Abstract
Adolescence is a time of marked changes across sleep, circadian rhythms, brain function, and alcohol use. Starting at puberty, adolescents’ endogenous circadian rhythms and preferred sleep times shift later, often leading to a mismatch with the schedules imposed by secondary education. This mismatch induces circadian misalignment and sleep loss, which have been associated with affect dysregulation, increased drug and alcohol use, and other risk-taking behaviors in adolescents and adults. In parallel to developmental changes in sleep, adolescent brains are undergoing structural and functional changes in the circuits subserving the pursuit and processing of rewards. These developmental changes in reward processing likely contribute to the initiation of alcohol use during adolescence. Abundant evidence indicates that sleep and circadian rhythms modulate reward function, suggesting that adolescent sleep and circadian disturbance may contribute to altered reward function, and in turn, alcohol involvement. In this review, we summarize the relevant evidence and propose that these parallel developmental changes in sleep, circadian rhythms, and neural processing of reward interact to increase risk for alcohol use disorder (AUD).
Keywords: circadian rhythms, sleep, reward function, adolescence, alcohol use disorders
Introduction
Adolescence is a time of marked changes across sleep, circadian rhythms, brain function, and alcohol use. Starting at puberty, adolescents’ endogenous circadian rhythms and preferred sleep times shift later. This can lead to a mismatch with the schedules imposed by secondary education. Consequently, adolescents often suffer from circadian misalignment, sleep disturbance, and sleep loss. In parallel, adolescent brains are undergoing structural and functional changes in the circuits subserving the pursuit and processing of rewards. Alcohol use initiation typically occurs during adolescence, resulting in risk for alcohol use disorder (AUD). We propose that these parallel developmental changes in sleep, circadian rhythms, and neural processing of reward interact to increase risk for AUD.
Circadian rhythms primer
We live on a rotating planet, with light and dark periods that alternate over the 24-h day. Accordingly, most organisms have evolved to experience internal rhythms with approximately 24-h periods, known as circadian rhythms. Circadian rhythms exist in many physiological, behavioral, and psychological processes, including the sleep-wake cycle, and serve to organize these processes for optimal interaction with the environment. In humans and other mammals, these rhythms, which exist in tissues throughout the brain and body, are kept in time by a central clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN coordinates rhythms of various peptides and hormones, as well as temperature, which serve as internal messengers and synchronize rhythms in other brain areas and the periphery (Hastings, Reddy, & Maywood, 2003; Reppert & Weaver, 2002). The clock is primarily entrained – that is, synchronized to the environment – by light. Light is the most powerful entraining cue, or zeitgeber (“time giver” in German). Other cues, such as food, social interaction, activity, and drugs, also can entrain rhythms (Mistlberger & Skene, 2005).
Disruptions in regular exposure to light and, to a lesser degree, disruptions in other zeitgebers, can lead to internal desynchrony among various rhythms. Sustained desynchrony can have adverse effects on health and well-being. In shift work and jet lag, the sleep-wake cycle and exposure to the external light/dark cycle are misaligned from internal timing, requiring re-entrainment to the new schedule. In the case of night shift work, some individuals never entrain to the reversed schedule, and chronically exhibit circadian misalignment (Sack et al., 2007a). Jet lag is another example. After plane travel to other time zones, re-entrainment can require multiple days and proceeds at different rates in various internal processes. Circadian misalignment compromises function across physiological systems, and is likely responsible for the health ills ranging from gastrointestinal distress and affective disturbance associated with jet lag, to diabetes and cancer associated with long-term shift work (Davis & Mirick, 2006; Drake, Roehrs, Richardson, Walsh, & Roth, 2004; Monk & Buysse, 2013; Rogers & Reilly, 2002).
