Abstract
BK polyomavirus (BKPyV) is a known kidney tropic virus that has been detected at high levels in HIV-associated salivary gland disease (HIV-SGD), one of the most important AIDS associated oral lesions. BKPyV has been detected in HIV-SGD patient saliva and replicates in salivary gland cells in vitro. BKPyV antivirals are currently in wide use to guard against BKPyV mediated organ rejection in kidney transplant recipients. The goal of this study was to investigate the inhibitory effects of three such antiviral agents, ciprofloxacin, cidofovir, and leflunomide in BKPyV infected salivary gland cells. Human salivary gland cells, and Vero cells, were infected with BKPyV, treated with antiviral drugs and assessed for BKPyV gene expression and viral replication for up to 5 days post infection. The kinetics of BKPyV replication were different in salivary gland cells compared to kidney cells. Ciprofloxacin and cidofovir had minimal effect on metabolic activity and host cell DNA replication, however, cell toxicity was detected at the protein level with leflunomide treatment. Ciprofloxacin decreased BKV T Ag and VP1 mRNA expression by at least 50% in both cell types, and decreased T Ag protein expression at days 3 and 4 post infection. A 2.5 – 4 log decrease in intracellular DNA replication and a 2 – 3 log decrease in progeny release were detected with ciprofloxacin treatment. Cidofovir and leflunomide also inhibited BKPyV gene expression and DNA replication. The three drugs diminished progeny release by 30–90% and 2 – 6 fold decreases in infectious virus were detected post drug treatment by fluorescence focus assay. Additionally, three clinical BKPyV isolates were assessed for their responses to these agents in vitro. Cidofovir and leflunomide, but not ciprofloxacin treatment resulted in statistically significant inhibition of BKPyV progeny release from salivary gland cells infected with HIV-SGD BKPyV isolates. All three drugs decreased progeny release from cells infected with a transplant derived viral isolate. In conclusion, treatment of human salivary gland cells with each of the three drugs produced modest decreases in BKPyV genome replication. These data highlight the need for continued studies to discover more effective and less toxic drugs that inhibit BKPyV replication in salivary gland cells.
Keywords: Polyomavirus; HIV-associated salivary gland disease, BKPyV, Leflunomide, Cidofovir, Ciprofloxacin, Salivary gland cells
INTRODUCTION
BK virus (BKPyV) belongs to the polyomavirus family and is ubiquitous in the human population (1). BKPyV infection typically occurs during childhood, is mostly asymptomatic and followed by a state of non-replicative infection in various tissues, with the urogenital tract as the principal site (2). In the setting of relative or absolute cell-mediated immunosuppression, dramatic increase in BKPyV viral replication occurs, resulting in the lytic destruction of infected uroepithelial cells, which in turn induces the influx of inflammatory immune cells (3). Destruction of kidney cells most often occurs in 5–8% of kidney transplants resulting in organ loss in half of these cases and is termed BKPyV-associated nephropathy (BKPyVN) (4) (5–7). BKPyV is also the cause of BKPyV-associated hemorrhagic cystitis which occurs in over 10% of bone marrow transplant recipients (8, 9). BKPyV-associated disease incidence is increasing as a result of the growing number of immunocompromised patients, including transplant and HIV patients, and development of more potent immunosuppressive drugs.
Currently there is no FDA approved antiviral drug to treat BKPyV infections despite its importance in transplant and immunocompromised patients. Several in vitro and in vivo studies however, have been performed to test for their efficacy against BKPyV including cidofovir, CMX001, leflunomide, ciprofloxacin, and lactoferrin (10–16). All of these drugs are effective against other DNA viruses including hepadnaviruses, herpesviruses, adenovirus, papillomavirus, polyoma- and poxviruses (9, 17–20) but have shown mixed results against BKPyV, both in vitro and in vivo in kidney cells (13, 14, 21, 22). Ciprofloxacin (CPRO) is a synthetic antibiotic of the fluoroquinolone drug class. Ciprofloxacin’s antibacterial activity functions by inhibiting type II and IV topoisomerases and has been shown to inhibit T antigen helicase activity (20). Cidofovir (CDV) is a nucleoside analog that inhibits viral DNA polymerase activity, however BKPyV does not encode for a DNA polymerase. CDV has been shown to inhibit BKPyV activity in vitro in human embryonic lung fibroblast cells (WI-38) (12) and in primary human renal proximal tubular epithelial cells (RPTECs) (14). In RPTECs, CDV inhibited BKPyV replication but also decreased host cellular DNA replication and metabolic activity (14). Although CDV has shown in vitro activity against BKPyV, there are conflicting reports of in vivo activity (23, 24). In addition, CDV has been shown to be nephrotoxic and must be given intravenously. Most recently, CMX001, a hexadecyloxypropyl lipid conjugate of CDV has been shown to inhibit polyomaviruses JCV and BKPyV in human kidney and brain progenitor-derived astrocytes (11, 25). Leflunomide (LEF) is an anti-inflammatory drug known to inhibit dihydroorotate dehydrogenase, tyrosine kinase and pyrimidine synthesis (12). LEF has been approved to treat rheumatoid arthritis and has shown activity against cytomegalovirus and herpesvirus with conflicting reports against BKPyV (13, 26, 27).
We have previously shown that BKPyV DNA can be detected at high levels in the saliva of HIV patients diagnosed with salivary gland disease compared to patients without the disease (28–30). HIV-associated salivary gland disease (HIV-SGD) has been universally established as one of the most important AIDS-associated oral lesions. Oral lesions are important clinical indicators for HIV/AIDS, indicating clinical disease progression and predicting development of AIDS (31). In developing countries the incidence of HIV-SGD has been reported to be as high as 48% among HIV-1 infected patients (32). Importantly, in 1–2% of HIV-SGD patients, malignant lymphomas have been described in association with their glandular lesions, making HIV-SGD a premalignant lesion (33, 34). We have shown that BKPyV can productively infect salivary gland cells in vitro model described by Jeffers et al (28, 30). Further, our group has shown that the BKPyV in HIV-SGD has a non-coding control region that is distinct from archetype. The OPQPQQS architecture is consistently detected in throat wash samples of HIV-SGD positive samples (30) unlike the OPQRS archetype that is often detected in kidney derived BKPyV. It has been determined that the replication kinetics of BKPyV in salivary gland cells differ compared to kidney cells (28–30).
Assays to test anti-BKPyV drugs have previously been performed in kidney or lung cells (12, 35, 36). Noting the importance of BKPyV in HIV-SGD and the detection of efficient BKPyV replication in salivary gland cells in vitro (28, 29), it is imperative that an in vitro drug screen against BKPyV replication in human salivary gland cells be tested to identify effective viral targets for drug treatment for salivary gland derived BKPyV. The aim of this study was to investigate the effect of three drugs, ciprofloxacin, cidofovir and leflunomide on BKPyV replication in human salivary gland cells, using the previously described BKPyV-infected salivary gland in vitro culture system (28), the primary target cells in HIV-SGD.
MATERIAL AND METHODS
Subjects, sample collection and cell culture
HIV-SGD patients were recruited from UNC hospitals dental clinic to participate in the IRB approved study, where throat wash was collected from patients for viral detection and isolation. Urine from a lung transplant patient was kindly donated by Dr. Volker Nickeleit for viral detection and isolation of BK virus. HSG cells, an epithelial cell line from human submandibular salivary gland (37), were obtained as a gift from Dr. B. Baum (NIH) and cultured in McCoy’s 5A medium (Sigma). African kidney monkey cells or Vero cells (American Type Culture Collection [ATCC]) were also cultured in DMEM (Sigma). All cell types were grown in medium supplemented with 10% fetal bovine serum (FBS) (Sigma), unless otherwise stated and 1% penicillin-streptomycin (pen/strep) (Gibco) and maintained in a humidified 37°C CO2 chamber. BKPyV Gardner strain was obtained from ATCC (VR-837).
