Abstract
Background
In the United States, half of men with prostate cancer harbor the androgen-regulated gene fusion TMPRSS2:ERG. We hypothesized that men with TMPRSS2:ERG positive tumors are more responsive to androgen deprivation therapy (ADT).
Methods
We studied a cohort of 239 men with prostate cancer from the Physicians’ Health Study and Health Professionals Follow-up Study who received ADT during their disease course. Fusion status was assessed on available tumor tissue by immunohistochemistry for ERG protein expression. We used Cox models to calculate hazard ratios (HRs) and 95 percent confidence intervals (CIs) for assessment of prostate cancer-specific mortality after ADT initiation.
Results
Roughly half of the men had stage T3 or higher tumors at diagnosis and 39% had Gleason 8–10 tumors. During an average follow-up of 10.2 years, 42 men died from prostate cancer. There was a non-significant inverse association between positive fusion status and time to death from prostate cancer after ADT (multivariable HR: 0.76; 95% CI: 0.40–1.45). Harboring the TMPRSS2:ERG fusion was associated with a statistically significant lower risk of prostate cancer mortality among men who were treated with orchiectomy (multivariable HR: 0.13; 95% CI: 0.03–0.62), based on 15 events.
Conclusions
Our results, combined with those from earlier studies, provide suggestive evidence that men with TMPRSS2:ERG positive tumors may have longer prostate cancer survival after ADT. Larger cohorts are needed for more robust results and to assess whether men with tumors harboring the fusion benefit from treatment with ADT in the (neo)adjuvant or metastatic setting specifically.
INTRODUCTION
Prostatic tumor growth is strongly regulated by androgenic hormones and androgen receptor signaling. For decades, hormonal ablation has been the standard approach to treat prostate cancer patients with newly metastatic disease [1], and has also been used in combination with radiation or surgery for men with locally advanced cancer [2]. Androgen deprivation therapy (ADT), by surgical or chemical castration, suppresses androgens, which in turn decreases androgen receptor signaling, ultimately resulting in slowed tumor growth and prolonged survival. In the metastatic setting, men typically respond to ADT for 18 to 24 months before ultimately becoming castration-resistant, although there is considerable variability in response time [3,4].
In the United States, roughly half of men with prostate cancer harbor a somatic gene event known as the TMPRSS2:ERG fusion [5,6]. The fusion involves TMPRSS2, a gene regulated by androgenic hormones [7], and ERG, a member of the erythroblast transformation specific (ETS) family of transcription factors, which is a set of genes that are critically involved in oncogenic pathways and play key roles in the regulation of cellular proliferation, differentiation and apoptosis [8]. In TMPRSS2:ERG positive prostate cancer, the fusion of TMPRSS2 with ERG renders its oncogenic function under androgen regulation [9].
Given this feature, tumors harboring the fusion may be more dependent on androgen signaling and men with such tumors may therefore be more responsive to the effects of ADT than men with tumors lacking the fusion. While some studies have supported the hypothesis [10,11], other studies have not seen an association between TMPRSS2:ERG status and responsiveness to ADT (Table 1) [9,12]. In the largest analysis to-date, we investigated whether the TMPRSS2:ERG fusion is associated with prolonged survival among men with prostate cancer treated with ADT from the prospective Physicians’ Health Study and the Health Professionals Follow-up Study.
Table 1.
Comparison of studies (including the current study) having evaluated responsiveness to ADT in the context of TMPRSS2:ERG
| Study | Year | Study Design | Sample Size | Participant & Study Characteristics | Findings |
|---|---|---|---|---|---|
| Attard, et al.[10] | 2009 | Case Study | 1 | Tumor demonstrating rearrangements involving both ERG and ETV1; GnRH agonist & anti-androgen in response to biochemical recurrence following radical prostatectomy & salvage radiation | Long-lasting response to multiple courses of ADT |
| Boormans, et al.[9] | 2010 | Cohort | 71 | Pelvic lymph node dissection ± radical prostatectomy, assessment of TMPRSS2:ERG in lymph node metastases | No difference in prostate cancer-specific survival (P:0.22), overall survival (P:0.71), or median duration of response to ADT (P:0.70) |
| Leinonen, et al.[12] | 2010 | Cohort | 178 | ADT as a primary treatment, assessment of TMPRSS2:ERG in biopsy tissue | No difference in progression-free survival (P:0.62) |
| Karnes, et al.[11] | 2010 | Cases from a Case-Control Study | 152 | Treatment with radical prostatectomy ± adjuvant ADT, cases developed metastases or died from prostate cancer within 6 years following treatment, controls did not progress within 8 years of treatment | Suggestive, but non-significant, interaction between treatment with adjuvant ADT and ERG status on time to development of metastases or prostate cancer-specific death |
| Graff, et al. | 2014 | Cohort | 239 | Assessment of TMPRSS2:ERG in radical prostatectomy or TURP tissue | No difference in prostate cancer-specific (P:0.40) or overall survival (P:0.59) |
Abbreviations: ADT – Androgen Deprivation Therapy
MATERIALS AND METHODS
Study population
This study included men diagnosed with prostate cancer from the Physicians’ Health Study and the Health Professionals Follow-up Study for whom tumor tissue was available, ERG status was assessed and who underwent ADT during their clinical course. The Physicians’ Health Study consisted of two randomized primary prevention trials of aspirin and dietary supplements among US male physicians ages 40 to 84; the Physicians’ Health Study I included 22,071 at randomization in 1982 and an additional 7,000 men enrolled in the Physicians’ Health Study II in 1995 (clinicaltrials.gov Identifier: NCT00270647) [13,14]. The Health Professionals Follow-up Study is an ongoing prospective study of risk factors for cancer and other diseases among 51,529 male health professionals ages 40 to 75 at enrollment in 1986. Participants in each cohort responded to a baseline questionnaire concerning their medical histories and known or suspected contributors to cancer and other chronic diseases. Follow-up questionnaires are mailed regularly to update information on potential risk factors and to identify newly diagnosed illnesses.
