Abstract
Midline defects account for approximately 5% of congenital abnormalities observed at birth. However, the molecular mechanisms underlying the formation of the ventral body wall are not well understood. Recent studies linked mutations in Porcupine—an O-acetyl transferase mediating Wnt ligand acylation—with defects in the thoracic body wall. We hypothesized that anomalous Wnt signaling is involved in the pathogenesis of defective closure of the thoracic body wall. We generated a mouse model wherein Wntless (Wls), which encodes a cargo receptor mediating secretion of Wnt ligands, was conditionally deleted from the developing mesenchyme using Dermo1Cre mice. Wlsf/f;Dermo1Cre/+ embryos died during mid-gestation. At E13.5, skeletal defects were observed in the forelimbs, jaw, and rib cage. At E14.5, midline defects in the thoracic body wall began to emerge: the sternum failed to fuse and the heart protruded through the body wall at the midline (ectopia cordis). To determine the molecular mechanism underlying the phenotype observed in Wlsf/f;Dermo1Cre/+ embryos, we tested whether Wnt/β-catenin signaling was operative in developing the embryonic ventral body wall using Axin2LacZ and BatGal reporter mice. While Wnt/β-catenin signaling activity was observed at the midline of the ventral body wall before sternal fusion, this pattern of activity was altered and scattered throughout the body wall after mesenchymal deletion of Wls. Mesenchymal cell migration was disrupted in Wlsf/f;Dermo1Cre/+ thoracic body wall partially due to anomalous non-canonical Wnt signaling as determined by in vitro assays. Deletion of Lrp5 and Lrp6 receptors, which mediate Wnt/β-catenin signaling in the mesenchyme, partially recapitulated the phenotype observed in the chest midline of Wlsf/f;Dermo1Cre/+ embryos supporting a role for Wnt/β-catenin signaling activity in the normal formation of the ventral body wall mesenchyme. We conclude that Wls-mediated secretion of Wnt ligands from the developing ventral body wall mesenchyme plays a critical role in fusion of the sternum and closure of the secondary body wall. Thus, impaired Wls activity in the ventral body wall mesenchyme is a mechanism underlying ectopia cordis and unfused sternum.
Keywords: Midline defects, Sternum, Wls, ectopia cordis, Pentalogy of Cantrell
INTRODUCTION
Midline defects of the ventral body wall are observed in 1:2500 live births. The Pentalogy of Cantrell (PC) is a rare disorder (approximate incidence of 1:65000 live births) typified by a constellation of congenital malformations affecting the heart, diaphragm, sternum, abdominal wall, and pericardium (Cantrell et al., 1958). Even with advances in surgical interventions, morbidity and mortality of this disorder remain high (O'Gorman et al., 2009). The abnormalities associated with PC vary considerably, but ectopia cordis, absent or cleft sternum and abdominal wall defects are commonly observed anomalies. While the anatomic findings of PC are well established, the pathogenesis or genetic causes of PC are unknown. Recent studies identified mutations in Porcupine (PORCN) in individuals with PC and Focal Dermal Hypoplasia (also known as Goltz-Gorlin Syndrome) (Smigiel et al., 2011) or with body-wall complex syndrome (Maas et al., 2009).
Porcupine is an O-acyltransferase involved in the acylation of Wnt ligands; this post-translational modification is required for secretion and biological activity of most Wnt ligands (Coombs et al., 2010; Komekado et al., 2007). Porcupine, located on the X chromosome, has been linked to Focal Dermal Hypoplasia (FDH), a condition primarily affecting skin and in some cases, the abdominal wall (Wang et al., 2007). Moreover, deletion of Porcupine (Porcn) in mice caused FDH and abnormal development of the body wall (Barrott et al., 2011). It is presently unclear whether PORCN mutations are also the cause of PC. Mouse studies have demonstrated that deletion of various components of the Wnt/β-catenin signaling pathway affects formation of the sternum. Conditional deletion of either co-receptors Lrp5 Lrp6 or the Wnt nuclear transducer β-catenin in mesenchymal tissue causes a dysmorphic sternum that failed to fuse at the midline (Joeng et al., 2011). Similarly, conditional deletion of GSK-3β, a Wnt/β-catenin signaling modulator, causes midline defects including bifid sternum and delayed sternal ossification (Hoeflich et al., 2000; Liu et al., 2007). Moreover, Wnt/β-catenin activity is detected in developing dermis and sternum during normal development (Chen et al., 2012; Ohtola et al., 2008). Taken together, these clinical and experimental data support the concept that Wnt/β-catenin signaling participates in formation of the ventral body wall.
Previous studies, including our own investigations, demonstrated that Wntless (Wls) is a critical component of the Wnt signaling pathway and is required for the morphogenesis of various organs including lung and skin (Augustin et al., 2013; Carpenter et al., 2010b; Cornett et al., 2013; Fu and Hsu, 2013; Huang et al., 2012). As a cargo receptor that mediates Wnt ligand secretion from producing cells, Wls acts downstream of PORCN-mediated acylation of Wnt ligands (Coombs et al., 2010; Herr and Basler, 2012). Given the finding that Porcn is present in Wnt-producing cells of the midline dermis (Ohtola et al., 2008) and that mutations in Porcn have been associated with midline defects, we sought to test whether secretion of Wnt ligands, mediated by Wls, affects ventral body wall formation.
In this study, we utilized Dermo1Cre mice (Yu et al., 2003) to conditionally delete Wls in the embryonic mesenchyme-specifically, the lateral plate mesoderm, somites and dermis of developing body wall-(Li et al., 1995). Wls expression in the mesenchyme of the ventral body wall is required for migration of mesenchymal cells to the midline, closure of the secondary body wall and formation of the sternum. Thus, we provide evidence supporting the concept that anomalous production or activity of Wnt ligands from the mesenchyme is involved in the pathogenesis of congenital midline malformations such as ectopia cordis and unfused sternum.
MATERIAL AND METHODS
Mouse Breeding and Genotyping
Animals were housed in pathogen-free conditions and handled according to protocols approved by CCHMC Institutional Animal Care and Use Committee (Cincinnati, OH USA). Generation of the Wntless (Wls) conditional knockout (CKO) mouse has been described (Carpenter et al., 2010a). Wlsf/f;Dermo1Cre/+ embryos were generated by breeding Wlsf/f mice with Dermo1Cre/+ mice (Sosic et al., 2003) and recrossing resultant mice with Wlsf/f. Wlsf/f;Dermo1Cre/+;Axin2LacZ and Wlsf/f;Dermo1Cre/+; BatGal mice were obtained by mating Wlsf/+ Dermo1Cre/+ with Axin2LacZ (Joeng et al., 2011; Lustig et al., 2002) or BatGal mice (Maretto et al., 2003), respectively. Wlsf/f;Dermo1Cre/+;Rosa mT/mG embryos were generated by breeding Wlsf/+;Dermo1Cre+/− with Rosa mT/mG mice (Muzumdar et al., 2007). Wlsf/f;Col2a1Cre/+ were generated by breeding Wlsf/f mice with Col2a1Cre/+ mice (Ovchinnikov et al., 2000) and breeding resultant mice with Wlsf/f. Lrp5/6f/f;Dermo1Cre/+ were obtained by crossing Lrp5f/f Lrp6f/f mice (Joeng et al., 2011) with Dermo1Cre/+ and recrossing the offspring to Lrp5f/f;Lrp6f/f mice. Sox9f/f;Dermo1Cre/+ mice were obtained by breeding Sox9f/f with Dermo1Cre/+ mice and mating resultant mice to Sox9f/f mice. Wlsf/f;Dermo1Cre/+;Sox9EGFP embryos were generated by breeding Wlsf/f mice with Sox9EGFP mice (Gong et al., 2003) and breeding resultant mice with Wlsf/+;Dermo1Cre/+. Genotypes of transgenic mice were determined by PCR with genomic DNA isolated from mouse tail or embryonic tissue. Primers utilized for genotyping have been provided as Supplementary Material (Table S1).
Histology, Immunohistochemistry and Immunofluorescence Staining
Embryonic tissue was fixed and embedded in paraffin or frozen using OCT. Sections (6um) were processed for H&E or DAB staining as described (Mucenski et al., 2003). For immunofluorescence, sections were blocked with normal serum and incubated with a mix of primary antibodies overnight at 4°C. Fluorochrome-conjugated anti-IgG antibodies were applied for one hour at room temperature, and sections were preserved in VECTASHIELD mounting medium with DAPI (Vector) to visualize nuclei. Primary antibodies included Sox9 (Millipore), α Smooth Muscle Actin (αSMA, Sigma), antiBrdU, antiGFP and phosphohistone-H3 (PHH3) (Santa Cruz).