Whole Genome BKPyV Cloning
BKPyV VP1 gene forward (BKPVWGF; 5’-GCGGGATCCAGATGAAAACCTTAGG-3’) and reverse primers (BKPyVWGR; 5’-GCGGGATCCCCCATTTCTGG-3’) including the naturally occurring BamH1 restriction fragment recognition sites were used to amplify the whole genome (wg) of BKPyV via PCR from throat wash of HIVSGD patients, and the urine of a lung transplant patient using the Expand Long Range dNTPack (Roche) as described by manufacturer. Amplified wg BKPyV products were purified by QIAquick PCR Purification Kit (QIAGEN) as described by manufacturer. Purified wg BKPyV DNA was cloned via the TOPO TA Cloning Kit (Invitrogen) as described by manufacturer. Constructs were isolated via QIAfilter Plasmid Midi Kit (QIAGEN) post-24hrs incubation of bacterial cells for clone DNA amplification at 37 °C.
Infection and drug treatment
Ciprofloxacin (Sigma), Leflunomide/A771726 (Calbiochem) and Cidofovir (Gilead) were dissolved to 150ug/ml, 20ug/ml and 40ug/ml, respectively in McCoy’s 5A medium. HSG cells were infected at about 50% confluence with 64 HAUs of BKPyV as described by Jeffers et al. (28). As previously described by Jeffers et al, HSG cells were infected with BKPyV or supernatant from BKPyV-infected cells for 24h for optimal viral entry (28). At 24h post infection virus was removed from the culture medium, washed with 1× PBS and replaced with fresh medium with or without drug. At various times post infection the cell monolayers or supernatant were further processed for IFA, protein, RNS or DNA isolation, as described below.
Cell viability and cell proliferation assay
The mitochondrial metabolic activity was monitored by the colorimetric WST-1 assay (Roche), measuring the reduction of the tetrazolium salt WST-1 by mitochondrial dehydrogenases. DNA synthesis was quantified by the colorimetric measurement of bromodeoxyuridine (BrdU) incorporation into DNA using the cell proliferation enzyme-linked immunosorbent assay (ELISA) BrdU kits (Roche). Both tests were performed in fresh medium with or without BKPyV Gardner in the presence or absence of drug at 3, 4 and 5 dpi.
RNA isolation and real-time RT-PCR amplification
Total RNA was extracted using TRizol (Invitrogen) as described by the manufacturer. Contamination DNAs were removed by use of RQ1 DNase kit (Promega) as described by the manufacturer. cDNA was generated using 20µg RNA, random primers and the SuperScript™ II Reverse Transcriptase (RT) Kit (Invitrogen) as described by the manufacturer. A non-RT enzyme reaction was performed for each sample as a negative control for cDNA synthesis. cDNA was then subjected to real-time PCR analysis using Roche LightCycler 480 Syber Green I Master Mix as a detector in the Roche Light Cycler 480. Primers for T Ag and VP1 were previously described in Dana et al (38) and Ding (39) et al, respectively. Gene expression values were normalized to the levels of β-actin transcripts, using the 2− ΔΔC(T) method, and are presented as the changes (n-fold) in T Ag and VP1 transcript levels, with the levels in BKPyV only (on drug) samples arbitrarily set to 1.
Fluorescent Focus Assay
Vero cells (50% confluence) were infected with supernatant from drug-treated BKV-infected HSG cells for 4 days. Vero cells were then fixed in 50:50 methanol-acetone, air dried, and stored at −80°C overnight. Cells were rehydrated with 1× phosphate-buffered saline (PBS) for 10 mins, followed by overnight incubation with anti-JC/BK polyomavirus primary antibody NCL JCBK (Novocastra, Leica) at a 1:10 dilution in PBS at 4°C. The secondary antibody, goat anti-mouse-Alexa Fluor 488 (Life Technologies) conjugate at a 1:100 dilution in PBS, was added for 1h at RT after the cells had been washed twice with 1× PBS. Cells were washed with PBS prior to addition of DAPI (4′,6-diamidino-2-phenylindole)-DNA stain (1:10,000) and fluorescence microscopy analysis (Olympus IX81). BKPyV-infected cells were counted at a ×20 magnification for a minimum of 10 fields per dish, and FFU were calculated as described in reference (40). FFU values were graphed for each treatment and are depicted as FFU/µl.
Immunoblotting
Total cell protein was extracted using RIPA buffer. Protein concentration was determined using the BioRad protein assay, and equal amounts of protein were electrophoresed on a 4–12% Bis-Tris polyacrylamide minigel (Invitrogen). PAb416 (1:200) (Genetex) in 5% NFDM/PBS-T was used to detect T Ag expression and Actin (C-11)-R sc-1615-R (1:1000) (Santa Cruz Biotechnology) in 1%BSA/TBS-T for actin expression. After washing in PBS/TBS-T, blots were probed with a horseradish peroxidase-conjugated secondary antibody (1:10,000) (Promega). Antibody complexes were detected using SuperSignal West Pico Chemiluminescent substrate (Thermo scientific) and exposed to film (Kodak).
Detection of DNase resistant viral particles
The detection of infectious virus being released from HSG cells were performed by collecting HSG cell supernatant at stated times post infection. To degrade free DNA not encapsidated within virions, supernatant was treated with 250U DNase (Promega) or PBS for 15 min at 56°C, followed by enzyme inactivation at 65°C for 10 min. To release viral DNA (vDNA) from capsids, proteinase K was used as described in the blood and body fluid spin protocol of the QIAamp DNA blood minikit (QIAGEN). DNA was eluted in 200 µl of sterile water. Levels of viral DNA was determined using primers for T Ag and/or VP1 in quantitative real time PCR as described above. A plasmid, pBKPyV containing the entire genome of BKPyV, a gift from Volker Nickeleit (UNC-CH) was used to derive standard curves for viral DNA quantitation.
Determination of effective and cytotoxic concentrations
Data for the extracellular BKPyV DNA load and BrdU incorporation in the presence of increasing drug concentrations were expressed as percent inhibition for both uninfected and infected HSG cells. CC50 and EC50 curves were plotted using the non-linear regression curve fitting in GraphPad Prism. The respective selectivity index (SI) was obtained by determining the CC50/EC50 ratios.
RESULTS
Kinetics of BKPyV replication in salivary gland cells are distinct from kidney cells
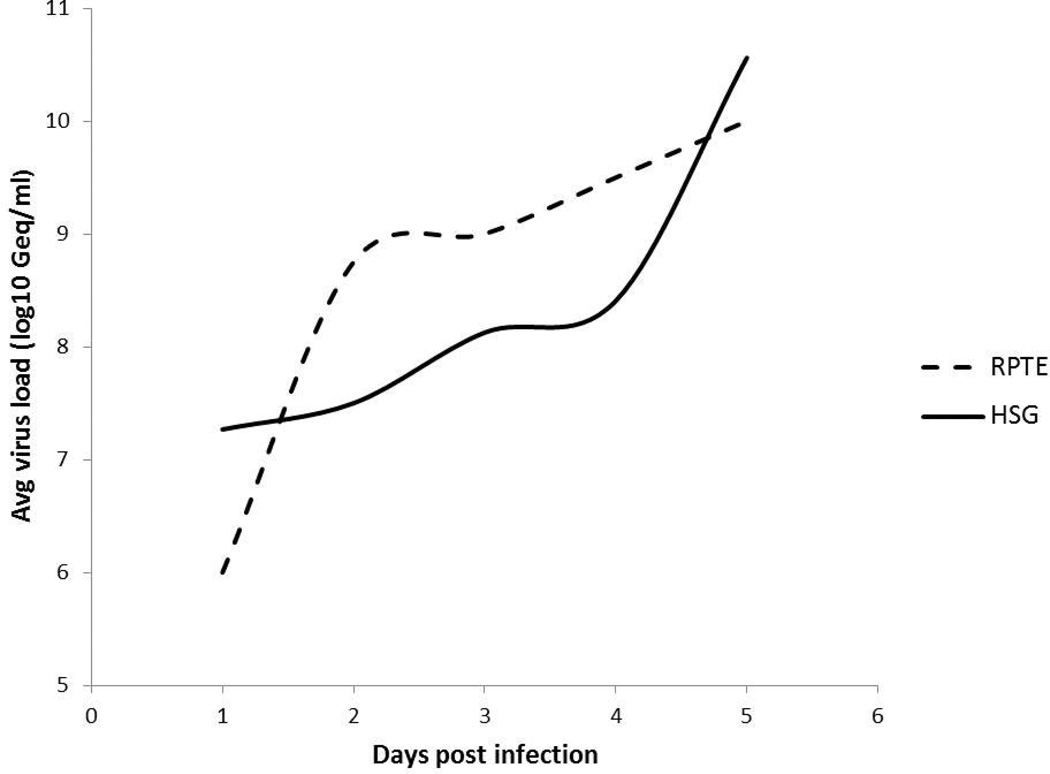
While BKPyV tropism is known to be nephrotropic (2), recent studies from our group have shown that BKPyV can replicate permissively in the salivary gland (28, 30). BKPy viral kinetics have been shown to be different in kidney cells compared to salivary gland cells (28). To directly compare kinetics of replication, data acquired in RPTE cells (41) was plotted with data acquired in salivary gland cells. Figure 1 shows that a significant increase in extracellular viral load occurred at 3.5 dpi (28) compared to kidney (RPTE) cells which occurred after only 1 dpi (28, 41) (Figure 1). As such, days 3 through 5 which exhibited significant replication in salivary gland cells were used in the experiments that followed.