Clinical data and prostate cancer follow-up
Since the beginning of follow-up, 3,837 cases of prostate cancer have been diagnosed in the Physicians’ Health Study and 7,129 have been diagnosed in the Health Professionals Follow-up Study. Diagnoses are confirmed by review of medical records and pathology reports. The study team reviews these records in order to abstract information on tumor stage, prostate specific antigen (PSA) level at diagnosis, and treatments. Since 2000, we have conducted biennial follow-up of men in the cohorts living with prostate cancer to collect detailed information including: cancer treatments, biochemical recurrence, development of metastases, and PSA levels. Development of metastatic disease is verified by medical records for the majority of cases. Deaths are ascertained via repeat mailings, telephone calls to non-respondents, and inspection of the National Death Index. An endpoints committee of physicians confirms all deaths through review of death certificates and medical records. Prostate cancer is defined as the cause of death when there is evidence of extensive metastatic disease, and no other more plausible cause of death. Follow-up for mortality in the cohorts is over 95 percent.
Whenever possible, we have retrieved archival diagnostic tumor tissue material from participants with prostate cancer having undergone radical prostatectomy or transurethral resection of the prostate (TURP). In total, we were able to collect tumor tissue from and assay ERG status for 439 men with prostate cancer in the Physicians’ Health Study and 843 in the Health Professionals Follow-up Study who were diagnosed between 1986 and 2004.
Identification of participants treated with ADT
This study included men treated with ADT at any point during their disease course. We considered treatment with ADT to include use of an anti-androgen (e.g., flutamide, bicalutamide), injection with a gonadotropin-releasing hormone (GnRH) agonist (e.g., leuprolide, goserelin), and/or surgical castration (orchiectomy). Whenever possible, the study team ascertained information from patient medical records about the timing and utilization of these treatments as well as disease status at the time of therapy. If data were unavailable from medical records, we supplemented with information from follow-up questionnaires sent to patients. Among those assayed for ERG status, 239 men were treated with ADT, 86 from the Physicians’ Health Study and 153 from the Health Professionals Follow-up Study. Of the men, 215 had radical prostatectomy tissue available and 24 had TURP tissue available.
For some cases, we had only the year of ADT initiation and assigned the month to be June (n = 121). We compared dates of all ADT treatments to assign the earliest class of ADT received by each participant. If two classes of therapy were received in the same time frame, we considered participants to have received combination therapy (n = 45). We used explicit information from medical records (n = 166) and comparisons of primary and ADT treatment dates (n = 73) to determine whether subjects were receiving ADT as a primary therapy (n = 19), (neo)adjuvant therapy (n = 92) or in response to disease progression (biochemical recurrence or development of metastases) (n = 124).
Tumor tissue and biomarker studies
The study pathologists (RTL, MF, ML) reviewed hematoxylin and eosin slides to provide uniform Gleason grading and other histopathological features, and to select areas of tumor for the construction of tumor tissue microarrays [15]. Tissue microarrays were constructed from archival prostate cancer tissue specimens by taking at least three 0.6-mm tumor cores from the primary tumor nodule or the nodule with the highest Gleason grade.
We characterized the presence or absence of TMPRSS2:ERG in tumors by immunohistochemical assessment of ERG protein expression as previously described [16]. While ERG does occasionally fuse with genes other than TMPRSS2, immunohistochemistry has been shown to provide an effective marker for the fusion; it has demonstrated high concordance with TMPRSS2:ERG fusion status as assessed by both fluorescence in situ hybridization (FISH) [17,18] and quantitative polymerase chain reaction [19]. Briefly, sections of each TMA were prepared for analysis and ERG antisera were applied at 1:100 for one hour. Detection of the primary ERG antibody was carried out and visualization of ERG was accomplished using the DAB substrate kit (Vector Laboratories Inc., Burlingame, CA). A study pathologist (RTL) manually analyzed tumor specimens for ERG expression. The presence of ERG staining in the vasculature endothelium served as a positive internal control. A case was considered to be TMPRSS2:ERG positive if at least one core from an individual case had positive ERG staining observed within prostate cancer epithelial cells.
