Abstract
Belief in the effectiveness of a placebo treatment is widely thought to be critical for placebo analgesia. Many types of placebo responses—even those that depend on conditioning—appear to be mediated by expectations that are strengthened as treatment cues are reinforced with positive outcomes. However, placebo effects may occur even when participants are aware they are receiving placebo. To address the question of whether conditioned placebo analgesia can persist in the absence of expectations, we studied the effects of long (4 days) vs. short (1 day) conditioning to a placebo treatment. After an initial placebo test, a “reveal” manipulation convincingly demonstrated to participants that they had never received an active drug. Placebo analgesia persisted after the reveal in the long conditioning group only. These findings suggest that reinforcing treatment cues with positive outcomes can create placebo effects that are independent of reported expectations for pain relief.
Keywords: Placebo, Pain, Conditioning, Expectancy, Reversal
Introduction
Placebo analgesia is pain relief observed following administration of a treatment that is not directly caused by the pharmacological properties of that treatment. Placebo analgesia is typically induced in the laboratory using a “response conditioning” paradigm, where treatment cues (e.g., a cream or injection) are paired with surreptitious reductions in the intensity of painful stimuli25, 33. Afterward, painful stimuli are presented under placebo (paired) and control (no pairing) conditions to test for placebo effects. This procedure is a model paradigm in the study of placebo analgesia and the influence of expectations on pain and other affective, perceptual, and physiological processes24, 31, 35.
Early studies concluded that the experience of pain relief was critical for reliably inducing placebo analgesia33, 34, but it is now generally understood that placebo analgesia is directly mediated by expectations and only indirectly relies on prior experiences2, 6, 19, 21, 24. Manipulations of expectations produce pain relief2, 7, and greater expectancies are associated with greater placebo analgesia18, 21, 22, 25, 37. Even within conditioning paradigms, expectancies appear to be critical: when subjects attribute pain relief to sources other than a placebo treatment they do not acquire placebo analgesia21, 38, and verbal suggestions of hyperalgesia can block conditioned placebo analgesic effects6, 7, 14. These findings fit within a broader literature suggesting that conditioning depends on the information value of cues rather than associative pairing per se26, and may reflect inferential rather that gradual learning processes12.
Expectancy theory implies that belief in the placebo is critical for placebo analgesia. This expectation need not be a belief in the chemical analgesic properties of the treatment, but may instead be a more general belief that a placebo treatment can relieve symptoms. This belief may allow placebos to serve as either dose extenders for chemically active treatments28, 29 or effective treatments on their own17. However, expectancy theory is challenged by demonstrations that placebo treatments can result in analgesia even when participants are unaware they are receiving a treatment2, 15. Other placebo manipulations that generate expectancy-independent placebo effects (e.g. conditioned immunosuppression) generally use multiple conditioning sessions1, 6, and increasing the number of conditioning sessions leads to placebo analgesia that is both stronger and more resistant to extinction10. A key question is whether enhanced placebo analgesia following multiple conditioning sessions also depends on expectancy. If not, this suggests the existence of a class of placebo analgesia that depends on conditioned associations3 and, like conditioned immunosuppression, is independent of expectations. These placebo effects should depend on the duration of conditioning, be independent of reported expectations, and persist when expectations are reversed.
In order to determine whether conditioned placebo analgesia persists despite subject knowledge of placebo treatment, pain response was tested both before and after a complete and convincing disclosure of the placebo manipulation (Placebo Reveal). To directly measure the role of associative learning in ‘open-label’ placebo effects, we varied the number of conditioning sessions and tested whether Post-Reveal placebo effects were greater for participants who had experienced more conditioning sessions. Critically, we measured expected pain relief both before and after the Placebo Reveal, as non-conscious cues may continue to elicit expectations for pain relief15. We hypothesized that participants who experienced more conditioning would engage mechanisms for placebo analgesia that were independent of reported expectancies, and would continue to show placebo analgesia even when aware that the treatment was a placebo.
Methods
Participants
Fifty-four participants (thirty female, age 18–55) were recruited via online advertisements on a recruitment website managed by the School of Medicine at the University of Colorado Anschutz Medical Campus. Data collection was planned to continue until 40 participants met inclusion criteria and completed the study. Twelve participants were excluded during an initial calibration because they did not find the thermal stimuli sufficiently painful (average pain rating below 30 on a 100-point visual-analog scale (VAS) for a 48 °C stimulus), and two participants stopped participation midway through the study due to discomfort from the heat. It was also required that participants’ pain ratings increased with higher stimulation temperatures during the initial calibration (R2 > .40), but no participants were excluded based on low temperature discriminability. A total of 40 participants were included in the final analysis, 20 in the long conditioning group (Long, 13 female) and 20 in the short conditioning group (Short, 14 female). All participants gave informed consent to participate in a study of treatment effects on pain relief and were fully debriefed at the conclusion of the study. This study was approved by the University of Colorado Boulder Institutional Review Board.
Materials and Procedures
Overview
Participants were informed that they were participating in a study to compare the analgesic effects of a topical cream with an active analgesic component (placebo cream) to a topical cream with no active ingredients (control cream). Following the initial calibration phase, subjects were randomized to long or short conditioning groups and began the conditioning phase of the study. Immediately following the conditioning phase, placebo analgesia was measured during the testing phase both before and after subjects were told the treatment was a placebo (Placebo Reveal) (Figure 1). Placebo analgesia was measured as the difference in reported pain between placebo and control stimulations at identical temperatures. All thermal pain stimulations were delivered from a 16×16 mm thermode (Medoc, Ltd.) and lasted for 20 seconds at peak temperature32.