Figure 1. Comparison of BKPYV replication trend over time in human kidney (RPTE) versus salivary gland (HSG) cells.
HSG cells show a significant increase in extracellular BKPYV load at 3dpi compared to RPTE cells which occurs earlier at 2dpi. RPTE trend was extracted from work performed by Li et al (41).
Effect of Cidofovir, Ciprofloxacin and Leflunomide on salivary gland cell proliferation and viability
Salivary gland cells (HSG) were treated with three known drugs that have demonstrated inhibition of BKPyV replication in kidney cells, CDV, CPRO and LEF. The metabolic activity of these drugs had not been previously assessed in salivary gland cells, to investigate this, HSG cells were drug-treated in the presence or absence of BKPyV infection. Host cell DNA replication and metabolic activity were measured using BrdU incorporation and WST-1 assays at increasing concentrations of each drug (Figure 2 A, B, C). As determined by BrdU incorporation, each drug reduced host cell DNA replication in a concentration-dependent manner (grey bars). Previous experiments using these drugs against BKPyV were all performed in kidney cells at similar ranges of drug concentrations but did not significantly alter kidney cell metabolism (13, 14, 22, 36). In kidney cells optimal drug concentrations were as follows, CPRO 150ug/ml, LEF 20mg/ml and CDV 40ug/ml. In salivary gland cells treated at these same drug levels, changes in host cell metabolism and DNA replication were detected. With CPRO drug treatment at 150ug/ml there was no significant change in metabolic activity but there was a decrease in host cell DNA replication (Figure 2A). For cells treated with 40ug/ml CDV, the level was decreased to 60–65% in both host cell DNA replication and cell metabolism compared to untreated, uninfected cells (Figure 2B). For cells treated with 20ug/ml LEF, there was approximately 20% increase in cell metabolism and the level was decreased to 60% in DNA replication compared to untreated, uninfected cells (Figure 2C). The percent of BKPyV inhibition was determined for CPRO, CDV and LEF in salivary gland cells by calculating the EC50, CC50 and SI values (Table 1/Supplemental Figure 1).
Figure 2. Effect of increasing concentrations of drug treatment on host cell DNA replication and metabolic activity.
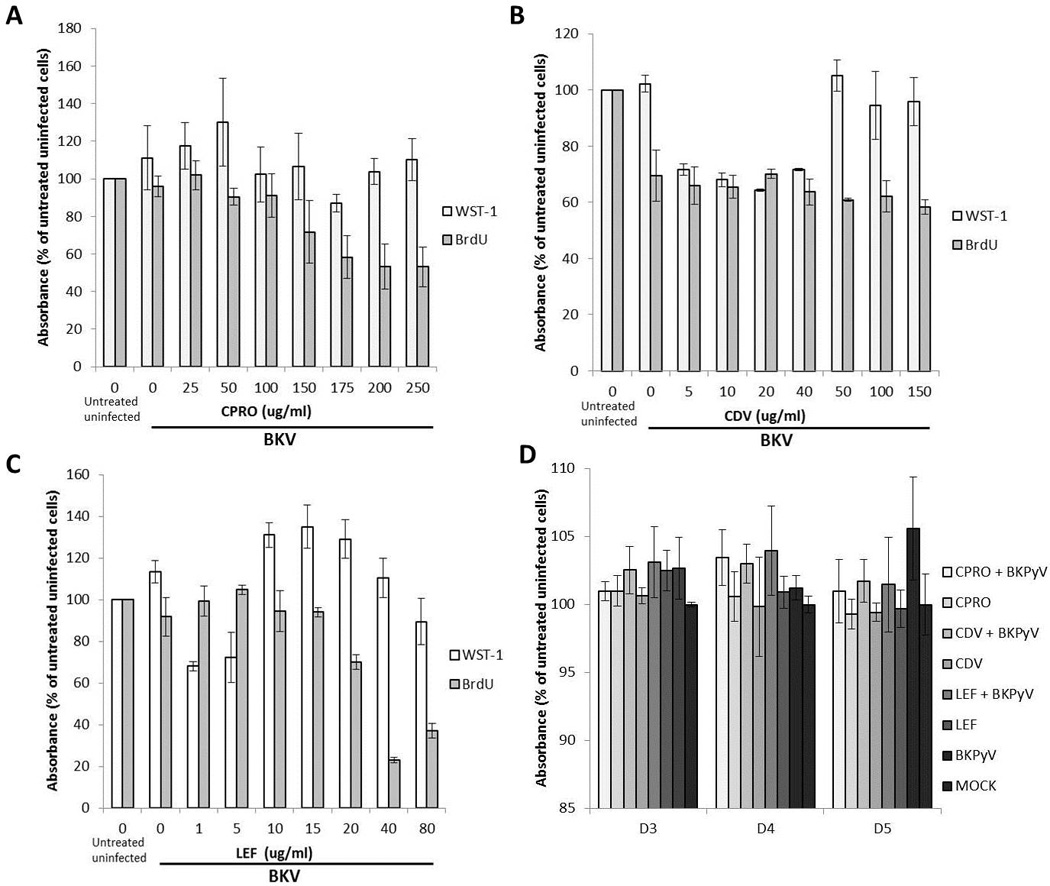
Cellular DNA replication (BrdU) and total metabolic activity (WST1) of (A) Ciprofloxacin, (B) Cidofovir and (C) Leflunomide treated BKPyV-uninfected and infected HSG cels were measured at 72 hpi. Mean values +/− SD are presented as percent of absorbance of untreated, uninfected (mock) cells. (D) Cellular DNA replication (BrdU) with or without CPRO (150mg/ml), LEF (20mg/ml) and CDV (40mg/ml) was measured 24 hpi and absorbance measured at indicated time points. Absorbance for untreated uninfected cells (mock) at each time point was set as 100%.
Table 1.
EC50, CC50 and SI values for each drug treatment on BKPyV-infected HSG cells
| Treatment | CC50/EC50 | SI |
|---|---|---|
| CPRO | 122/33 | 3.7 |
| CDV | 28/18 | 1.6 |
| LEF | 20.8/2.3 | 9.1 |
WST-1, a standard metabolic assay, was used for assessment of activity over 3 to 5 day period post BKPyV infection with a concentration of CPRO (150mg/ml), of LEF (20mg/ml) and of CDV (40mg/ml). These concentrations were based on physiologically relevant previous experiments in kidney cells performed by Bernhoff et al (14), Sharma et al (22) and Liacini et al (36) and were used to treat salivary gland cells in the following experiments. Compared to mock (uninfected untreated), BKPyV infection increased HSG cellular metabolic activity by 1.5–5% at each time point, with the greatest increase occurring at 5 days pi to 105%. Compared to mock (uninfected untreated), drug-treated cells did not significantly change metabolic activity at any time point investigated. With the exception of CPRO at day 3, all BKPyV infected drug-treated cells displayed increased metabolic activity compared to uninfected drug-treated cells at each time point (Figure 2D). In the presence of drug, the most significant increase in WST-1 activity occurred at day 4 pi, to 103% for CPRO+BKPyV, CDV+BKPyV and LEF+BKPyV.