Statistical analysis
We compared differences in clinical features by ERG status using t-tests for continuous variables, and Cochran-Armitage trend tests, Fisher’s exact tests or chi-squared tests to assess differences in categorical variables. We used Cox proportional hazards models to calculate hazard ratios (HRs) and 95 percent confidence intervals (CIs) for time to prostate cancer-specific death. Follow-up time was calculated as the date of ADT initiation to death from prostate cancer, censored death from other causes or end of follow-up, whichever occurred first. We also considered all-cause mortality as a primary outcome. Follow-up for death ended in June 2011 for the Physicians’ Health Study and in December 2011 for the Health Professionals Follow-up Study. In that disease progression may differ according to the tumor tissue assessed for the fusion (radical prostatectomy or TURP), we ran sensitivity analyses in which we excluded men with TURP tissue specimens. Because stage at diagnosis is associated with ERG status, we also ran an analysis restricted to men with T2 or T3 N0/NX tumors, and we ran analyses separately for the Physicians’ Health Study and Health Professionals Follow-up Study.
Next, we assessed the association between prostate cancer outcomes and ERG status for different reasons of ADT initiation. Among men who were treated with ADT as a (neo)adjuvant treatment for non-metastatic disease, we ran additional Cox models looking at time to biochemical recurrence. To examine whether the subtype of ADT matters, we also conducted analyses restricted to those ever treated with each of the classes of ADT.
All analyses were conducted using SAS version 9.2 (SAS Institute, Inc.). All tests were 2-sided with P < 0.05 considered to be statistically significant. This study was approved by the institutional review boards at the Harvard School of Public Health and Partners Health Care. Written informed consent was obtained from each subject.
RESULTS
The study cohort consisted of 239 men treated with androgen deprivation for whom ERG data were available (Table 2). The mean age at prostate cancer diagnosis was 65.8 years (SD: 6.5 years). Roughly half of the men had locally advanced or metastatic disease at diagnosis, and 39% had poorly differentiated Gleason 8–10 disease. Eight percent received ADT as primary treatment following diagnosis, 39% of men received ADT as adjuvant or neoadjuvant therapy, and 53% percent were treated in response to disease progression. More than half (57%) of patients were initially treated with a GnRH agonist. During a mean follow-up of 10.2 years from therapy initiation, 42 men died from prostate cancer and 61 died of other causes; the cumulative incidence of death from prostate cancer was 18%.
Table 2.
Clinical characteristics for all men and by ERG expression status among 239 men treated with ADT for prostate cancer, Physicians’ Health Study, and Health Professionals Follow-up Study cohorts 1986–2011
| Characteristic | Men Treated w/ADT |
ERG- Negative |
ERG- Positive |
% ERG- Positive |
Pa |
|---|---|---|---|---|---|
| Number | 239 | 123 | 116 | 49% | |
| Mean Age at Diagnosis, years (SD) | 65.8 (6.5) | 66.0 (6.1) | 65.5 (6.8) | -- | 0.50 |
| Mean Time from Diagnosis to Initiation of ADT, years (SD) | 3.3 (4.4) | 3.6 (4.7) | 3.0 (4.1) | -- | 0.36 |
| Mean Follow-up Time from ADT Initiation, years (SD) | 10.2 (4.9) | 10.2 (4.7) | 10.2 (5.1) | -- | 0.90 |
| Reason for ADT Initiationb | |||||
