgent as REST-IT has three recruiting sites, mandating consistency across sites in how maximally-tolerable suicide risk is defined. The first iteration of this operating rule was to exclude participants with a baseline Scale for Suicide Ideation score of > 16. This decision rule was based upon preliminary data that revealed that a score of > 16 was the best cut point to segregate stable outpatients with Major Depressive Disorder from patients who require emergency psychiatric hospitalization because of intense suicidality.25 The initial implementation of this rule led to some logistic and ethical dilemmas, as the rule excluded some potential participants who clearly lacked imminent intent to commit suicide (i.e., they did not need to be psychiatrically hospitalized). Many of these participants had applied to participate in the study because they lacked insurance, and as a result had minimal access to outpatient mental health services. Forcing this group of patients out of the study had the effect of (1) denying them their only ready source of outpatient care, (2) potentially precipitating a new crisis through the disappointment of being refused study participation, and (3) and hampering study recruitment. As a result, after conferring with our NIH Program Officer and our external Data Safety Monitoring Board, and gaining approval from our respective Institutional Review Boards, we modified our rule for ‘excessive suicidal ideation’ to be defined by the presence of imminent intent to commit suicide. This revision of our inclusion/exclusion criteria seemed more ethically defensible and had the added benefit of enhancing enrollment.
The Columbia Suicide Severity Rating Scale is an observer-rated instrument that stratifies suicidal ideation over several dimensions such as intensity, frequency, controllability, and the presence of deterrents.26 Along the intensity dimension, the Columbia Suicide Severity Rating Scale describes 5 stages of risk: (1) passive death wish, (2) thoughts of killing oneself, (3) making a plan, (4) some intent to carry out the plan, and (5) serious intent to carry out the plan. The stage of risk is determined by following structured questions, which assures some consistency across sites, but leaves to the judgment of the interviewer how to best interpret the patient's answers. For our new operating rule, we chose the fourth stage of risk as our exclusion criterion with the additional refinement that the risk had to be imminent and not at some future indeterminate point. This revision satisfies the 2001 NIH recommendation to “provide specific inclusion criteria and their measurement with regards to suicidality”.9
Additional qualifications for participation include a score of > 20 on the 24-item Hamilton Rating Scale for Depression,27 which is consistent with severe depressive symptoms, and a score > 7 on the Insomnia Severity Index, consistent with at least mild insomnia.28 Hopelessness, which is a core and consistent feature of suicide, was measured with the Beck Hopelessness Scale.29,30 The Beck Hopelessness Scale is comprised of 20 True/False statements.
We required patients to be free of all psychotropic medications for 7 days before completing the qualifying study-entry measures including the Scale for Suicide Ideation, Columbia Suicide Severity rating Scale, Hamilton Depression Rating Scale, and Insomnia Severity Index. Some potential participants were taking a variety of psychotropics at the time of the telephone screen, including antidepressants other than fluoxetine and anxiolytics. In many cases it was possible to taper the potential participant off of their ineffective medication regimen in order to complete the one-week psychotropic free period prior to baseline assessment. An ineffective medication regimen was defined as the presence of suicidal ideation and symptoms of depression. These taper periods were always conducted after consenting the patient to the study, providing detailed instructions on how to complete the taper, and with daily phone calls to the participants during the taper to assure their safety.
Recruitment and consent
Prospective participants’ capacity to consent to the study was assessed with a standard instrument, the Evaluation to Sign Consent. The Evaluation to Sign Consent was developed by Love in 1988 (unpublished; as cited in Deronzo, Conley, & Love, 1998).31 The Evaluation to Sign Consent assesses the factual understanding of subjects, is composed of five items, and can be adapted to any research design. This operating rule satisfies the 2001 NIH suggestion to: “consider the impact of suicidality on the study participant's capacity to give informed consent”.9
Participants are encouraged to bring loved ones or significant others to the first visit and to involve them in the study. This operating rule satisfies the 2001 NIH suggestion to: “as part of the consent process, consider having explicit discussion with relevant family members, guardians, or friends that includes the risks inherent when study participants are suicidal (risk of death, side effects of treatment)”.9
The consent form also states that an expressed high risk of suicidal intent may result in disclosure of this information to other providers, thus meeting the 2001 NIH guidelines recommendation to “consider and identify the limits of confidentiality with respect to suicidality, as well as other circumstances”.9
The most basic question regarding recruitment has been whether advertisements and initial screening phone calls should emphasize that participation requires that suicidal ideation be present at study entry, and further that suicidal ideation is the primary endpoint. The study's design is to recruit patients with active suicidal ideation that is incidental to their Major Depressive Disorder, rather than patients who are chronically engaging in self-injurious thoughts and behaviors irrespective of their mood state. The ethical prohibition against needless deception of research subjects requires that we inform patients that suicidal ideation is a topic of relevance to the study, but that still left considerable latitude in how much importance to give suicidal ideation in comparison to insomnia or other symptoms of depression. Our first approach was to avoid emphasizing suicidal ideation, but as a result we excluded several potential participants who expressed an inadequate degree of suicidality, and who, when they were told that they were disqualified for entry into the study, then claimed they had falsely minimized their symptoms for fear that they might be involuntarily hospitalized had they told the truth. This early experience led us to modify our approach during recruitment and consent and become explicit in detailing that suicidality be present for acceptance into the study, and that this was an outpatient study not designed to involuntarily hospitalize participants. There is no way to determine whether patients anxious to participate in the study might falsify the presence of suicidal ideation in order to qualify, but this type of moral hazard is endemic to studies using self-reported symptoms, and in an environment of challenging recruitment, it is justifiable to err on the side of inclusion.
