Abstract
Background
Castration-resistant progression of prostate cancer after androgen deprivation therapy remains a critical challenge in the clinical management of prostate cancer. Resurgent androgen receptor activity is an established driver of castration-resistant progression, and upregulation of androgen receptor expression has been implicated to contribute to the resurgent androgen receptor activity. We reported previously that methylselenocysteine can decrease the expression and activity of androgen receptor. Here we investigated the ability of methylselenocysteine to inhibit castration-resistant progression of prostate cancer.
Methods
The regrowth of LNCaP prostate cancer xenografts after castration was monitored. The levels of prostate-specific antigen in mouse serum were measured by ELISA. Tumor cell proliferation and apoptosis were analyzed via Ki-67 immunohistochemistry and TUNEL assay, respectively. Intratumoral angiogenesis was assessed by immunohistochemistry staining of vascular endothelial growth factor and CD31.
Results
We showed that methylselenocysteine delayed castration-resistant regrowth of LNCaP xenograft tumors after androgen deprivation. This was accompanied by decreased serum levels of prostate-specific antigen, inhibition of prostate cancer cell proliferation and tumor angiogenesis, as well as downregulation of androgen receptor and induction of apoptosis in the relapsed tumors.
Conclusions
The present study represents the first to show the preclinical efficacy of methylselenocysteine in delaying castration-resistant progression of prostate cancer. The findings provide a rationale for evaluating the clinical application of combining methylselenocysteine with androgen deprivation therapy for the treatment of advanced prostate cancer.
Keywords: methylselenocysteine, prostate cancer, castration resistance
INTRODUCTION
As the first-line treatment for disseminated prostate cancer, androgen deprivation therapy disrupts androgen receptor (AR) signaling through androgen ablation or AR antagonists. While this regimen is effective initially, progression to castration-resistant prostate cancer (CRPC) is almost inevitable [1]. Despite recent advances in prostate cancer therapy, metastatic CRPC remains incurable. Resurgent AR activity is an established driver of castration-resistant progression of prostate cancer [2, 3].
Prostate cancer can adapt to androgen deprivation therapy by mutating AR, amplifying AR, upregulating constitutively-active AR splice variants, activating AR by androgen-independent mechanisms, and/or increasing intra-tumoral androgen levels through de novo androgen synthesis [4–17]. Importantly, the expression of AR was shown to be prevalently upregulated in prostate tumors that relapsed after androgen deprivation [10]. Moreover, elevated expression of AR was shown to sensitize the receptor to low levels of androgen [18] and to convert prostate cancer growth from a castration-sensitive to a castration-resistant stage [10]. Knocking down AR by shRNA in xenograft models can delay the progression of prostate cancer to castration resistance [19]. Therefore, therapeutic approaches that can diminish the availability of AR should offer considerable benefit in preventing or delaying prostate cancer recurrence after androgen deprivation therapy.
Previously, we showed that methylselenocysteine (CH3-Se-CH2-CH(NH2)-COOH, MSC), a methyl-selenium compound, is effective in downregulating the expression of AR and AR target, such as the prostate-specific antigen (PSA), in both the castration-sensitive LNCaP prostate tumor xenografts and the castration-resistant 22Rv1 prostate tumor xenografts, leading to inhibited growth of these tumors [20, 21]. In the present study, we tested the hypothesis that MSC prevents or delays prostate cancer recurrence after androgen deprivation. It is important to point out that MSC has a very different biological and pharmacological activity than selenomethionine, the form of selenium used in the selenium and vitamin E chemoprevention trial [22–27], and that MSC has shown no evidence of toxicity in a single-dose Phase I trial [28].
MATERIALS AND METHODS
Cell Lines and Reagents
LNCaP cells were obtained from American Type Culture Collection at Passage 4. The cells used in this study were within 20 passages (~3 months of non-continuous culturing) and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 unit/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. MSC was obtained from PharmaSe.