An important individual difference in circadian timing is termed chronotype. Early chronotypes have relatively advanced circadian timing, defined as being predisposed to earlier sleep-wake schedules. Late chronotypes have relatively delayed circadian timing, defined as being predisposed to later sleep-wake schedules (Roenneberg, Wirz-Justice, & Merrow, 2003). Definitive determination of chronotype requires the measurement of endogenous circadian phase via a physiological circadian marker such as melatonin or core body temperature. However, this is not always practical for larger studies or clinical settings. As a result, self-report measures of chronotype, also termed morningness-eveningness or diurnal preference, have achieved wide usage (e.g., Adan et al., 2012).
Early and late chronotypes (or morning- and evening-types) not only differ on sleep and circadian variables, but also exhibit marked differences in other areas of physical and mental health. As described in more detail below, late chronotypes (evening-types) tend to report more disturbed sleep and more irregular sleep timing, more depression, and increased rates of drug and alcohol use (Adan, 1994; Broms et al., 2011; Drennan, Klauber, Kripke, & Goyette, 1991; Gau et al., 2007; Hasler, Allen, Sbarra, Bootzin, & Bernert, 2010; Negriff, Dorn, Pabst, & Susman, 2011; Pieters, Van Der Vorst, Burk, Wiers, & Engels, 2010; Wittmann, Dinich, Merrow, & Roenneberg, 2006). These differences have been attributed to a phenomenon called social jet lag (Wittmann et al., 2006), which is operationalized as the difference between weekday and weekend sleep timing (typically the midpoint of sleep). Based on the social jet lag hypothesis, the later sleep-wake schedules preferred by evening-types are poorly matched with schedules imposed by school or work. As a result, evening-types suffer sleep onset insomnia and sleep loss on school or work days (typically weekdays). They have trouble falling asleep when attempting to sleep at a point in the circadian cycle incompatible with sleep onset, and their sleep bouts are curtailed by early rise times. In contrast, on free days (typically weekends) they tend toward later sleep-wake schedules and longer sleep durations, which result, in turn, in delays in their circadian timing. The “jet lag” occurs as the evening-types try to shift back to an earlier schedule at the conclusion of the weekend.
Adolescent sleep and circadian rhythms – brief overview
Important sleep and circadian changes occur during adolescence. Bedtimes shift later during middle school and high school, reflecting an increasing preference for later sleep times, a.k.a. eveningness (Carskadon, Acebo, & Jenni, 2004; Crowley, Acebo, & Carskadon, 2007; Randler, 2008; Roenneberg et al., 2004). The increase in eveningness continues until approximately age 18–20, when sleep preference undergoes a long slow shift toward earlier sleep times, a.k.a. morningness (Frey, Balu, Greusing, Rothen, & Cajochen, 2009; Roenneberg et al., 2004). These changes reflect, in part, alterations in the endogenous circadian clock, which shift to a later (more delayed) phase during puberty (Carskadon, Vieira, & Acebo, 1993). Reduced sensitivity to the homeostatic sleep drive that builds during extended wakefulness may also contribute to later bedtimes in adolescents (Hagenauer, Perryman, Lee, & Carskadon, 2009).
In the absence of social-cultural constraints, these normative developmental changes in sleep and circadian rhythms might be adaptive. Indeed, some have suggested that these changes serve as an evolutionary adaption aimed at increasing autonomy and independence by driving adolescents to be engaging the world at times when their parents have already retired to bed (Ellis et al., 2012). Instead, adolescents are faced with school schedules that sharply conflict with their pre-disposed timing (Hansen, Janssen, Schiff, Zee, & Dubocovich, 2005). Consequently, adolescents are forced to adopt schedules that are too early during the school week, resulting in difficulty falling asleep at night, curtailed sleep duration, and daytime fatigue. Then, on the weekend, adolescents return to their preferred timing, staying up later, and sleeping into the late morning or afternoon to make up for sleep loss during the school week. Sleeping later has consequences for the circadian system, which responds to the later rise times and later exposure to light by delaying internal timing (Crowley & Carskadon, 2010). This maintains adolescents’ later circadian timing, resulting in insomnia and sleep loss as the school week begins. In other words, many adolescents must do the equivalent of traveling multiple time zones each Sunday night and Monday morning. This social jet lag, when operationalized via differences in weekday-weekend sleep timing, is associated with mood disturbance and drug and alcohol use in both adolescents (O’Brien & Mindell, 2005; Pasch, Laska, Lytle, & Moe, 2010) and adults (Levandovski et al., 2011; Wittmann et al., 2006). Increasingly prevalent use of social media and late-night exposure to the light from electronic devices may compound delays in sleep timing (Munezawa et al., 2011; Pieters et al., 2012; Van den Bulck, 2007; Wood, Rea, Plitnick, & Figueiro, 2013).