| Primary Therapy | 19 (8%) | 11 (9%) | 8 (7%) | 42% | |
| (Neo)Adjuvant Therapy | 92 (39%) | 47 (39%) | 45 (39%) | 49% | |
| Recurrence / Progression | 124 (53%) | 62 (52%) | 62 (54%) | 50% | 0.81 |
| Earliest Class of ADT Receivedc | |||||
| Anti-androgen | 14 (7%) | 10 (10%) | 4 (4%) | 29% | |
| GnRH Agonist | 116 (57%) | 58 (55%) | 58 (60%) | 50% | |
| Orchiectomy | 27 (13%) | 15 (14%) | 12 (12%) | 44% | |
| Combination | 45 (22%) | 22 (21%) | 23 (24%) | 51% | 0.46 |
| Treatment with Radiation Therapy | |||||
| Primary Therapy | 32 (13%) | 16 (13%) | 16 (14%) | 50% | |
| (Neo)Adjuvant / Salvage Therapy | 28 (12%) | 12 (10%) | 16 (14%) | 57% | |
| Other Course of Radiation | 37 (15%) | 23 (19%) | 14 (12%) | 38% | |
| Never Radiation | 142 (59%) | 72 (59%) | 70 (60%) | 49% | 0.46 |
| Pathological Tumor Staged | |||||
| T2 N0/NX | 102 (49%) | 58 (55%) | 44 (43%) | 43% | |
| T3 N0/NX | 87 (42%) | 41 (39%) | 46 (45%) | 53% | |
| T4/N1/M1 | 19 (9%) | 6 (6%) | 13 (13%) | 68% | 0.03 |
| Clinical Tumor Stagee | |||||
| T1/T2 | 15 (63%) | 13 (81%) | 2 (25%) | 13% | |
| T3/T4/N1/M1 | 9 (38%) | 3 (19%) | 6 (75%) | 67% | 0.02 |
| Gleason Score | |||||
| 2–6 | 20 (8%) | 13 (11%) | 7 (6%) | 35% | |
| 3+4 | 52 (22%) | 30 (24%) | 22 (19%) | 42% | |
| 4+3 | 73 (31%) | 33 (27%) | 40 (34%) | 55% | |
| 8–10 | 94 (39%) | 47 (38%) | 47 (41%) | 50% | 0.18 |
| PSA-Level at Diagnosis, ng/ml | |||||
| <4 | 23 (10%) | 12 (11%) | 11 (10%) | 48% | |
| 4–<10 | 103 (47%) | 49 (44%) | 54 (50%) | 52% | |
| ≥10 | 94 (43%) | 51 (46%) | 43 (40%) | 46% | 0.56 |
| Prostate Cancer Deaths | |||||
| Yes | 42 (18%) | 21 (17%) | 21 (18%) | 50% | |
| No | 197 (82%) | 102 (83%) | 95 (82%) | 48% | 0.83 |
| All-Cause Mortality | |||||
| Yes | 103 (43%) | 52 (42%) | 51 (44%) | 50% | |
| No | 136 (57%) | 71 (58%) | 65 (56%) | 48% | 0.79 |
Abbreviations: ADT – Androgen Deprivation Therapy; PSA – Prostate Specific Antigen; SD – Standard Deviation
Note: Numbers do not add up to 239 for characteristics missing information for some men; Percentages may not add up to 100% due to rounding
P-values based on t-tests for age at diagnosis, time from diagnosis to initiation of ADT & follow-up time, Cochran-Artmitage trend test for pathological tumor stage, Gleason score, & PSA-level at diagnosis, Fisher’s exact test for clinical tumor stage, & χ2 for reason for ADT initiation, earliest type of ADT received, treatment with radiation therapy, prostate cancer deaths and all-cause mortality
Among 235 participants for whom the reason for ADT initation can be determined
Among 202 participants for whom earliest class of ADT received can be determined
Restricted to 208 radical prostatectomy specimens with stage data available (out of 215 radical prostatectomy specimens total)
Restricted to 24 TURP specimens
The prevalence of the fusion in the cohort of men undergoing ADT was 49% percent (Table 2). Men with ERG-positive tumors were more likely to be diagnosed at a higher tumor stage (P = 0.03 for radical prostatectomy / pathological tumor stage, P = 0.02 for TURP / clinical tumor stage). ERG status was otherwise not significantly different for all characteristics evaluated, including the mean time from diagnosis to initiation of ADT (ERG-Positive: 3.0 years, ERG-Negative: 3.6 years, P = 0.36)
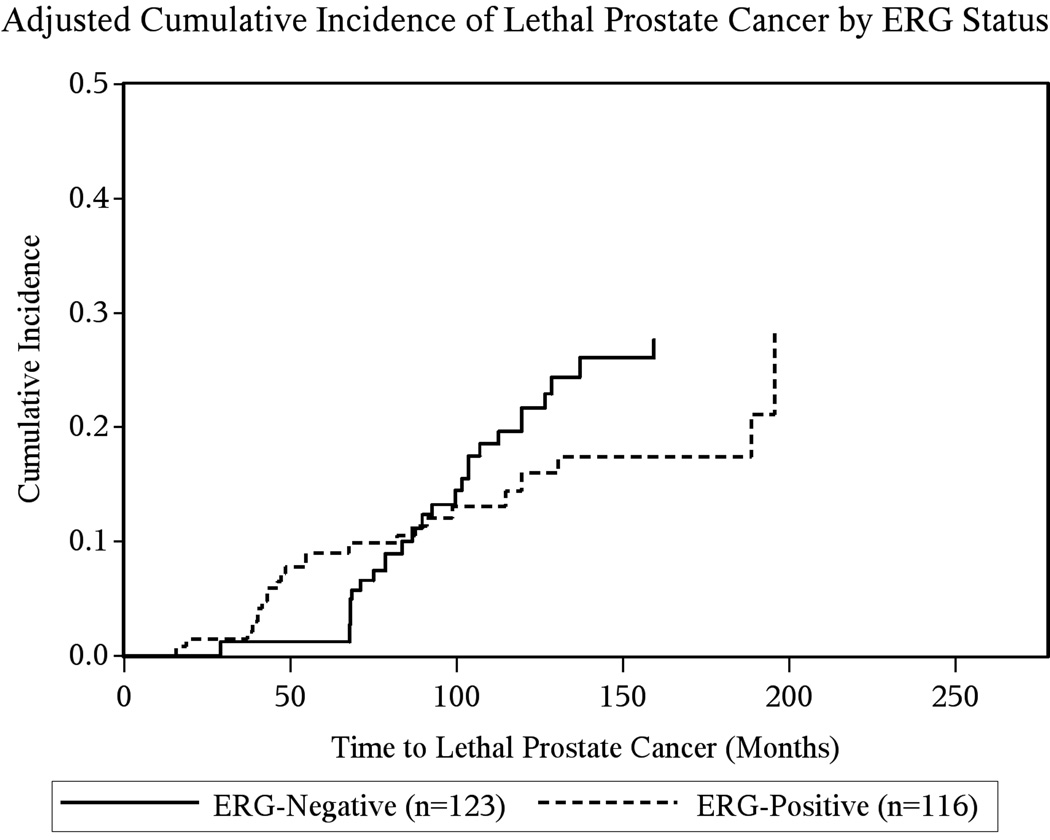