The doses used in these experiments did not significantly alter host cell metabolic activity or proliferation in kidney cells. Based on our observations, the impact on each of the three agents on salivary gland cell proliferation and metabolic activity was more significant than on kidney cells (Fig 2A–D). While the drug concentrations that were selected may have affected the salivary gland cell viability and/ or proliferation for this initial set of studies in a novel cell type it was important to utilize the same concentrations that had been tested in previous studies.
Effect of Cidofovir, Ciprofloxacin and Leflunomide on the BKPyV life cycle in salivary gland cells
To investigate the effect of CDV, CPRO and LEF on the BKPyV life cycle, BKPyV DNA replication (Figure 3), RNA transcript levels and protein expression (Figure 4) were measured between days 3 and 5 post drug treatment/viral infection of HSG cells. BKPyV DNA loads were measured at 3, 4 and 5 dpi/post drug treatment by qPCR (Figure 3). A plasmid encoding the BKPyV genome was used to construct a standard curve for determination of BKPyV copy number. CPRO-treated cells demonstrated the largest decrease in BKPyV genome replication over the three day period with a 2.5–4 log decrease in copy number compared to untreated infected cells. LEF decreased BKPyV genome replication by 0.5–1 log and CDV demonstrated an intermediate phenotype with a 2–3 log decrease over the three day period. Primers targeted toward T Ag (Figure 3) and VP1 (data not shown) regions of the genome provided similar results. An R2 value of 0.94 suggested good PCR efficiency for the detection of BKPyV DNA.
Figure 3. Effect of drug treatment on BKPyV genome replication in human salivary gland cells.
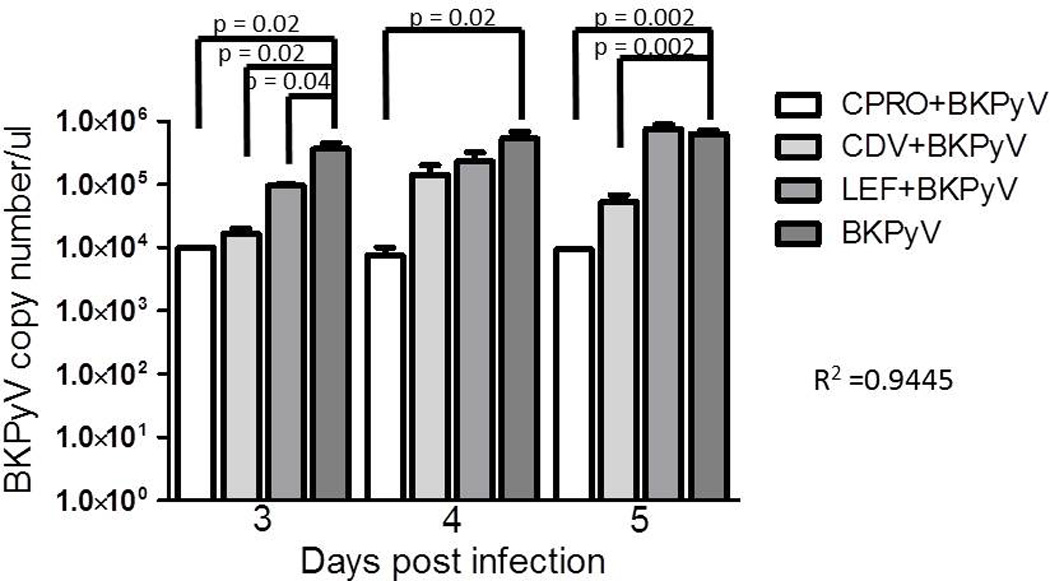
HSG cells were infected with BKPYV and treated with drug as described in the materials and methods. At stated times post infection cells were collected, DNA isolated, and qPCR performed for T Ag DNA copy no. A standard curve (data not shown) was constructed using a plasmid coding for BKPYV whole genome. The error bars represent the SD and p-value calculated using the t-test.
Figure 4. Effect of drug treatment on BKPy viral gene expression in human salivary gland and Vero cells.
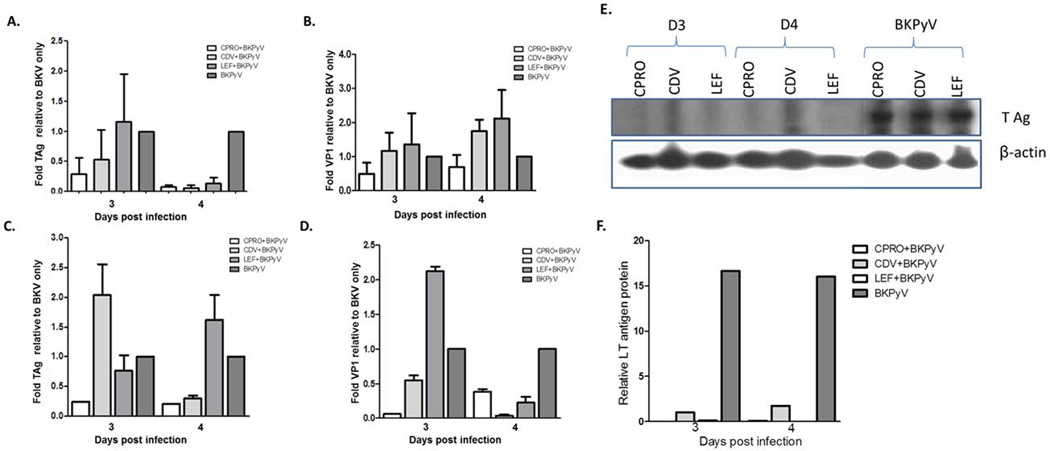
HSG (A and B) and Vero (C and D) cells were infected with BKPyV for 24h then treated with drug as described in the materials and methods. At stated times post infection cells were collected, RNA isolated, cDNA generated and qRTPCR performed for T Ag (A and C) or VP1 (B and D) viral transcripts. Gene expression values were normalized to the levels of β-actin transcripts, using 2−DDC(T) method and are represented as the changes (n-fold) in transcript levels with the levels in non-drug treated (BKPYV only) samples arbitrarily set to 1. At stated times post infection cell lysates were collected and used for immunoblotting as described in the materials and methods. Antibodies against T Ag and β-actin (E) were used (F). Relative L Tag protein expression compared to β-actin.
To investigate the effect of CDV, CPRO and LEF on BKPyV mRNA expression in salivary gland cells, T Ag and VP1 transcripts were measured at 3 and 4 dpi by qRTPCR (Figure 4A, B). The results shown represent a composite of 4 separate experiments with each condition carried out in triplicate. Results were normalized to β-actin using the 2−ΔΔC(T) method, and are presented as the changes (n-fold) in T Ag/VP1 transcript levels relative to untreated BKPyV-infected cells at each time point. Compared to salivary gland cells infected with BKPyV, a consistent 50–90% decrease was detected in T Ag expression in the presence of CPRO and CDV. Mean T Ag BKPyV levels were not changed with LEF at day 3, but an 80% decrease with LEF treatment was detected at day 4 (Figure 4A). Mean VP1 expression increased in the presence of both CDV and LEF at days 3 and 4 pi. However, a 50–75% decrease in VP1 expression was detected with CPRO treatment during each time point post infection (Figure 4B).
Monkey kidney cells have replication kinetics that are distinct from salivary gland cells (28–30). In Vero cells, that likely experience a life cycle similar to RPTE cells (Figure 1) with peak BKPyV replication at day 2 pi, CDV treatment cells resulted in a variable trend with enhanced T Ag expression on day 3 pi followed by a decrease on day 4. A slight decrease in T Ag expression on day 3 was detected with LEF treatment, followed by an increase on day 4 pi (Figure 4C). CPRO treatment demonstrated a >50% decrease in both T Ag and VP1 transcripts at both day 3 and 4 post infection. A decrease in VP1 expression was observed in the presence of CDV on both days 3 and 4 pi. LEF treatment on day 4 pi resulted in a significant decrease in VP1 expression compared to day 3 pi (Figure 4D).
To investigate the effect of CDV, LEF and CPRO on BKPyV T Ag protein expression, T Ag protein was measured at 3 and 4 days pi/post drug treatment by immunoblot (Figure 4E). Results were normalized to β-actin using densitometry (Figure 4F), and are presented as T Ag protein relative to untreated BKPyV-infected cells at each time point. A consistent decrease of over 15 fold T Ag protein expression was detected in the presence of all three drugs at days 3 and 4 pi.
