Abstract
Objectives
To examine preliminarily the effectiveness of a SMS text message-based social support intervention for reducing daily pain and pain interference levels, improving affect and perceptions of social support in patients with chronic non-cancer pain, and exploring the feasibility of a novel mobile application to track perceptions of pain and pain-interference.
Materials and Methods
Participants (17 men, 51 women) from two pain clinics in New York City downloaded a pain tracking App to their smartphone and employed it to record twice-daily pain, pain interference, and affect scores over the 4-week study period. Participants were randomly assigned to receive standard care (control) or standard care along with receipt of twice-daily supportive SMS text messages delivered during the 2nd and 3rd week of the study (intervention). Demographic and clinical data were obtained at baseline, and social support measures were administered at baseline and at 4-weeks. Statistical analysis was carried out using general linear mixed models taking into account variances associated with time of assessments and with patients.
Results
The social support intervention reduced perceptions of pain and pain interference and improved positive affect for chronic non-cancer pain patients assigned to the intervention condition in comparison to controls. Participants completed approximately 80% of the daily measurements requested.
Discussion
These findings establish the feasibility of collecting daily pain data using a mobile tracking App and provide significant implications and insight into a nuanced approach to reducing the daily experience of pain via mobile technology, especially because of its accessibility.
Keywords: Chronic pain, social support, mobile applications
Research shows that social support (i.e., perceived and actual support from known others, often during aversive experiences)1 is beneficial for coping, recovery, rehabilitation, and adaptation to chronic diseases.2 Social support is associated with reduced pain perceptions across several health conditions, including cardiovascular disease,3 asthma, 4 postoperative pain,5 and chronic pain (e.g., rheumatoid arthritis).6 Previous studies show that there is a causal relationship between social support and pain such that social support reduces pain perceptions (i.e., self-reported perceptions of the severity of pain), even when the support provided is minimal.7,8
Decades of research showing positive effects of social support on health outcomes2–6 has motivated the development of social support interventions for a number of health conditions (e.g., rheumatoid arthritis, substance abuse treatment, weight loss, eating disorder treatment, depression).9 One study of patients with rheumatoid arthritis found that participants who received family support along with cognitive-behavioral therapy experienced less joint swelling than those who received cognitive-behavioral therapy alone.10 Another study of patients undergoing surgery found that participants who received a support and education intervention needed less anesthesia and pain medication than those receiving standard care.11 In the context of pain, Master et al.8 found that viewing a photograph of a romantic partner significantly reduced pain perceptions compared to viewing photographs of a stranger or object.
In the context of chronic non-cancer pain, social support can be particularly beneficial in reducing perceived pain severity because it provides individuals positive experiences and can buffer and attenuate the effects of stress.12 Lazarus argued that the positive effects of social support result from the activation of psychosocial resources that alter people’s appraisal of their coping abilities.13 Formative research in this area shows that cognitive reappraisal of anxiety-provoking events prior to surgery helped individuals have fewer negative experiences pre and post surgery and request less pain medication and sedatives following surgery.14
In recent years there have been an increasing number of social support interventions using smart phone applications in an effort to improve pain management.15 Social support interventions have been developed for individuals with pain,10,11 but researchers have yet to develop social support interventions delivered through mobile technology using short message service (SMS) capabilities. However, a number of effective SMS-based interventions have been developed for other health conditions (e.g., smoking cessation, weight loss).16–20
This study sought to administer and evaluate a SMS text message-based social support intervention for patients with chronic non-cancer pain. Although social support has several dimensions and has been conceptualized in various ways, this study focuses on whether four specific types of social support, i.e., 1) network (i.e., interpersonal social support that emphasizes access, presence, and companionship of other individuals); 2) emotional (i.e., relational social support, affection, sympathy, and understanding); 3) esteem (i.e., encompasses compliments and validation); and 4) informational (i.e., involves suggestions, advice, referral, and situation appraisal),21 delivered daily via SMS messages can influence individuals’ perceptions of pain, pain interference, affect, and social support over time. In addition to being easily translated to SMS, these dimensions of social support should provide patients with psychosocial resources for engaging in cognitive reappraisal processes that help in pain management.13,14
To examine the effects of a social support intervention among patients with chronic pain, it is important to track pain levels and other key outcomes. Ecological momentary assessment (EMA) is a data collection method that allows researchers to collect in situ data.22 Participants report on behaviors immediately after the fact and do so frequently with minimal disruption to routines, helping to reduce recall bias and reporting errors. Mobile health applications (Apps) have been used successfully for EMA by allowing patients to track pain variables via diary methods.15 These Apps often help individuals cope with and manage their pain. As a result, there has been increasing popularity of Apps for pain management.15 Therefore, a key related goal of this study was to establish the feasibility of a novel mobile App to track daily chronic pain and related variables in situ (e.g., will participants respond to twice daily prompts and input study data?) to determine the effectiveness of the social support intervention.
In this pilot randomized controlled study involving patients with chronic non-cancer pain, we sought to evaluate whether a social support intervention delivered via daily SMS text messages could: 1) reduce daily pain and pain interference levels and increase positive affect, 2) improve perceptions of social support and connectedness, and 3) be feasibly delivered using mobile phone technology.
MATERIALS AND METHODS
Participants
Participants (N = 68) received longitudinal pain care at New York Presbyterian’s Pain Medicine Center or the Pain Management Practice located at the Hospital for Special Surgery. Both practices deliver multidisciplinary care to a broad range of patients with diverse chronic pain disorders. All participants reported experiencing chronic non-cancer pain on most days of every month over the preceding three-month period and did not start using any new medication during the study. All participants were English speakers, between the ages of 30 and 80, New York state residents and owners of an Android or iPhone smartphone capable of downloading the novel pain tracking App developed for the study and receiving SMS texts. Participants were compensated with $25 for their participation to help defray the cost of receiving SMS texts. The Weill Cornell Medical College Institutional Review Board approved the study.