Adjusted for age at diagnosis and cohort (Table 3), there was no significant association between positive ERG status and time from initiation of ADT to death from prostate cancer in the overall cohort undergoing ADT (HR: 0.96, 95% CI: 0.52–1.77). Among men who died from prostate cancer, there was a small but not significant difference in time from ADT initiation to death by ERG status (ERG-positive: 76 months, ERG-negative: 95 months, P = 0.14). Adjusting Cox models for stage at diagnosis made the hazard ratio more inverse, (HR: 0.76, 95% CI: 0.40–1.45), but it remained non-significant. These results were confirmed by the cumulative incidence curves depicted in Figure 1. There was no difference between ERG status and cancer survival stratified by stage at diagnosis (data not shown). In an a-posteriori analysis, we adjusted the association between ERG status and death from prostate cancer for a propensity score based on age, cohort, stage, Gleason score and PSA level at diagnosis. Results remained null (HR: 0.82, 95% CI: 0.44–1.54). Similarly, there was no association between ERG status and time to all-cause mortality among men undergoing ADT. Analyses restricted to radical prostatectomy specimens, to men with T2 and T3 tumors, and within each cohort, all returned comparable results (data not shown).
Table 3.
Hazard ratios and 95% confidence intervals for time from ADT initiation to outcomes by ERG status among 239 men treated with ADT for prostate cancer, Physicians’ Health Study, and Health Professionals Follow-up Study cohorts 1986–2011
| Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|
| Number of Events |
Person-years of Follow-up |
HR | 95% CI | HR | 95% CI | |
| All Men Treated with ADT | ||||||
| Prostate Cancer Death | ||||||
| ERG-Negative | 21 | 1,249 | 1.00 | -- | 1.00 | -- |
| ERG-Positive | 21 | 1,187 | 0.96 | (0.52, 1.77) | 0.76 | (0.40, 1.45) |
| All-cause Mortality | ||||||
| ERG-Negative | 52 | 1,249 | 1.00 | -- | 1.00 | -- |
| ERG-Positive | 51 | 1,187 | 0.98 | (0.66, 1.45) | 0.89 | (0.60, 1.34) |
| Results Stratified by Reason for ADT Initiation | ||||||
| Prostate Cancer Death Among Neoadjuvant / Adjuvant Therapy Recipients (n = 92) | ||||||
| ERG-Negative | 7 | 594 | 1.00 | -- | 1.00 | -- |
| ERG-Positive | 5 | 600 | 0.54 | (0.17, 1.77) | 0.44 | (0.13, 1.50) |
| Prostate Cancer Death Among Men who Received ADT for Recurrence/Progression (n= 124) | ||||||
| ERG-Negative | 9 | 526 | 1.00 | -- | 1.00 | -- |
| ERG-Positive | 10 | 542 | 1.00 | (0.40, 2.51) | 0.95 | (0.36, 2.50) |
| Results Stratified by ADT Type | ||||||
| Prostate Cancer Death Among Ever Users of Anti-androgens (n = 61)c | ||||||
| ERG-Negative | 3 | 323 | 1.00 | -- | 1.00 | -- |
| ERG-Positive | 6 | 246 | 1.90 | (0.42, 8.57) | 1.57 | (0.27, 9.20) |
| Prostate Cancer Death Among Ever Users of GnRH Agonists (n = 157)c | ||||||
| ERG-Negative | 11 | 811 | 1.00 | -- | 1.00 | -- |
| ERG-Positive | 9 | 737 | 0.89 | (0.36, 2.23) | 0.91 | (0.36, 2.30) |
| Prostate Cancer Death Among Recipients of Orchiectomy (n = 37)c | ||||||
| ERG-Negative | 9 | 165 | 1.00 | -- | 1.00 | -- |
| ERG-Positive | 6 | 206 | 0.54 | (0.17, 1.67) | 0.13 | (0.03, 0.62) |
Abbreviations: ADT – Androgen Deprivation Therapy
Adjusted for age at diagnosis (<60, 60–64, 65–69, 70+), and cohort (Physicians’ Health Study, Health Professionals Follow-up Study)
Adjusted for age at diagnosis (<60, 60–64, 65–69, 70+), cohort (Physicians’ Health Study, Health Professionals Follow-up Study), and stage (T2, T3, T4/N1/M1)
Includes men who also received other classes of ADT as part of their treatment regime
Figure 1.