Sample size
The effect of the intervention will be assessed by comparing the average weekly post-treatment difference in Scale for Suicide Ideation between the two groups. The primary analysis will be a mixed model analysis of covariance with treatment and baseline Scale for Suicide Ideation as fixed covariates. Under the assumption of compound symmetry, this test is equivalent to an analysis of covariance on the mean post-treatment Scale for Suicide Ideation with the baseline level as the covariate. Thus, the sample size needed to detect a clinically meaningful difference between treatment groups can be determined approximately using formulae based on t-tests, where the standard deviation for the post-treatment mean is adjusted by its correlation with baseline levels. To ensure that the trial is not continued if one therapy is clearly superior, a three-stage group sequential design (i.e., two interim analyses and one final analysis) will be employed to allow interim monitoring of the data while the trial is ongoing using stopping boundaries that are intermediate to those proposed by Pocock32 and O'Brien and Fleming33 in the likelihood of stopping early. The S-Plus module S+SeqTrial 2 (2006) was used to determine the stopping boundaries and the sample size required at each stage. Based on our preliminary data, the standard deviation for Scale for Suicide Ideation for the patients meeting the eligibility criteria for this study was 3.1 .34 Assuming conservatively that the standard deviation will be 3.1 in the proposed study, and additionally assuming two interim looks and a drop-out rate of 20%, the total sample size needed to detect a 2.0 unit difference in Scale for Suicide Ideation between treatment groups with 90% power at the 5% two-sided level of significance was 138. In our previous pilot study,34 the adjusted treatment difference was 1.3, but the upper bound on the confidence interval was 3.5, so an effect of 2 units is consistent with the pilot data. Further, differences in Scale for Suicide Ideation >2 represent risk for future suicide death.10
Safety features after enrollment
Sedative hypnotics are generally contraindicated in untreated severe obstructive sleep apnea, so all participants complete a single-night of screening for sleep apnea via either a home or inlab sleep study which measured airflow and oximetry to exclude patients with more than mild obstructive sleep apnea. Mild sleep apnea was defined as 5-10 apnea/hypopnea-desaturation events per hour of recording. Thereafter participants are immediately prescribed study medication and given a one week supply (plus 3 days), as lethal overdose would be nearly impossible with a week supply of fluoxetine or zolpidem either alone or in combination. Follow-up clinic visits are scheduled at 1, 2, 4, 6, and 8 weeks post-randomization. Only enough study drug is provided at each visit to last until the next scheduled visit, plus 3 days. Research staff routinely call study participants at the mid-point between scheduled visits, and additional clinic visits are scheduled on an as-needed basis when study staff are concerned about suicide risk, thus satisfying the NIH guidelines recommendation to “describe procedures in the protocol for managing increases in suicidality, and how research staff is trained and available to provide clinical management”.9
At each visit, blinded study physicians are provided the Scale for Suicide Ideation, Hamilton Rating Scale for Depression, and Insomnia Severity Index scores, and then they perform a Columbia Suicide Severity Rating Scale assessment. The Scale for Suicide Ideation, Hamilton Rating Scale for Depression, and Columbia Suicide Severity Rating Scale data provide the basis for assessing the safety of continuing the patient in the study. If the patient is not making progress toward reduced suicidal ideation and resolution of depression (i.e. Hamilton Rating Scale for Depression > 15 after 4 weeks), then the study physician offers to withdraw the patient and refer them to care-as-usual, thus satisfying the 2001 NIH guidelines to “specify the criteria for withdrawal from the treatment trial with regard to increased suicidality, increased related symptoms, lack of treatment response, and treatment side effects, and what alternative treatment or referral will be offered.”9 The study physician also assesses the presence or absence of drowsy driving or drowsiness-related accidents since the last visit, consistent with the 2013 FDA guidance on surveillance for drowsy driving in patients taking hypnotic medications,35 and doses of zolpidem are adjusted accordingly.