Cross-sectional associations between sleep, circadian rhythms, and alcohol involvement
Sleep problems/disorders
Sleep problems include disturbed sleep continuity (difficulty falling or staying asleep), insufficient sleep duration (sleep deprivation or restriction), and hypersomnia (excessive sleep is desired and/or required). Although there are important distinctions among these categories, all have been associated with alcohol problems, suggesting that any sleep disturbance increases risk for AUDs.
Multiple studies have demonstrated cross-sectional associations between sleep problems and alcohol use/AUDs in adolescents and adults. Past-year insomnia symptoms were associated with increased use of alcohol, cannabis, and other drugs, after controlling for sex effects, in a sample of 4494 12–18-year-olds (Roane & Taylor, 2008). In 703 South African adolescents, overall sleep problems (trouble falling or staying sleep, tiredness in the morning, and/or daytime sleepiness) were associated with greater lifetime alcohol and drug use, and this association was independent of the effect of learning difficulties (Fakier & Wild, 2011). Comparing adolescents with AUDs and a reference group, Clark and colleagues (Clark, Lynch, Donovan, & Block, 2001) found self-reported sleep problems (5 items) to be significantly associated with AUD, negative emotionality, and tobacco involvement. The association between AUD and sleep problems remained statistically significant after controlling for negative emotionality and tobacco involvement. Other studies have linked sleep problems to consequences of alcohol use. Self-reported sleep quality over the past month was associated with greater binge drinking and more alcohol-related consequences (e.g., “Had a fight, argument, or bad feelings with a friend”) in a sample of 261 college students (Kenney, LaBrie, Hummer, & Pham, 2012). Furthermore, in that study and a larger follow-up study, the authors reported compounding effects of sleep problems and alcohol involvement. Poorer sleep quality was associated with greater alcohol-related consequences in heavy drinkers (Kenney et al., 2012). The combination of poorer sleep quality and higher coping motives (e.g., to forget your worries) was associated with worse alcohol-related consequences (Kenney, Lac, Labrie, Hummer, & Pham, 2013). Findings such as these suggest a complex, synergistic, and likely bidirectional relationship between sleep and alcohol use.
The sleep restriction that adolescents experience during school days appears to be associated with increased use of alcohol and other drugs. Based on data from 12,154 US high-school students in the 2007 Youth Risk Behavior survey, the 68.9% of teens reporting typical school night sleep durations under 8 h were more likely to have engaged in alcohol, marijuana, and cigarette use in the preceding 30 days, even after adjusting for age, sex, and ethnicity (McKnight-Eily et al., 2011). This is generally consistent with adult data. Adults with AUDs report shorter sleep times, and this may persist for years into abstinence (Roehrs & Roth, 2001). Interestingly, there may be a U-shaped association between sleep duration and alcohol use in adulthood. Some epidemiological studies link shorter sleep durations to increased alcohol consumption and more frequent binge drinking (Krueger & Friedman, 2009), while others link longer sleep durations (usually > 9 h) to greater alcohol use (e.g., Gottlieb et al., 2006; Patel et al., 2004). At least one study specifically reported a U-shaped association (Patel, Malhotra, Gottlieb, White, & Hu, 2006). This U-shaped relationship between sleep duration and various other measures of morbidity and mortality has been repeatedly reported, but not yet explained (e.g., see metanalysis by Cappuccio, Cooper, D’Elia, Strazzullo, & Miller, 2011).