In-Situ Hybridization
Procedure was performed according to a protocol developed by Advanced Cell Diagnostics (ACD) (Wang et al., 2012). In situ probes were designed by ACD. In brief, slides were baked and deparaffinized. Each slide was then permeabilized and washed. In situ probes were added to the slides and hybridization was performed for 2 hours at 40°C followed by several rounds of amplification steps. Finally, chromogenic reaction using DAB and counter staining with Hematoxylin was performed on slides. After mounting with permanent mounting media, slides were photographed using a wide field Nikon i90 microscope.
Whole Mount X-Galactosidase Staining
Whole embryos were fixed in 4% paraformaldehyde (PFA) in PBS for 30 minutes, then washed in PBS and incubated for two to three hours in X-gal staining solution. To stop the reaction, explants were washed in 3% dimethyl sulfoxide-PBS, rinsed in PBS, and stored in 70% ethanol. Before imaging, explants were dehydrated in methanol and cleared in methyl salicylate. Samples were then dehydrated in a graded series of ethanol, and processed for paraffin embedding and sectioning.
Cell Proliferation and Cell Death
Sections from E11.5 embryos were labeled with PHH3 antibody to identify mitotic cells in body wall and flanks. Labeled cells and total cells were counted per each field photograph at 20× and ratios of proliferating to total cell numbers were calculated. Average mitotic index was determined for three different samples. Cell proliferation was also determined by incorporation of BrdU. Dams were injected with BrdU (1mg/g body weight) at E11.5. Embryos were harvested at E13.5 and processed for paraffin embedding. Sections were stained with antiBrdU antibody and Sox9 or αSMA antibody. Average mitotic index was determined as previously described. Cell death was determined by TUNEL assay performed on sections of E13.5 and E14.5 embryos using a commercially available detection kit (Roche).
MEF Cell Isolation and Culture
Embryos were harvested at E13.5. Chest flank regions of at least three embryos of the same genotype (Wlsf/f or Wlsf/f ;Dermo1Cre/+) were isolated, washed in PBS, minced in trypsin and incubated for 10 minutes at 37C. After incubation, tissue was pipetted until cell suspension formed. Cells were seeded in flasks containing MEF tissue culture media composed of DMEM, 1% penicillin/streptomycin and 20% non-heat inactivated FBS.
Migration Assay
MEF cells were seeded as monolayer in 24 well plates. Using a 200ul pipet tip, a wound was induced in the monolayer. Culture media were removed and replaced with media containing low percentage of non-heat inactivated FBS and recombinant Wnt ligands Wnt5a or Wnt3a (100 ng/ml) (R&D), Ca2+/ calmodulin-dependent protein kinase inhibitor KN93 (10 uM) (Millipore), or JNK inhibitor II (25uM) (Millipore). Images were acquired at 0, 7, 11 and 24 hours post-scratch using an Olympus inverted microscope. Migration was determined as the ratio of covered surface to initial wound surface using Image J (NIH) software.
RNA Extraction and RT-PCR
Gene expression was determined by quantitative RT-PCR. RNA was isolated from embryonic tissue using a commercially available kit (RNAeasy mini kit or micro kit, Qiagen-Promega). Four body wall samples were pooled for RNA extraction, while three flank samples were pooled for RNA extraction. Reverse transcription was performed according to manufacturer instructions (Verso Fisher Sci), and Taqman probes (Life Technologies) were utilized to detect differential expression using a StepOnePlus RT-PCR System.
Statistics
Quantitative data were presented as mean plus standard error. Experiments were repeated at least twice with a minimum of three biological replicates for each group. Statistically significant differences were determined by paired T-test, one-way ANOVA followed by Bonferroni’s multiple comparisons test using Graph Pad Prism. Significance was set at p<0.05.
RESULTS
Deletion of Wls from embryonic mesenchymal progenitors causes skeletal anomalies
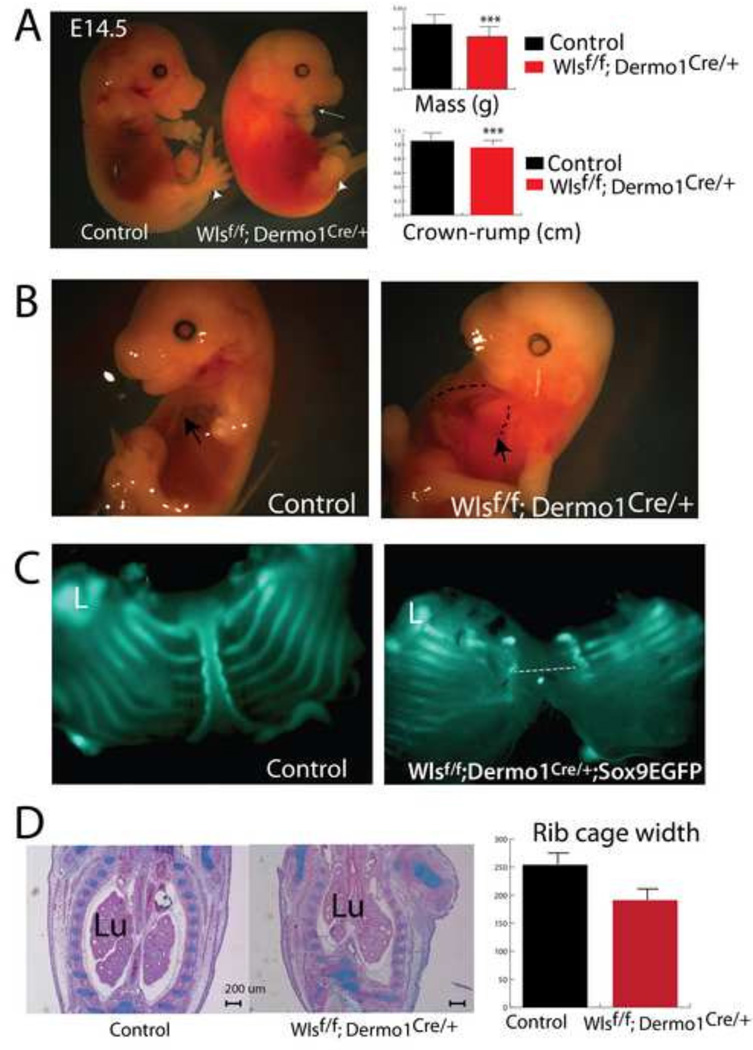
To test whether secretion of Wnt ligands from mesenchymal progenitors is required for the formation of the ventral body wall, we conditionally deleted Wls using Dermo1Cre mice. While morphological abnormalities were not observed at E12.5, several congenital abnormalities were readily observed in Wlsf/f;Dermo1Cre/+ embryos at E13.5. At this developmental stage, no digits were detected in the forelimbs, and the jaw was underdeveloped (micrognathia) (arrow Fig1A). At E14.5, mass and crown-rump length were reduced in Wlsf/f;Dermo1Cre/+ embryos. Embryos were hemorrhagic and had ectopia cordis (exposed heart) along with malformed sternum, which failed to fuse in the midline of the chest cavity (Fig. 1A and 1B, dotted line). The abnormal thoraxes had decreased width, as seen in the coronal plane of embryos (Fig. 1C). Conversely, the lungs, where Dermo1 (Twist2) is broadly expressed, were only marginally affected by deletion of Wls. While early cell differentiation was not perturbed (Cornett et al, 2013), Wlsf/f;Dermo1Cre/+ lungs were elongated, presumably as a secondary effect due to the abnormal chest cavity observed in these embryos (Fig. 1B,C and Supplementary Fig. 1).. No live embryos were recovered after E14.5 indicating embryonic lethality. These data demonstrate that conditional deletion of Wls from mesenchymal progenitors impaired formation of skeletal structures and the thoracic body wall.
Figure 1. Congenital malformations after Wls deletion from mesenchymal progenitors.