Age, cohort and stage adjusted cumulative incidence of death from prostate cancer for the time from ADT initiation to outcomes by ERG status among 239 men treated with ADT for prostate cancer, Physicians’ Health Study, and Health Professionals Follow-up Study cohorts 1986–2011
Among those who were treated with ADT as a (neo)adjuvant treatment, multivariable results indicated a non-statistically significant inverse association between positive ERG status and death from prostate cancer (HR: 0.44, 95% CI: 0.13–1.50) (Table 3) as well as biochemical recurrence (HR: 0.43, 95% CI: 0.14–1.29). Results were null for time to prostate cancer mortality among men who initiated ADT after disease progression. After multivariable adjustment, there was a lower risk of death from prostate cancer associated with ERG-positive prostate cancer among men who were treated with orchiectomy (HR: 0.13, 95% CI: 0.03–0.62). It is important to note that there were only 15 total prostate cancer deaths in these analyses.
DISCUSSION
In this study of prostate cancer patients treated with ADT, there was no significant difference in the time to death from prostate cancer or all-cause mortality by TMPRSS2:ERG status. Moreover, there was no association between TMPRSS2:ERG and time to initiation of hormonal therapy after diagnosis. There was a non-significant inverse association between ERG status and death from prostate cancer among men who had received (neo)adjuvant ADT. There was also an inverse association between ERG status and prostate cancer mortality among men treated by orchiectomy.
It seems likely that the association between ERG status and response to ADT among men treated with orchiectomy is due to chance, but there may also be alternative explanations. Surgical castration is an irreversible procedure, making the effects of androgen deprivation lifelong. In contrast, chemical castration may be administered for varying durations and its effects may be reversed. It is possible that longer courses of therapy, either surgical or chemical, benefit men with ERG-positive prostate cancer. Our data did not permit assessment of each participant’s duration on these therapies because of lacking information about ADT stop dates.
We had hypothesized that ERG-positive tumors may be more responsive to androgen deprivation than ERG-negative tumors. When androgen receptor is activated, it regulates the transcription of TMPRSS2. When the oncogene ERG is fused to TMPRSS2, it also becomes androgen-regulated [20,21]. Disease with TMPRSS2:ERG is thus a unique model of cancer in which an oncogene is regulated by androgens. We speculated that androgen deprivation of ERG-positive cancers could diminish their oncogenic potential.
Despite the biological rationale, few studies have evaluated the benefits of ADT in the context of TMPRSS2:ERG (Table 1). In 2009, Attard and colleagues published a case report of a patient with especially hormone-sensitive cancer [10]. The ERG-positive patient was treated with several rounds of ADT and maintained a long-lasting response to hormonal treatment despite poor prognostic factors at diagnosis. The following year, Boormans and colleagues assessed the association between ERG status and biochemical recurrence in a cohort of 71 men with initially hormone-naïve node-positive prostate cancer [9]. They found no difference in the time from start of endocrine therapy to biochemical recurrence according to ERG status. Leinonen and colleagues found similarly null results in their analysis of progression-free survival in 178 patients [12]. A case-control study of men treated with radical prostatectomy from Karnes and colleagues evaluated the interaction between ERG status and receipt of adjuvant ADT on time to systemic progression. Among ERG-positive participants, they found that treatment with adjuvant ADT was associated with a longer time to systemic progression, but their results were not statistically significant [11]. Our secondary analysis of men treated with (neo)adjuvant hormonal therapy indicated a possible protective association between positive ERG status and death from disease, but results did not reach significance. Overall, our study is the largest analysis to-date using fatal prostate cancer as the primary endpoint and results are consistent with those of the null studies.