Tumor Xenograft Models
Male nude mice were obtained from the Vital River Laboratories Co. Ltd. at 5–6 weeks of age. After one week of adaptation, mice were inoculated subcutaneously with 4×106 LNCaP cells suspended in 50% Matrigel on the right flank. When the tumor size reached ~65 mm3, castration was performed via a scrotal approach. The day following castration, the mice were randomly assigned to two groups, viz., vehicle control (saline) and 5 mg/kg/day MSC through oral gavage [21, 29, 30]. The tumor dimensions and body weights were measured weekly. Tumor volume was calculated as 0.524 x width2 x length [31]. At the termination of the experiment, mice were anesthetized with Ketamine/Xylazine, blood collected for serum PSA determination, and euthanized by CO2. Tumors were removed, weighed, and fixed in 10% formalin for paraffin embedding and histological analyses. All animal procedures were approved by the Jilin University Institutional Animal Care and Use Committee.
Determination of Serum PSA Levels
Serum PSA levels were quantified with the use of the quantitative ELISA kit (United Biotech) per instruction of the manufacturer.
Immunohistochemical Analyses
Immunohistochemistry (IHC) was conducted as described previously [32] with a Ki-67 (NovoCastra), vascular endothelial growth factor (VEGF; abcam), or CD31 (NovoCastra) primary antibody at a 1:200, 1:500, or 1:500 dilution, respectively. As the negative control, the primary antibody was replaced with a non-immune IgG at the same concentration, and no reactivity was detected. Apoptosis was detected by the TUNEL assay using the DeadEnd™ Colorimetric TUNEL System (Promega) per manufacturer’s protocol. Five random microscopic fields were captured for each tumor section at 10x magnification, and the images were analyzed by using the ImmunoRatio online analysis tools [33]. For Ki-67 and VEGF staining as well as the TUNEL results, the percentage of positively stained cells was calculated for each image. The data are presented as average % of positively stained cells in each group. For CD31 staining, the ratio of vessel area to total analyzed area was calculated by using the Microvessel Analysis V1 software.
Statistical Analysis
The Student’s two-tailed t test was used to determine the mean differences between treatment and control, and P < 0.05 was considered statistically significant. Data are presented as mean ± SEM.
RESULTS
MSC Inhibition of Castration-Resistant Progression of LNCaP Tumors
We evaluated the effect of MSC on prostate cancer recurrence after androgen deprivation therapy in the LNCaP xenograft model. LNCaP is an androgen-dependent human prostate cancer cell line [34]. The development of LNCaP xenograft tumors requires androgens, and castration causes an initial decelerated growth of the xenografts [35, 36]. However, similar to human prostate cancers, the xenografts eventually become castration resistant and resume growth [35–39]. Male nude mice were implanted with LNCaP cells on the right dorsal flank. Castration was performed when the tumors reached ~65 mm3. Castrated mice were then divided into two groups receiving either saline as control or 5 mg/kg/day MSC through oral gavage. The dose of MSC was the same as we previously used in the study with castration-sensitive LNCaP tumor xenografts [20]. Treatment was initiated the day following castration.