Deletion of Wls in embryonic mesenchyme causes craniofacial (white arrow, A), skeletal (white arrowhead,A) and midline defects (C). Weight and crown-rump length are diminished in Wlsf/f;Dermo1Cre/+ embryos (A). Malformed sternum and exposed heart (ectopia cordis) are reminiscent of Pentalogy of Cantrell (B). Extent of the lack of fusion of the sternum (dotted line, C) as determined by GFP fluorescence in the Wlsf/f;Dermo1Cre/+;Sox9EGFP mouse at E14.5 is shown (C). H&E staining depicts the anomalous shape of the rib cage after mesenchymal deletion of Wls in E13.5 embryos (D). The widest region of the rib cage is diminished in Wlsf/f;Dermo1Cre/+ embryos (D). Lu=Lung, L=Limb, N=4 ***p< 0.001
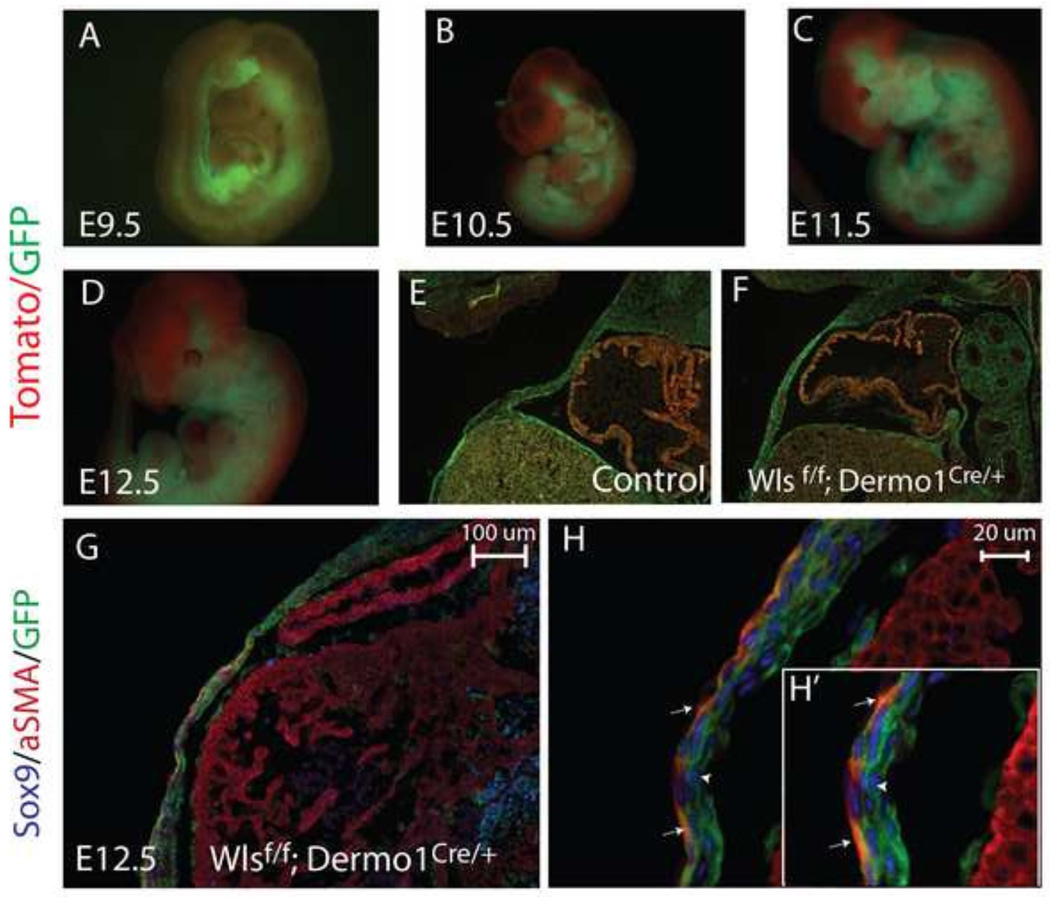
To formally demonstrate the extent of the mesenchymal deletion of Wls in ventral body wall, we analyzed the efficiency of recombination of Dermo1Cre/+ using the reporter mouse Rosa mT/mG (Tomato/GFP). As early as E9.5, we detected Cre-mediated recombination in cells expressing Dermo1, as indicated by expression of GFP in somites and lateral plate mesoderm (Fig. 2A,B,C). In this model, cells that failed to undergo Cre mediated recombination expressed tdTomato fluorescent protein (Muzumdar et al., 2007). As development progressed, GFP was detected in ventral body wall dermis—derivative of mesenchymal progenitors—but was excluded from the cardiac atrium and ventricle tissue (Fig.2 D). Similar levels and specificity of recombination were observed in both control and Wlsf/f;Dermo1Cre/+;Rosa mT/mG embryos, as indicated by GFP expression (Fig.2 D,E,F). Dermo1Cre mediated recombination was observed in both Sox9 and αSMA (smooth muscle actin) expressing cells present in the ventral body wall of Wlsf/f;Dermo1Cre/+;Rosa mT/mG embryos (Fig.2 G, H). Hence, deletion of Wls using Dermo1Cre mice is a useful model to study the Wls-mediated signaling from mesenchymal cells of the ventral body wall.
Figure 2. Efficiency and distribution of Dermo1Cre-mediated mesenchymal recombination.
Whole mount images (A–D) and longitudinal sections (E, F) of Wlsf/f;Dermo1Cre/+;Rosa mT/mG embryos are shown. Dermo1Cre-mediated recombination is detected in the mesenchyme as early as E9.5 and progressively expands throughout the mesenchyme as development progresses (A–D). At E12.5, Dermo1Cre-mediated recombination in the body wall is extensive, as demonstrated by the GFP signal in both control and Wlsf/f;Dermo1Cre/+ embryos (E,F). Cre recombination, detected using GFP, was present in both aSMA (white arrows) and Sox9 (white arrowhead) stained cells of the ventral body wall (G,H and H’ insert).
Wnt/β-catenin signaling is operative before sternal fusion
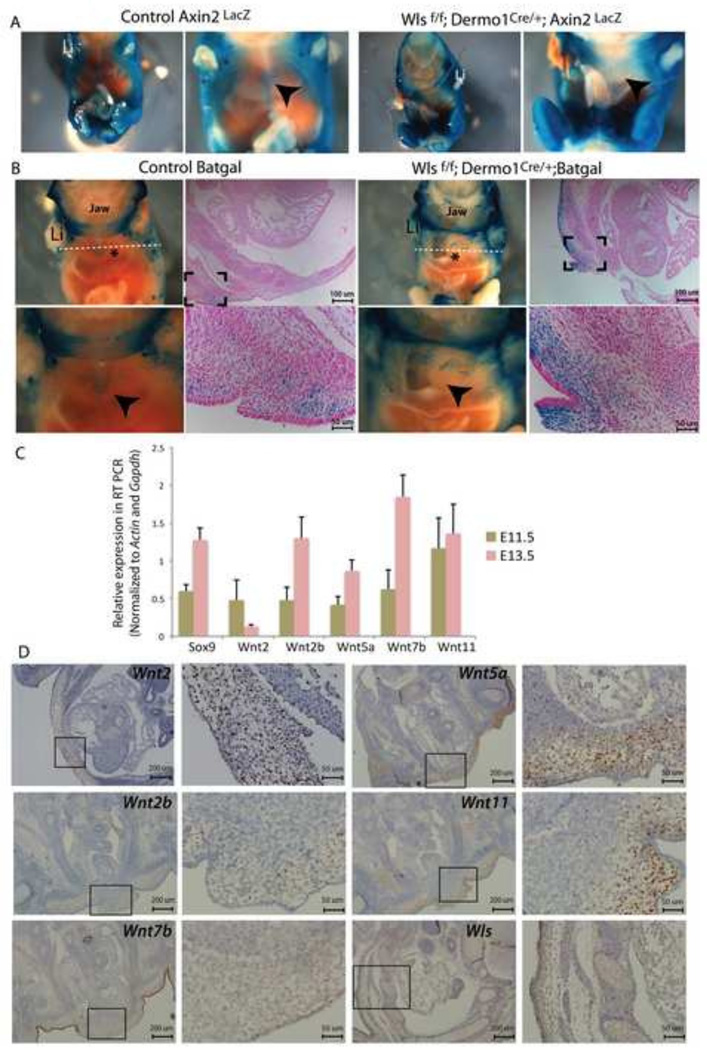
Since Wls mediates secretion of Wnt ligands from producing cells, we reasoned that the lack of fusion of the sternum in Wlsf/f;Dermo1Cre/+ mice was the result of impaired Wnt signaling activity in the ventral body wall. To test whether Wnt/β-catenin signaling was active in the developing dermis of the ventral body wall, we crossed Axin2LacZ and Batgal reporter mice with Wlsf/f;Dermo1Cre/+ mice (Fig. 3A and B). At E13.5, we detected Lac-Z staining in the ventral body wall of control embryos that converged at the midline as a definitive stripe. In contrast, in the Wlsf/f;Dermo1Cre/+;Axin2LacZ or Wlsf/f;Dermo1Cre/+;BatGal mice, Lac-Z staining was scattered throughout the ventral body wall and well-defined midline staining was not detected. The staining in ventral body walls of Wlsf/f;Dermo1Cre/+ was more intense than in ventral body wall of control embryos. Analysis of cross sections determined that in Wlsf/f;Dermo1Cre/+ embryos, Lac-Z staining was present in mesenchymal cells and to a lesser extent in the epidermal layer of developing secondary body wall (Fig. 3B). These data support the concept that Wnt/β-catenin signaling is active in the mesenchyme of the developing ventral body wall of both control and Wlsf/f;Dermo1Cre/+ embryos and that mesenchymal deletion of Wls does not completely abrogate the capability of body wall mesenchymal cells to respond to Wnt/β-catenin signaling.