Our study was limited by its sample size, even though it is the largest study of its kind to-date. Based on 42 deaths from prostate cancer, we had only 57% power to detect an HR of 0.50 at an alpha of 0.05. Our study population was heterogeneous with respect to timing and classes of ADT treatments received, but we were able to assess the association within specific subtypes of ADT. We were not able to further sub-classify (neo)adjuvant therapy into neoadjuvant versus adjuvant therapy (because of missing months of treatment) or therapy in response to biochemical recurrence versus metastatic progression (because of lacking data from medical records). Misclassification of treatment months was not likely differential between ERG-positive and ERG-negative patients. Our study did not include men who were treated with alternative forms of hormonal therapy used predominantly in castration-resistant prostate cancer. For example, we were unable to evaluate the prognostic value of TMPRSS2:ERG in the setting of abiraterone treatment; our cohort had low prevalence of abiraterone use given that it was not FDA-approved until 2011. As such, anti-androgens, GnRH agonists and orchiectomy were the first lines of therapy administered for the treatment of advanced prostate cancer. While Attard and colleagues found that ERG rearrangement was associated with the magnitude of maximal PSA decline on abiraterone acetate [22], Danila and colleagues did not find that TMPRSS2:ERG predicts response to treatment [23]. We were unable to evaluate the time from treatment to castration-resistance in addition to death. Mortality, however, is a key clinical outcome used in clinical trials. Lastly, our study may have benefited from a test of interaction between treatment with ADT and ERG status. However, a large majority of men who die from prostate cancer are treated with ADT at some point during their clinical course. As such, we would expect to see very few cases of fatal prostate cancer in the set of men who do not receive ADT. We thus elected to limit our study population to those who had been treated with ADT.
CONCLUSIONS
Treatment strategies targeted toward unique biological features of prostate cancer specific to certain subsets of patients have remained elusive. Upwards of 100,000 ERG-positive prostate cancers are diagnosed in the United States each year [24]; there would be key public health implications if they were more susceptible to treatment with ADT. Our results do not rule out the possibility that ERG status could be associated with responsiveness to hormonal therapy in certain settings, but larger cohorts are needed for more robust results. In particular, they should be well powered to evaluate the prognostic significance of TMPRSS2:ERG in patient subgroups defined by timing and class of ADT.
ACKNOWLEDGEMENTS
We would like to thank the participants and staff of the Health Professionals Follow-up Study and the Physicians' Health Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH. The authors assume full responsibility for analyses and interpretation of these data. We would like to acknowledge the contributions of several members of the research team for their efforts: Li Moy, Elizabeth Frost-Hawes, Lauren McLaughlin, and Chungdak Li. We would also like to acknowledge Mary Downer for her help with coding and Miguel Hernán for his feedback on the study. We are grateful to an anonymous donor for support of the ToPCaP collaboration. The tissue microarrays were constructed by the Tissue Microarray Core Facility at the Dana Farber/Harvard Cancer Center.
Grant Acknowledgment: This work was supported by the Dana-Farber/Harvard Cancer Center Specialized Programs of Research Excellence (SPORE) in Prostate Cancer (5P50CA090381), the National Institutes of Health and the National Cancer Institute (CA34944, CA136578, CA141298, CA40360, CA097193, PO1 CA055075, UM1CA167552, U01CA098233, HL26490, HL34595). REG was supported by R25 CA098566 from the National Cancer Institute. LAM, JRR and PLN are Prostate Cancer Foundation Young Investigators. PLN was also supported by a grant from an anonymous family foundation, Fitz’s Cancer Warriors, and David and Cynthia Chapin. AP was supported by the Swedish Research Council (Reg. No. 2009–7309) and the Royal Physiographic Society in Lund.
Footnotes
Disclosures: Nothing to disclose.
REFERENCES
- 1.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, Middleton R, Sharp SA, Smith TJ, Talcott J, Taplin M, Vogelzang NJ, Wade JL, 3rd, Bennett CL, Scher HI. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 2.Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Schmid HP, van der Kwast T, Wiegel T, Zattoni F, Heidenreich A. EAU guidelines on prostate cancer. part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Actas Urol Esp. 2011;59:572–583. doi: 10.1016/j.acuro.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 3.De Sy WA, De Wilde P, De Meyer JM, Casselman J, Desmet R, Renders G, Schelfhout W. Long term experience in the treatment of advanced prostatic cancer with decapeptyl, compared to orchiectomy. Acta Urol Belg. 1988;56:581–588. [PubMed] [Google Scholar]
