To the Editor:
Chronic lymphocytic leukemia (CLL) is an incurable disease identified most commonly in elderly patients. Interphase cytogenetics, IGHV status and ZAP70 protein expression/methylation have been used to risk-stratify patients relative to likelihood of disease progression or treatment assignment when CLL becomes symptomatic Reviewed in 1. microRNAs are short non-coding RNAs that post-transcriptionally regulate mRNAs for degradation or translational block. Recently, microRNA (miR) expression profiling in CLL has been used to identify miR signatures that predict disease progression and explain disease biology. miR expression profiling in CLL has been able to identify miRs that correlate with shorter time of diagnosis to treatment, such as miR-155 (high), miR-29c (low), and miR-181a (high)2. In patients treated with front-line therapy fludarabine, miR profiling identified variable expression of miR-148a, miR-21 and miR-222 at pretreatment could be predicative of response3. One of the most studied miRs, miR-155 and its host gene BIC, have been previously indicated to be overexpressed in CLL and has been found to be leukemogeneic when overexpressed under a B cell specific promoter in mice4,5. To further elucidate the clinical impact of baseline miR-155 expression, we examined clinical outcome of previously untreated patients treated with chemoimmunotherapy. A previous study using the CLL MEC1 cell line demonstrated targeting miR-155 lead to inhibition of proliferation6. miR-155 is critical for B cell development and can be enhanced by antigen receptor stimulation7,8. miR-155 has been found to be up-regulated in B cell receptor (BCR) stimulated B cells. Also increased miR-155 expression has been linked to a BCR activated phenotype characterized by un-mutated IGVH and high ZAP70 expression2,7,8,9. Given the relationship of between active BCR signaling and miR-155 expression, we examined the influence of the BCR targeted therapy, Bruton's tyrosine kinase inhibitor, ibrutinib, on expression of this onco-miR in vivo among patients at different time points during their treatment.
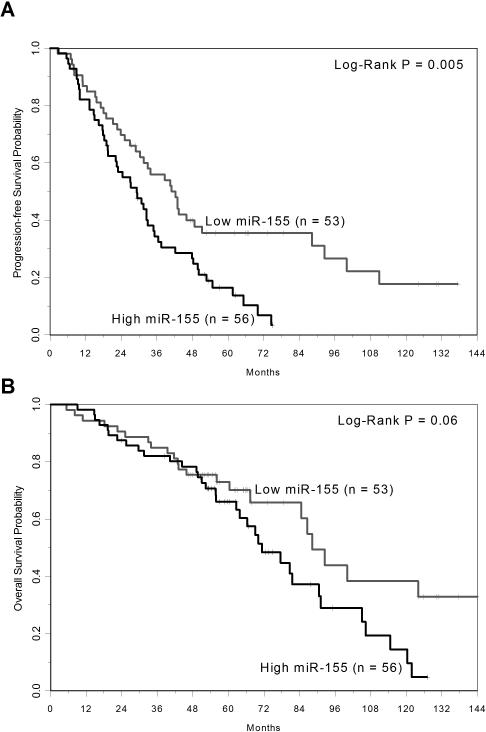
miR-155 expression in 109 patients who had been previously treated with fludarabine and rituximab (CALGB 9712) or fludarabine and rituximab followed by alemtuzumab (CALGB 10101) was measured10,11. Baseline samples were procured from these patients and miR-155 analysis was done by Nanostring Technologies’ nCounter platform. miR-155 levels were background corrected, normalized by quantile normalization, and the log(2) expression values for each patient were calculated. Nanostring analysis showed the expression of miR-155 was above the background threshold in all patients. Patients were dichotomized as high (n=53) and low expressers (n=56) using the median value of miR-155 expression (median intensity = 1154; range: 110-3265). The expression of miR-155 was not significantly associated with the majority of baseline demographic, clinical and cytogenetic characteristics, including age, Rai stage and high-risk cytogenetics del(17p)/del(11q) (all p>0.15). However, high miR-155 expression was significantly associated with IGHV un-mutated disease (p=0.03) and ZAP70 methylation <20% (p<0.001). Among the high miR-155 expressers, 81% had IGHV un-mutated disease and 94% had low ZAP70 methylation, compared to low miR-155 expressers with 58% IGHV un-mutated disease and 65% with low ZAP70 methylation. With respect to clinical outcome, patients with high miR-155 expression had a significantly shorter progression free survival (PFS) (p=0.005) and tended toward shorter overall survival (OS) (p=0.06) compared to those with low miR-155 expression (Figures 1A-B). The high miR-155 expressers had an estimated median PFS of 29 months (95% CI: 20-35) and an OS of 71 months (95% CI: 63-91), respectively, versus low expresser with an estimated median PFS 42 months (95% CI: 29-51) and OS of 88 months (95% CI: 67-not reached). In a multivariable model for PFS, high miR-155 remained significantly associated with higher risk of relapse or death (HR=1.82, 95% CI: 1.13-2.94, p=0.01) when adjusting for high-risk cytogenetics and increased WBC. For OS, there was evidence of non-proportional hazards, where the risk of death increased with longer follow-up. In a model adjusting for hemoglobin, the risk of death in the first 4 years on study was not significantly different according to miR-155 expression (HR=0.95, 95% CI: 0.41-2.19, p=0.91), but thereafter, higher miR-155 expression was associated with increased risk of death (HR=3.25, 95% CI: 1.46-7.21, p=0.004).