Sample Assembly
Prospective participants were first approached while in the waiting room of their pain clinic. Of the 272 patients approached, 115 (42%) were ineligible: 95 (35%) did not own a smartphone, 10 (4%) were non-English speakers, 3 (1%) had cancer pain, 2 (1%) were older than 80-years-old, 1 (.4%) was not a NY state resident, and 1 (.4%) would not be receiving further care at the clinic. Eligibility criteria could not be determined for 11 (4%) patients who reported that they did not have time to answer screening questions.
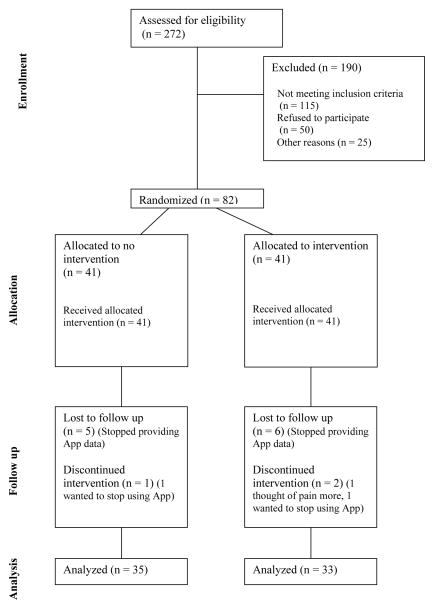
A total of 146 patients met eligibility criteria. Of these, 50 (18%) did not want to participate, 13 (5%) had technical difficulties with the App or their smartphone, and 1 (.4%) did not want to download the App to their work phone (see Figure 1).
Figure 1.
Flowchart of participants’ progress through phases of the trial.
Patients who met eligibility criteria and were interested in participating provided written informed consent and completed the baseline questionnaire online using a laptop computer. All participants then downloaded an App to their smartphone and were provided instructions on how to use the App; the research assistants (RAs) helped participants download the App if anyone had trouble locating the App in their App store or needed clarification with the instructions sheet. The RAs also provided assistance with showing the participants each measure in the App that they would need to fill out daily to become familiar with the layout and formatting of the App. Further assistance was provided throughout the study as well in regards to technical support (e.g. forgetting the log-in id or password). Overall however, the vast majority of the recruited participants needed little to no support in downloading the App, and therefore there is no evidence that the participants who needed extra help at the time of recruitment responded more favorably in terms of adherence to filling out the measures. Participants in the intervention condition were told that they would be receiving additional text messages during weeks 2 and 3 of the study. At the end of the study participants completed the exit questionnaire online.
Eighty-two participants enrolled, and 14 (5%) dropped out before the study ended.
Social Support Intervention
A series of 14 supportive text messages were crafted based on the following dimensions of social support from an established taxonomy of social support:21 emotional support (e.g., ‘You are a strong and courageous person. You have made it through many struggles and will not give up.’); network support (e.g., ‘Make plans to spend time with a friend or family member today by phone or video chat.’); esteem support (e.g., ‘Do not feel guilt about the changes in your life caused by your pain. Your loved ones and doctors support you.’); and informational support (e.g., ‘Spend time exploring an online health community for people with chronic pain. You might find useful information about managing your pain.’) (see Appendix for full list of messages). All dimensions from Cutrona and Suhr’s21 taxonomy were represented except for tangible support (e.g., providing loans, helping with tasks, etc.), which could not be represented via text message.
Participants in the intervention condition received each supportive message twice per day during weeks 2 and 3 resulting in 28 total messages received. Participants were sent each of the 14 messages in random order until they had received all 14, at which time messages cycled through a second time, at random, until all 14 messages were received again. Each day, participants received the first message at a random time between 9 am and noon and the second message at a random time between 3 and 6 pm. All participants received all messages, though not in the same order.
Trial Design
Participants were randomly assigned to one of two study conditions. Control participants received their customary treatments along with twice-daily reminder messages to complete pain, pain interference and affect ratings using the App throughout the 4-week study. Intervention participants continued their standard treatments, received the same twice-daily reminder messages as control participants did, and additionally received automated, twice-daily supportive SMS text messages that they were told came from a clinician during weeks 2 and 3.
Patient-Level Background Data
Demographic data provided via an online questionnaire at baseline included participant age, gender, race/ethnicity, education, and marital status. In addition, participants provided data on whether they experienced any of 14 chronic health conditions. Participants’ medical records were reviewed by trained research assistants to identify the primary type of pain they were experiencing and the number and type of pain medications (e.g., opioid, NSAID, anticonvulsant) that they were taking at the time of enrollment.
Primary Outcome Measures
During the 4-week study, all participants received SMS reminders daily at noon and 6pm prompting them to complete various study measures. Participants were instructed to complete the measurements based on how they were feeling at that moment. When they opened the App, participants were prompted to complete the following pain and pain interference measures where responses varied from 0 to 10: 1) ‘Indicate your current pain level’, 2) extent to which pain is interfering with your general activities, 3) extent to which pain is interfering in your relations with others, and 4) extent to which pain is interfering with your sleep (see Figure 2, for example). Participants completed these ratings on a concentric scale, rather than a line, so that each number on the scale was large enough for participants to see and touch without difficulty. Lastly, participants rated their affect choosing a photo that best captured how they felt at that moment (see Figure 3) using a validated tool appropriate for use with iPhone technology.23 This tool calculates a positive affect rating between 1 and 16 based on participants’ choice of photo (where 1 represents the lowest level of positive affect and 16 represents the highest level).23
Figure 2.