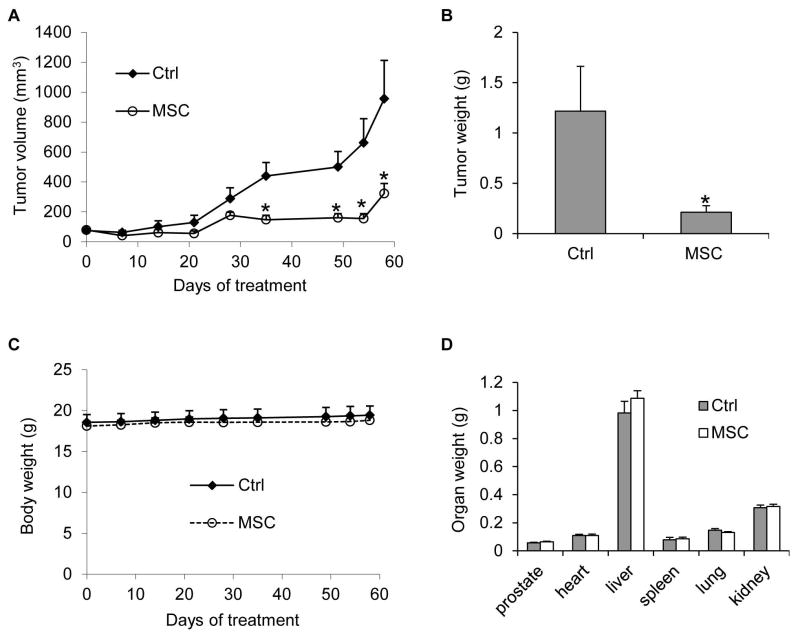
The tumors in the control group resumed growth starting from day 21 after castration as a result of acquiring castration resistance, whereas the tumors in the MSC group remained small (Figure 1A). From day 35, the difference in the average tumor size between the two groups became statistically significant. At the termination of the experiment, the average tumor size was 956 mm3 in the control group, but 323 mm3 in the MSC group, indicating ~66% inhibition of tumor regrowth by MSC (Figure 1A). In line with this result, the average tumor weights were 1.2 g and 0.2 g in control and MSC groups, respectively (Figure 1B). During the 58-day period of treatment, MSC did not appear to cause toxicity in the mice as indicated by no decrease in body weight (Figure 2A) or weights of various organs, including the prostate, compared to the control group (Figure 2B). Collectively, the data showed the potential of MSC to inhibit prostate cancer relapse after androgen deprivation therapy without causing overt toxicity.
Figure 1. MSC inhibition of castration-resistant progression of LNCaP xenograft tumors.
LNCaP cells were inoculated into gonad-intact nude mice, and surgical castration was performed when the tumors reached ~ 65 mm3. Treatment with 5 mg/kg/day MSC through oral gavage was initiated the day following castration (n = 5). A. Tumor volumes. B. Tumor weights at the conclusion of the experiment. C. Mouse body weights. D. Weights of various organs at the conclusion of the experiment. *, P < 0.05 from the control group. Error bars, SEM.
Figure 2. MSC suppression of rise in serum PSA levels during castration-resistant progression of LNCaP xenograft tumors.
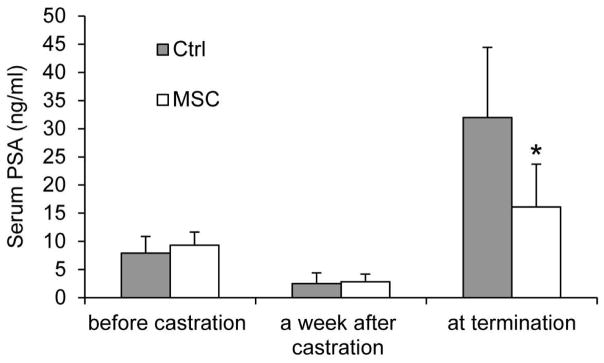
Serum PSA levels were determined by ELISA before castration, a week after castration, and at the conclusion of the study. *, P < 0.05 from the control group. n = 5. Error bars, SEM.
MSC Reduction of Serum PSA Levels
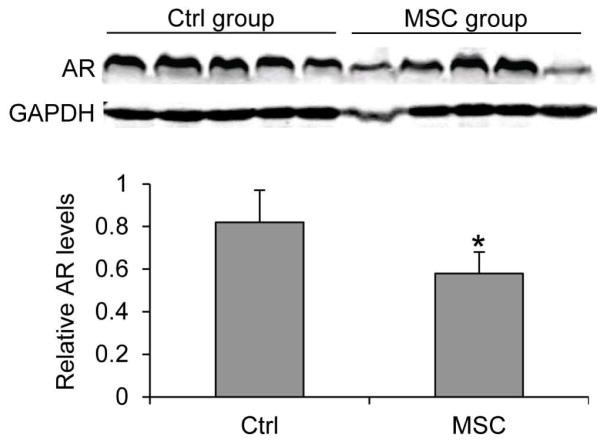
Prostate tumor relapse after castration is associated with elevated PSA levels in the serum [40] and sustained or increased AR levels in the tumors [2, 3]. We measured the levels of PSA in mouse serum using ELISA. As shown in Figure 2, MSC supplementation led to an almost 50% drop in mean serum PSA level at the termination of the experiment, from 32 ng/ml in the control group to 16 ng/ml in the MSC group. We also measured AR protein expression by Western blotting. As shown in Figure 3, MSC treatment decreased the mean level of AR protein in the tumors by ~30%. The data thus support the ability of MSC to suppress rise in serum PSA levels after androgen deprivation therapy and to reduce AR protein levels in castration-resistant prostate tumors.