Whether treating sleep problems reduces risk for new problems with alcohol and other drugs remains an open question. Extant published studies have focused on relapse prevention. In a small trial (18 adolescent participants) of a multicomponent sleep intervention based on sleep restriction, stimulus control, and mindfulness meditation, participants reported reduced substance use. Increased sleep duration was associated with reductions in substance-related problems (Britton et al., 2010). Adult sleep treatment studies in recovering drinkers consistently report improvements in sleep, but either indeterminate effects on alcohol outcomes in cognitive-behavioral treatment studies (Arnedt et al., 2007; Arnedt, Conroy, Armitage, & Brower, 2011; Currie, Clark, Hodgins, & El-Guebaly, 2004) or worse alcohol outcomes following pharmacological treatment (Friedmann et al., 2008).
Eveningness and other “circadian” proxies (e.g., social jet lag)
Eveningness, or the tendency toward a late chronotype, has been consistently linked with increased alcohol involvement in adults (Adan, 1994; Wittmann et al., 2006) and adolescents (Gau et al., 2007; Negriff et al., 2011; Pieters et al., 2010; Saxvig, Pallesen, Wilhelmsen-Langeland, Molde, & Bjorvatn, 2012; Tavernier & Willoughby, 2013; Urbán, Magyaródi, & Rigó, 2011)). A recent study of 215 university students reported that eveningness was associated with increased endorsement of various drinking motivations, including drinking for social reasons (e.g., to enjoy a party), for enhancement (e.g., to get high), for conformity (e.g., to be liked), and, most notably, increased endorsement of drinking to cope (e.g., to forget about problems or improve mood) (Currie et al., 2004). In contrast, eveningness was associated with lower endorsement of problem- or emotion-focused coping with stress, as well as worse subjective sleep quality. Furthermore, eveningness (and/or delayed sleep timing) has been consistently associated with psychiatric conditions, most prominently mood disorders (e.g., Chelminski, Ferraro, Petros, & Plaud, 1999; Drennan et al., 1991; Hasler et al., 2010; Kitamura et al., 2010). A smaller literature has extended this association to attention-deficit and hyperactivity disorder, eating disorders, and schizophrenia (Caci, Bouchez, & Baylé, 2009; Hofstetter, Mayeda, Happel, & Lysaker, 2003; Natale, Ballardini, Schumann, Mencarelli, & Magelli, 2008; Voinescu, Szentagotai, & David, 2012). Affective, motivational, and/or personality constructs, as well as sleep/circadian processes pathways, have all been proposed as putative mechanisms (e.g., Hasler et al., 2010; Kitamura et al., 2010). The role of social jet lag as a risk factor for affective and substance use disorders, in particular, has gained increasing attention.
Social jet lag may be associated with substance use and depression in adolescents and adults. Relevant studies have used several different, but overlapping, measures of weekday-weekend differences, including differences in sleep timing (bedtime, midsleep, risetime) and sleep duration (or total sleep time). Taken together, the studies suggest that instability in sleep timing is related to risks for alcohol and other drug use that are independent and perhaps stronger than those with sleep duration. Two adult studies assessing weekday-weekend difference in midsleep reported associations with elevated smoking (Wittmann et al., 2006) and depression (Levandovski et al., 2011). Pasch and colleagues (Pasch et al., 2010) studied a sample of 242 9th–11th graders (M age = 16.4), reporting on cross-sectional associations between self-reported sleep, substance use, and depression. Both weekday-weekend difference in bedtime and weekday-weekend difference in rise time were significantly associated with higher rates of smoking, alcohol involvement, and marijuana use, after controlling for demographic variables and sleep duration. Neither difference measure was related to depressive symptoms. In contrast, weekday and weekend sleep duration (as well as their difference) were unrelated to substance use, but shorter weekday sleep duration was significantly associated with depressive symptoms. These findings partially converge with an earlier study in 388 9th–12th graders (O’Brien & Mindell, 2005), which reported that weekday-weekend differences in bedtime were significantly associated with the use of tobacco, alcohol, marijuana, and other drugs, as well as sexual behaviors. Similar to the above study, weekday-weekend differences in total sleep time were unrelated to substance use, while less sleep on school nights was associated with depressed mood. Interestingly, less sleep on school nights was also associated with increased alcohol use, but not with other substance use.