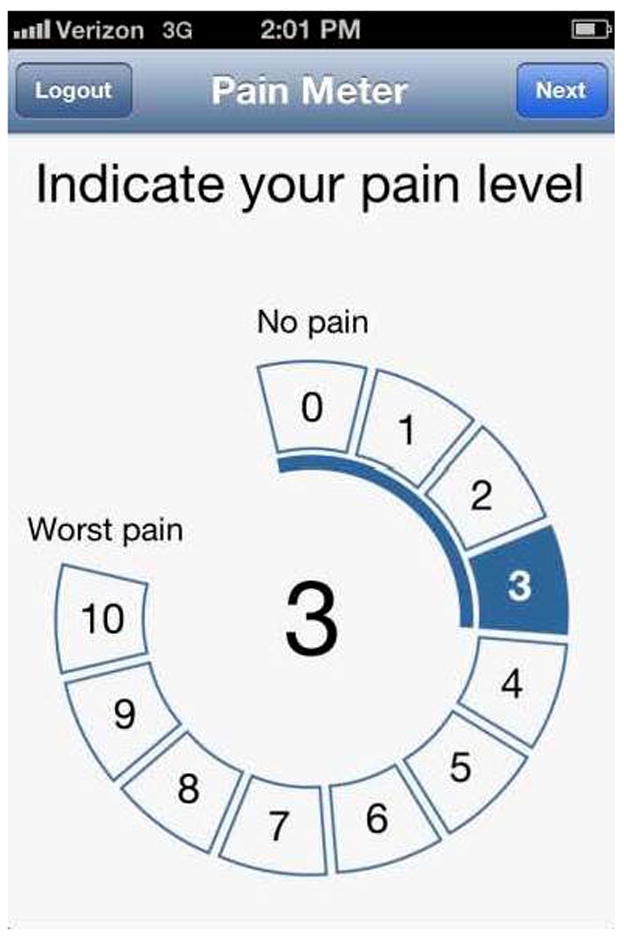
Tracking App screen shots for pain measures.
Figure 3.
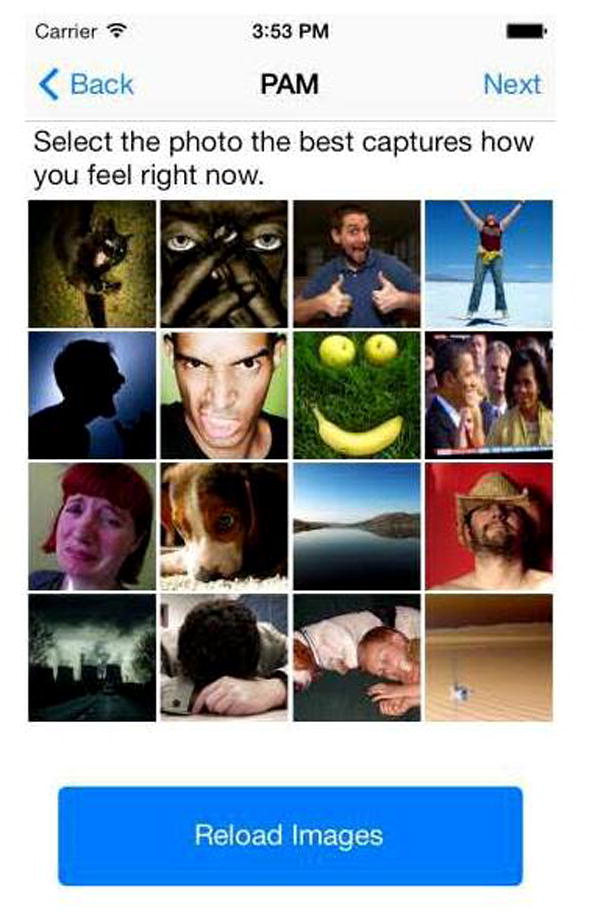
Tracking App screen shots for affect measure.
Participants also completed all items from two established scales assessing their perceptions of social support24 and connectedness25 (5-point likert scales, 1 = strongly disagree, 5 = strongly agree) at baseline and in an exit questionnaire at the end of the 4-week study to determine whether the intervention led to changes in perceived social support and connectedness over time. Social connectedness and support scales (baseline ratings) demonstrated satisfactory internal consistency, with coefficient α’s ranging from .94 to .96.
Statistical Analysis
Pain and other assessments were obtained on each patient twice per day for 28 days. The first week of observations provided a baseline before the intervention. The intervention was implemented during weeks 2 and 3. Week 4 provided information on post-intervention effects.
The core statistical model includes treatment (control versus intervention) and weeks (week 1 versus weeks 2 and 3 versus week 4) as fixed classification factors; the interaction of these 2 factors; time of the observation (the 2 × 28 observations) as levels of a random classification factor; and individuals as level of a random classification factor. An unstructured error assumption was specified, and denominator degrees of freedom computed by the first-order Kenward-Rogers method.26 There were a number of possible specifications involving the multiple observations on individuals, and the core model was chosen based on fit and coherence after examination of a number of alternatives. These alternatives included various autoregressive structures on time of observation, time as a fixed factor, and models excluding the factor for weeks.
A number of other classification factors (sex, race/ethnicity (Hispanic, non-Hispanic white, African American and other), and marital status of the patient (married/partnered versus not)) and covariates (baseline social support scale, baseline social connectedness scale, education, age, and number of chronic medical conditions) were also examined for inclusion in the model. A complete examination of interactions among these additional variables and the classification factors for treatment and weeks was carried out (testing homogeneity of regressions of outcome variables on the covariates for levels of treatment by weeks).
Two primary models were used for presentation of the results. The first was a model with the core variables as described above (treatment, weeks, the interaction of these 2 factors, and the random specification derived as best representing the data) and a priori inclusion of all additional classification factors and covariates. The only interaction of consequence involves marital status, and the second model for presentation of results adds the interaction of treatment, weeks, and marital status to the first model.