Figure 3. MSC downregulation of AR expression in castration-resistant LNCaP xenograft tumors.
AR protein levels in the tumors were determined by Western blotting. Densitometric quantitation of the Western data is shown below the blots. *, P < 0.05 from the control group. Error bars, SEM.
MSC Inhibition of Cell Proliferation and Induction of Apoptosis
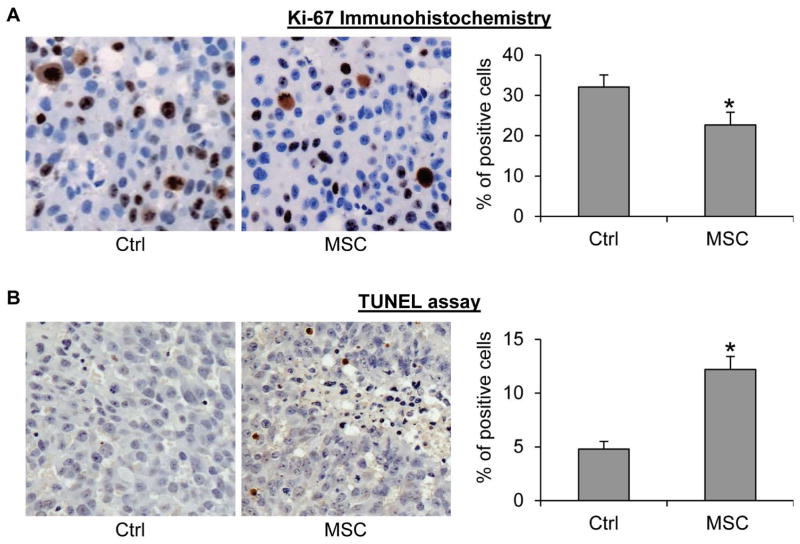
We further evaluated the effect of MSC on proliferation and apoptosis in the tumors via immunohistochemistry using an antibody against Ki-67, a marker of cell proliferation, and TUNEL assay, respectively. As shown in Figure 4, MSC supplementation caused an approximately 30% reduction of the percentage of Ki-67-positive cells and a 2.5-fold induction of the percentage of apoptotic cells in the tumors. The data thus indicate that inhibition of cell proliferation and induction of apoptosis may underlie MSC inhibition of prostate cancer relapse after androgen deprivation therapy.
Figure 4. MSC inhibition of cell proliferation and induction of apoptosis in castration-resistant LNCaP xenograft tumors.
A. Ki-67 immunohistochemical staining of the tumor sections. B. TUNEL assay of the tumor sections. Left panels, representative images. Right panels, quantitation of the data. *, P < 0.05 from the control group. Error bars, SEM.
MSC Inhibition of Angiogenesis
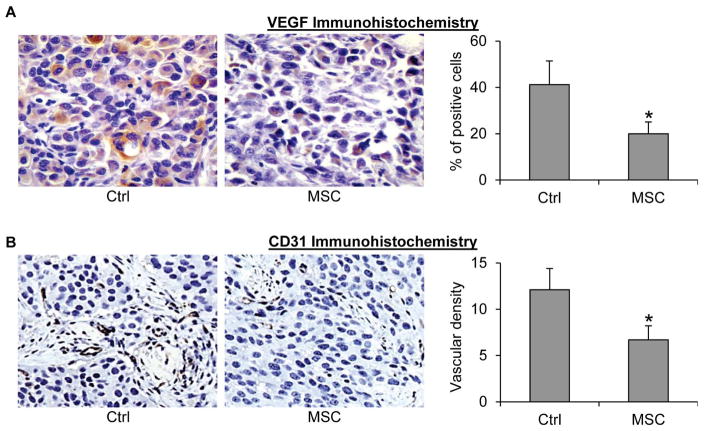
Angiogenesis plays an important role for supporting the growth of prostate tumors, and a recent study reported that castration-resistant prostate cancer is associated with increased angiogenesis [41]. We therefore evaluated the effect of MSC on intratumoral angiogenesis via IHC using VEGF and CD31 as markers. As shown in Figure 5, MSC treatment caused a ~50% reduction of the percentage of VEGF-positive cells and microvessel density, suggesting that inhibition of tumor angiogenesis may represent an additional mechanism by which MSC prevents prostate cancer relapse after androgen deprivation therapy.