Figure 3. Wnt/β-catenin signaling is operative in ventral body wall.
Canonical Wnt signaling was assessed in E13.5 Wlsf/f;Dermo1Cre/+ embryos. In control embryos, LacZ staining was detected in the ventral body wall converging at the midline as a well-defined stripe (arrowheads in A and B control). In the Wls Wlsf/f;Dermo1Cre/+;Axin2LacZ (A) and Wlsf/f;Dermo1Cre/+;BatGal embryos (B), staining was scattered throughout the ventral body wall, and definitive midline staining was not detected (arrowhead in A and B Wlsf/f;Dermo1Cre/+ panels). Cross sections of the whole mounts show the extent of Wnt/β-catenin signaling in the ventral body wall of control and Wlsf/f;Dermo1Cre/+;BatGal embryos, (B). White dotted lines indicate the plane of section (B). Levels of Wnt ligand mRNA in body wall tissue were determined at E11.5 and E13.5 by RT-PCR (C). Cross sections of E13.5 embryos were hybridized with riboprobes to detect mRNA for Wnt ligands Wnt2, Wnt2a and Wnt7b, Wnt5a and Wnt11, as well as Wls transcripts. Low magnification and high magnification of the areas in squares are shown for each staining. Wls mRNA was detected in both epithelium and mesenchyme, including developing ribs and muscle of the thoracic body wall (D). Li= limb*=heart
Wnt ligands are expressed in ventral wall dermis
Since Wnt/β-catenin signaling is active in the ventral body wall dermis, we sought to identify which Wnt ligands were expressed in the anterior body wall using a candidate approach. At E11.5 and E13.5, Wnt2, Wnt2b, Wnt5a, Wnt7b and Wnt11 mRNA were detected in ventral body wall tissue by qRT-PCR (Fig. 3C). To determine the precise spatial expression pattern we performed in situ hybridization. At E13.5, Wnt2 and Wnt2b mRNAs were detected in the mesenchyme of the ventral body wall. Wnt2 transcripts were also detected in the epithelium of the body wall. Wnt7b was primarily detected in the epithelium (ectoderm) of the body wall and to a lesser extent was detected in the body wall mesenchyme. Wnt5a and Wnt11, which are known to mediate Wnt/β-catenin independent signaling, were detected at high levels in the mesenchymal cells of the body wall; Wnt5a expression was not prominent in the epidermis of the body wall (Fig. 3D). qRT-PCR and in situ hybridization assays revealed no significant differences in Wnt ligand mRNA levels in the midline tissues of E11.5 control and Wlsf/f;Dermo1Cre/+ embryos (Supplementary Fig2). Fzd receptors, Fzd1, 4, 7, and 10, which are necessary for transduction of Wnt signals, were expressed in the flanks and midline of the body wall (Supplementary Fig2). Wls mRNA was present in epithelium (ectoderm), muscle, cartilage, and mesenchyme of the secondary body wall of control embryos (Fig. 3D). Taken together, these data support the concept that cells of the ventral body wall are capable of producing and responding to β-catenin dependent and β-catenin independent Wnt signaling. Wnt ligands that typically elicit a β-catenin independent response were expressed primarily in the mesenchyme of the body wall while Wnt ligands eliciting Wnt/β-catenin signaling are expressed in the epithelial cells (ectoderm) of the body wall. These data also support the concept that transcription of Wnt ligands was not affected by mesenchymal deletion of Wls.
Faulty closure of the thoracic body wall in Wlsf/f;Dermo1Cre/+ embryos is associated with decreased apoptosis at the midline
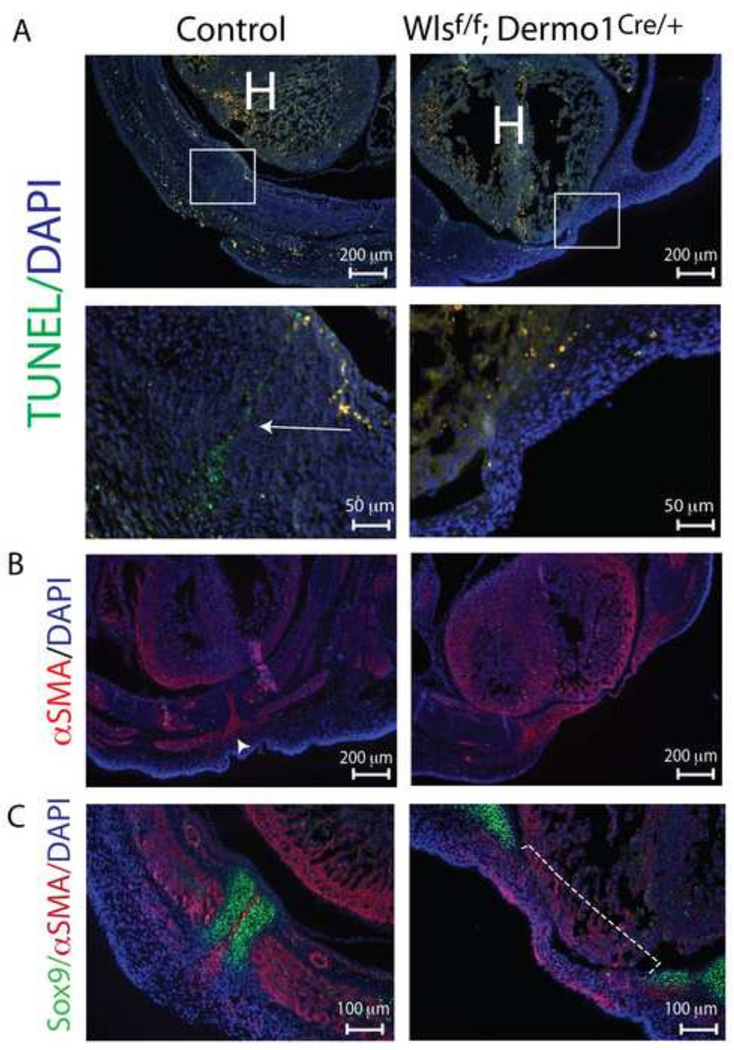
To test whether the lack of fusion of the sternal bars in the Wlsf/f;Dermo1Cre/+ embryo was related to diminished cell proliferation, mitotic index was determined by incorporation of BrdU in cells of the body wall. Dams were injected with BrdU at E11.5 and sacrificed at E13.5 when incorporation of the synthetic nucleoside was analyzed in developing body wall. Cell proliferation was not altered in Sox9 stained cells (which labels chondroblasts) or in αSMA stained cells (Supplementary Fig.3, Supplementary Fig.4). Previous studies have shown that anomalous formation of the secondary body wall was associated with increased cell death (Brewer and Williams, 2004; Ohtola et al., 2008). At E13.5, a low percentage of apoptotic cells (less than 2%) was identified in control and Wlsf/f;Dermo1Cre/+ embryos by TUNEL assay and no significant differences in apoptosis were observed (data not shown). Interestingly, we detected apoptotic cells located between the converging sternal bars of E14.5 control embryos (Fig.4 A, arrow head). These cells localize in the same region where αSMA stained cells are found at the midline of control ventral body wall (Figure 4B). In contrast, no apoptotic cells were detected in body wall midline of E14.5 Wlsf/f;Dermo1Cre/+ embryos since the sternal bars failed to converge; however, a few number of apoptotic cells were detected in the flanks of E14.5 Wlsf/f;Dermo1Cre/+ embryos. Taken together, mesenchymal deletion of Wls did not impair the proliferative capability of cells within the body wall; however, Wls was required for the induction of apoptosis of cells located between sternal bars at E14.5 that were not detected in body wall of Wlsf/f;Dermo1Cre/+ embryos.
Figure 4. Decreased apoptosis in ventral body wall and impaired migration to midline after mesenchymal loss of Wls.