The intervention was evaluated for the social support and connectedness scales in a model with treatment, time of assessment (baseline versus 4 weeks), the interaction of these classification factors, and individuals as levels of a random classification factor.
Analysis of all models was carried out in general linear mixed models. The key test for evaluation of the intervention is the test of the interaction of treatment by week. We partitioned from this interaction key pre-specified contrasts of interest—specifically, the test of treatment by week 1 versus weeks 2 and 3 and week 1 versus week 4 (2 × 2 contrasts). The interaction of other variables such as marital status of patient with treatment and week, and the analogous partitionings of week, allow for the examination of whether treatment effects were conditioned by these other variables. The tables show means and probabilities for these contrasts.
RESULTS
Sample Characteristics
The final sample consisted of 68 participants, 51 of whom were women (75%). The mean age of participants was 48.55 ± 11.58. Most participants were married or had a partner (n = 38, 56%). The majority of participants had an undergraduate education or greater (n = 32, 47%), followed by participants with some college (n = 22, 32%), and those with a high school education or less (n = 14, 21%). Most participants were white, non-Hispanic (n = 43, 63%) followed by Hispanic (n = 13, 19%), black, non-Hispanic (n = 9, 13%), and people of other races and ethnicities, (including one mixed race participant) (n = 3, 4%). The mean number of chronic conditions in the sample was 2.28 ± 1.98. Most participants’ primary pain disorder was of musculoskeletal origin (n = 39, 48.1), 6 (7.4%) were diagnosed with neuropathic pain, 1 (1.2%) had headaches, 4 (4.9%) had chronic abdominal pain, while the remaining 31 (38.3%) had other types of chronic pain (Table 1).
Table 1.
Sociodemographic Characteristics by Intervention Condition (N = 68)
| Experimental Condition
| |||
|---|---|---|---|
| p-value | |||
| Characteristics | Control (n = 35) | Intervention (n = 33) | |
| Age (M ± SD) (y) | 48.50 ± 12.69 | 48.59 ± 10.47 | .343 |
| Sex (N [%]) | .484 | ||
| Female | 25 (71.4) | 26 (78.8) | |
| Male | 10 (28.6) | 7 (21.2) | |
| Marital status | .458 | ||
| Married/Partner | 17 (48.6) | 21 (63.6) | |
| Widowed/divorced/Separated | 9 (25.7) | 6 (18.2) | |
| Never married | 9 (25.7) | 6 (18.2) | |
| Education level (N [%]) | .167 | ||
| High School or Less | 7 (20.0) | 7 (21.2) | |
| Some College or Technical School | 8 (22.9) | 14 (42.4) | |
| Undergraduate Degree or Higher | 20 (57.1) | 12 (36.4) | |
| Race/Ethnicity | .586 | ||
| White, Non-Hispanic | 24 (68.6) | 19 (57.6) | |
| Black, Non-Hispanic | 3 (8.6) | 6 (18.2) | |
| Other, Non-Hispanic | 1 (2.9) | 2 (6.1) | |
| Hispanic | 7 (20.0) | 6 (18.2) | |
| Number of chronic health conditions (M ± SD) | 2.09 (2.02) | 2.48 (1.95) | .612 |
| Primary pain disorder | .232 | ||
| Musculoskeletal pain | 22 (62.9) | 17 (51.5) | |
| Neuropathic pain | 3 (8.6) | 3 (9.1) | |
| Headache | 1 (2.9) | 0 (0) | |
| Chronic abdominal pain | 0 (0) | 4 (12.1) | |
| Other | 9 (25.7) | 9 (27.3) | |
| Number of pain medications (M ± SD) | 2.39 (1.41) | 3.07 (1.25) | .052 |
| Taking an opiod | 23 (65.7) | 22 (66.7) | .560 |
| Taking an NSAID | 9 (25.7) | 10 (30.3) | .537 |
| Taking anticonvulsant | 10 (28.6) | 17 (51.5) | .053 |
Numbers in parentheses are the percentages of participants within each group who have that characteristic.
Feasibility Assessment of Pain Tracking App
Participants completed 44.34 ± 9.13 (79%) of the measurements requested (total possible = 56) via the twice-daily reminder SMS messages. Furthermore, 68% of participants completed 75% or more of the total measurements requested. The number of measurements completed did not differ between the intervention (43.33 ± 9.52) and control conditions (45.29 ± 8.79), t(66) = .879, P = .382.
Intervention Effects on Pain, Pain Interference and Affect
Table 2 shows for the first model (a priori specification of additional socio-demographic and health variables) means for treatment and weeks and mean differences and tests of mean differences. In the tables and results described below ‘visual pain’ refers to pain ratings, ‘general pain’ refers to pain interference with general activity, ‘relation pain’ refers to pain interference with relations with others, ‘sleep pain’ refers to pain interference with sleep, and ‘positive affect’ refers to perceived level of positive affect. There were significant treatment effects at weeks 2 and 3 for the social support intervention for visual, general, relation and sleep pain and positive affect, as seen in the interaction of treatment and time (week 1 versus weeks 2 and 3). At 4 weeks, there were significant effects for general and relation pain.
Table 2.