Figure 5. MSC inhibition of angiogenesis in castration-resistant LNCaP xenograft tumors.
A. VEGF immunohistochemical staining of the tumor sections. B. CD31 immunohistochemical staining of the tumor sections. Left panels, representative images. Right panels, quantitation of the data. *, P < 0.05 from the control group. Error bars, SEM.
Discussion
We demonstrated previously that MSC inhibits the growth of the castration-sensitive LNCaP prostate tumor xenografts in gonad-intact mice and castration-resistant 22Rv1 prostate tumor xenografts and downregulates AR expression and activity in these tumors [20, 21]. In the present study, we extended the observations to a castration-resistant progression model, showing, for the first time, the potential of using MSC to prevent or delay the development of castration-resistant prostate cancer and to suppress rise in serum PSA levels after androgen deprivation therapy. Mechanistically, MSC downregulates AR expression, inhibits prostate cancer cell proliferation and tumor angiogenesis, as well as induces apoptosis in prostate tumors. The findings provide a strong rationale for a combination therapy using MSC to improve the therapeutic outcome of androgen deprivation therapy.
Selenium has been shown to be an effective anticancer agent in cancers of various sites, including the prostate, by numerous preclinical studies, epidemiological observations, and clinical trials [23, 42]. Although the interim analysis of the Selenium and Vitamin E Chemoprevention Trial (SELECT) indicated that supplementation of healthy individuals with nutritional dose of selenium did not reduce prostate cancer risk [25], the finding should not have been entirely unexpected. The anticancer efficacy of selenium depends on the form and dosage of selenium administered and the stage of the disease progression [22–24, 26, 43]. In fact, animal studies using xenograft models have shown that selenomethionine, the form of selenium used in the SELECT, is ineffective against prostate cancer growth, whereas “second-generation” selenium compounds, including MSC, are very effective in inhibiting tumor growth [24, 26]. This is in concordance with the fact that selenomethionine is converted to the active metabolite, methylselenol, much less efficiently than the much more potent new selenium compounds [22, 23, 27]. Additionally, in most of the preclinical studies that showed a positive association between selenium administration and tumor inhibition, the experiments were conducted by using pharmacological doses of selenium, not the nutritional dose that was used in the SELECT. Therefore, the negative SELECT finding underscores the urgency of studying the efficacy of the new selenium compounds, particularly at pharmacological doses, for prostate cancer intervention. Selenium has a low toxicity profile. While the tolerable upper intake level for selenium is set at 400 μg a day for adults [44], prostate cancer patients supplemented with 3,200 μg selenium per day for an average of one year did not develop any serious selenium-related toxicities [45]. Therefore, selenium supplementation at pharmacological doses will be well tolerated.
Our previous studies showed that MSC, at pharmacological doses, can effectively reduce the abundance of both the full-length and/or the constitutively-active splice variants of AR in LNCaP and 22Rv1 prostate tumor xenografts [20, 21]. Increased expression of the full-length and splice variants of AR has been indicated to be one mechanism of resistance to traditional androgen deprivation therapy and the new androgen deprivation drugs abiraterone and enzalutamide [10, 15, 46–50]. To date, none of the anti-androgens currently used in clinics can target AR directly to reduce its availability. In addition to MSC, several other compounds have been shown pre-clinically to reduce the levels of AR and to inhibit the growth of castration-resistant prostate cancer cells [21, 51–56]. These compounds may serve as an effective antidote to overcoming resistance to androgen deprivation therapies. Determining the combinatory efficacies and the best sequences of treatments is an area of our ongoing research.