TUNEL staining was performed on cross and longitudinal sections of E14.5 control and Wlsf/f;Dermo1Cre/+ embryos (A). Note the cell death at the midline (corresponding to the region between the fusing sternal bars) in the control body wall (arrow). This pattern is absent in mutants. Representative images and higher magnifications of areas in square are shown. The midline region were apoptosis takes place in control embryos (A) overlaps with a region that stains positive for αSMA (B). Cross sections of E14.5 embryos stained with Sox9 and αSMA antibodies demonstrate the extent of the lack of fusion of sternal bars in mutants (dotted line) and the position of αSMA stained cells between the sternal bars in control embryos (C). H=heart.
Cell migration towards the midline is impaired after deletion of Wls from mesenchyme
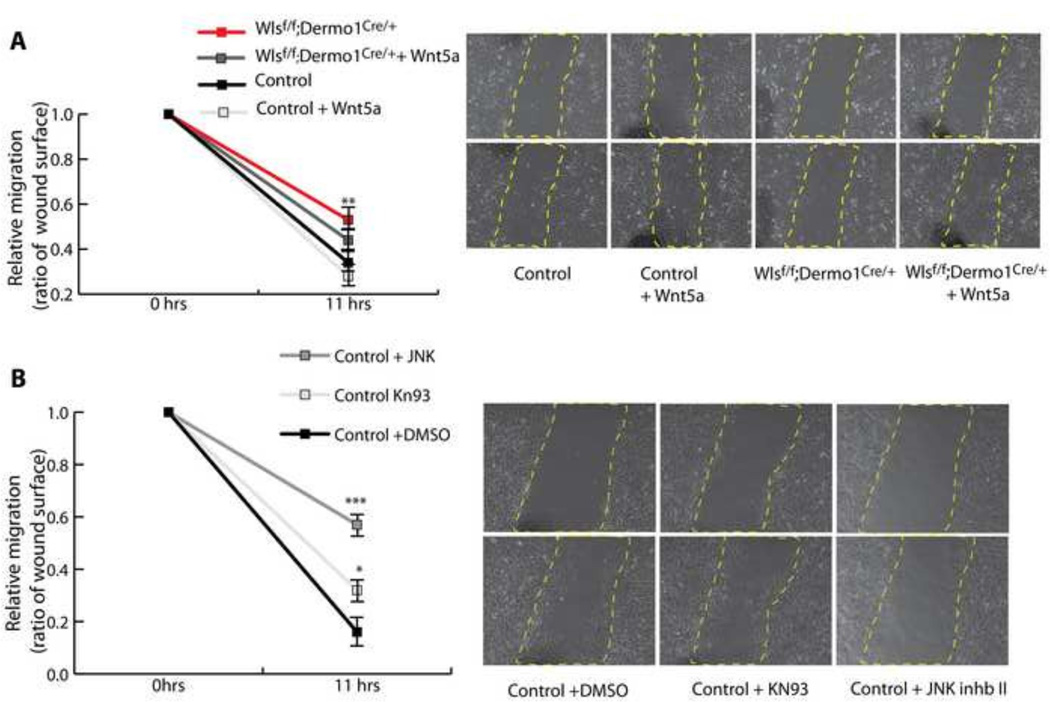
The lack of midline fusion of the sternal bars in Wlsf/f;Dermo1Cre/+ embryos at the midline was not the product of altered cell proliferation or specification. Accordingly, this failure of midline fusion suggests that in absence of mesenchymal Wnt ligand secretion, cells within the mesenchyme of the body wall do not migrate properly (Fig.4C). Therefore, we analyzed in vitro the migratory capacity of mesenchymal cells isolated from the E13.5 embryonic flanks. Flank cells of both control and Wlsf/f;Dermo1Cre/+ embryos were able to migrate to produce wound closure. Cells isolated from flanks of Wlsf/f;Dermo1Cre/+ embryos migrated slower than control cells. To test the hypothesis that Wnt ligands secreted by mesenchymal cells mediate cell migration, we performed wound-healing assays in the presence of Wnt ligands Wnt3a and Wnt5a, ligands known to induce Wnt/β-catenin dependent and independent responses respectively. While Wnt3a did not affect the migratory ability of the cells isolated from control or Wlsf/f;Dermo1Cre/+ flanks, addition of Wnt5a partially improved cell migration of Wlsf/f;Dermo1Cre/+ cells (Fig. 5A). Since Wnt5a and Wnt11 are highly expressed in the mesenchyme of the ventral body wall and these ligands are known to activate Wnt signaling independently of β-catenin, we tested whether inhibition of the “Ca++” or “PCP” Wnt pathways, impaired migration of mesenchymal cells. The calcium inhibitor KN93 impaired cell migration (Sumi et al., 1991), while addition of JNK inhibitor II, (Han et al., 2001), strongly impaired cell migration (Fig. 5B). Taken together, these data support the concept that Wnt ligand secretion from the body wall mesenchyme is required for cell migration. In vitro, β-catenin independent Wnt signaling mediates migration of mesenchymal cells isolated from the flanks of control and Wlsf/f;Dermo1Cre/+ embryos.
Figure 5. Wnt/β-catenin independent signaling is required for migration of mesenchymal flank cells in vitro.
Wound healing assays were performed on Control and WlsDermo1Cre cells isolated from the ventral flanks of the thoracic cavity. Mesenchymal cells from WlsDermo1Cre embryos migrate slower than cells isolated from control flanks. Addition of Wnt5a partially improved the migratory ability of the WlsDermo1Cre cells (A). Inhibition of the PCP Wnt signaling pathway, using JNK inhibitor II, impaired the migratory ability of control mesenchymal cells, while inhibition of Calcium Wnt signaling using KN93, had a lesser effect on cell migration (B). Representative images at initiation of the assay and 11hrs post wound are shown per each treatment. N=6 ANOVA Bonferroni’s multiple comparisons test; *p<0.05 vs Control, **p<0.01 vs Control, p***<0.001 vs Control.
Midline mesenchymal cells in the ventral body wall are required for fusion of the sternum
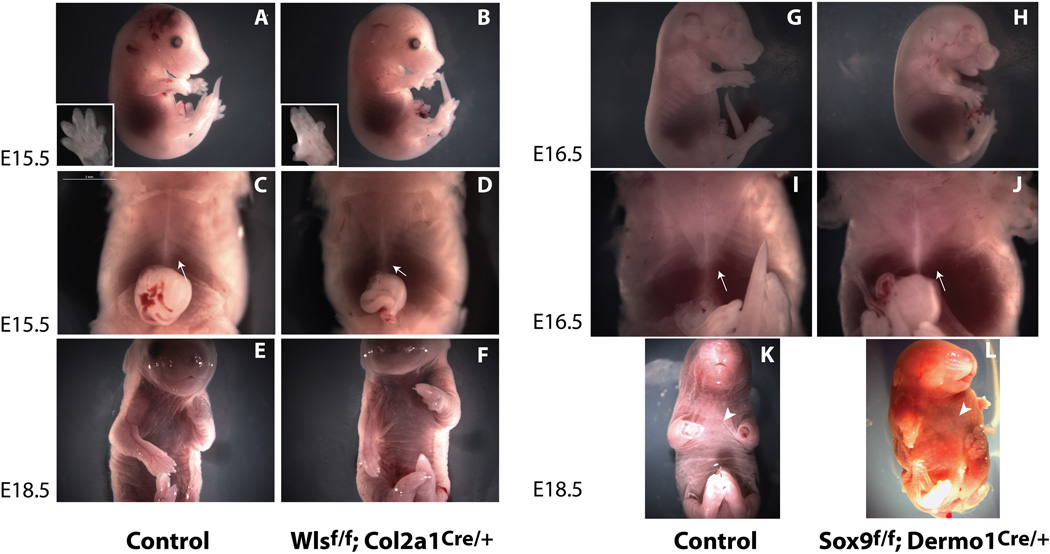
Dermo1Cre mediated recombination was observed throughout the body wall mesenchyme in myoblasts, connective tissue and chondrocytes of developing ribs and sternum. Therefore, we sought to identify Wnt-producing cells in the body wall mesenchyme that are required for fusion of the sternum. We assessed expression of Sox9 in differentiating chondrocytes to test whether lack of Wls-mediated signaling affects their differentiation. Sox9 staining was similar in Wlsf/f;Dermo1Cre/+ and control embryos (Fig. 4C, Supplementary Fig. 3 and 4). We deleted Wls from chondrocytes using Col2a1Cre mice and detected defects in digits of fore and hindlimbs at E15.5 (Fig.6A and B). Deletion of Wls from chondrocytes did not affect closure of the thoracic body wall (Fig. 6C and D). Moreover, E18.5 Wlsf/f;Col2a1Cre/+ embryos were recovered at expected Mendelian ratios without midline defects (Fig.6 E and F). Efficiency and specificity of recombination was confirmed by PCR and by mating the Wlsf/f;Col2a1Cre/+ mice to the reporter mice Rosa mT/mG (data not shown). We also deleted Sox9 using Dermo1Cre to target chondrocytes progenitors in the body wall. While several skeletal defects including short limbs, and smaller rib cage were observed in Sox9f/f;Dermo1Cre/+ embryos (Fig. 6 G and H), the ventral body wall closed normally (Fig. 6 arrow in J and arrow head in L). Analysis of αSMA staining of skeletal and smooth muscle was similar in control and Wlsf/f;Dermo1Cre/+ embryos (Fig.4A and B, Supplementary Fig. 3). These data support the concept that differentiation of chondrocytes and smooth muscle fibroblasts was maintained after deletion of Wls from mesenchymal body wall precursors. These data also suggest that Wnt ligands produced by chondrocyte progenitors are either not essential to or play a redundant role in the formation and closure of the thoracic body wall.