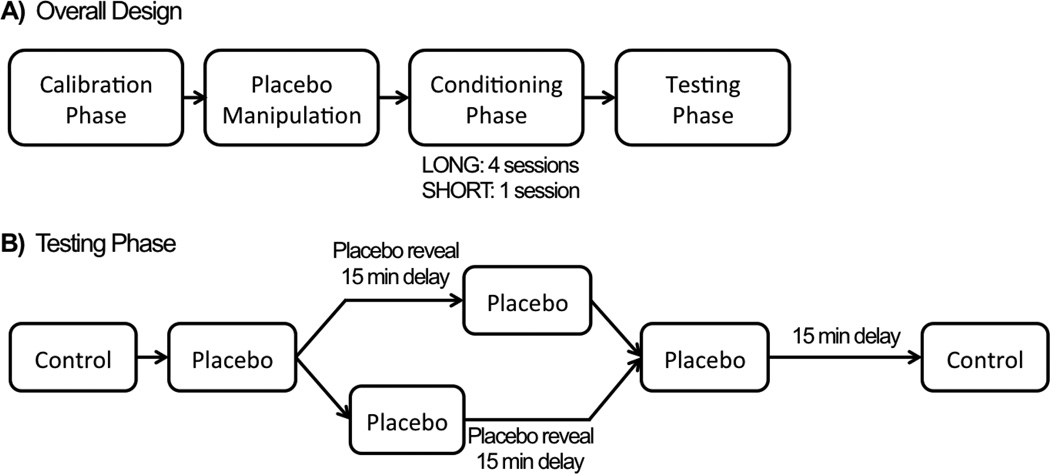
Figure 1.
Study Design. A) Participants in the LONG group had four sessions during the conditioning phase and participants in the SHORT group had a single session. B) During the testing phase, the placebo reveal occurred after the first placebo run for half of all subjects and after the second placebo run for the remaining subjects.
Calibration Phase
During the initial calibration, participants received 16 thermal stimulations on their left forearm at eight different sites. Each site received one high temperature stimulus (45, 46, 47, or 48 °C) and one low temperature stimulus (41, 42, 43, or 44 °C). During stimulation, participants were asked to continuously report how much pain they were experiencing on a on a 100-point VAS, where 0 was ‘no pain experienced’ and 100 was ‘the most pain imaginable’. These continuous pain ratings were averaged within each trial to create a single pain value (equivalent to an area-under-the-curve measure up to scaling) associated with each stimulation. The overall ratings were regressed onto temperature, and the four sites with the lowest residual errors were used for the remainder of the experiment. The regression was used to derive six temperatures for each participant to be used in the remainder of the experiment: two low (ratings from 10–20), two medium (ratings from 30–40), and two high (ratings from 50–60). This difference in pain level between the low and high stimulations has been shown to elicit strong placebo effects24.
Placebo Manipulation
Two creams were used in the study: a control cream and a placebo cream. Both creams were an identical petroleum-based jelly; the only difference between them was the addition of blue food coloring to the placebo cream. Participants were told the placebo cream contained an active analgesic, and were instructed on the nature of the analgesic, including its use, warnings, and potential side effects. Following each application of either cream, participants reported whether they were experiencing any side effects (e.g. drowsiness, swelling, labored breathing) as a result of the cream. During debriefing, all participants indicated that they had believed the placebo cream contained active analgesic components prior to the experimenter revealing otherwise.
Conditioning Phase
During conditioning, experience with the placebo treatment was manipulated by adjusting how many conditioning sessions each participant completed. Participants in the Long group participated in four separate conditioning sessions, while participants in the Short group only participated in a single conditioning session. Conditioning sessions given to participants in the Long group were each given on separate days, with a maximum of seven days of separation between sessions (Mean intervening time = 2.42 +/− 1.0 days). During each conditioning session placebo and control creams were administered block-wise, with the order of the blocks counterbalanced across participants such that half of participants were first presented with control cream blocks during conditioning (Control First) while the other half were first presented with placebo cream blocks (Placebo First), counterbalanced with conditioning group. Participants were fully aware of which cream they had received at all times. Following each cream application, participants rated their expectancies for pain relief from that cream using a 0–100 VAS, where 0 was ‘no pain relief’ and 100 was ‘the most pain relief imaginable’. After a five-minute waiting period the cream was cleaned off of the arm and the thermal stimulation runs were initiated. Each run consisted of two stimulations on each of four sites for a total of eight trials per run. Placebo stimulations used the two low temperatures, and control stimulations used the two high temperatures. Conditioning sessions on days 1–3 for the long group contained two runs within each cream block (16 trials total per cream). However, on the final conditioning day (day 4 for Long, day 1 for Short) there was only a single run of eight stimulations in each cream block. This was designed to reduce the likelihood of habituation during the subsequent testing phase while still providing enough conditioning to develop placebo analgesia in the short group10.
Testing Phase
The testing phase began 15 minutes after the end of the conditioning phase and involved five runs of eight medium temperature stimulations each. The first and last run used the control cream, (runs 1 and 5) while the middle three runs used the placebo cream (runs 2–4). Previously learned beliefs, such as those learned during the conditioning phase, tend to persist even when subjects are given subsequent information that those beliefs may be inaccurate27, 30, so prior to each run, participants were asked to rate how much pain relief they expected from the cream using the same 0 to 100 point VAS discussed previously. Midway through the placebo runs, the true nature of the treatment was revealed and subjects were informed that the placebo treatment was not a pain-relieving cream. In order to ensure that participants truly believed that both treatment creams were inert, the placebo reveal incorporated both demonstration and verbal information, both of which were designed to lead subjects to attribute their previous pain relief to another source. Specifically, participants were told that: a) both creams were identical with the exception of blue food coloring, b) the stimulation temperatures during conditioning had been lowered for the placebo cream, and c) neither cream possessed active analgesic ingredients. Following the reveal, a 15-minute waiting period was imposed prior to resuming the experiment. During this time, the experimenter demonstrated how the placebo cream was made from the control cream to encourage belief in the reveal. Control cream was removed from the canister, mixed with blue food coloring, and then placed into the placebo cream canister. All subjects reported being convinced by this demonstration during debriefing. The placebo reveal and subsequent delay occurred following either the first or second placebo run (counterbalanced, overall testing run 2 or 3). There was an additional 15-minute delay between the fourth and fifth testing run designed to reduce any potential carry-over analgesic effects from placebo to control blocks (Figure 1B).