Effect of Cidofovir, Ciprofloxacin and Leflunomide on encapsidated BK viral progeny release and infectivity in salivary gland cells
To determine the effect of these agents on the release of encapsidated BKPyV virions into the supernatant of HSG, DNase resistant particles were quantified by qPCR and BKPyV DNA loads measured at 3, 4 and 5 dpi/post drug treatment (Figure 5). A standard curve was constructed using a plasmid encoding for BKPyV genome to determine BKPyV copy number. Amplification was performed with both T Ag (Figure 5A) and VP1 (data not shown) primer sets, both providing similar results. In media from both BKPyV only cell types, the virion release increased from day 3 to day 5. In HSG media, treatment with all of the agents resulted in decrease of 1–1.5 logs at day 3 and a statistically significant 2–3 logs decrease at day 5 (Figure 5A). An R2 value of 0.98 suggests optimal PCR efficiency in the detection of encapsidated BKV. These assays were also performed in Vero cells and similar results were obtained (data not shown). The efficacy of these agents on the treatment of wild type clinical isolate-derived viruses from patients with HIV associated Salivary Gland Disease (HIV-SGD 1 and 2) and from an organ transplant patient (transplant) was assessed 5 days pi of HSG cells. Statistically significant decreases in HIV-SGD-derived released virions were detected in the presence of CDV and LEF but not CPRO (Figure 5B). Treatment with either of the drug treatments for the lung transplant-derived virus did not result in a statistically significant decrease in the levels of released virions (Figure 5C). The laboratory adapted viral strain (MM) treated with CDV and LEF showed a statistically significant decrease in released virions but CPRO treatment did not. BKVPy DNA amplification was performed with both T Ag (Figure 5B) and VP1 (data not shown) primer sets, both provided similar results. PCR for the detection of BKPyV DNA was efficient as determined by R2 value of 0.9.
Figure 5. Effect of drug treatment on lab-strain and patient-derived BKPy virus progeny release from human salivary gland cells.
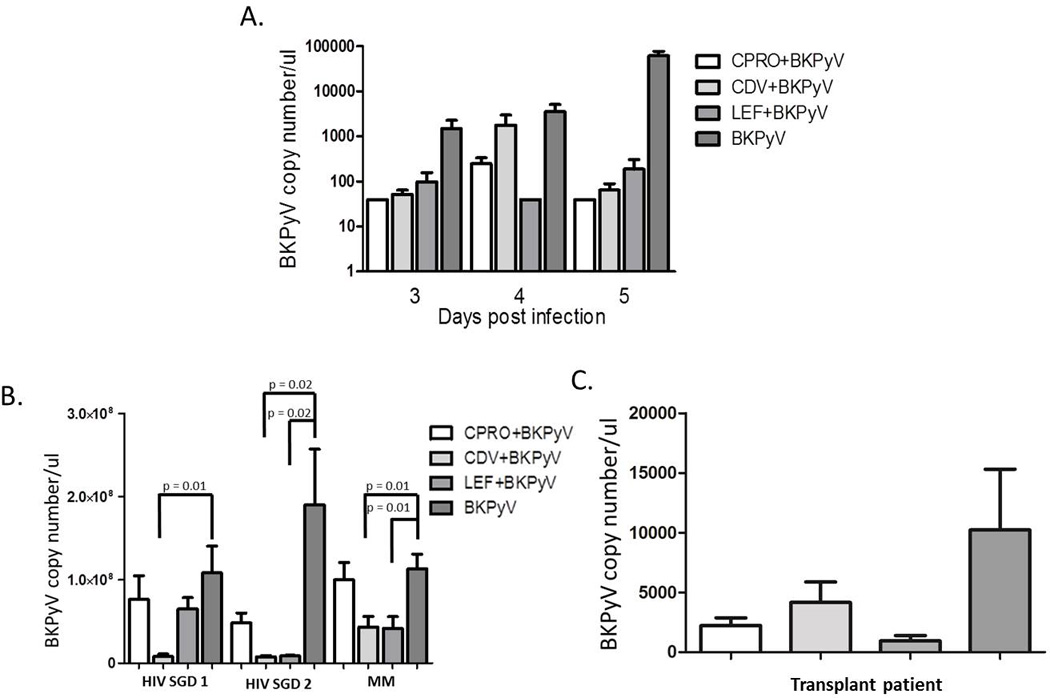
HSG cells were infected with (A) lab-strain BKPyV (VR837) or (B) BKPyV isolated from the saliva of two HIVSGD patients and a lab-adapted virus strain (MM), or (C) BKPyV isolated from urine of a lung transplant patient, and treated with drug as described in the materials and methods. At stated times post infection supernatant was collected, Dnase-treated and qPCR performed for TAg and VP1 (data not shown) DNA copy no. A standard curve (data not shown) was constructed using a plasmid coding for BKPyV whole genome. The error bars represent the SD and p-value calculated using the t-test.
To investigate the infectivity of virions released from drug treated BKPyV infected salivary gland cells, supernatants from HSG cells were used to infect naive Vero cells in a florescence focus assay (FFA) (Figure 6). At 5 days post Vero cell infection, cells were fixed for FFA analysis. Immunofluorescence staining determined that supernatant from HSG cells treated with CPRO, CDV or LEF significantly decreased infectivity of released virions (Figure 6). Virions from BKV infected HSG cells that were not subject to drug treatment demonstrated infectivity of 66 FFU/ul while those treated with CPRO, CDV or LEF demonstrated 37, 11, and 22 FFU/ul respectively. These data suggest that human salivary gland cells treated with CPRO, CDV or LEF indeed reduce BKPyV replication and subsequent infectivity.
Figure 6. Effect of drug treatment on infectious BKPyV progeny release in human salivary gland cells.
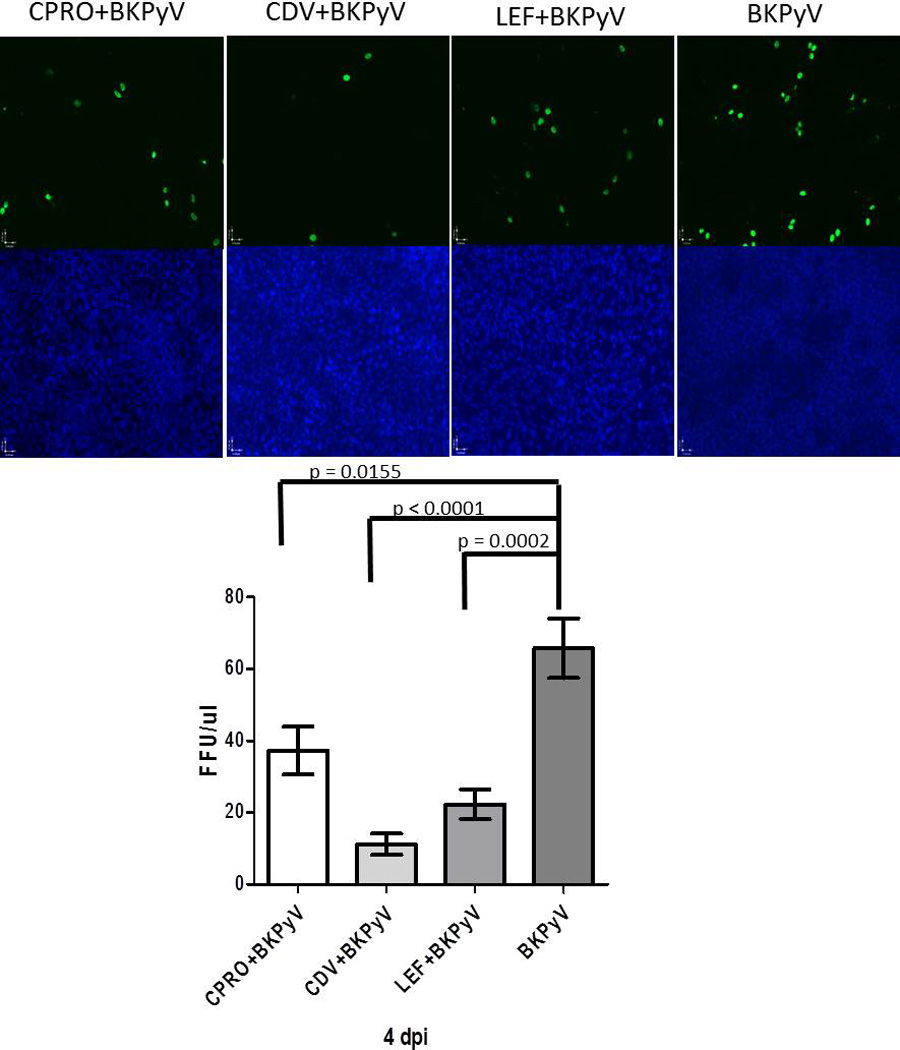
Immunofluorescence of Vero cells treated with HSG cell supernatant collected at 4 dpi and drug treatment BKPyV only, LEF, CDV, CPRO at 10× magnification. Infected cells (green) and nuclei (blue) were quantified, and FFU/µl values were calculated. FFU values were graphed for each treatment and are depicted as FFU/µl.