Treatment Differences by Time of Assessment
| Outcome | Week 1 | Weeks 2 and 3 | Week 4 | Week 2, 3 – Week 1 | Week 4 – Week 1 |
|---|---|---|---|---|---|
|
| |||||
| Mean (p-value) | Mean (p-value) | ||||
| Visual Pain | |||||
| Control | 5.85 | 5.92 | 5.92 | .07 (.633) | .07 (.767) |
| Treatment | 5.89 | 5.56 | 5.48 | −.33 (.027) | −.41 (.110) |
| Treatment - Control | .04 (.759) | −.36 (.121) | −,44 (.257) | (.055) | (.171) |
| General Pain Interference | |||||
| Control | 5.29 | 5.44 | 5.72 | .15 (.349) | .43 (.096) |
| Treatment | 5.30 | 4.71 | 4.61 | −.59 (.000) | −.69 (.013) |
| Treatment - Control | .01 (.972) | −.73 (.003) | −1.11 (.008) | (.001) | (.003) |
| Interference with Relations | |||||
| Control | 4.45 | 4.57 | 4.86 | .12 (.449) | .41 (.125) |
| Treatment | 4.58 | 4.04 | 3.98 | −.55 (.001) | −.60 (.038) |
| Treatment - Control | .13 (.366) | −.53 (.040) | −.88 (.045) | (.004) | (.010) |
| Sleep Pain | |||||
| Control | 5.36 | 5.42 | 5.38 | .06 (.764) | .02 (.945) |
| Treatment | 6.06 | 5.52 | 5.59 | −.54 (.004) | −.47 (.154) |
| Treatment - Control | .70 (<.0001) | .10 (.732) | .21 (.673) | (.024) | (.277) |
| Positive Affect | |||||
| Control | 6.31 | 6.10 | 6.58 | −.22 (.444) | .27 (.541) |
| Treatment | 6.79 | 7.68 | 7.38 | .88 (.002) | .59 (.191) |
| Treatment - Control | .48 (.103) | 1.58 (<.0001) | .80 (.221) | (.007) | (.603) |
The model includes treatment (control versus intervention) and weeks (week 1 versus weeks 2 and 3 versus week 4) as fixed classification factors; the interaction of these 2 factors; time of the observation (the 2 × 28 observations) as levels of a random classification factor; individuals as level of a random classification factor; and the baseline social support scale, baseline connectedness scale, education, age, and number of chronic medical conditions as covariates and gender, living with spouse/partner versus living alone, and race/ethnicity (Hispanic versus non-Hispanic white versus African American and other) as fixed classification factors. Table entries are means or mean differences, with probabilities in parentheses. The key tests of treatment effects are tests partitioned from the treatment-by-weeks interaction. These are given in the lines for Treatment – Control under the 2 rightmost columns.
Patients receiving social support messages reported lower visual, general, relation and sleep pain and higher levels of positive affect during the intervention period (weeks 2 and 3) compared to baseline ratings in Week 1 (P = .027; .0001; .001; .004; .002). Ratings of visual, general, relation and sleep pain, and positive affect for patients in the control condition did not differ between the intervention period (weeks 2 and 3) and baseline (week 1) (P = .633; .349; .449; .764; .444).
Was the mean difference in pain ratings between the baseline and intervention period also different when comparing the intervention and control conditions? There was a difference in visual pain between the intervention and control conditions over time (Weeks 2 & 3 – Week 1), but this change did not reach significance (P = .055). Significant differences emerged for all pain interference variables such that perceptions of pain interference were lower in the intervention condition compared to control over time (Weeks 2 & 3 – Week 1), (general P = .001; relation P = .004; sleep P = .024). Positive affect ratings were higher for participants in the intervention condition compared to control over time (P = .007).
Did the pain attenuating effects of the social support intervention extend beyond the 2-week intervention? While there was no difference in visual pain between intervention and control conditions over time when comparing week 1 to week 4 (P = .171), a difference emerged for pain interference with general and relation pain between intervention and control over time when comparing week 1 to week 4 (P = .003; P = .010), suggesting that the intervention effects extended beyond the intervention period for pain interference with general and relation pain.
The second model, which includes the interaction of marital status (married/partnered versus single), shows that the treatment effects are essentially the result of differences for the married/partnered group. Table 3 gives the means and mean differences for this group. Treatment differences are highly significant for all outcomes at 2 and 3 weeks as well as for visual pain, general and relation pain at 4 weeks (with sleep pain and positive affect showing trends at 4 weeks). Similar effects did not hold for participants who were not married or partnered. These effects were likely due to higher baseline levels of perceived social support among participants who were married/partnered 4.48 ± .58, compared to those who were not 3.75 ± 1.29, t(66) = 3.11, P = .003.
Table 3.
Treatment Differences by Time of Assessment for Patients who are Married or have Partners (N = 38)
| Outcome | Week 1 | Weeks 2 and 3 | Week 4 | Week 2, 3 – Week 1 | Week 4 – Week 1 |
|---|---|---|---|---|---|
|
| |||||
| Mean (p-value) | Mean (p-value) | ||||
| Visual Pain | |||||
| Control | 6.21 | 6.40 | 7.02 | .19 (.353) | .81 (.017) |
| Treatment | 6.36 | 5.76 | 5.73 | −.60 (.001) | −.63 (.050) |
| Treatment - Control | .14 (.419) | −.64 (.039) | −1.29 (.013) | (.004) | (.002) |
| General Pain Interference | |||||
| Control | 5.51 | 5.82 | 6.48 | .31 (.145) | .97 (.007) |
| Treatment | 4.92 | 4.03 | 3.91 | −.89 (<.0001) | −1.01 (.003) |
| Treatment - Control | −.59 (.002) | −1.79 (<.0001) | −2.57 (<.0001) | (<.0001) | (<.0001) |
| Interference with Relations | |||||
| Control | 4.75 | 4.83 | 5.44 | .08 (.701) | .69 (.057) |
| Treatment | 3.94 | 3.05 | 2.99 | −.89 (<.0001) | −.95 (.008) |
| Treatment - Control | −.81 (<.0001) | −1.78 (<.0001) | −2.45 (<.0001) | (.001) | (.001) |
| Sleep Pain | |||||
| Control | 5.08 | 5.26 | 5.40 | .18 (.472) | .32 (.455) |
| Treatment | 5.64 | 4.77 | 4.99 | −.87 (.0003) | −.65 (.109) |
| Treatment - Control | .56 (.011) | −.49 (.215) | −.41 (.534) | (.002) | (.100) |
| Positive Affect | |||||
| Control | 5.86 | 5.27 | 5.34 | −.59 (.132) | |
| Treatment | 6.34 | 7.51 | 7.14 | 1.17 (.001) | .80 (.157) |
| Treatment - Control | .48 (.214) | 2.24 (<.0001) | 1.80 (.039) | (.001) | (.112) |
The model includes marital status (living with spouse/partner versus living alone), treatment (control versus intervention), and weeks (week 1 versus weeks 2 and 3 versus week 4) as fixed classification factors; the interaction of these 3 factors; time of the observation (the 2 × 28 observations) as levels of a random classification factor; individuals as level of a random classification factor; and the baseline social support scale, baseline connectedness scale, education, age, and number of chronic medical conditions as covariates and gender, and race/ethnicity (Hispanic versus non-Hispanic white versus African American and other) as fixed classification factors. The table shows results for contrasts on the married/partnered patients. Table entries are means or mean differences, with probabilities in parentheses. The key tests of treatment effects are tests partitioned from the treatment-by-weeks interaction. These are given in the lines for Treatment – Control under the 2 rightmost columns.