While the manipulation of belief in the placebo cannot be easily counterbalanced, several aspects of this design mitigate the issue of convolving block order with the reveal2, 16, 23. Using the control cream during the first and final blocks allows the test of whether there is a habituation effect over the entirety of the testing period. If there is no habituation effect, this provides confidence that differences in pain reports following the reveal are not due to simple habituation mechanisms. Additionally, we controlled for any extinction differences in placebo pain reports due to simple repetition of thermal stimulations by adjusting the timing of the reveal between participants. The second placebo run occurs Pre-Reveal for half of the participants and Post-Reveal for the other half. Comparing pain reports during the second placebo run between subjects before and after the reveal allows testing of whether the instructions were effective at increasing placebo pain reports, controlling for the total number of thermal stimulations.
Analysis
Differences in expected analgesia [Placebo - Control] were analyzed using a hierarchical mixed-effects GLM over Reveal State [Pre-Reveal - Post-Reveal] and Conditioning Group [Long - Short]. In addition to the hierarchical GLM, we performed two planned, independent-samples t-tests. The first was used to evaluate differences in Pre-Reveal expected analgesia by Conditioning Group, and the second was used to evaluate differences in Post-Reveal expected analgesia by Conditioning Group.
Placebo analgesia, calculated as [Placebo - Control] differences in pain, was analyzed using a mixed-effects GLM: a 2-within [Placebo - Control] × [Post-Reveal - Pre-Reveal] by 2-between [Long - Short] × [Control First - Placebo First] model, with temperature (two medium-intensity values that varied by subject based on their prior calibration) included as a covariate of no interest. Planned contrasts included testing placebo analgesia both Pre-Reveal and Post-Reveal, the interaction of analgesia with Reveal State, and the interaction of all of the above with Conditioning Group. Given that these contrasts are not orthogonal to each other, we ran two variants of the pain GLM. The first variant was designed to implement a standard ANOVA design, estimating main effects for each factor and all interactions between them. The second variant was identical to the first, save that Pre-Reveal and Post-Reveal analgesia were estimated separately in lieu of the [Placebo - Control] main effect and [Placebo - Control] × [Post-Reveal - Pre-Reveal] interaction, providing planned comparisons of placebo effects Pre-Reveal and Post-Reveal.
We also implemented tests in the hierarchical GLM for Short and Long conditioning groups separately and Control First and Placebo first groups separately. Conditioning order effects [Control First - Placebo First] were not part of our planned contrasts and were originally included in the model to control for order effects. However, conditioning order was strongly predictive of subsequent placebo analgesia, and so we also report results from the [Placebo - Control] × [Post-Reveal - Pre-Reveal] × [Short vs. Long Conditioning] model in the Control First group, which showed the strongest placebo analgesia.
We ran an additional mixed-effects GLM to test for differences in placebo pain Pre-Reveal and Post-Reveal, controlling for the total number of stimulus presentations. This model was a 3-between [Long - Short] × [Control First - Placebo First] × [Post-Reveal - Pre-Reveal] GLM. This model only used pain reports collected from the third testing run where half of the participants were in the Pre-Reveal phase and the other half were in the Post-Reveal phase. The planned contrasts for this model were whether placebo pain was different Pre-Reveal compared to Post-Reveal, and whether that difference varied by conditioning group [Long - Short]. All mixed-effects models of pain were fit using the ‘lme4’ package in R, allowing the within-subject intercepts and slopes to vary as random effects4. When reporting statistics for mixed-effects models, we report F-statistics using the most conservative estimates of degrees of freedom.
Finally, we tested whether placebo analgesia was correlated with expected analgesia both Pre-Reveal and Post-Reveal, and whether that correlation was different between conditioning groups [Long - Short].
Results
Habituation
During the testing phase, pain ratings for control stimulations did not change following the Placebo Reveal (F1,38 = 0.04, p = 0.84) and this effect was not different between conditioning groups (F1.38 = 0.16, p = 0.70). These results suggest an absence of overall habituation or sensitization effects across time during the testing phase.