DISCUSSION
Currently, there is no agreed upon standard treatment of BKPyV viremia in kidney transplant patients. Given that there has been some benefit with CDV, CPRO or LEF (42, 43), these agents were considered an important starting point for potential therapeutics targeting BKPyV associated HIV-SGD. HIV-SGD (29) is AIDS defining in pediatric HIV infection and increasing in the adult HIV population (44). In 1–2% of HIV-SGD patients malignant lymphomas have been described in association with glandular lesions making this disease a premalignant lesion (33, 34). Treatment for HIV-SGD thus far has been palliative due to the lack of an identified pharmacologic target. However, our group has recently shown that BKPyV infection is associated with HIV-SGD (28, 29).
All previous BKPyV anti-viral experiments have been performed in human or monkey kidney cells or lung fibroblasts (12, 35, 36). Based on our observation that BKPyV can also infect salivary gland cells in vitro and is associated with pathology in vivo in humans (28, 29, 45, 46), three drugs with demonstrated anti BKPyV activity in kidney cells, CPRO, CDV and LEF were assessed for BKPyV inhibition in salivary gland cells in vitro. It is clear that the kinetics of salivary gland infection are delayed compared to BKPyV infection in kidney cells (Figure 1), thus the response to the agents may vary based on time post infection. These drugs effectively halt viral replication activity by either inhibiting topoisomerase activity (CPRO), interfering with the helicase activity of BKPyV large T antigen (LTAg) (CPRO), potentially disrupting DNA polymerases by an unknown mechanism (CDV), or modulate pyrimidine/tyrosine kinase activity (LEF) (20, 47). The concentration of drugs used to treat cells were based on previous experiments performed by Bernhoff et al (14), Sharma et al (22) and Liacini et al (36). While these concentrations were not optimal for salivary gland BKPyV infection, as determined by WST-1, Brdu and CC50/EC50 (Fig 2, Table 1) they are physiologically relevant in kidney cells and were previously described concentrations in the literature. CPRO inhibited BKPyV activity in vitro in kidney cells (48) and reduced BKPyV viremia when used in combination with immunosuppression reduction in kidney transplant patients (16). CPRO inhibits type II and IV topoisomerase activity during bacterial DNA replication and given the similarities between the bacterial and polyoma viral topoisomerases is thought to inhibit BKPyV T Ag viral helicase activity (20). We show that CPRO inhibited BKPyV early and late gene expression in both human salivary gland and monkey kidney cells (Figure 4). Likewise, CPRO inhibited DNA replication (Figure 3) and infectious progeny release in salivary gland cells (Figure 5A). However when BKPyV patient-derived clinical isolates were used to infect salivary gland cells, CPRO was not as effective as CDV against HIV-SGD derived viruses but was most effective against the transplant derived virus (Figure 5C). CPRO had minimal effect on metabolic activity and host cell DNA replication at the concentration used for these experiments over the three to five day time period tested (Figure 2D). A gradual decrease in host cell DNA replication was observed with CPRO concentrations over 150ug/ml (Figure 2A). A low selectivity index of 3.4, defined as the ratio of the 50% reduction in host cell replication value to the 50% virus inhibitory concentration value, suggested a modest anti-BKPyV effect.
CDV treatment is currently approved for CMV-induced retinitis in HIV infected patients (49) and has been used at low doses to treat transplant patients resulting in decreased BKPyV viremia and viruria (24). There are reports however, of the deleterious effects of reduced renal function and increased viral load in cidofovir-treated BKPyVN patients (23). CDV inhibits CMV viral DNA polymerase activity, yet it's mode of action against BKPyV is currently unknown. In vitro, CDV inhibits BKPyV activity in human kidney and lung fibroblast cells (12, 14) but has not previously been tested in human salivary gland cells. In this study, CDV inhibited BKPyV early gene expression but not late gene expression in human salivary gland cells (Figure 4A, B). In monkey kidney (Vero) cells, CDV inhibited early gene expression at 4dpi (Figure 4C) and late gene expression at both 3 and 4 dpi (Figure 4D). CDV inhibited DNA replication (Fig 3), diminished progeny release from salivary gland cells (Figure 5A), and significantly decreased replication of both the HIVSGD patient-derived viruses (HIVSGD 1 and 2) and the laboratory adapted strain (MM) (Figure 5B). CDV was more effective than CPRO for the aforementioned patient-derived viruses but not the urine derived virus (transplant patient) (Figure 5C). Of the three agents, CDV was the most effective in the FFA assay demonstrating a 6 fold decrease in infectious progeny virus. CDV had minimal effect on metabolic activity and host cell DNA replication at the concentration used for these experiments over the three to five day time period tested (Figure 2D). However, at 72hpi with increasing drug concentration a seventy percent decrease in host cell DNA replication was observed. In the presence of viral infection with and without drug, host cell metabolism decreased at lower drug concentrations (5–40ug/ml) and increased at higher drug concentrations (50–150ug/ml) (Figure 2B). The SI of 1.6 suggested a lower anti-BKPyV effect in HSG cells compared to the SI’s of CDV in human embryonic lung fibroblasts (WI-38) and human renal proximal tubular epithelial cells 2.3 (12) and 4.9 respectively (50). Although CDV inhibited BKPyV replication in salivary gland cells, there are clinical limitations for its use due to its nephrotoxicity and limited oral bioavailability (23).
LEF is an anti-inflammatory drug approved to treat rheumatoid arthritis and other autoimmune conditions. LEF has been shown to inhibit pyrimidine synthesis, tyrosine kinase and dihydroorotate dyhydrogenase activity (12). Like CDV, there is conflicting data with regards to its activity against BKPYV in vitro (12, 13). In vivo studies have detected LEF activity against CMV and HSV. LEF has been used in combination with other immunosuppressive agents for treatment of BKPyVN (12, 26). The mechanism of LEF’s antiviral activity against CMV is thought to occur through interference with viral assembly, specifically on the viral envelope (27). However this mechanism is unlikely for BKPyV as it is a non-enveloped virus. Serum steady-state levels of the principal metabolite of LEF are 8.8, 18, and 63 µg/mL after 24 days of therapy with a 5, 10, or 25 mg daily dose, respectively (12) (Physicians Desk Reference, accessed at http://www.micromedex.com), therefore the concentrations used in our experiments were in the clinically relevant range. LEF had minimal effect on metabolic activity and host cell DNA replication at the concentration used for these experiments over the three to five day time period tested (Figure 2C). At the level of transcription, our data showed that LEF inhibition was inconsistent over the two days tested in both salivary gland cells and in monkey kidney (Vero) cells (Figure 4C, D). It is possible that the observed variability in transcript levels in the presence of LEF may be due to drug toxicity. At the protein level, LEF consistently decreased T Ag expression, albeit beta actin levels were markedly lower with LEF treatment, suggesting cell toxicity (Figure 4E). LEF appeared to be the least effective drug with regard to inhibiting BKPyV DNA replication (Fig 3) and release of encapsidated virus from salivary gland cells (Figure 5). However, LEF treatment resulted in a threefold decrease in infectious progeny virus as determined by FFA (Figure 6). Likewise, LEF consistently inhibited both HIV-SGD and transplant patient-derived progeny release in salivary gland cells compared to the other drug types (Figure 5B) and showed significant decrease in lab-adapted MM virus progeny release (Figure 5B). Given its relatively high SI of 9.1 (Table 1), a significant anti BKPyV effect was expected in salivary gland cells, however the cellular toxicity and potential off target effect dampen enthusiasm for the use of LEF in the treatment of HIV-SGD.