- 4.Moreau JP, Delavault P, Blumberg J. Luteinizing hormone-releasing hormone agonists in the treatment of prostate cancer: a review of their discovery, development, and place in therapy. Clin Ther. 2006;28:1485–1508. doi: 10.1016/j.clinthera.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Tomlins S, Rhodes D, Perner S, Dhanasekaran S, Mehra R, Sun X, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie J, Shah R, Pienta K, Rubin M, Chinnaiyan A. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 6.Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, Rubin MA, Schalken JA. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56:275–286. doi: 10.1016/j.eururo.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 7.Nam RK, Sugar L, Yang W, Srivastava S, Klotz LH, Yang LY, Stanimirovic A, Encioiu E, Neill M, Loblaw DA, Trachtenberg J, Narod SA, Seth A. Expression of the TMPRSS2:ERG fusion gene predicts cancer recurrence after surgery for localised prostate cancer. Br J Cancer. 2007;97:1690–1695. doi: 10.1038/sj.bjc.6604054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seth A, Watson DK. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41:2462–2478. doi: 10.1016/j.ejca.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Boormans JL, Hermans KG, Made AC, van Leenders GJ, Wildhagen MF, Collette L, Schroder FH, Trapman J, Verhagen PC. Expression of the androgen-regulated fusion gene TMPRSS2-ERG does not predict response to endocrine treatment in hormone-naive, node-positive prostate cancer. Eur Urol. 2010;57:830–835. doi: 10.1016/j.eururo.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Attard G, Jameson C, Moreira J, Flohr P, Parker C, Dearnaley D, Cooper CS, de Bono JS. Hormone-sensitive prostate cancer: a case of ETS gene fusion heterogeneity. J Clin Pathol. 2009;62:373–376. doi: 10.1136/jcp.2008.061515. [DOI] [PubMed] [Google Scholar]
- 11.Karnes RJ, Cheville JC, Ida CM, Sebo TJ, Nair AA, Tang H, Munz JM, Kosari F, Vasmatzis G. The ability of biomarkers to predict systemic progression in men with high-risk prostate cancer treated surgically is dependent on ERG status. Cancer Res. 2010;70:8994–9002. doi: 10.1158/0008-5472.CAN-10-1358. [DOI] [PubMed] [Google Scholar]
- 12.Leinonen K, Tolonen T, Bracken H, Stenman U, Tammela T, Saramaki O, Visakorpi T. Association of SPINK1 expression and TMPRSS2:ERG fusion with prognosis in endocrine-treated prostate cancer. Clin Cancer Res. 2010;16:2845–2851. doi: 10.1158/1078-0432.CCR-09-2505. [DOI] [PubMed] [Google Scholar]
- 13.Hennekens CH, Eberlein K. A randomized trial of aspirin and beta-carotene among U.S. physicians. Prev Med. 1985;14:165–168. doi: 10.1016/0091-7435(85)90031-3. [DOI] [PubMed] [Google Scholar]
- 14.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, Ma J, Fiorentino M, Kurth T, Loda M, Giovannucci EL, Rubin MA, Mucci LA. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27:3459–3464. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, Stack EC, Martin NE, Kunz L, Penney KL, Ligon AH, Suppan C, Flavin R, Sesso HD, Rider JR, Sweeney C, Stampfer MJ, Fiorentino M, Kantoff PW, Sanda MG, Giovannucci EL, Ding EL, Loda M, Mucci LA. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1497–1509. doi: 10.1158/1055-9965.EPI-12-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaux A, Albadine R, Toubaji A, Hicks J, Meeker A, Platz EA, De Marzo AM, Netto GJ. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. Am J Surg Pathol. 2011;35:1014–1020. doi: 10.1097/PAS.0b013e31821e8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park K, Tomlins SA, Mudaliar KM, Chiu YL, Esgueva R, Mehra R, Suleman K, Varambally S, Brenner JC, MacDonald T, Srivastava A, Tewari AK, Sathyanarayana U, Nagy D, Pestano G, Kunju LP, Demichelis F, Chinnaiyan AM, Rubin MA. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12:590–598. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Leenders GJ, Boormans JL, Vissers CJ, Hoogland AM, Bressers AA, Furusato B, Trapman J. Antibody EPR3864 is specific for ERG genomic fusions in prostate cancer: implications for pathological practice. Mod Pathol. 2011;24:1128–1138. doi: 10.1038/modpathol.2011.65. [DOI] [PubMed] [Google Scholar]
- 20.Bastus NC, Boyd LK, Mao X, Stankiewicz E, Kudahetti SC, Oliver RT, Berney DM, Lu YJ. Androgen-induced TMPRSS2:ERG fusion in nonmalignant prostate epithelial cells. Cancer Res. 2010;70:9544–9548. doi: 10.1158/0008-5472.CAN-10-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin C, Yang L, Tanasa B, Hutt K, Ju BG, Ohgi K, Zhang J, Rose DW, Fu XD, Glass CK, Rosenfeld MG. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell. 2009;139:1069–1083. doi: 10.1016/j.cell.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, A'Hern R, Levink R, Coumans F, Moreira J, Riisnaes R, Oommen NB, Hawche G, Jameson C, Thompson E, Sipkema R, Carden CP, Parker C, Dearnaley D, Kaye SB, Cooper CS, Molina A, Cox ME, Terstappen LW, de Bono JS. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 23.Danila DC, Anand A, Sung CC, Heller G, Leversha MA, Cao L, Lilja H, Molina A, Sawyers CL, Fleisher M, Scher HI. TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur Urol. 2011;60:897–904. doi: 10.1016/j.eururo.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]