Expectancy
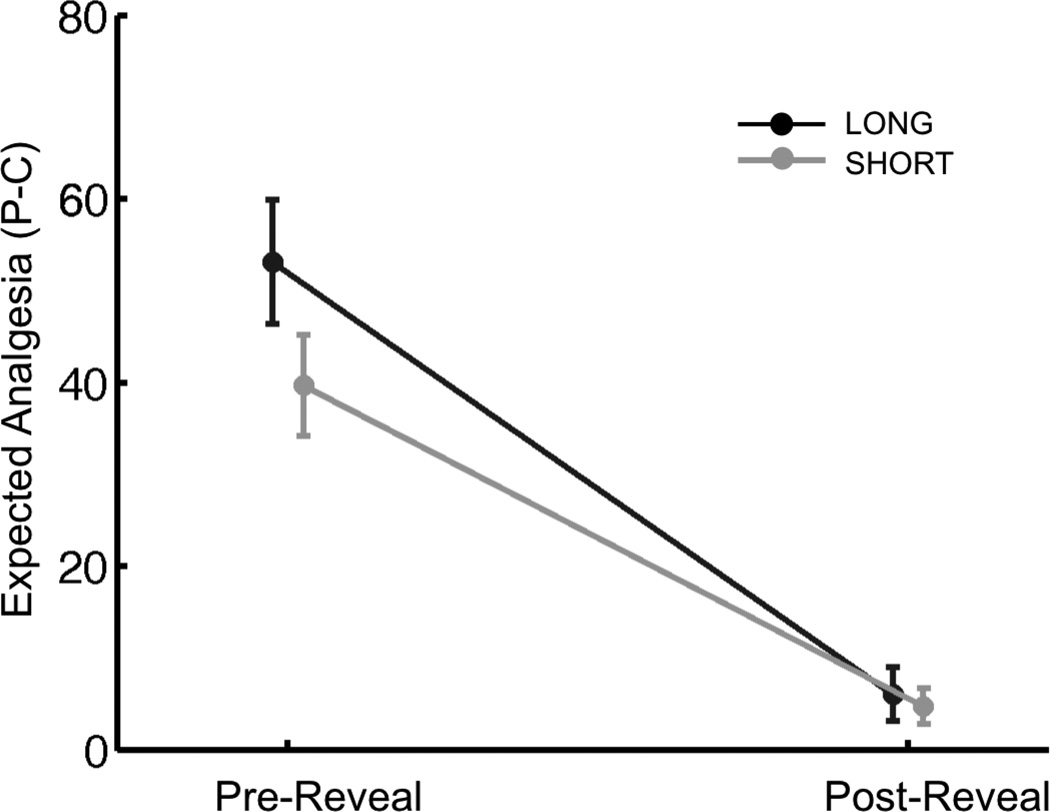
VAS ratings of expected analgesia [Placebo - Control] were higher for the placebo cream compared to the control cream Pre-Reveal (Difference scores of 53.1 ± 13.2 and 39.7 ± 10.7 out of 100 points for Long and Short groups, respectively), and were not different between conditioning groups (t38 = 1.54, p = 0.13). Differences in expected analgesia dropped 41 points on average to near-zero levels (6.0 ± 5.7 and 4.7 ± 3.9 for Long and Short groups, respectively) following the reveal (F1,38 = 81.27, p < .001), and this decrease was not significantly different between conditioning groups (F1,38 = 1.79, p = 0.19). Post-Reveal expected analgesia was not significantly different between conditioning groups (t38 = 0.36, p = 0.72) (Figure 2). The reduction in expected analgesia following the reveal was primarily driven by changes in expectancy for the placebo cream (F1,38 = 87.86, p < 0.001), with no significant changes in expectancy for the control cream (F1,38 = 0.06, p = 0.81) (Supplementary Data 1).
Figure 2.
Expectancy. Expected Analgesia [Placebo - Control] significantly decreased following the reveal in both long and short conditioning groups, with no significant differences between groups. The solid dark grey and light grey lines represent the Long and Short group, respectively. Data are presented as means ± sem.
Placebo Analgesia
The main effect of placebo analgesia [Placebo - Control] was significant (F1,36 = 5.30, p = 0.027) and marginally greater within the Long conditioning group (F1,36 = 4.07, p = 0.051). Overall, placebo analgesia decreased marginally following the reveal (F1,36 = 3.69, p = 0.063). This change was not different between conditioning groups (F1,36 = 0.20, p = 0.66). Removing the temperature covariate from the model had no effect on the significance of these or other contrasts of placebo analgesia discussed below.
Placebo × Reveal State
We found that the reveal resulted in increased pain under Placebo conditions by 1.9 points on average (F1,36 = 11.30, p = 0.002). This increase was not significantly different between Long and Short participants (F1,36 = 0.05, p = 0.83).
Pre-Reveal
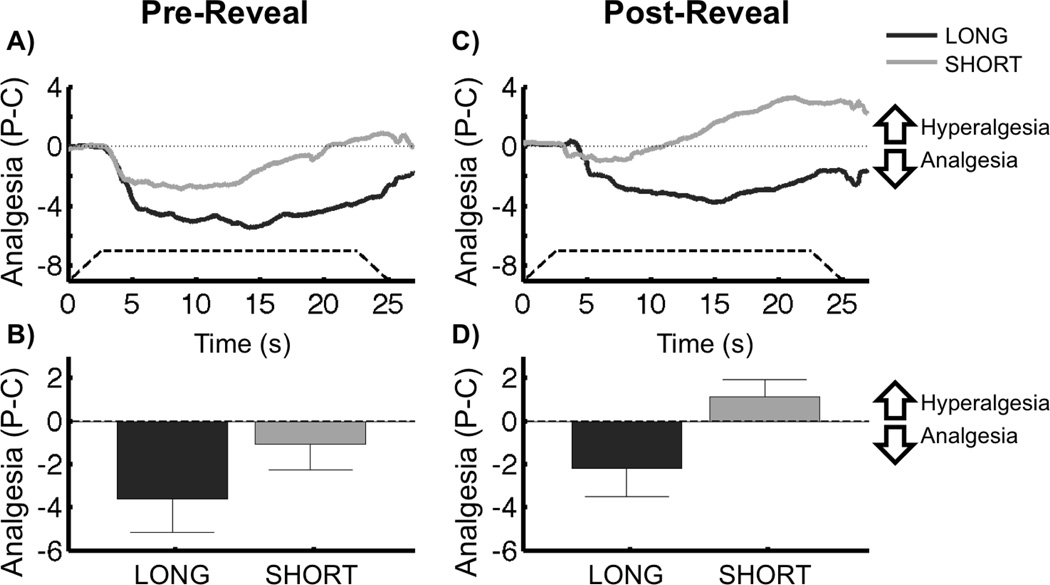
Pre-Reveal, placebo pain ratings were lower than control pain ratings on average (F1,36 = 7.89, p = 0.008), and were not significantly different between conditioning groups (F1,36 = 1.88, p = 0.18). However, Pre-Reveal placebo analgesia was significant in the Long group (F1,36 = 8.74, p = 0.005), but not the Short group (F1,36 = 1.03, p = 0.32) (Figure 3A,B). A post-hoc analysis revealed that Pre-Reveal Placebo Analgesia was not correlated with the time interval between conditioning sessions in the Long conditioning group (F1,35 = 0.03, p = 0.88).