Figure 1.
A. Kaplan-Meier curves of progression-free survival according to low and high levels of miR-155 expression in relapse/refractory CLL patients prior to treatment with chemoimmunotherapy B. Kaplan-Meier curves of overall survival according to low and high levels of miR-155 expression in relapse/refractory CLL patients prior to treatment with chemoimmunotherapy.
Given the potential oncogenic role of miR-155 and contribution of its over-expression to shortened PFS and OS with chemoimmunotherapy in CLL, we next sought to determine if this could be therapeutically targeted. The BCR activated phenotype is known to be closely associated with patients with poor prognostic factors, such as high ZAP70 expression and un-mutated IGHV status9. Associations between miR-155 expression, ZAP70 expression and IGHV mutational status and BCR activation have been made in recent literature2,12. It has been observed that CLL patients with high miR-155 are generally more responsive to BCR ligation, showing a greater amount of anti-μ induced calcium flux, compared to patients with low miR-15512. The distinct relationship between BCR activation and miR-155 led to the hypothesis that BCR targeted therapies could potentially modulate miR-155 expression. Ibrutinib, an irreversible inhibitor, which binds cysteine 481 of Bruton's tyrosine kinase (BTK) has been previously shown to decrease pro-survival signaling, such as AKT, ERK and NFκB13. BTK has been found to be an integral kinase in the BCR pathway and important to the development and survival of leukemic B cells in the Eμ-TCL1 mouse model and also in in vitro analysis of primary CLL cells14. Ibrutinib has also been shown to be highly effective as a treatment in relapsed or refractory CLL15. It was found that patients with un-mutated IGHV disease, who are likely to have greater dependence on BCR stimulation, were found to clear their lymphocytosis earlier and meet traditional criteria for response15. While multiple BCR targeted genes were shown to be decreased by ibrutinib, the influence on miR-155 expression was not examined.
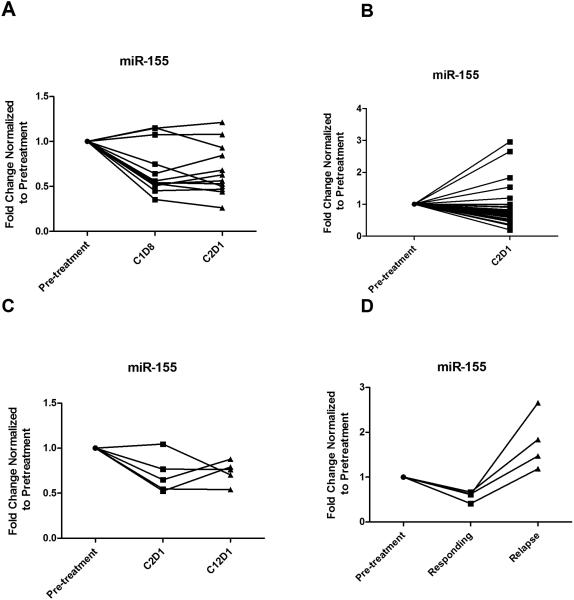
To investigate the close association between B cell receptor activation and miR-155 in CLL, we explored regulation of BCR pathways through ibrutinib inhibition of BTK and its ability to modulate miR-155. We initially examined blood samples from 12 CLL patients prior to receiving ibrutinib (420mg/day) and after 1 week (C1D8) and 29 days (C2D1) with treatment on OSU-1005316. RNA was extracted using the Trizol and purified with the miRVana kit (Ambion). miR-155 expression was assessed by quantitative real time PCR (RT-PCR). Quantitative RTPCR expression was normalized to housekeeping gene RNU44 using the 2-ΔCT method and fold changes found by normalizing each patient's C1D8 and C2D1 values relative to the pre-treatment value are presented. Analysis by t-tests using mixed effects models to account for repeated measures over time showed significantly down-regulated miR-155 expression at C1D8 (p< 0.001) and C2D1 (p=0.001) relative to baseline (Figure 2A). To confirm this, we examined lymphocytes from 34 additional patients treated with ibrutinib on OSU-11133 (NCT01589302), and found that on average, miR-155 expression post-treatment with ibrutinib was 0.71 times the expression prior to therapy (95% CI: 0.59-0.85, p=0.0006; Figure 2B)17. miR-155 expression was down-regulated at C2D1 in 29 (85%) of the patients studied.