A final important question that this research explored was whether the intervention changed perceptions of social support21 and connectedness25 for participants in the intervention condition compared to control. There were no treatment effects for either social support or connectedness. P-values for the test of treatment by time (baseline versus 4 weeks) interaction are .80 and .78, respectively.
DISCUSSION
The pilot randomized controlled trial expands on prior research by testing a novel approach to social support interventions (e.g.,10,11) for patients with chronic non-cancer pain. Our findings provide preliminary evidence that a simple, social support intervention delivered daily via SMS text message can reduce perceived pain and pain interference with general activity, relations with others and sleep among patients with chronic non-cancer pain. Perceptions of pain and pain interference decreased between baseline and the intervention period for participants in the intervention condition. Positive treatment effects are shown by a significant treatment-by-time interaction, such that perceived pain interference with general activity decreased during the intervention period (compared to baseline) for participants in the treatment condition but not for participants in the control condition.
Positive affect was also higher during the intervention than baseline for participants in the intervention condition. These effects also emerged when comparing the intervention and control conditions by time, such that positive affect ratings increased during the intervention period (compared to baseline) for participants in the treatment (but not control) condition. These findings suggest that in addition to pain attenuation effects, the text messages also helped intervention patients feel more emotionally positive.
Perceptions of social support and connectedness did not change between baseline and post-test, suggesting that the intervention did not change the degree to which chronic pain patients felt supported by and connected to family and friends over the 4-week period. It is also possible that the effects of the intervention decayed by the time participants completed the post-test questionnaire due to the 1-week washout period between the end of the intervention and completion of the post-test questionnaire.
An interaction of treatment, time, and marital status indicates that the effects of the intervention held only for married/partnered participants (not those who were never married, widowed, separated or divorced). This finding is consistent with research showing that married people have easier access to social support and larger social support networks.27 In addition, married/partnered participants perceived significantly higher levels of social support24 at baseline than participants who were not in a relationship. The sample size for this pilot study was small, and further examination of this interaction in a larger study would be valuable.
While this research demonstrates that a simple SMS-based social support intervention can attenuate pain and pain interference, further exploration of which dimensions of social support are most effective will be important to understand the mechanisms underlying these effects. In this study, we included messages conveying network (i.e., interpersonal support emphasizing access, presence, and companionship of other individuals), emotional (i.e., relational support, affection, sympathy, and understanding), esteem (i.e., compliments, validation), and informational support (i.e., suggestions, advice, referral, and situation appraisal),21 but could not isolate individual effects of these types of support.
It is also important to understand how the quality of social support influences the pain experience. Though social support has undoubtedly positive effects for health outcomes,2–6 social relationships can contribute to increased stress and strain28,29 that ultimately lead to negative health outcomes.30,31 Given this caveat, it will be important for future research to explore how the quality of social support influences the positive effects of social support interventions for chronic pain.
This pilot research suggests that people can experience pain-attenuating effects of social support delivered via a mobile SMS-based intervention, providing important contributions to the pain and social support literatures.7,8,11 To the best of our knowledge, this is the first study to demonstrate evidence of the effects of a minimal, SMS-based social support intervention for chronic pain. This is important because this type of intervention provides chronic pain patients with access to a non-pharmacological aid that does not require physical co-presence of social support. This intervention could easily be used with simple instructions and little to no involvement from a clinician, provided that the patient owns a smartphone. Interventions with this level of flexibility are especially useful for patients who would not otherwise have access to social support for pain management. While our pilot findings will need to be validated in a subsequent, larger clinical trial, they suggest the importance of incorporating social support into pain management programs.
This study also demonstrates the feasibility of using a mobile App to track daily pain and positive affect. More than half of participants (68%) completed at least 75% of the twice-daily measurements requested, and there was no difference in compliance between the intervention and control conditions. This suggests that the mobile App developed for the study presents a simple and viable means of tracking pain and affect for studies testing the effects of interventions among chronic pain patients. With the ubiquity of mobile pain Apps,15 the implementation of social support SMS messages to existing pain management Apps could be beneficial for this patient population.
Finally, it is worth noting that intervention patients were taking a substantially greater number of pain medications, including anticonvulsant medications relative to control patients. This may reflect underlying between group differences in pain etiology. Future research should examine whether social support interventions have differential effects based on pain etiology (i.e., nociceptive, neuropathic, and mixed).
Limitations
This study has several limitations that warrant consideration. First, the small sample size along with the sample having been drawn from the same geographic area and a largely female population limits the generalizability of our findings. Second, because this was a pilot study with a brief and minimal intervention (2 supportive SMS texts a day for 2 weeks) the differences observed in this study were small. Future studies should explore the implementation and effects of SMS text message-based social support interventions in a larger sample drawn from various clinics and geographic locations, with different clinicians, different healthcare access, and different familiarity with mobile technology and Apps.