Figure 3.
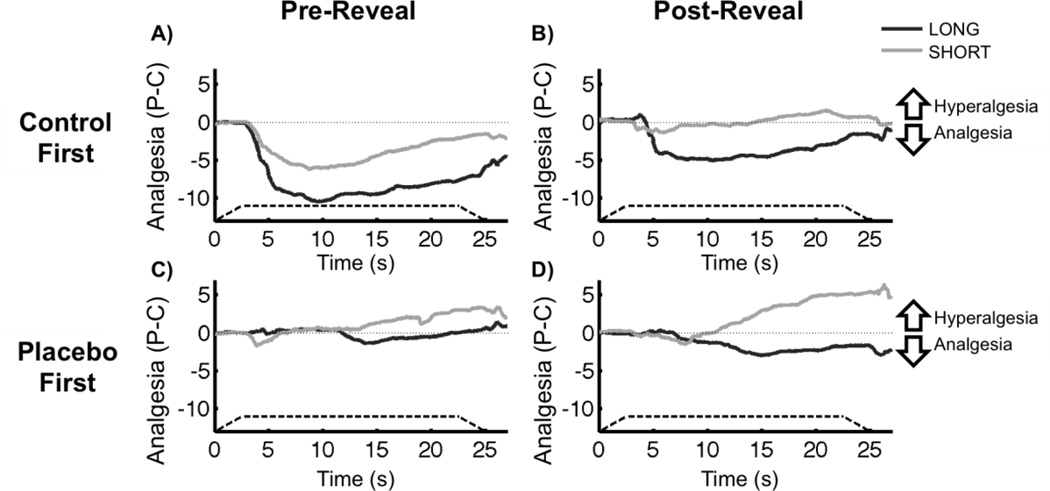
Placebo Analgesia × Conditioning Group. A) Average time course of Pre-Reveal placebo analgesia [Placebo – Control] during painful stimulation. Negative values indicate reduced pain with the placebo (analgesia), whereas positive values indicate increased pain with the placebo (hyperalgesia). The solid dark grey and light grey lines represent the average of subjects within the Long and Short conditioning groups, respectively. The dashed line shows the onset and offset of the painful stimulus, as well as the duration of peak temperature. B) Average time course of Post-Reveal placebo analgesia during painful stimulation. The lines are defined as in panel A. C) Mean Pre-Reveal placebo analgesia. Analgesia scores are generated by averaging over the placebo effect [Placebo-Control] time course for each subject. Long subjects had significant placebo analgesia Pre-Reveal, but Short subjects did not. Placebo analgesia was not different between groups. D) Mean Post-Reveal placebo analgesia. Long subjects continued to demonstrate placebo analgesia Post-Reveal, and had significantly greater placebo analgesia than the Short group. There was no significant Post-Reveal placebo effect in the Short group. Error bars represent sem.
Post-Reveal
Post-Reveal, placebo analgesia was not significant on average across both conditioning groups (F1,36 = 0.90, p = 0.35). However, the Long group demonstrated placebo analgesia (F1,36 = 4.55, p = 0.040) and this analgesia was significantly greater than that reported by the Short group (F1,36 = 4.27, p = 0.046) who did not have a significant placebo response (F1,36 = 0.62, p = 0.44) (Figure 3C,D). A post-hoc analysis revealed that Post-Reveal Placebo Analgesia was not correlated with the time interval between conditioning sessions in the Long conditioning group (F1,35 = 0.15, p = 0.70).