Figure 2.
A. miR-155 expression at pre-ibrutinib treatment. miR-155 down-modulation at 8 days (C1D8) (p<0.0001), and 29 days (C2D1) (p=0.001) of treatment with ibrutinib. B. miR-155 expression at pre-treatment and 29 days (C2D1) of treatment with ibrutinib; miR-155 expression was significantly down-regulated at C2D1 (p=0.0006) relative to pre-treatment. n=34 C. miR-155 expression in patients with a partial response with persistent blood lymphocytosis: at pre-treatment, 29 days (C2D1) and 1 year (C12D1) of therapy n=5; miR-155 expression was significantly decreased at C2D1 (p=0.005) and at C12D1 (p=0.013) relative to pre-treatment. D. miR-155 expression in ibrutinib relapse patients: at pre-treatment, time of response, and time of relapse n=4; miR-155 expression was significantly decreased at time of response (p=0.002) but significantly increased at relapse (p=0.002) relative to pre-treatment.
The response pattern observed with ibrutinib includes traditional partial and complete responses, but also patients who have dramatic node disease reduction but persistent blood lymphocytosis that remains asymptomatic for an extended period of time without evidence of active proliferation18. In contrast, patients who relapse after responding to ibrutinib typically have proliferative disease19. Expression of miR-155 was measured in serial samples from patients with a partial response with persistent lymphocytosis at 1 year as well as in patients responding to ibrutinib with subsequent progressions to determine if expression patterns were similar or different. In patients with lymphocytosis, miR-155 expression decreased with 29 days of ibrutinib treatment and remained at this lower expression level at 1 year relative to baseline (p=0.013; Figure 2C). In contrast, patients who relapsed with ibrutinib treatment showed elevated miR-155 expression relative to baseline (p=0.002; Figure 2D), despite an initial decrease in expression with response.
Herein, we provide the evidence indicating that miR-155 is predictive of outcome in CLL patients treated with frontline chemoimmunotherapy, independent of high-risk cytogenetic abnormalities and common clinical characteristics. We also demonstrate that high miR-155 expression correlates with low ZAP70 methylation and confirm the finding of others relative to its association with un-mutated IGHV disease. Our findings further associate BCR regulation with the expression of miR-155 by demonstrating that the irreversible BTK inhibitor ibrutinib treatment can down-regulate miR-155 within the first cycle of therapy. For patients without progression, including those with partial response with peripheral lymphocytosis, the down-modulation is persistent. In contrast, those patients that progress on ibrutinib therapy do not maintain miR-155 down-regulation. The relevance of our findings are significant as they use cellular miR-155 levels to risk stratify CLL patients receiving chemoimmunotherapy and also identify an effective therapeutic, ibrutinib, which targets the critical BCR signaling pathway, which can down modulate a leukemogenic miR.
Acknowledgements
The authors wish to thank the families who provided samples for this work. This work was supported by Specialized Center of Research from the Leukemia and Lymphoma Society, P50- CA140158, P01 CA95426, and R01 CA177292 from the National Cancer Institute, The D. Warren Brown Foundation, Four Winds Foundation, The Sullivan Chronic Lymphocytic Leukemia Research Fund, Mr. and Mrs. Michael Thomas, Mr. and Mrs. Al Lipkin, and The Harry T. Mangurian Foundation.