Figure 6. Wls activity in chondroblasts is not necessary for midline closure.
E15.5 Wlsf/f;Col2a1Cre/+ embryos did not display gross morphological anomalies compared to control (A–F), except for abnormal digits observed in forelimbs and hindlimbs (insets A, B). Deletion of Wls in chondroblasts did not impair body wall closure or formation of the sternum (C, D). E18.5 Wlsf/f;Col2a1Cre/+ embryos in ventral view show no gross abnormalities in the ventral body wall (E,F). At E16.5, Sox9f/f;Dermo1Cre/+ embryos exhibit several skeletal defects including shorter limbs and decreased crown-rump length (G, H). No defects in body wall closure were detected in Sox9f/f;Dermo1Cre/+ embryos at E16.5 (I, J arrows) or at E18.5 (K, L arrowheads).
Wnt ligands known to induce Wnt/β-catenin signaling are required for body wall formation
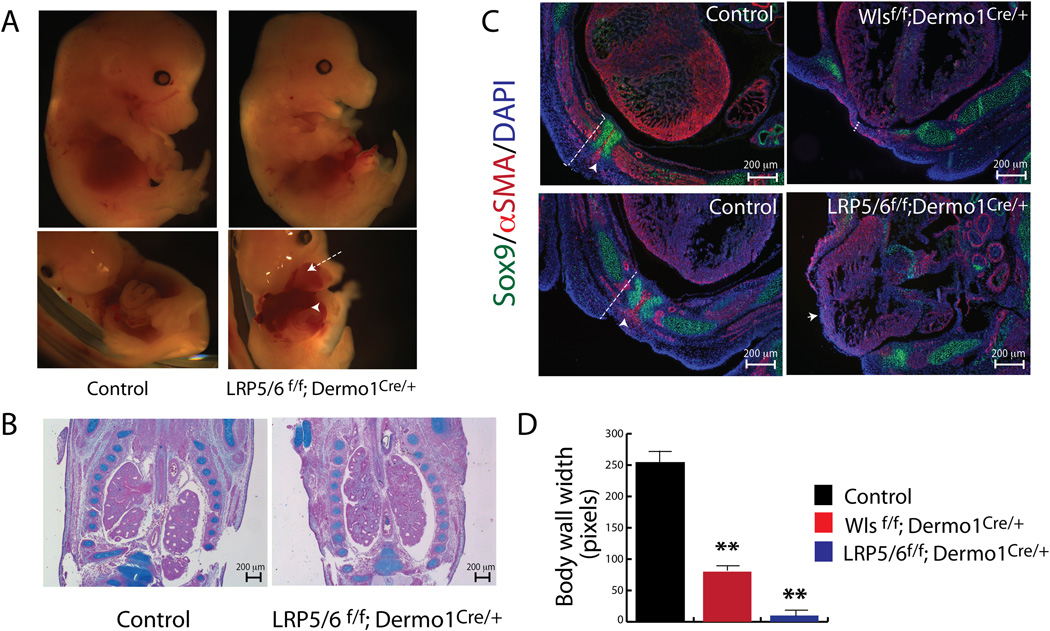
To test the role of Wnt ligands that mediate Wnt/β-catenin activity in ventral body wall formation, we used Dermo1Cre mice to deleted co-receptors Lrp5 and Lrp6 which mediate Wnt/β-catenin signaling. In agreement with previous studies (Joeng et al., 2011) and with present observations in Wlsf/f;Dermo1Cre/+ embryos, the chest wall was abnormal and the heart was exposed in association with failure of sternal fusion (Fig.7A, arrow). In contrast to findings in Wlsf/f;Dermo1Cre/+ embryos, mesenchymal deletion of Lrp5 and Lrp6 prevented the closure of the abdominal wall exposing the liver outside of the abdomen (Fig. 7A arrowhead). In comparison to the abnormalities observed in Wlsf/f;Dermo1Cre/+ embryos (Fig.1), chest cavity shape in Lrp5/6f/f;Dermo1Cre/+ embryos was unaltered (Fig. 7B) and lung morphology was normal (Fig.7B). Similar indices of cell proliferation were detected in the flanks of control and Lrp5/6f/f;Dermo1Cre/+ embryos, at E11.5 (Supplementary Fig.4). Specification of chondroblasts and myoblasts occurred properly (Fig.7C and Supplementary Fig.4). At E14.5, the width of the ventral body wall was reduced in both Lrp5/6f/f;Dermo1Cre/+ and Wlsf/f;Dermo1Cre/+ embryos; however, in Lrp5/6f/f;Dermo1Cre/+ a reduction of the body wall width was detected as early as E11.5. At E14.5, the mesenchyme of the ventral body wall was almost absent, making it difficult to differentiate the body wall from the cardiac tissue (Fig.7 C, D Supplementary Fig.4). Deletion of Lrp5 and Lrp6 did not cause significant changes in levels of Wnt mRNAs, as determined by in situ hybridization and qRT-PCR (Supplementary Fig. 5). Taken together, these data support the concept that deletion of Lrp5 and Lrp6 impaired differentiation of mesenchymal dermal cells of the primary body wall and cell migration of myoblasts and chondroblats towards body wall midline.
Figure 7. Conditional deletion of Lrp5 and Lrp6 caused midline defects.
Deletion of genes encoding Lrp5 and Lrp6 from mesenchyme causes craniofacial anomalies and defects in the ventral body wall (white arrow, A), partially resembling the phenotype seen after deletion of Wls in mesenchyme. Midline defects extend to the abdominal body wall, where failure of midline closure leaves the liver exposed (white arrowhead, A). In contrast to findings in the Wlsf/f;Dermo1Cre/+ embryos, no anomalies in lung morphogenesis were observed in Lrp5/6f/f; Dermo1Cre/+ embryos (B). Cross sections of E14.5 Wlsf/f;Dermo1Cre/+ and Lrp5/6f/f; Dermo1Cre/+ embryos were stained with Sox9 and αSMA antibodies (C). Chondrocytes and myocytes were present in the flanks and ventral body wall of control embryos. Myocytes appear as leading cells between the almost fused sternal bars in controls (arrowheads in control, C); however, only poorly organized αSMA positive cells are present in Wlsf/f;Dermo1Cre/+ and LRP5/6 Dermo1Cre ventral body wall (C). Dotted white lines indicate width of the body wall, which is difficult to distinguish from the cardiac tissue in Lrp5/6f/f; Dermo1Cre/+ embryos (C). Body wall width was extremely reduced in Lrp5/6f/f; Dermo1Cre/+ embryos when compared to control and Wlsf/f;Dermo1Cre/+ embryos (D). N= 3 to 6 **p<0.01 vs Control.
DISCUSSION
In this study, we sought to identify the molecular mechanisms underlying midline defects of the body wall and their relationship to the congenital anomalies associated with the Pentalogy of Cantrell. Failure of sternal fusion and resulting ectopia cordis, two features of the Pentalogy of Cantrell, were caused by deletion of Wls in mesenchymal precursors of the embryonic body wall. Wnt/β-catenin activity, as determined by reporters Axin2LacZ and Batgal, was observed in the thoracic body wall and was restricted to the midline of the ventral body wall of control embryos during normal development of the sternum. Wnt/β-catenin activity was dispersed in the thoracic body wall after deletion of Wls from mesenchymal precursors. While specification of cell types present in the thoracic body wall was not affected by deletion of Wls from mesenchymal precursors, cell migration to the midline was disrupted. Addition of Wnt5a in vitro partially restored migration of in mesenchymal cells lacking Wls, likely via Wnt/β-catenin independent signaling. Thus, Wls promotes migration of myoblasts and chondroblasts towards the midline to form the thoracic body wall, at least in part by activating Wnt/β-catenin independent signaling.