Participants are given a phone number for an on-call psychiatrist for after-hours emergencies, and each site is affiliated with a university medical school emergency department and inpatient psychiatric unit, thus meeting the 2001 NIH guidelines recommendation to “have a procedure for emergency coverage that is clearly understood by the clinical research staff, study participants, and families”.9
The chairman of the external Data Safety Monitoring Board was involved in the penultimate iterations of the protocol before it was launched and provided commentary on all revisions to the protocol and to the study's conduct. The membership of the Data Safety Monitoring Board includes a suicidologist, an expert in depression clinical trials, an expert in hypnotic clinical trials, a biostatistician, an ethicist, and a patient advocate. The Data Safety Monitoring Board has operating rules that describe the circumstances under which the study could be terminated prematurely, thus satisfying the 2001 NIH suggestion to “consider situations in which a trial would be terminated prematurely”.9
Secured clinical hand-offs at the end of study participation
The risks associated with suicidal ideation demand that participants have secured care-as-usual clinic visits within a month after their last study visit. The anticipation of delays in obtaining a care-as-usual clinic visit led us to the strategy of discussing the participant's preference for care-as-usual visits as soon as they entered the study in order to assure no lags in care at the end of study participation. At the end of study participation, participants are provided with sufficient fluoxetine to last until the first care-as-usual visit. For obvious reasons of safety and maintenance of the study blind, no continuation hypnotic medication is offered upon study completion. Furthermore, we have previously reported that in most cases hypnotics can be discontinued after 8 weeks of concurrent selective serotonin reuptake inhibitor therapy with no substantial loss of benefit.36 Study staff call participants weekly for 2 weeks after study participation to assure that the after-care plan is in motion, and to administer the Scale for Suicide Ideation over the telephone.
Results
At the time of this writing, 584 potential participants have undergone phone screening. The three recruiting sites varied in their approach to recruiting potential participants to complete the initial telephone screen. In descending rank order, Wisconsin had its best success with flyers posted at University of Wisconsin primary care offices, followed by emails to university staff, ads in the local paper, flyers in community mental health clinics, advertisements on departmental websites, and emails to university providers. Duke has had its best success in recruiting for telephone screening with newspaper ads, followed by radio ads, campus flyers, and community mental health center flyers. Medical College of Georgia had its best recruitment success with ads in local health magazines, followed by referrals to the departmental outpatient clinic, newspaper ads, flyers on the university campus, then radio ads. Sixty-seven% of those who completed a phone screen failed, most often because of no expressed suicidal ideation (26% of the telephone screens). Over time, the study staff learned that during the phone screen it was important to put potential participants at ease, by explaining that suicidal ideas were common in persons who were depressed. One hundred and twelve persons appeared for a face-to-face baseline assessment and consented to participate; 64% of these failed to proceed to randomization, most commonly because of no clinically significant suicidal ideation (51% of those excluded at baseline). Forty consented participants were tapered off of psychotropic medications without incident before completing the baseline assessment, including 7 who were withdrawn from psychotropics other than selective serotonin reuptake inhibitors before randomization. One participant was offered and accepted voluntary psychiatric hospitalization in lieu of study participation. Thus far, 40 participants have been randomized into the study; 28 completed the 8 weeks of randomized care, 9 left the study before 8 weeks, and 3 are still active at this time (Figure 1). The drop our rate of 22% is slightly higher than the 15% drop out that we have reported in our pilot studies.34 Participants have thus far completed 88.7% of all scheduled visits during randomized care, with 93.8% adherence to fluoxetine, and 91.6% adherence to randomized hypnotic versus placebo. At baseline, the participants are severely depressed, suffer from extreme insomnia and a moderate degree of hopelessness, and are moderately suicidal, with about one-third reporting at least one prior suicide attempt (Table 2). Nevertheless, none of the randomized participants have required hospitalization, had a suicide attempt, or had any amnestic behavior. One patient had an episode of drowsy driving and a drowsy-related motor vehicle accident after having taken a prohibited psychotropic medication at 7 AM. This participant was administratively dropped from the study because of inability to follow instructions. Another patient had an unanticipated pregnancy during the last week of the study despite stating at the time of recruitment that she was sexually abstinent. This was coded as a serious adverse event.