Placebo Analgesia × Expectancy
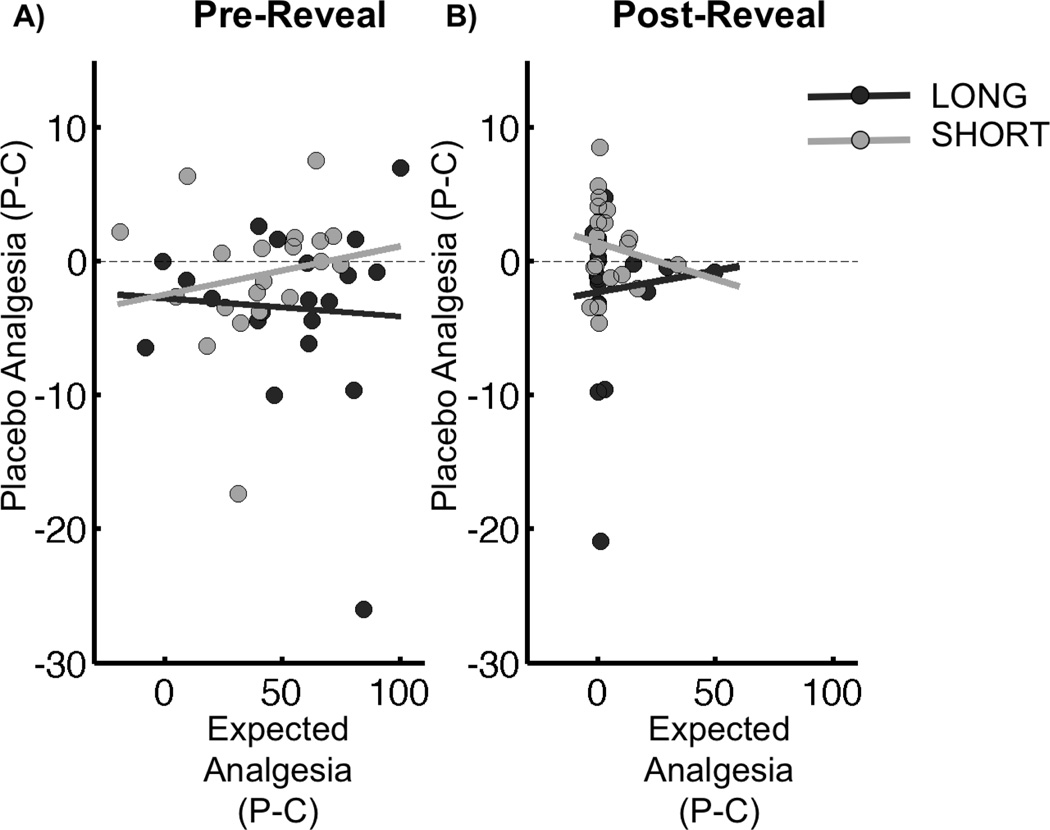
There was no relationship between expected analgesia and placebo analgesia either Pre-Reveal (t38 = −0.14, p = 0.89) or Post-Reveal (t38 = −0.04, p = 0.96) (Figure 4).
Figure 4.
Placebo Analgesia × Expected Analgesia. In both conditioning groups, placebo analgesia [Placebo – Control] was not correlated with expected analgesia [Placebo-Control] either A) Pre-Reveal or B) Post-Reveal. In both panels, the dark grey line represents the relationship between expected and reported analgesia in the Long group, with the dark grey circles representing individual subjects. Similarly, the light grey line represents the relationship between expected and reported analgesia in the Short group, with light grey circles representing individual subjects.
Conditioning Order
Overall, subjects who were exposed to the control cream (and high pain) before the placebo cream (and low pain) during the initial conditioning sessions [Control First - Placebo First] demonstrated stronger placebo effects than subjects with the reversed conditioning order (F1,36 = 7.10, p = 0.011). This conditioning order effect was significantly greater Pre-Reveal compared to Post-Reveal (F1,36 = 4.78, p = 0.035). Specifically, having a Control First conditioning order was associated with stronger Pre-Reveal placebo effects on average (F1,36 = 10.44, p = 0.003) (Figure 5). Post-Reveal, there was no effect of conditioning order on placebo analgesia (F1,36 = 1.26, p = 0.27). These effects were not different between conditioning groups either Pre-Reveal (F1,36 = 0.50, p = 0.48) or Post-Reveal (F1,36 < 0.01, p = 0.96).
Figure 5.
Placebo Analgesia by Conditioning Order, Conditioning Group, and Reveal State. A) Average time course of Pre-Reveal placebo analgesia [Placebo – Control] during painful stimulation for subjects who received the Control First order. Negative values indicate reduced pain with the placebo (analgesia), whereas positive values indicate increased pain with the placebo (hyperalgesia). The solid dark grey and light grey lines represent the average of subjects within the Long and Short conditioning groups, respectively. The dashed line shows the onset and offset of the painful stimulus, as well as the duration of peak temperature. On average, subjects who received the Control First order had significant placebo analgesia, and this effect was not significantly different between conditioning groups (Long – Short). B) Average time course of Post-Reveal placebo analgesia during painful stimulation for subjects who received the Control First order. The lines are defined as in panel A. Post-Reveal, Long subjects, but not Short subjects, who received the Control First order continued to demonstrate placebo analgesia. C) Average time course of Pre-Reveal placebo analgesia during painful stimulation for subjects who received the Placebo First order The lines are defined as in panel A. Pre-Reveal, there was no significant placebo effect for subjects who received the Placebo First conditioning order. D) Average time course of Post-Reveal placebo analgesia during painful stimulation for subjects who received the Placebo First order. The lines are defined as in panel A. Post-Reveal, Placebo First subjects did not show significant analgesia or hyperalgesia on average.
Subjects in the Control First order demonstrated significant Pre-Reveal placebo analgesia (F1,36 = 18.24, p < 0.001) that did not vary by Conditioning Group (F1,36 = 2.16, p = 0.15), while subjects in the Placebo First order had no Pre-Reveal placebo response (F1,36 = 0.09, p = 0.77). Therefore, it was critical to conduct a secondary post-hoc test to examine how placebo analgesia changed Post-Reveal specifically among Control First subjects. Post-Reveal, within the Control First order group, Long subjects continued to demonstrate placebo analgesia (F1,36 = 4.19, p = 0.048), while Short subjects did not (F1,36 < 0.01, p = 0.98). There was no significant difference between these groups (F1,36 = 2.04, p = 0.16). Within the Placebo First order, there was a non-significant trend of Post-Reveal hyperalgesia among the Short conditioning group (F1,36 = 1.30, p = 0.26), and no Post-Reveal placebo effect in the Long subjects (F1,36 = 0.94, p = 0.34).