Footnotes
Conflict of Interest:
We declare that there are no conflicts of interest pertaining to this work.
Contributions
DG conceived of the research, performed experiments, wrote the paper and approved the final manuscript. AR performed the statistical analysis, assisted in writing the paper and approved the final version. KM, SG, GM, AG, TL, and RL contributed to acquiring samples for this trial, reviewed drafts of the paper and approved the final version. EH, JW, and AJ contributed to the design of the experiments, reviewed drafts of the paper and approved the final version. JCB conceived of the research, guided the experiments, wrote the paper and approved the final manuscript.
References
- 1.Hallek M. Chronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatment. Am J Hematol. 2013;88:803–816. doi: 10.1002/ajh.23491. [DOI] [PubMed] [Google Scholar]
- 2.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, et al. A MicroRNA Signature Associated with Prognosis and Progression in Chronic Lymphocytic Leukemia. N Engl J Med. 2005;353:1793–801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 3.Ferracin M, Zagatti B, Rizzotto L, et al. MicroRNAs involvement in fludarabine refractory chronic lymphocytic leukemia. Mol Cancer. 9:123. doi: 10.1186/1476-4598-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. PNAS. 2005;102:3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costinean S, Zanesi N, Pekarsky Y, Tilli E, Volinia S, Heerema N, et al. Pre-B cell proliferation and lymphoblastic leukemia high-grade lymphoma in Eμ-miR155 transgenic mice. PNAS. 2006;103:7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Roccaro AM, Rombaoa C, Flores L, Obad S, Fernandes SM, et al. LNA-mediated anti-miR-155 silencing in low-grade B cell lymphomas. Blood. 2012;120:1678–1686. doi: 10.1182/blood-2012-02-410647. [DOI] [PubMed] [Google Scholar]
- 7.Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of MIR155 host gene in physiological and pathological processes. Gene. 2013;532:1–12. doi: 10.1016/j.gene.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Mraz M, Kipps TJ. MicroRNAs and B cell receptor signaling in chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54:1836–1839. doi: 10.3109/10428194.2013.796055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiestner A, Rosenwald A, Barry TS, Wright G, Davis RE, Henrickson SE, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–4951. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 10.Byrd JC, Peterson BL, Morrison VA, Park K, Jacobson R, Hoke E, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 11.Lin TS, Donohue KA, Byrd JC, Lucas MS, Hoke EE, Bengtson EM, et al. Consolidation therapy with subcutaneous alemtuzumab after fludarabine and rituximab induction therapy for previously untreated chronic lymphocytic leukemia: Final Analysis of CALGB 10101. J Clin Oncol. 2010;28:4500–4506. doi: 10.1200/JCO.2010.29.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui B, Chen L, Zhang S, Mraz M, Fecteau JF, Yu J, et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood. 2014;124:546–554. doi: 10.1182/blood-2014-03-559690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woyach JA, Bojnik E, Ruppert AS, Stefanovski MR, Goettl VM, Smucker KA, et al. Bruton's tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014;123:1207–1213. doi: 10.1182/blood-2013-07-515361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaglowski SM, jones JA, flynn JM, andritsos LA, maddocks KJ, blum KA, et al. A phase ib/ii study evaluating activity and tolerability of btk inhibitor PCI-32765 and ofatumumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (cll/sll) and related diseases. J clin oncol. 2012;30 (suppl; abstr 6508) [Google Scholar]
- 17.Maddocks K, Flynn JM, Andritsos LA, Awan F, Woyach JA, Grever MR, et al. A phase 2 study of the BTK inhibitor ibrutinib in genetic risk-stratifed relapsed and refractory patients with chronic lymphocytic leukemia (CLL)/ small lymphocytic lymphoma (SLL). EHA. 2014 (abstr S1342) [Google Scholar]
- 18.Woyach JA, Smucker K, Smith LL, Lozanski A, Zhong Y, Ruppert AS, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woyach JA, Ruppert As, Lozanski G, Lozanski A, Heerema Na, Zhao Weiqiang, et al. Association of disease progression on ibrutinib therapy with the acquisition of resistance mutations: a single-center experience of 267 patients. J Clin Oncol. 2014;32:5s. [Google Scholar]