Discussion
Suicide is a major public health concern, with only meager information to direct clinicians in their management of individuals with suicidality. Clinical trials that expressly recruit suicidal outpatients and have suicidality as an a priori endpoint are exceptionally uncommon. Reducing Suicidal Ideation Through Insomnia Treatment (REST-IT) is a first step toward addressing this deficiency, and its implementation has incorporated significant safety features in its study design and conduct. In the early phases of this study, our team has succeeded in recruiting patients with moderate degrees of suicidality and retaining them for the majority of scheduled visits without serious adverse events related to suicide. Each of the three recruiting sites has found its own formulae for eliciting telephone screens, and the telephone screen-to-randomized –participant ratio is less than 1:10. Although a high degree of hopelessness might be predicted to lead to early drop out, we believe that our use of open label selective serotonin reuptake inhibitors, a flexible dosing regimen for zolpidem, with potential dose increases for the hypnotic after week 1 of randomization, and potential increases in the antidepressant after week 4, gives our participants reason to hope and remain in the study even if they do not perceive early improvement, and this may explain the observed high rates of adherence to scheduled visits during the randomized phase of the study. We have been able to enhance our recruitment by allowing for a psychotropic medication tapering period prior to the baseline assessment, and this process has been successfully pursued without emergence of psychiatric crises during the tapering period.
The recruitment for REST IT is limited to adult participants, in part because zolpidem is not approved for use in children or adolescents. Suicide is equally important in children and adolescents as in adults. While the absolute suicide death rate in children and adolescents is lower than in adults, the rank-order for suicide as a cause of mortality in children and adolescents is higher than adults, constituting the third most common cause of death in young people.37 The risk factors for suicide in young people are mostly the same as in adults, including depressed mood, substance abuse, hopelessness, and impulsivity. On the other hand, some risk factors are more relevant to the psychosexual development of young people as compared to adults, including sexual orientation.37 Remarkably, sleep disturbance is a risk factor for suicidal ideation, similar to what has been reported in adults.38
Limitations
This is a small 3-recruitment-site clinical trial in adults, and the lessons learned in its launch may not apply to larger scale studies, or to children and adolescents. Also, REST IT does not address the value of treatment of sleep disturbances in suicidal persons who suffer with schizophrenia, bipolar disorder, post-traumatic stress disorder, or substance abuse. The study is still in its early phases and may continue to evolve with experience.
Future directions
There is a desperate need for more clinical trials addressing suicidality in actively suicidal outpatients, as a means of generating evidence-based interventions for these individuals. It is possible that the safety features and other considerations that have been developed for REST-IT could be applied to other interventions in suicidal patients.
Figure 1.
Consort Diagram
Table 1.
Summary of National Institute of Mental Health “Issues to consider in intervention research with persons at high risk for suicidality”.9
| 1. Identify specific inclusion criteria and their measurement with regard to suicidality. Exclusion criteria, including those relevant to suicidality, should also be specified. |
| 2. Specify the criteria for withdrawal from the treatment trial with regard to increased suicidality, increased related symptoms, lack of treatment response, and treatment side effects, and what alternative treatment or referral will be offered. |
| 3. Consider and establish criteria for hospitalization, where the hospitalization should take place, and procedures within the hospital that provide additional safety. |
| 4. Describe procedures in the protocol for managing increases in suicidality, and how research staff is trained and available to provide such clinical management. |
| 5. Have a procedure for emergency coverage that is clearly understood by the clinical research staff, study participants and families. Consider providing a written document describing this coverage. |
| 6. As part of the consent process, consider having explicit discussion with relevant family members, guardians, or friends that includes the risks inherent when study participants are suicidal (risk of death, side effects of treatments); the procedures for handling increases in suicidality; the criteria for withdrawal from the study; the risks and benefits of the treatment and control conditions offered, and the limits of confidentiality. |
| 7. Consider and identify the limits to confidentiality with respect to suicidality, as well as other circumstances. |
| 8. Consider the impact of suicidality on the study participants' capacity to give informed consent. |
| 9. Determine whether additional safeguards are needed to ensure the safety of the study participants. This includes procedures available to individual study participants, such as study participant advocates, or those relevant to the overall conduct of the study, such as a Data Safety Monitoring Board. |
| 10. Consider situations in which a trial would be terminated prematurely. A Data Safety and Monitoring Board may independently review the trial's progress and relevant safety concerns, and address “stopping rules.” |
Table 2.