Circadian data
Relevant adolescent studies that include physiological circadian measures remain scarce. The most widely used measure of circadian phase is dim light melatonin onset (DLMO). In a sample of 21 adolescents (14–19 years) with a history of substance abuse and current sleep disturbance (Hasler, Bootzin, Cousins, Fridel, & Wenk, 2008), circadian alignment was quantified as the interval between DLMO and their wake-up time, based on wrist actigraphy. The misalignment between the adolescents’ sleep-wake schedule and their endogenous circadian phase (i.e., DLMO) was positively correlated with symptoms of substance-use disorder over the preceding 30 days. Furthermore, the DLMO-wake time phase angle was unrelated to measures of sleep continuity, suggesting that circadian factors, rather than sleep disturbance per se, contributed to the increased substance abuse.
Several studies have examined physiological circadian measures in adult samples. In adults with AUD, disturbed or misaligned circadian rhythms occur during alcohol withdrawal (see Hasler, Smith, Cousins, & Bootzin, 2012 for review). We are not aware of any such studies in AUD adults with ongoing alcohol use. Conroy and colleagues (2012) compared 52 alcohol-dependent adults to 19 age- and sex-matched healthy controls on sleep and circadian characteristics. The alcohol-dependent group had been abstinent for 3–12 weeks. The alcohol-dependent group reported more insomnia and worse sleep quality, and suggestive evidence was present of delayed circadian timing (based on DLMO) and altered sleep-circadian alignment similar to that in the adolescent study (Hasler, Bootzin, et al., 2008). However, methodological limitations in the melatonin collection preclude a definitive interpretation of the data. Another study used bioluminescence methods to examine the rhythmicity of circadian gene expression (Per2) in skin fibroblasts collected from 19 alcohol-dependent adults (McCarthy, Fernandes, Kranzler, Covault, & Welsh, 2013). Rhythm amplitude and period in the alcohol-dependent group were similar to those of a control group of 10 age- and race-matched adults. Within the alcohol-dependent group, the period of Per2 rhythms correlated with the number of AUD symptoms. These findings suggest that circadian disruption may be differentially related to alcohol problems in sensitive individuals. An emerging literature also links mutations in circadian genes (e.g., Period and Clock) to human alcohol involvement, consistent with findings in the more substantive animal literature (reviewed in Logan, Williams, & McClung, 2014).
Longitudinal studies of sleep, circadian rhythms, and alcohol involvement
Longitudinal studies relating sleep, circadian rhythms, and alcohol use remain scant, particularly in adolescent samples. Extant findings are mixed, but are generally consistent with sleep complaints and circadian disturbance conferring risk for the development of new alcohol problems or relapse to recurrent alcohol problems.