Which cells within the ventral mesoderm produce the Wnt ligands?
Several cell types are present in the developing body wall, including chondrocytes, muscle cells and connective tissue cells, the latter forming tendons and ligaments. In the present study deletion of Wls from chondroblasts using a Col2a1Cre driver did not alter the formation of the sternum. Likewise, mesenchymal deletion of Sox9, a key regulator of chondroblast and osteoblast differentiation, did not alter ventral body wall formation. Therefore, Wls-mediated secretion of Wnt ligands by chondroblast precursors in the ventral body wall is not required for closure of the body wall. Present findings support the concept that mesenchynal cells that are precursors of muscle and connective tissue likely produce the Wnt ligands necessary for body wall formation. Furthermore, we observed that myoblasts seem to be the leading cells migrating into the midline of the chest wall and are present between the sternal bars before sternal fusion.
Mesenchymal Wls controls cell migration and cell survival in the thoracic body wall midline
Formation of the primary body wall depends upon multiple processes including cell migration, proliferation and differentiation (Sadler, 2010). Present studies demonstrate that migration of chondroblasts and myoblasts towards the midline of the thoracic body wall was abnormal after deletion of Wls from mesenchymal precursors. Our in vitro studies demonstrated that cells isolated from the flanks of Wlsf/f;Dermo1Cre/+ embryos have impaired migratory capability that can be partially rescued by exogenous addition of Wnt5a. Thus, Wnt signaling from mesenchymal cells plays a critical role in the fusion of the sternal bars and the closure of the thoracic body wall.
Previous studies support the role of defects in cell proliferation and/or increased cell death in anomalous body wall formation (Brewer and Williams, 2004). In the present study cell proliferation was unaltered in Wlsf/f;Dermo1Cre/+ mutant embryos. Apoptotic cells were present between the fusing sternal bars of control mice at E14.5 creating a pattern resembling the apoptotic process in leading cells during the normal fusion of palatal shelves (Cuervo et al., 2002). This apoptotic pattern was not present in the thoracic body wall of the Wlsf/f;Dermo1Cre/+ embryos. Decreased apoptosis at the thoracic body wall midline after deletion of Wls was associated with the abnormal migration of mesenchymal cells from the flanks of the body wall to the midline.
Cell differentiation is another critical aspect of body wall formation. Multiple mesenchymal cell types, including chondroblasts and α-SMA stained myofibroblasts were present in the primary body wall of control and Wlsf/f;Dermo1Cre/+ embryos even before formation of the secondary body wall. These findings support the concept that differentiation of these cell types does not depend on Wnt ligands produced by mesenchymal fibroblasts. Other tissues, such as the ectoderm, where Wnt2 and Wnt7b are expressed, may provide the Wnt signals required for mesenchymal cell differentiation. Wls is expressed in both ectodermal and mesenchymal tissues of the body wall. Deletion of Wls from ectodermal cells does not affect the thoracic body wall, but does impair formation of the abdominal body wall muscle (Zhang et al., 2014). While midline defects were readily observed in the Wlsf/f;Dermo1Cre/+ model, we did not detect severe abnormalities in the abdominal wall. Thoracic body wall and the abdominal body wall have different developmental origins, i.e. thoracic somites versus abdominal somites (Liem and Aoyama, 2009), then Wnt signaling may play distinct roles in the formation of these two regions of the body wall.
Wls modulates Wnt/β-catenin signaling activity during body wall formation
There is a strong consensus that Wls plays an important role in Wnt signaling by escorting Wnt ligands to the cell surface of producing cells (Banziger et al., 2006; Hausmann et al., 2007). It remains unclear whether Wls balances the activity of the β-catenin dependent or independent branches of the Wnt signaling pathway by differentially favoring the secretion of specific Wnt ligands. Present data demonstrated that deletion of Wls in mesenchymal tissue partially resembles the lack of sternal fusion observed after deletion of co-receptors Lrp5 and Lrp6 and β-catenin from developing embryonic mesenchyme (Joeng et al., 2011), supporting the concept that development of the sternum relies upon Wnt/ β-catenin signaling. Studies by Bryja and colleagues demonstrated that deletion of Lrp5 and Lrp6 promotes Wnt/β-catenin independent signaling induced by increase of Wnt5a activity without changes in Wnt5a expression (Bryja et al., 2009). We did not detect increased expression of Wnt5a or Wnt11 in thoracic body wall of Lrp5/6f/f; Dermo1Cre/+ embryos; however, it is possible that a shift in the molecular ratio of Wnt5a/Wnt11 to Lrp5/6 molecules resulted in ectopic Wnt/β-catenin independent signaling. This excess in Wnt/β-catenin independent signaling may account, at least partially, for the aberrant body wall phenotype observed in Lrp5/6f/f;Dermo1Cre/+ embryos. Ectopic expression of Wnt5a in embryonic tissue did not cause same midline defects as seen after mesenchymal deletion of Lrp5 and Lrp6, but led to mild lower sternum malformations (bifid sternum) while the secondary thoracic body wall closed normally (van Amerongen et al., 2012). Present studies demonstrated the requirement of Lrp5/Lrp6 (Fig.7) and Wnt/β-catenin independent signaling (Fig.5) during formation of the thoracic body wall. Bryja and colleagues demonstrated that interactions between Lrp5/Lrp6 and Wnt ligands inducing Wnt/β-catenin independent signaling are physiologically important and necessary for normal development (Bryja et al., 2009). Therefore, it is likely that balanced levels of Lrp5/Lrp6 (required for Wnt/β-catenin signaling) and Wnt5/Wnt11 (ligands eliciting Wnt/β-catenin independent signaling) may influence migration of cells towards the midline of the body wall; deviations to this balance will result in faulty migration and midline defects. Further analyses are required to formally demonstrate this hypothesis.
Mesenchymal deletion of Lrp5 and Lrp6 caused a sharp reduction in the amount of mesenchymal tissue of the primary body wall, a feature that was not as extensive as in the primary body wall of Wlsf/f;Dermo1Cre/+ embryos. Lrp5 and Lrp6 are required for reception of Wnt ligands, regardless of it source, to promote Wnt/β-catenin signaling; it is likely that ectodermal ligands such as Wnt2 and Wnt7b, and to lesser extent mesenchymal Wnt2 and Wnt2b, may signal to the mesoderm to promote differentiation and growth of dermal progenitors of the primary body wall that in turn influence migration of myoblast and chondroblast to the midline. This potential role for Wnt ligands produced by the ectoderm is supported by studies demonstrating that ectodermal Wls is required for differentiation of the abdominal wall muscle (Zhang et al., 2014).
In Wlsf/f;Dermo1Cre/+ embryos, Wnt/β-catenin signaling—as determined by the reporter mice Axin2LacZ and Batgal—was not abrogated by mesenchymal deletion of Wls; however, the distribution of Wnt signaling was detected as a scattered pattern throughout the thoracic body wall without a defined signal at the midline. A plausible explanation for the anomalous pattern of Wnt/β-catenin activity in the thoracic body wall is that deletion of Wls from mesenchymal progenitors influenced the ability of cells to respond to Wnt ligands Wnt2, and Wnt7b, produced by the ectoderm. It should be also considered that mesenchymal Wnt5a and Wnt11, known to induce β-catenin independent signaling, might play a role in modulating Wnt/β-catenin signaling during ventral body wall formation, thus balancing Wnt signaling pathway activities. Studies on developing limb and hair follicle have shown that Wnt5a antagonizes Wnt/β-catenin signaling to promote differentiation of these structures (Topol et al., 2003; van Amerongen et al., 2012).