Conclusions
In summary, the present study represents the first to show the preclinical efficacy of methylselenocysteine in delaying castration-resistant progression of prostate cancer. The findings not only provide a rationale for further developing MSC or its analogue for intervention of CRPC, but also substantiate reducing AR availability as a viable approach to improve therapeutic outcome of androgen deprivation therapy.
Acknowledgments
This work was supported by the National Cancer Institute of the National Institutes of Health grant K01CA114252, American Cancer Society grant RSG-07-218-01-TBE, Department of Defense grants W81XWH-12-1-0112 and W81XWH-12-1-0275, National Natural Science Foundation of China Projects 81272851, 30973587, 81102383, 81302206, 81302226, and 81430087, Jilin Provincial Science and Technology Department of China Project 20130101164JC, and Jilin Provincial Education Department of China Project 2013191.
Footnotes
Disclosure Statement: The authors declare no competing financial interests.
References
- 1.Lamont KR, Tindall DJ. Minireview: Alternative Activation Pathways for the Androgen Receptor in Prostate Cancer. Mol Endocrinol. 2011;25:897–907. doi: 10.1210/me.2010-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan A, Dong Y, Zhang H, Qi Y, Balk SP, Sartor O. Castration-resistant prostate cancer: Adaptive responses in the androgen axis. Cancer Treat Rev. 2014;40:426–433. doi: 10.1016/j.ctrv.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, Feldman D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 5.Culig Z, Hobisch A, Hittmair A, Peterziel H, Cato AC, Bartsch G, Klocker H. Expression, structure, and function of androgen receptor in advanced prostatic carcinoma. Prostate. 1998;35:63–70. doi: 10.1002/(sici)1097-0045(19980401)35:1<63::aid-pros9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 7.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 8.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 11.Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, Miyamoto M. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–153. [PubMed] [Google Scholar]
- 12.Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GG, Jenster G, Trapman J, Brinkmann AO, Mulder E. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665–669. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida T, Kinoshita H, Segawa T, Nakamura E, Inoue T, Shimizu Y, Kamoto T, Ogawa O. Antiandrogen Bicalutamide Promotes Tumor Growth in a Novel Androgen-Dependent Prostate Cancer Xenograft Model Derived from a Bicalutamide-Treated Patient. Cancer Research. 2005;65:9611–9616. doi: 10.1158/0008-5472.CAN-05-0817. [DOI] [PubMed] [Google Scholar]
- 14.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF, Pretlow TG, Kung HJ. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res. 2002;62:6606–6614. [PubMed] [Google Scholar]
- 18.Waltering KK, Helenius MA, Sahu B, Manni V, Linja MJ, Janne OA, Visakorpi T. Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res. 2009;69:8141–8149. doi: 10.1158/0008-5472.CAN-09-0919. [DOI] [PubMed] [Google Scholar]
- 19.Cheng H, Snoek R, Ghaidi F, Cox ME, Rennie PS. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 2006;66:10613–10620. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- 20.Lee SO, Yeon CJ, Nadiminty N, Trump DL, Ip C, Dong Y, Gao AC. Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA) Prostate. 2006;66:1070–1075. doi: 10.1002/pros.20329. [DOI] [PubMed] [Google Scholar]