In conclusion, BKPyV genome replication in human salivary gland cells was decreased by all three drug types. The mechanism of these drugs against BKPyV are currently up for debate, however it is possible that BKPyV inhibition in salivary gland cells may be due to manipulation of a host intracellular signaling pathway critical to BKPyV replication, for example the Akt or mTOR. With regard to patient treatment, CDV has been shown to be cytotoxic in kidney cells in vivo and is therefore not a favorable candidate for HIV-SGD treatment in patients. In kidney transplant patients, LEF is used as an immunomodulatory agent to lower the incidence of organ rejection, but given its toxicity in salivary gland cells and potential off target effect may not be optimal for treatment of salivary gland infection. CPRO’s wide availability and profile as a well-tolerated agent enhances its appeal for clinical use. Until better drugs become available and more in vitro and in vivo studies are performed, it would be reasonable to test CPRO in clinical trials to evaluate its efficacy in salivary gland disease in HIV patients.
The consistent association and potential causal relationship between HIV-SGD and BKPyV infection of the salivary gland highlight the importance of a targeted anti-BKPyV therapy in the salivary gland (28–30). Our data highlight the need for continued studies to discover more effective and less toxic drugs that can inhibit BKPyV replication in salivary gland cells.
Supplementary Material
Supplemental 1. Determination of EC50 and CC50 values by curve fitting. EC50- the effect of increasing drug concentration on BKPyV supernatant loads at 72 hpi and CC50- the effect of increasing drug concentration on BrdU incorporation at 72 hpi were plotted using the non-linear regression curve fitting in GraphPad Prism. The data displayed are mean values of triplicate assay results.
Highlights.
BKPyV is an emerging pathogen in the HIV community, associated with HIV-SGD.
BKPyV-infected salivary gland in vitro culture system is used.
BKPyV replication is inhibited by ciprofloxacin, leflunomide and cidofovir.
Our data highlight the need for continued studies to discover more effective and less toxic drugs that can inhibit BKPyV replication in salivary gland cells.
ACKNOWLEDGEMENT
We would like to thank Webster-Cyriaque Laboratory members for useful discussions and comments about this work. We are also thankful to Gilead Inc for supplying Cidofovir reagent, Bruce Baum and Corrine Goldsmith (NIDCR, NIH) for providing us with HSG cells. We would also like to thank Robert Bagnell and the UNC microscopy services for their assistance with all of our microscopy needs. This work was supported by R21DE023046 NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Imperiale MJ, Major EO. Polyomaviruses, p. 3091. In: Field AM, Knipe DM, Howley PM, editors. Fields' Virology. 5th ed. Vol. 2. Philadelphia: Wolers Kluwer Health/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Hirsch HH. BK virus: opportunity makes a pathogen. Clin Infect Dis. 2005;41:354–360. doi: 10.1086/431488. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 4.Kuypers DR. Management of polyomavirus-associated nephropathy in renal transplant recipients. Nat Rev Nephrol. 2012;8:390–402. doi: 10.1038/nrneph.2012.64. [DOI] [PubMed] [Google Scholar]
- 5.Dugan AS, Eash S, Atwood WJ. Update on BK virus entry and intracellular trafficking. Transpl Infect Dis. 2006;8:62–67. doi: 10.1111/j.1399-3062.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 7.Nickeleit V, Klimkait T, Binet IF, Dalquen P, Del Zenero V, Thiel G, Mihatsch MJ, Hirsch HH. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N Engl J Med. 2000;342:1309–1315. doi: 10.1056/NEJM200005043421802. [DOI] [PubMed] [Google Scholar]
- 8.Ahsan N, Shah KV. Polyomaviruses and human diseases. Adv Exp Med Biol. 2006;577:1–18. doi: 10.1007/0-387-32957-9_1. [DOI] [PubMed] [Google Scholar]
- 9.Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41:11–18. doi: 10.1038/sj.bmt.1705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longhi G, Pietropaolo V, Mischitelli M, Longhi C, Conte MP, Marchetti M, Tinari A, Valenti P, Degener AM, Seganti L, Superti F. Lactoferrin inhibits early steps of human BK polyomavirus infection. Antiviral Res. 2006;72:145–152. doi: 10.1016/j.antiviral.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Rinaldo CH, Gosert R, Bernhoff E, Finstad S, Hirsch HH. 1-O-hexadecyloxypropyl cidofovir (CMX001) effectively inhibits polyomavirus BK replication in primary human renal tubular epithelial cells. Antimicrob Agents Chemother. 2010;54:4714–4722. doi: 10.1128/AAC.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farasati NA, Shapiro R, Vats A, Randhawa P. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation. 2005;79:116–118. doi: 10.1097/01.tp.0000149338.97084.5f. [DOI] [PubMed] [Google Scholar]
- 13.Bernhoff E, Tylden GD, Kjerpeseth LJ, Gutteberg TJ, Hirsch HH, Rinaldo CH. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol. 2010;84:2150–2156. doi: 10.1128/JVI.01737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernhoff E, Gutteberg TJ, Sandvik K, Hirsch HH, Rinaldo CH. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am J Transplant. 2008;8:1413–1422. doi: 10.1111/j.1600-6143.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 15.Leung AY, Chan MT, Yuen KY, Cheng VC, Chan KH, Wong CL, Liang R, Lie AK, Kwong YL. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:528–537. doi: 10.1086/427291. [DOI] [PubMed] [Google Scholar]
- 16.Gabardi S, Waikar SS, Martin S, Roberts K, Chen J, Borgi L, Sheashaa H, Dyer C, Malek SK, Tullius SG, Vadivel N, Grafals M, Abdi R, Najafian N, Milford E, Chandraker A. Evaluation of fluoroquinolones for the prevention of BK viremia after renal transplantation. Clin J Am Soc Nephrol. 2010;5:1298–1304. doi: 10.2215/CJN.08261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa K, Motsuchi W, Tanaka S, Dosako S. Inhibition with lactoferrin of in vitro infection with human herpes virus. Jpn J Med Sci Biol. 1994;47:73–85. doi: 10.7883/yoken1952.47.73. [DOI] [PubMed] [Google Scholar]
- 18.Hara K, Ikeda M, Saito S, Matsumoto S, Numata K, Kato N, Tanaka K, Sekihara H. Lactoferrin inhibits hepatitis B virus infection in cultured human hepatocytes. Hepatol Res. 2002;24:228. doi: 10.1016/s1386-6346(02)00088-8. [DOI] [PubMed] [Google Scholar]
- 19.Evers DL, Wang X, Huong SM, Andreoni KA, Huang ES. Inhibition of human cytomegalovirus signaling and replication by the immunosuppressant FK778. Antiviral Res. 2005;65:1–12. doi: 10.1016/j.antiviral.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Ali SH, Chandraker A, DeCaprio JA. Inhibition of Simian virus 40 large T antigen helicase activity by fluoroquinolones. Antivir Ther. 2007;12:1–6. [PubMed] [Google Scholar]
- 21.Rinaldo CH, Hirsch HH. Antivirals for the treatment of polyomavirus BK replication. Expert Rev Anti Infect Ther. 2007;5:105–115. doi: 10.1586/14787210.5.1.105. [DOI] [PubMed] [Google Scholar]