The effect of conditioning order on placebo analgesia was not explainable in terms of habituation or other adaptation effects as in previous studies36, because participants in both order conditions received the same Control – Placebo – Placebo – Control sequence during the test session. Similarly, Expectancy (Placebo - Control) was not different based on conditioning order either Pre-Reveal (t38 = −1.3, p = 0.20) or Post-Reveal (t38 = 1.0, p = 0.32) suggesting that the observed conditioning order effects cannot be explained by differences in expected analgesia.
Discussion
This study demonstrates that multiple sessions of conditioning can lead to placebo analgesia that persists even when the true nature of the placebo treatment is convincingly revealed. Furthermore, there were no detectable differences in Post-Reveal expected analgesia between the Long and Short groups, even though Post-Reveal placebo analgesia was significantly greater in the Long group. Together, these results suggest that processes not explicitly associated with reported expectancies can mediate conditioned placebo analgesia.
These results parallel emerging evidence from other studies demonstrating that placebo analgesia may occur when subjects know they have received a placebo. In one study, placebo treatments reduced symptoms of irritable bowel syndrome and measures of clinical function even when participants were told the medication was an inert placebo17, while in another study, informing subjects that previous tests were performed using a placebo did not inhibit subsequent placebo analgesia, where subjects were again told they were receiving a real analgesic8. However, in both studies subjects were encouraged to believe the placebo to be effective by either imagining it to assist their own healing processes or actually believing it to be an active treatment. A critical difference between the current study and previous studies is that subjects knew the treatment was a placebo, were explicitly told that it had no analgesic properties, and were not led to expect relief from a placebo treatment.
Other studies suggest that initial experiences create persistent beliefs that are subsequently hard to reverse27, 30, and such studies highlight the resistance of placebo effects to potentially disconfirming information. However, as most “open-label placebo” studies17 and studies demonstrating conditioned physiological effects1,30, 13 have not explicitly measured belief in the placebo, these studies may demonstrate the persistence of beliefs themselves. Our results provide new information by demonstrating conditioned placebo analgesia in response to a treatment that participants believe to be ineffective.
The observed conditioning order effects are very important with respect to future studies on conditioned placebo analgesia, as the conditions under which placebo effects are maximized are not well understood. Previous work has demonstrated that eliminating the association between placebo cues and pain relief during the initial conditioning session inhibited acquisition of the placebo effect even when subsequent presentations of the placebo cues were paired with pain relief9. Here, we found that even if the initial placebo cues are associated with pain relief, subsequent placebo analgesia is impaired if those cues are presented before receiving the high pain stimulations experienced during control blocks. In particular, subjects in the Short conditioning group who received the Placebo First order appeared to have a mild hyperalgesic response to the placebo. While not significant, this hyperalgesia likely contributed to the finding that Short subjects reported less placebo analgesia than Long subjects Post-Reveal. Critically, both Long and Short subjects in the Control First order reported analgesia Pre-Reveal, though only the Long subjects continued to experience analgesia Post-Reveal.
The mechanisms underlying the effect of conditioning order on analgesia are unclear and deserve further investigation at both the psychological and neural level. Acquisition of placebo analgesia may rely on a reduction in stress that favors subsequent learning effects20. The feeling of relief when transitioning from the control cream to the placebo cream could enhance placebo analgesia11, while the stress of transitioning from the placebo cream condition to the more painful control cream condition could inhibit acquisition of placebo analgesia20, and even induce hyperalgesia5. The reduced placebo analgesia in the Placebo First group may also result from a relative judgment effect. Experiencing the more painful control stimuli followed by less painful placebo conditioning stimuli invites a relative comparison that focuses on pain reduction with the placebo, while beginning with the mildly painful placebo stimulus instead highlights the painfulness of the placebo stimulation absent the context of the more painful control stimuli. Focus on the painfulness of the placebo stimuli may inhibit acquisition of placebo analgesia similar to when the pain is not reduced for the placebo9.
There were several limitations to this study. Given that participants had participated in all experimental sessions with the same experimenter, the experimenter was never blinded to which conditioning group subjects were in. This could be mitigated in the future by having a separate experimenter conduct the conditioning portion of the study. A second limitation is that there is no titration of expectation in our manipulation as we believed it was important for subjects to believe they had received an analgesic during conditioning given that certainty in a treatment typically leads to stronger placebo analgesia than uncertainty22. Future studies could include subject groups who are told they “may have” or “have not” received an analgesic during conditioning sessions. There is some difficulty in interpreting the relationship between expectancy and analgesia Post-Reveal, as expected analgesia was near zero with little variance. Finally, subjects who received the Placebo First order did not acquire placebo analgesia Pre-Reveal. Future studies aiming to condition placebo analgesia should use a Control First, as opposed to Placebo First, order9.
To our knowledge, this is the first study to demonstrate reduced pain to a conditioned stimulus in healthy subjects who are fully aware that they are receiving an inert treatment without the use of any pharmacological agents. Here, we have demonstrated that we can use different levels of conditioning to elicit reliably different effects from an expectation reversal, and demonstrate that conditioning can suppress the effects of a reversal of beliefs even when no difference in expectations is observed between conditioning groups. This has several implications for medical contexts. We speculate that some variant of the design used in this study may be used to wean patients with acute pain (e.g. post-operative) off of painkillers in a way that could lower the potential for future addiction by substituting a placebo for a chemically active treatment while simultaneously keeping pain reduced. We suspect that the repeated administration of a (potentially non-opioid) analgesic2 combined with a subsequent enhancement of expected analgesia from a placebo treatment17 would yield the strongest placebo effects. In addition to differences in analgesia by conditioning group, differences by conditioning order may explain why people experience either relief or symptom worsening from a variety of medical treatments based on differences in beliefs and prior learning, highlighting the importance of psychology in medicine.
Supplementary Material
Perspective.