Baseline Characteristics of Randomized Participants N=40
| Characteristic | Overall N(%) or Mean ± sd |
|---|---|
| Age | 41.7 ± 12.5 |
| Female | 21 (52.5%) |
| Race/Ethnicity | |
| Non-Hispanic White | 29 (72.5%) |
| Black or African American | 8 (20%) |
| Hispanic | 1 (2.5%) |
| Asian | 1 (2.5%) |
| Native Hawaiian or Pacific Islander | 1 (2.5%) |
| Employment Status | |
| Professional | 11 (27.5%) |
| Unprofessional/Skilled | 5 (12.5%) |
| Retired/Disabled/Student | 5 (12.5%) |
| Homemaker | 2 (5%) |
| Unemployed | 15 (37.5%) |
| Never worked in Paid Employment | 2 (5%) |
| Suicide Severity Index | |
| Total Score | 10.5 ± 4.5 |
| % with at least one suicide attempt | 14 (35%) |
| Columbia Suicide Severity Rating Scale - Severity of ideation score | 1.9 ± 1.3 |
| Hamilton Rating Scale for Depression total score | 29.7 ± 5.5 |
| Insomnia Severity Index total score | 21.1 ± 3.8 |
| Beck Hopelessness Score | 13.9 ± 4.5 |
Acknowledgements
Supported by NIMH award R01 MH095776
Footnotes
Conflict of Interest Statement:
Dr. McCall is a scientific advisor for Merck & Co. pharmaceutical company and for Otusak pharmaceutical company. He receives research support from Merck & Co and the NIMH. He receives honoraria from Wolter Kluwer publishing.
Dr Benca is a consultant for Merck & Co and Jazz Pharmaceuticals, and receives grant support from Merck
Dr Rumble receives research support from Merck & Co Pharmaceuticals and from NIMH
Dr Krystal receives grant support from NIH, Teva, Sunovion, Astellas, Abbott, Neosync, Brainsway, Janssen, ANS St Jude, Novartis and is a consultant to Abbott, Astellas, Astrazeneca, Attentiv, BMS, Teva, Eisai, Eli Lilly, GlaxoSmithKline, Jazz, Janssen, Merck, Neurocrine, Otsuka, Lundbeck, Roche, Sanofi-Aventis, Somnus, Sunovion, Takeda, Transcept and Vantia
ClinicalTrials.gov Identifier: NCT01689909
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Kochanek KD, Murphy SL, et al. NCHS data brief, no 168. National Center for Health Statistics; Hyattsville, MD: 2014. Mortality in the United States, 2012. [PubMed] [Google Scholar]
- 3.Grunebaum MF, Ellis S, Duan N, et al. Pilot randomized clincial trial of an SSRI vs bupropion: effects on suicidal behavior, ideation, and mood in major depression. Neuropsychopharmacology. 2012;37:697–706. doi: 10.1038/npp.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischmann A, Bertolote JM, Wasserman D, et al. Effectiveness of brief intervention and contact for suicide attempters: a randomized controlled trial in five countries. Bull World Health Organ. 2008;86:703–709. doi: 10.2471/BLT.07.046995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv. 2001;52:828–833. doi: 10.1176/appi.ps.52.6.828. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia. Arch Gen Psychiatry. 2003;60:82–91. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]
- 7.Iltis AS, Misra S, Dunn L, et al. Addressing risks to advance mental health research. JAMA Psychiatry. 2013;70:1363–1371. doi: 10.1001/jamapsychiatry.2013.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oquendo M, Mann JJ. Intervention research for suicidal behavior. Lancet. 2003;362:844–845. doi: 10.1016/S0140-6736(03)14347-4. [DOI] [PubMed] [Google Scholar]
- 9.Pearson JL, Stanley B, King C, et al. Issues to consider in intervention research with persons at high risk for suicidality. National Institue of Mental Health. 2001 http://www.nimh.nih.gov/health/topics/suicide-prevention/issues-to-consider-in-intervention-research-with-persons-at-high-risk-for-suicidality.shtml. [PubMed]
- 10.Brown GK, Beck AT, Steer RA, et al. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol. 2000;68:371–377. [PubMed] [Google Scholar]
- 11.Maris R. Suicide. Lancet. 2002;360:319–326. doi: 10.1016/S0140-6736(02)09556-9. [DOI] [PubMed] [Google Scholar]
- 12.Coryell W, Young EA. Clinical predictors of suicide in primary major depressive disorder. J Clin Psychiatry. 2005;66:412–417. doi: 10.4088/jcp.v66n0401. [DOI] [PubMed] [Google Scholar]