- 21.Zhan Y, Cao B, Qi Y, Liu S, Zhang Q, Zhou W, Xu D, Lu H, Sartor O, Kong W, Zhang H, Dong Y. Methylselenol prodrug enhances MDV3100 efficacy for treatment of castration-resistant prostate cancer. Int J Cancer. 2013;133:2225–2233. doi: 10.1002/ijc.28202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- 23.Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998;128:1845–54. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 24.Li GX, Lee HJ, Wang Z, Hu H, Liao JD, Watts JC, Combs GF, Jr, Lu J. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis. 2008;29:1005–1012. doi: 10.1093/carcin/bgn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, III, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Bonorden MJ, Li GX, Lee HJ, Hu H, Zhang Y, Liao JD, Cleary MP, Lu J. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev Res (Phila Pa) 2009;2:484–495. doi: 10.1158/1940-6207.CAPR-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohta Y, Kobayashi Y, Konishi S, Hirano S. Speciation analysis of selenium metabolites in urine and breath by HPLC- and GC-inductively coupled plasma-MS after administration of selenomethionine and methylselenocysteine to rats. Chem Res Toxicol. 2009;22:1795–1801. doi: 10.1021/tx900202m. [DOI] [PubMed] [Google Scholar]
- 28.Marshall JR, Ip C, Romano K, Fetterly G, Fakih M, Jovanovic B, Perloff M, Crowell J, Davis W, French-Christy R, Dew A, Coomes M, Bergan R. Methyl selenocysteine: single-dose pharmacokinetics in men. Cancer Prev Res (Phila) 2011;4:1938–1944. doi: 10.1158/1940-6207.CAPR-10-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10:2561–2569. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 30.Jiang W, Jiang C, Pei H, Wang L, Zhang J, Hu H, Lu J. In vivo molecular mediators of cancer growth suppression and apoptosis by selenium in mammary and prostate models: lack of involvement of gadd genes. Mol Cancer Ther. 2009;8:682–691. doi: 10.1158/1535-7163.MCT-08-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gleave ME, Hsieh JT, Wu HC, von Eschenbach AC, Chung LW. Serum prostate specific antigen levels in mice bearing human prostate LNCaP tumors are determined by tumor volume and endocrine and growth factors. Cancer Res. 1992;52:1598–1605. [PubMed] [Google Scholar]
- 32.Cao B, Liu X, Li J, Liu S, Qi Y, Xiong Z, Zhang A, Wiese T, Fu X, Gu J, Rennie PS, Sartor O, Lee BR, Ip C, Zhao L, Zhang H, Dong Y. 20(S)-protopanaxadiol-aglycone downregulation of the full-length and splice variants of androgen receptor. Int J Cancer. 2013;132:1277–1287. doi: 10.1002/ijc.27754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuominen VJ, Ruotoistenmaki S, Viitanen A, Jumppanen M, Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010;12:R56. doi: 10.1186/bcr2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 35.Jackson JK, Gleave ME, Yago V, Beraldi E, Hunter WL, Burt HM. The suppression of human prostate tumor growth in mice by the intratumoral injection of a slow-release polymeric paste formulation of paclitaxel. Cancer Res. 2000;60:4146–4151. [PubMed] [Google Scholar]
- 36.Miyake H, Pollak M, Gleave ME. Castration-induced up-regulation of insulin-like growth factor binding protein-5 potentiates insulin-like growth factor-I activity and accelerates progression to androgen independence in prostate cancer models. Cancer Res. 2000;60:3058–3064. [PubMed] [Google Scholar]
- 37.Lamoureux F, Thomas C, Yin MJ, Kuruma H, Fazli L, Gleave ME, Zoubeidi A. A novel HSP90 inhibitor delays castrate-resistant prostate cancer without altering serum PSA levels and inhibits osteoclastogenesis. Clin Cancer Res. 2011;17:2301–2313. doi: 10.1158/1078-0432.CCR-10-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubik AA, Gunter JH, Hendy SC, Locke JA, Adomat HH, Thompson V, Herington A, Gleave ME, Pollak M, Nelson CC. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res. 2011;71:5754–5764. doi: 10.1158/0008-5472.CAN-10-2470. [DOI] [PubMed] [Google Scholar]
- 39.Wafa LA, Cheng H, Plaa N, Ghaidi F, Fukumoto T, Fazli L, Gleave ME, Cox ME, Rennie PS. Carbidopa abrogates L-dopa decarboxylase coactivation of the androgen receptor and delays prostate tumor progression. Int J Cancer. 2012;130:2835–2844. doi: 10.1002/ijc.26287. [DOI] [PubMed] [Google Scholar]