This article demonstrates a form of placebo analgesia that relies on prior conditioning rather than current expected pain relief. This highlights the importance of prior experience on pain relief, and offers insight into the variability of placebo effects across individuals.
Acknowledgments
Research reported in this publication was supported by the National lnstitute of Mental Health of the National lnstitutes of Health under Award Number R01MH076136, by the National Institutes of Health under Grant Number 5F31DA034516-03 and by the Intramural Program of the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Albring A, Wendt L, Benson S, Witzke O, Kribben A, Engler H, Schedlowski M. Placebo effects on the immune response in humans: the role of learning and expectation. PloS one. 2012;7:e49477. doi: 10.1371/journal.pone.0049477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanzio M, Benedetti F. Neuropharamcological Dissection of Placebo Analgesia: Expectation-Activated Opiod Systems versus Conditioning-Activated Specific Subsystems. The Journal of Neuroscience. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Au Yeung ST, Colagiuri B, Lovibond PF, Colloca L. Partial reinforcement, extinction, and placebo analgesia. Pain. 2014;155:1110–1117. doi: 10.1016/j.pain.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0.6. 2014 [Google Scholar]
- 5.Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious Expectation and Unconscious Conditioning in Analgesic, Motor, and Hormonal Placebo/Nocebo Responses. The Journal of Neuroscience. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science translational medicine. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 8.Chung SK, Price DD, Verne GN, Robinson ME. Revelation of a personal placebo response: its effects on mood, attitudes and future placebo responding. Pain. 2007;132:281–288. doi: 10.1016/j.pain.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124:126–133. doi: 10.1016/j.pain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151:430–439. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaten MA, Aslaksen PM, Lyby PS, Bjorkedal E. The relation of emotions to placebo responses. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366:1818–1827. doi: 10.1098/rstb.2010.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13124–13131. doi: 10.1073/pnas.0404965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goebel MU, Trebst AE, Steiner J, Xie YF, Exton MS, Frede S, Canbay AE, Michel MC, Heemann U, Schedlowski M. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 2002;16:1869–1873. doi: 10.1096/fj.02-0389com. [DOI] [PubMed] [Google Scholar]
- 14.Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia--when the spine echoes what the brain expects. Pain. 2007;130:137–143. doi: 10.1016/j.pain.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jepma M, Jones M, Wager TD. The dynamics of pain: Evidence for simultaneous site-specific habituation and site-nonspecific sensitization in thermal pain. Pain. 2014;15:734–746. doi: 10.1016/j.jpain.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, Kowalczykowski M, Miller FG, Kirsch I, Lembo AJ. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PloS one. 2010;5:e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirsch I, Kong J, Sadler P, Spaeth R, Cook A, Kaptchuk TJ, Gollub R. Expectancy and conditioning in placebo analgesia: Separate or connected processes? Psychology of Consciousness: Theory, Research, and Practice. 2014;1:51–59. doi: 10.1037/cns0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirsch I, Lynn SJ, Vigorito M, Miller RR. The role of cognition in classical and operant conditioning. Journal of clinical psychology. 2004;60:369–392. doi: 10.1002/jclp.10251. [DOI] [PubMed] [Google Scholar]
- 20.Lyby PS, Aslaksen PM, Flaten MA. Is fear of pain related to placebo analgesia? Journal of psychosomatic research. 2010;68:369–377. doi: 10.1016/j.jpsychores.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 22.Pollo A, Amanzio M, Arslanian A, Casadio C, Giuliano M, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 23.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annual review of psychology. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 25.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 26.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Classical conditioning II: Current research and theory. In: Black AH, Prokasy WF, editors. Appleton-Century-Crofts. 1972. pp. 64–99. [Google Scholar]
- 27.Ross L, Lepper MR, Hubbard M. Perseverance in self-perception and social perception: Biased attributional processes in the debriefing paradigm. Journal of Personality and Social Psychology. 1975;32:880–892. doi: 10.1037//0022-3514.32.5.880. [DOI] [PubMed] [Google Scholar]
- 28.Sandler AD, Bodfish JW. Open-label use of placebos in the treatment of ADHD: a pilot study. Child: care, health and development. 2008;34:104–110. doi: 10.1111/j.1365-2214.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 29.Sandler AD, Glesne CE, Bodfish JW. Conditioned placebo dose reduction: a new treatment in attention-deficit hyperactivity disorder? Journal of developmental and behavioral pediatrics : JDBP. 2010;31:369–375. doi: 10.1097/DBP.0b013e3181e121ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staudinger MR, Buchel C. How initial confirmatory experience potentiates the detrimental influence of bad advice. NeuroImage. 2013;76:125–133. doi: 10.1016/j.neuroimage.2013.02.074. [DOI] [PubMed] [Google Scholar]
- 31.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychological bulletin. 2004;130:324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- 32.Vase L, Petersen GL, Riley JL, 3rd, Price DD. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain. 2009;145:36–44. doi: 10.1016/j.pain.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Voudouris NJ, Peck CL, Coleman G. Conditioned Placebo Responses. Journal of Personality and Social Psychology. 1985;48:47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- 34.Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- 35.Wager TD, Fields HL. Placebo Analgesia. In: Wall PD, Melzack R, editors. Textbook of Pain. 2013. pp. 362–373. [Google Scholar]
- 36.Wager TD, Matre D, Casey KL. Placebo effects in laser-evoked pain potentials. Brain, behavior, and immunity. 2006;20:219–230. doi: 10.1016/j.bbi.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson A, El-Deredy W, Bentley DE, Vogt BA, Jones AK. Categories of placebo response in the absence of site-specific expectation of analgesia. Pain. 2006;126:115–122. doi: 10.1016/j.pain.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AK. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.