- 22.Sharma BN, Li R, Bernhoff E, Gutteberg TJ, Rinaldo CH. Fluoroquinolones inhibit human polyomavirus BK (BKV) replication in primary human kidney cells. Antiviral Res. 2011;92:115–123. doi: 10.1016/j.antiviral.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Safrin S, Cherrington J, Jaffe HS. Clinical uses of cidofovir. Rev Med Virol. 1997;7:145–156. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Savona MR, Newton D, Frame D, Levine JE, Mineishi S, Kaul DR. Low-dose cidofovir treatment of BK virus-associated hemorrhagic cystitis in recipients of hematopoietic stem cell transplant. Bone Marrow Transplant. 2007;39:783–787. doi: 10.1038/sj.bmt.1705678. [DOI] [PubMed] [Google Scholar]
- 25.Gosert R, Rinaldo CH, Wernli M, Major EO, Hirsch HH. CMX001 (1-O-hexadecyloxypropyl-cidofovir) inhibits polyomavirus JC replication in human brain progenitor-derived astrocytes. Antimicrob Agents Chemother. 2011;55:2129–2136. doi: 10.1128/AAC.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Josephson MA, Gillen D, Javaid B, Kadambi P, Meehan S, Foster P, Harland R, Thistlethwaite RJ, Garfinkel M, Atwood W, Jordan J, Sadhu M, Millis MJ, Williams J. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81:704–710. doi: 10.1097/01.tp.0000181149.76113.50. [DOI] [PubMed] [Google Scholar]
- 27.Knight DA, Hejmanowski AQ, Dierksheide JE, Williams JW, Chong AS, Waldman WJ. Inhibition of herpes simplex virus type 1 by the experimental immunosuppressive agent leflunomide. Transplantation. 2001;71:170–174. doi: 10.1097/00007890-200101150-00031. [DOI] [PubMed] [Google Scholar]
- 28.Jeffers LK, Madden V, Webster-Cyriaque J. BK virus has tropism for human salivary gland cells in vitro: implications for transmission. Virology. 2009;394:183–193. doi: 10.1016/j.virol.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Jeffers L, Webster-Cyriaque JY. Viruses and salivary gland disease (SGD): lessons from HIV SGD. Adv Dent Res. 2011;23:79–83. doi: 10.1177/0022034510396882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burger-Calderon R, Madden V, Hallett RA, Gingerich AD, Nickeleit V, Webster-Cyriaque J. Replication of oral BK virus in human salivary gland cells. J Virol. 2014;88:559–573. doi: 10.1128/JVI.02777-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patton LL, van der Horst C. Oral infections and other manifestations of HIV disease. Infect Dis Clin North Am. 1999;13:879–900. doi: 10.1016/s0891-5520(05)70114-8. [DOI] [PubMed] [Google Scholar]
- 32.McArthur CP, Subtil-DeOliveira A, Palmer D, Fiorella RM, Gustafson S, Tira D, Miranda RN. Characteristics of salivary diffuse infiltrative lymphocytosis syndrome in West Africa. Arch Pathol Lab Med. 2000;124:1773–1779. doi: 10.5858/2000-124-1773-COSDIL. [DOI] [PubMed] [Google Scholar]
- 33.DiGiuseppe JA, Corio RL, Westra WH. Lymphoid infiltrates of the salivary glands: pathology, biology and clinical significance. Curr Opin Oncol. 1996;8:232–237. doi: 10.1097/00001622-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Ioachim HL, Ryan JR. Salivary gland lymphadenopathies associated with AIDS. Hum Pathol. 1988;19:616–617. doi: 10.1016/s0046-8177(88)80220-x. [DOI] [PubMed] [Google Scholar]
- 35.Acott PD, O'Regan PA, Lee SH, Crocker JF. Utilization of vero cells for primary and chronic BK virus infection. Transplant Proc. 2006;38:3502–3505. doi: 10.1016/j.transproceed.2006.10.163. [DOI] [PubMed] [Google Scholar]
- 36.Liacini A, Seamone ME, Muruve DA, Tibbles LA. Anti-BK virus mechanisms of sirolimus and leflunomide alone and in combination: toward a new therapy for BK virus infection. Transplantation. 2010;90:1450–1457. doi: 10.1097/TP.0b013e3182007be2. [DOI] [PubMed] [Google Scholar]
- 37.Shirasuna K, Sato M, Miyazaki T. A neoplastic epithelial duct cell line established from an irradiated human salivary gland. Cancer. 1981;48:745–752. doi: 10.1002/1097-0142(19810801)48:3<745::aid-cncr2820480314>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Rollison DE, Utaipat U, Ryschkewitsch C, Hou J, Goldthwaite P, Daniel R, Helzlsouer KJ, Burger PC, Shah KV, Major EO. Investigation of human brain tumors for the presence of polyomavirus genome sequences by two independent laboratories. Int J Cancer. 2005;113:769–774. doi: 10.1002/ijc.20641. [DOI] [PubMed] [Google Scholar]
- 39.Ding R, Medeiros M, Dadhania D, Muthukumar T, Kracker D, Kong JM, Epstein SR, Sharma VK, Seshan SV, Li B, Suthanthiran M. Noninvasive diagnosis of BK virus nephritis by measurement of messenger RNA for BK virus VP1 in urine. Transplantation. 2002;74:987–994. doi: 10.1097/00007890-200210150-00016. [DOI] [PubMed] [Google Scholar]
- 40.Moriyama T, Sorokin A. BK virus (BKV): infection, propagation, quantitation, purification, labeling, and analysis of cell entry. Curr Protoc Cell Biol. 2009;Chapter 26(Unit 26):22. doi: 10.1002/0471143030.cb2602s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li R, Sharma BN, Linder S, Gutteberg TJ, Hirsch HH, Rinaldo CH. Characteristics of polyomavirus BK (BKPyV) infection in primary human urothelial cells. Virology. 2013;440:41–50. doi: 10.1016/j.virol.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 42.Kwon HJ, Kang JH, Lee JW, Chung NG, Kim HK, Cho B. Treatment of BK virus-associated hemorrhagic cystitis in pediatric hematopoietic stem cell transplant recipients with cidofovir: a single-center experience. Transpl Infect Dis. 2013;15:569–574. doi: 10.1111/tid.12136. [DOI] [PubMed] [Google Scholar]
- 43.Zaman RA, Ettenger RB, Cheam H, Malekzadeh MH, Tsai EW. A novel treatment regimen for BK viremia. Transplantation. 2014;97:1166–1171. doi: 10.1097/01.TP.0000441825.72639.4f. [DOI] [PubMed] [Google Scholar]
- 44.Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ., Jr Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:299–304. doi: 10.1016/s1079-2104(00)70092-8. [DOI] [PubMed] [Google Scholar]
- 45.Robaina TF, Mendes GS, Benati FJ, Pena GA, Silva RC, Montes MA, Janini ME, Camara FP, Santos N. Shedding of polyomavirus in the saliva of immunocompetent individuals. J Med Virol. 2013;85:144–148. doi: 10.1002/jmv.23453. [DOI] [PubMed] [Google Scholar]
- 46.Robaina TF, Mendes GS, Benati FJ, Pena GA, Silva RC, Montes MA, Otero R, Castro GF, Camara FP, Santos N. Polyomavirus in Saliva of HIV-infected Children, Brazil. Emerg Infect Dis. 2013;19:155–157. doi: 10.3201/eid1901.120563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett SM, Broekema NM, Imperiale MJ. BK polyomavirus: emerging pathogen. Microbes Infect. 2012;14:672–683. doi: 10.1016/j.micinf.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portolani M, Pietrosemoli P, Cermelli C, Mannini-Palenzona A, Grossi MP, Paolini L, Barbanti-Brodano G. Suppression of BK virus replication and cytopathic effect by inhibitors of prokaryotic DNA gyrase. Antiviral Res. 1988;9:205–218. doi: 10.1016/0166-3542(88)90004-6. [DOI] [PubMed] [Google Scholar]
- 49.FDA approves cidofovir for treatment of CMV retinitis. Food and Drug Administration. J Int Assoc Physicians AIDS Care. 1996;2:30. [PubMed] [Google Scholar]
- 50.Topalis D, Lebeau I, Krecmerova M, Andrei G, Snoeck R. Activities of different classes of acyclic nucleoside phosphonates against BK virus in primary human renal cells. Antimicrob Agents Chemother. 2011;55:1961–1967. doi: 10.1128/AAC.01809-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental 1. Determination of EC50 and CC50 values by curve fitting. EC50- the effect of increasing drug concentration on BKPyV supernatant loads at 72 hpi and CC50- the effect of increasing drug concentration on BrdU incorporation at 72 hpi were plotted using the non-linear regression curve fitting in GraphPad Prism. The data displayed are mean values of triplicate assay results.