- 40.Freedland SJ. Screening, risk assessment, and the approach to therapy in patients with prostate cancer. Cancer. 2011;117:1123–1135. doi: 10.1002/cncr.25477. [DOI] [PubMed] [Google Scholar]
- 41.Tomic TT, Gustavsson H, Wang W, Jennbacken K, Welen K, Damber JE. Castration resistant prostate cancer is associated with increased blood vessel stabilization and elevated levels of VEGF and Ang-2. Prostate. 2012;72:705–712. doi: 10.1002/pros.21472. [DOI] [PubMed] [Google Scholar]
- 42.Meuillet E, Stratton S, Prasad CD, Goulet AC, Kagey J, Porterfield B, Nelson MA. Chemoprevention of prostate cancer with selenium: an update on current clinical trials and preclinical findings. J Cell Biochem. 2004;91:443–458. doi: 10.1002/jcb.10728. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Stampfer MJ, Giovannucci EL, Morris JS, Willett WC, Gaziano JM, Ma J. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst. 2004;96:696–703. doi: 10.1093/jnci/djh125. [DOI] [PubMed] [Google Scholar]
- 44.Institute of Medicine FaNB. Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 45.Reid ME, Stratton MS, Lillico AJ, Fakih M, Natarajan R, Clark LC, Marshall JR. A report of high-dose selenium supplementation: response and toxicities. Journal of Trace Elements in Medicine and Biology. 2004;18:69–74. doi: 10.1016/j.jtemb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. Resistance to CYP17A1 Inhibition with Abiraterone in Castration-Resistant Prostate Cancer: Induction of Steroidogenesis and Androgen Receptor Splice Variants. Clinical Cancer Research. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao B, Qi Y, Zhang G, Xu D, Zhan Y, Alvarez X, Guo Z, Fu X, Plymate SR, Sartor O, Zhang H, Dong Y. Androgen receptor splice variants activating the full-length receptor in mediating resistance to androgen-directed therapy. Oncotarget. 2014;5:1646–1656. doi: 10.18632/oncotarget.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2012;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Cao B, Liu X, Fu X, Xiong Z, Chen L, Sartor O, Dong Y, Zhang H. Berberine Suppresses Androgen Receptor Signaling in Prostate Cancer. Molecular Cancer Therapeutics. 2011;10:1346–1356. doi: 10.1158/1535-7163.MCT-10-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Liu Z, Xu X, Blair CA, Sun Z, Xie J, Lilly MB, Zi X. Kava components down-regulate expression of AR and AR splice variants and reduce growth in patient-derived prostate cancer xenografts in mice. PLoS One. 2012;7:e31213. doi: 10.1371/journal.pone.0031213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mashima T, Okabe S, Seimiya H. Pharmacological targeting of constitutively active truncated androgen receptor by nigericin and suppression of hormone-refractory prostate cancer cell growth. Mol Pharmacol. 2010;78:846–854. doi: 10.1124/mol.110.064790. [DOI] [PubMed] [Google Scholar]
- 54.Narizhneva NV, Tararova ND, Ryabokon P, Shyshynova I, Prokvolit A, Komarov PG, Purmal AA, Gudkov AV, Gurova KV. Small molecule screening reveals a transcription-independent pro-survival function of androgen receptor in castration-resistant prostate cancer. Cell Cycle. 2009;8:4155–4167. doi: 10.4161/cc.8.24.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita S, Lai KP, Chuang KL, Xu D, Miyamoto H, Tochigi T, Pang ST, Li L, Arai Y, Kung HJ, Yeh S, Chang C. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia. 2012;14:74–83. doi: 10.1593/neo.111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zengerling F, Streicher W, Schrader AJ, Schrader M, Nitzsche B, Cronauer MV, Hopfner M. Effects of sorafenib on C-terminally truncated androgen receptor variants in human prostate cancer cells. Int J Mol Sci. 2012;13:11530–11542. doi: 10.3390/ijms130911530. [DOI] [PMC free article] [PubMed] [Google Scholar]