In addition, this research was unable to differentiate the effects of different types of supportive messages on pain and affect. Future research should be conducted to determine which types of supportive messages are more effective for reducing pain and improving affect. For example, do messages that encourage patients to access network support (i.e., interpersonal support) have more influence on pain than those providing esteem support (i.e., compliments, validation)?
Several studies have suggested the need for research on the impact, benefits, and disadvantages of pain-related mobile Apps on patients.15 This study provides information on the effect of social support SMS text messages on pain attenuation. However, there is a substantial and increasing number of health-focused Apps available for patients. Therefore, participants in this study could have simultaneously used other pain management mobile Apps that could have contributed to pain reduction during the study period. This lack of regulation on smartphone App usage as well as lack of evidence of sole App effectiveness is worth noting. Future studies could ask participants what other mobile health Apps they are currently using simultaneously with the App provided by the study.
This study’s findings are informative about the effect of social support SMS-text messaging and were captured using quantitative data. However, there may be several underlying factors that are not captured in this study and could be explored through personal narratives from participants. Future studies could employ qualitative methods to explore the effects of social support on pain attenuation. For example, in-depth interviews could shed light on different types and levels of social support provided by social networks of patients, including family and friends. There could be different effects of social support received from different individuals from the patient’s social network (i.e., spouse, friend). These unexplored factors could help identify a more nuanced approach to providing social support via mobile technology by helping to tailor different types and amounts of social support to different patients.
Additionally, while this research shows that supportive SMS messages had a salutary effect on pain and positive affect, this study did not address the specificity of the mechanisms underlying these associations.15 Social support could attenuate pain because of the alteration in appraisals, decreasing negative affect and increasing positive affect, or altering pain expectations.8,32,33 Future studies could evaluate the mechanisms and the interplay of different mechanisms that influence emotional, mental, and physiological factors associated with pain and affect.
In addition to the aforementioned factors, there is opportunity for future studies to investigate the social context of pain, specifically existing interpersonal relationships that could shape pain-related preferences for social support and pain outcomes. The support provided in this study was from a clinician and future research will be important to determining whether and how pain outcomes differ when support is provided by friends or family members.
Conclusion
The use of mobile Apps in pain management has become increasingly popular. This pilot study examined the effectiveness of a SMS text message-based social support intervention via mobile technology for pain attenuation and improving positive affect in a sample of patients with chronic non-cancer pain. Findings show that this novel social support intervention reduced perceptions of pain and pain interference and improved positive affect among patients randomized to the intervention condition. These findings were driven by married/partnered patients, suggesting that those who already have access to social support were better able to derive benefit from supportive messages. These findings provide significant implications and insight into a nuanced approach to reducing the daily experience of pain via mobile technology, especially because of its accessibility and reach.
Acknowledgments
This research was supported by grants from the National Institute on Aging (P30AG022845), Agency for Healthcare Quality and Research (R01HS020648), and the National Cancer Institute (R25CA113710).
APPENDIX
Supportive Text Messages
Informational Support
Why not do something new to distract you from your pain today? Do some research on new hobbies or activities that you’d like to pursue.
Spend time exploring an online health community for people with chronic pain. You might find useful information about managing your pain.
Think about new books or magazines that you’d like to read today. Pick one up at a bookstore or download one to your phone or tablet and start reading!
Esteem Support
There is nothing wrong with feeling frustrated about your pain. It is normal to feel this way.
You are in control of your health and happiness. Don’t let the small setbacks you experience discourage you.
Do not feel guilt about the changes in your life caused by your pain. Your loved ones and doctors support you.
It’s ok to feel anger about your pain. You are experiencing a difficult time in your life.
You are a special person who has touched the lives of many people in important ways. Remind yourself of this today.
Emotional Support
You are a strong and courageous person. You have made it through so many struggles and will not give up.
Remember that there are many people who love and support you. Remind yourself of this whenever you feel discouraged today.
Don’t forget how important physical contact can be to your well-being. Try to shake someone’s hand, spend time with a pet, or give a hug to a loved one.
Network Support
Why don’t you get in touch with a friend today by sending them an email (or text message)?
Make plans to spend time with a friend or family member today.
Spend some time researching social support groups for chronic pain that meet in your area. Plan to attend a session next week if you feel comfortable.
Write a letter to a friend today and drop it in the mail. They will be glad to hear from you and everyone loves getting mail.
Pick up the phone today to call a loved one. They will be very happy to hear from you.
Think of a friend you’d like to reconnect with. Send them an email or give them a call today.
Contributor Information
Jamie Guillory, Center for Tobacco Control Research & Education, University of California, San Francisco.
Pamara Chang, Department of Communication, Cornell University.
Charles R. Henderson, Jr., Department of Human Development, Cornell University.
Rouzi Shengelia, Department of Medicine, Weill Cornell Medical College.
Sonam Lama, Department of Medicine, Weill Cornell Medical College.
Marcus Warmington, Department of Medicine, Weill Cornell Medical College.
Maryam Jowza, Pain Medicine Center & Weill Cornell Anesthesiology Associates, Weill Cornell Medical College.
Geri Gay, Departments of Communication & Information Science, Cornell University.