Conclusions
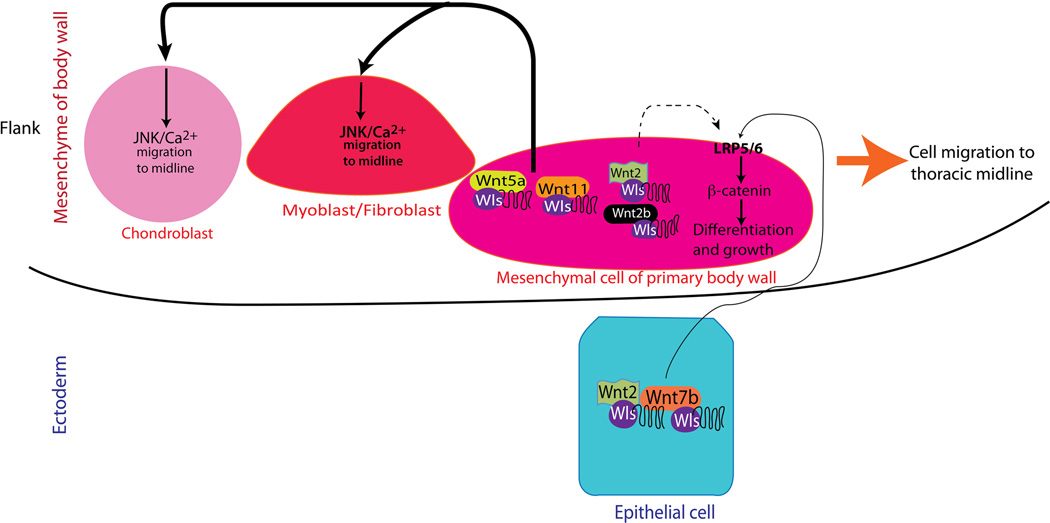
Wls activity in mesenchymal progenitors of connective and muscle tissue of the embryonic body wall is required for formation of the thoracic body wall. The model presented in this study supports the concept that Wnt mediated migration of cells towards the midline is critical for formation of the thoracic body wall. Midline migration is controlled by secretion of Wnt ligands from mesenchymal cells of the thoracic body wall. Ectodermal and to lesser degree mesenchymal Wnt ligands promote Wnt/β-catenin activity required for differentiation and growth of fibroblasts of the primary body wall wherein myoblasts and chondroblasts migrate. Migration of myoblasts, connective tissue precursors and chondroblasts towards the midline is partially mediated via Wnt/β-catenin independent signaling. During the closure of thoracic body wall, myofibroblast migration precedes migration of chondroblasts towards the midline (Fig. 8). Thus, impaired activity of Wls in mesenchymal precursors of the body wall may underlie the pathogenesis of unfused sternum and ectopia cordis, both of which are characteristic of the midline condition Pentalogy of Cantrell.
Figure 8. Model for thoracic body wall formation.
Wls activity in the developing mesenchyme of the body wall is required for normal formation of the sternum and secondary body wall. Wnt ligands produced by mesenchymal myofibroblasts promote migration of myoblasts and chondroblasts to the midline of the thoracic body wall via JNK and Ca++ Wnt signaling. Wnt2, Wnt2b and Wnt7b primarily from the ectoderm, via LRP5/6 and Wnt/β-catenin signaling, are necessary for differentiation and growth of the ventral body wall mesenchyme where myoblasts and chondrocytes migrate to the midline.
Supplementary Material
Highlights.
Mesenchymal Wls activity is required for formation of the thoracic body wall.
Canonical Wnt signaling is active at the body wall midline before sternum fusion.
Mesenchymal Wnt ligands promote cell migration towards thoracic body wall midline.
Apoptosis of mesenchymal cells at the midline precedes fusion of sternal bars.
Acknowledgments
We acknowledge the assistance of Ms. Gail Macke with cryosections and Mr. Chuck Crimmel with graphic design. We also thank Dr. Rulang Jiang for useful comments on this project, and Jacqueline Akech (ACD) for advice on in situ hybridization protocol. This work was partially supported by March of Dimes (Basil O’Connor No. 5-FY12-59 to DS) and National Institutes of Health (K01HL115447 to D.S. and U01 HL110964 to J.A.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Augustin I, Gross J, Baumann D, Korn C, Kerr G, Grigoryan T, Mauch C, Birchmeier W, Boutros M. Loss of epidermal Evi/Wls results in a phenotype resembling psoriasiform dermatitis. J Exp Med. 2013;210:1761–1777. doi: 10.1084/jem.20121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci U S A. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer S, Williams T. Loss of AP-2alpha impacts multiple aspects of ventral body wall development and closure. Dev Biol. 2004;267:399–417. doi: 10.1016/j.ydbio.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Bryja V, Andersson ER, Schambony A, Esner M, Bryjova L, Biris KK, Hall AC, Kraft B, Cajanek L, Yamaguchi TP, Buckingham M, Arenas E. The extracellular domain of Lrp 5/6 inhibits noncanonical Wnt signaling in vivo. Mol Biol Cell. 2009;20:924–936. doi: 10.1091/mbc.E08-07-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell JR, Haller JA, Ravitch MM. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynecol Obstet. 1958;107:602–614. [PubMed] [Google Scholar]
- Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010a:1–5. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010b;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522–1533. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, Utomo V, Banerjee N, Zhang ZH, Jadulco RC, Concepcion GP, Bugni TS, Harper MK, Mihalek I, Jones CM, Ireland CM, Virshup DM. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett B, Snowball J, Varisco BM, Lang R, Whitsett J, Sinner D. Wntless is required for peripheral lung differentiation and pulmonary vascular development. Dev Biol. 2013;379:38–52. doi: 10.1016/j.ydbio.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo R, Valencia C, Chandraratna RA, Covarrubias L. Programmed cell death is required for palate shelf fusion and is regulated by retinoic acid. Dev Biol. 2002;245:145–156. doi: 10.1006/dbio.2002.0620. [DOI] [PubMed] [Google Scholar]
- Fu J, Hsu W. Epidermal Wnt controls hair follicle induction by orchestrating dynamic signaling crosstalk between the epidermis and dermis. J Invest Dermatol. 2013;133:890–898. doi: 10.1038/jid.2012.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how WNT proteins are secreted. Nat Rev Mol Cell Biol. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev Biol. 2012;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Huang S, Zhu X, Liu Y, Tao Y, Feng G, He L, Guo X, Ma G. Wls is expressed in the epidermis and regulates embryonic hair follicle induction in mice. PLoS One. 2012;7:e45904. doi: 10.1371/journal.pone.0045904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol. 2011;359:222–229. doi: 10.1016/j.ydbio.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Li L, Cserjesi P, Olson EN. Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev Biol. 1995;172:280–292. doi: 10.1006/dbio.1995.0023. [DOI] [PubMed] [Google Scholar]
- Liem IK, Aoyama H. Body wall morphogenesis: limb-genesis interferes with body wall-genesis via its influence on the abaxial somite derivatives. Mech Dev. 2009;126:198–211. doi: 10.1016/j.mod.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Liu KJ, Arron JR, Stankunas K, Crabtree GR, Longaker MT. Chemical rescue of cleft palate and midline defects in conditional GSK-3[bgr] mice. Nature. 2007;446:79–82. doi: 10.1038/nature05557. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas SM, Lombardi MP, van Essen AJ, Wakeling EL, Castle B, Temple IK, Kumar VK, Writzl K, Hennekam RC. Phenotype and genotype in 17 patients with Goltz-Gorlin syndrome. J Med Genet. 2009;46:716–720. doi: 10.1136/jmg.2009.068403. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- O'Gorman CS, Tortoriello TA, McMahon CJ. Outcome of children with Pentalogy of Cantrell following cardiac surgery. Pediatr Cardiol. 2009;30:426–430. doi: 10.1007/s00246-009-9410-9. [DOI] [PubMed] [Google Scholar]
- Ohtola J, Myers J, Akhtar-Zaidi B, Zuzindlak D, Sandesara P, Yeh K, Mackem S, Atit R. beta-Catenin has sequential roles in the survival and specification of ventral dermis. Development. 2008;135:2321–2329. doi: 10.1242/dev.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–146. [PubMed] [Google Scholar]
- Sadler TW. The embryologic origin of ventral body wall defects. Semin Pediatr Surg. 2010;19:209–214. doi: 10.1053/j.sempedsurg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Smigiel R, Jakubiak A, Lombardi MP, Jaworski W, Slezak R, Patkowski D, Hennekam RC. Co-occurrence of severe Goltz-Gorlin syndrome and pentalogy of Cantrell - Case report and review of the literature. Am J Med Genet A. 2011;155A:1102–1105. doi: 10.1002/ajmg.a.33895. [DOI] [PubMed] [Google Scholar]
- Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt 5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Reid Sutton V, Omar Peraza-Llanes J, Yu Z, Rosetta R, Kou YC, Eble TN, Patel A, Thaller C, Fang P, Van den Veyver IB. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li H, Yu J, Cao J, Chen H, Zhao H, Zhao J, Yao Y, Cheng H, Wang L, Zhou R, Yao Z, Guo X. Ectodermal Wnt signaling regulates abdominal myogenesis during ventral body wall development. Dev Biol. 2014;387:64–72. doi: 10.1016/j.ydbio.2013.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.