- 13.Vaughn McCall W. Insomnia is a risk factor for suicide - what are the next steps? Sleep. 2011;34:1149–1150. doi: 10.5665/SLEEP.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCall WV, Blocker JN, D'Agostino R, Jr, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010;11:822–827. doi: 10.1016/j.sleep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCall WV, Black CG. The link between suicide and insomnia: theoretical mechanisms. Current Psychiatry Rep. 2013;15:389. doi: 10.1007/s11920-013-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weissman M, Bland R, Canino G, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- 17.McCall WV, Reboussin BA, Cohen W. Subjective measurement of insomnia and quality of life in depressed inpatients. J Sleep Res. 2000;9:43–48. doi: 10.1046/j.1365-2869.2000.00186.x. [DOI] [PubMed] [Google Scholar]
- 18.Baglioni C, Feige B, Soiegelhlder K, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiologic studies. J Affect Disord. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Alvaro P, Roberts R, Harris J. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36:1059–1068. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam R. Onset, time course and trajectories of improvement with antidepressants. Eur Neuropsychopharmacol. 2012;22:S492–S498. doi: 10.1016/j.euroneuro.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60:221–225. doi: 10.4088/jcp.v60n0403. [DOI] [PubMed] [Google Scholar]
- 22.McCall WV, Fleischer AB, Jr, Feldman SR. Diagnostic codes associated with hypnotic medications during outpatient physician-patient encounters in the United States from 1990-1998. Sleep. 2002;25:221–223. doi: 10.1093/sleep/25.2.221. [DOI] [PubMed] [Google Scholar]
- 23.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta- analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 24.Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA. 2005;294:2064–2074. doi: 10.1001/jama.294.16.2064. [DOI] [PubMed] [Google Scholar]
- 25.McCall WV, Batson N, Webster M, et al. A psychometric cut-point to separate emergently suicidal depressed patients from stable outpatients. Indian J Psychiatry. 2013;55:283–286. doi: 10.4103/0019-5545.117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: utility and limitations of research instruments. In: First MB, editor. Standardized Evaluation in Clinical Practice. APPI Press; Washington, DC: 2003. pp. 103–130. [Google Scholar]
- 27.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Beck A, Steer R. Beck Hopeless Scale Manual. Psychological Corporation; New York: 1988. [Google Scholar]
- 30.Beck A, Brown G, Berchick R, et al. Relationship between hopelessness and ultimate suicide: A replication with psychiatric outpatients. Am J Psychiatry. 1990;147:190–195. doi: 10.1176/ajp.147.2.190. [DOI] [PubMed] [Google Scholar]
- 31.Deronzo E, Conley R, Love R. Assessment of capacity to give consent to research participation: State of the art and beyond. J Health Care Law Policy. 1998;1:66–87. [PubMed] [Google Scholar]
- 32.Pocock S. Group sequential methods in the design and analysis of clinical trials. Biometrika. 2014;64:191–199. [Google Scholar]
- 33.O'Brien P, Fleming T. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 34.McCall WV, Blocker JN, D'Agostino RB, Jr, et al. Treatment of insomnia in depressed insomniacs: effects on health-related quality of life, objective and self-reported sleep, and depression. J Clin Sleep Med. 2010;6:322–329. [PMC free article] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration US Department of Health and Human Services. FDA requiring lower recommended dose for certain sleep drugs containing zolpidem. 2013 www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm334798.htm.
- 36.Krystal A, Fava M, Rubens R, et al. Evaluation of eszopiclone discontinuation after cotherapy with fluoxetine for insomnia with coexisting depression. J Clin Sleep Med. 2007;3:48–55. [PubMed] [Google Scholar]
- 37.Spirito A, Esposito-Smythers C. Attempted and completed suicide in adolescence. Annu Rev Clin Psychol. 2006;2:237–266. doi: 10.1146/annurev.clinpsy.2.022305.095323. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan G, Ali SK, Simpson B, et al. Associations between sleep disturbance and suicidal ideation in adolescents admitted to an inpatient psychiatric unit. Int J Adolesc Med Health. 2014;26:411–416. doi: 10.1515/ijamh-2013-0318. [DOI] [PubMed] [Google Scholar]