References
- 1.Sarason IG, Sarason BR, Shearin EN, Pierce GR. A brief measure of social support: Practical and theoretical implications. Journal of social and personal relationships. 1987;4(4):497–510. [Google Scholar]
- 2.Wallston BS, Alagna SW, DeVellis BM, DeVellis RF. Social support and physical health. Health psychology. 1983;2(4):367–391. [Google Scholar]
- 3.Berkman LF. Social network analysis and coronary heart disease. Adv Cardiol. 1982;29:37–49. doi: 10.1159/000406195. [DOI] [PubMed] [Google Scholar]
- 4.de Araujo G. Life change, coping ability and chronic intrinsic asthma. J Psychosom Res. 1973;17(5):359–363. doi: 10.1016/0022-3999(73)90045-7. [DOI] [PubMed] [Google Scholar]
- 5.Kulik JA, Mahler HI. Emotional support as a moderator of adjustment and compliance after coronary artery bypass surgery: A longitudinal study. J Behav Med. 1993;16(1):45–63. doi: 10.1007/BF00844754. [DOI] [PubMed] [Google Scholar]
- 6.Holtzman S, Newth S, Delongis A. The role of social support in coping with daily pain among patients with rheumatoid arthritis. J Health Psychol. 2004;9(5):677–695. doi: 10.1177/1359105304045381. [DOI] [PubMed] [Google Scholar]
- 7.Brown JL, Sheffield D, Leary MR, Robinson ME. Social support and experimental pain. Psychosom Med. 2003;65(2):276–283. doi: 10.1097/01.psy.0000030388.62434.46. [DOI] [PubMed] [Google Scholar]
- 8.Master SL, Eisenberger NI, Taylor SE, Naliboff BD, Shirinyan D, Lieberman MD. A picture’s worth: Partner photographs reduce experimentally induced pain. Psychol Sci. 2009;20(11):1316–1318. doi: 10.1111/j.1467-9280.2009.02444.x. [DOI] [PubMed] [Google Scholar]
- 9.Hogan BE, Linden W, Najarian B. Social support interventions: Do they work? Clin Psychol Rev. 2002;22(3):381–440. doi: 10.1016/s0272-7358(01)00102-7. [DOI] [PubMed] [Google Scholar]
- 10.Radojevic V, Nicassio PM, Weisman MH. Behavioral intervention with and without family support for rheumatoid arthritis. Behavior therapy. 1993;23(1):13–30. [Google Scholar]
- 11.Schmitt FE, Wooldridge PJ. Psychological preparation of surgical patients. Nurs Res. 1973;22(2):108–115. [PubMed] [Google Scholar]
- 12.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357. [PubMed] [Google Scholar]
- 13.Lazarus R, Folkman S. Cognitive appraisal processes. In: Lazarus RFS, editor. Stress, appraisal, and coping. New York, NY: Springer; 1984. pp. 22–52. [Google Scholar]
- 14.Langer EJ, Janis IL, Wolfer JA. Reduction of psychological stress in surgical patients. J Exp Soc Psychol. 1975;11(2):155–165. [Google Scholar]
- 15.Rosser BA, Eccleston C. Smartphone applications for pain management. J Telemed Telecare. 2011;17(6):308–312. doi: 10.1258/jtt.2011.101102. [DOI] [PubMed] [Google Scholar]
- 16.Abroms LC, Boal AL, Simmens SJ, Mendel JA, Windsor RA. A randomized trial of Text2Quit: A text messaging program for smoking cessation. Am J Prev Med. 2014;47(3):242–250. doi: 10.1016/j.amepre.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrick K, Raab F, Adams MA, et al. A text message-based intervention for weight loss: Randomized controlled trial. J Med Internet Res. 2009;11(1):e1. doi: 10.2196/jmir.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis MA, Uhrig JD, Bann CM, et al. Tailored text messaging intervention for HIV adherence: A proof-of-concept study. Health Psychology. 2013;32(3):248–253. doi: 10.1037/a0028109. [DOI] [PubMed] [Google Scholar]
- 19.Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: Systematic review. J Med Internet Res. 2009;11(2):e16. doi: 10.2196/jmir.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36(2):165–173. doi: 10.1016/j.amepre.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Cutrona CE, Suhr JA. Controllability of stressful events and satisfaction with spouse support behaviors. Communication Research. 1992;19(2):154–174. [Google Scholar]
- 22.Stone AA, Shiffman S. Capturing momentary, self-report data: A proposal for reporting guidelines. Annals of Behavioral Medicine. 2002;24(3):236–243. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- 23.Pollak JP, Adams P, Gay G. PAM: A photographic affect meter for frequent, in situ measurement of affect. 2011. pp. 725–734. [Google Scholar]
- 24.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. J Pers Assess. 1988;52(1):30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 25.Lee RM, Robbins SB. Measuring belongingness: The social connectedness and the social assurance scales. Journal of Counseling Psychology. 1995;42(2):232–241. [Google Scholar]
- 26.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997:983–997. [PubMed] [Google Scholar]
- 27.House JS, Umberson D, Landis KR. Structures and processes of social support. Annual review of sociology. 1988:293–318. [Google Scholar]
- 28.Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychol Bull. 2001;127(4):472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 29.Walen HR, Lachman ME. Social support and strain from partner, family, and friends: Costs and benefits for men and women in adulthood. Journal of Social and Personal Relationships. 2000;17(1):5–30. [Google Scholar]
- 30.Mirowsky J, Ross CE. Social causes of psychological distress. Transaction Publishers; 2003. [Google Scholar]
- 31.Nagasawa M, Smith MC, Barnes JH, Fincham JE. Meta-analysis of correlates of diabetes patients’ compliance with prescribed medications. Diabetes Educ. 1990;16(3):192–200. doi: 10.1177/014572179001600309. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S. Psychosocial models of the role of social support in the etiology of physical disease. Health psychology. 1988;7(3):269–297. doi: 10.1037//0278-6133.7.3.269. [DOI] [PubMed] [Google Scholar]
- 33.Epley SW. Reduction of the behavioral effects of aversive stimulation by the presence of companions. Psychol Bull. 1974;81(5):271–283. doi: 10.1037/h0036389. [DOI] [PubMed] [Google Scholar]