Wong and colleagues (Wong, Brower, Fitzgerald, & Zucker, 2004; Wong, Brower, Nigg, & Zucker, 2010) have reported that parent-rated childhood sleep problems, assessed between ages 3 and 8, and including “trouble sleeping” and “overtiredness”, predict alcohol problems during early adolescence (12–14 years) and late adolescence/young adulthood (18–20 years). The respective study samples were comprised mostly of boys. The authors also investigated potential mediators of these associations, reporting that response inhibition (based on a behavioral task) during adolescence statistically mediated the relationship between childhood sleep problems and young-adult alcohol problems. In contrast, depression and anxiety symptoms during late childhood (age 9–11) did not significantly account for variance in the association between sleep and alcohol problems. Interestingly, self-reported sleep problems during adolescence per se did not predict later alcohol outcomes over and above the effect of childhood sleep. Adolescent sleep problems were a risk factor for problems with other drugs. Similarly, Roane & Taylor (2008) reported that insomnia complaints (based on a single item assessing the past year) during adolescence (12–18 years) did not predict alcohol use during young adulthood (18–25 years) over and above the effects of gender. In a sample of adolescents (age 12–19) followed for 5 years, 347 adolescents with AUD reported more symptoms of insomnia and hypersomnia during follow-up visits relative to a reference group (n = 349) (Hasler, Martin, Wood, Rosario, & Clark, 2014). At baseline, adolescents with AUDs reported greater variability in their weekday-weekend sleep duration. Among the reference adolescents without AUD, both baseline insomnia and variability in sleep duration increased risk for subsequent alcohol symptoms.
Adult studies have more consistently demonstrated that sleep disturbances confer risk for alcohol problems. Breslau, Roth, Rosenthal, & Andreski (1996) reported that, in a sample of 979 young adults (21–30 years) meeting diagnostic criteria for hypersomnia predicted an increased AUD risk (OR = 3.92) over the course of 3.5 years. Insomnia was not significantly associated with an increased risk for AUD (OR = 1.72). Analyses accounted for sex effects, and sleep- and alcohol-related diagnoses were based on DSM-III criteria and assessed via telephone interviews. Similarly, sleep problems related to worry predicted alcohol problems a decade later among 1,537 adults ranging in age from 18 to over 65 (Crum, Storr, Chan, & Ford, 2004). Finally, several studies have reported that persistent symptoms of insomnia during abstinence from alcohol confer an increased risk of relapse in adults in remission from AUDs (Brower, Aldrich, & Hall, 1998; Brower, Aldrich, Robinson, Zucker, & Greden, 2001; Drummond, Gillin, Smith, & DeModena, 1998).
Alcohol effects on sleep and circadian rhythms
A large body of evidence documents the effect of acute alcohol use on human sleep. Acute alcohol consumption tends to reduce the latency to sleep onset, increase slow wave sleep (deep sleep) and sleep consolidation, and decrease rapid eye movement (REM) during the first half of the sleep period (see Ebrahim, Shapiro, Williams, & Fenwick, 2013 for review). However, the metabolic elimination of alcohol during the second half of the sleep period increases sleep disruption, which tends to outweigh the benefits in the first half of the night. A study in mice suggests that even the limited sleep-promoting effects of alcohol are short-lived, with alcohol-induced increases in non-REM sleep disappearing by the second day of alcohol self-administration (Sharma, Sahota, & Thakkar, 2014). While this finding requires replication in a human sample, it underscores the adverse effects of alcohol as a sleep aid. Consistent with this concern, adults with AUDs report more difficulty falling asleep and reduced total sleep time, although interpretation of such reports is complicated by the fact that most of these data come from individuals in various stages of abstinence (Brower, 2001).
Limited work has attempted to disentangle how the biphasic stimulating and sedative properties of alcohol may be differentially related to sleep and/or circadian factors. In a study with young adults, the time-of-day of alcohol use influenced the extent of stimulation and sedation (Van Reen, Rupp, Acebo, Seifer, & Carskadon, 2013). Specifically, alcohol was more stimulating in the evening (increasing onset latency to sleep), consistent with evidence that adolescents are relatively less sensitive to alcohol’s sedating properties (Spear, 2000). A study in 24 late adolescents (18–21 years old, 12 women) supports this hypothesis, in that sleep onset latency was not reduced following alcohol consumption relative to a placebo control, and alcohol consumption was associated with increased wakefulness during the rest of the night (Chan, Trinder, Andrewes, Colrain, & Nicholas, 2013).
Studies evaluating the impact of acute alcohol administration on the circadian system in healthy adults have produced mixed findings, though most studies have reported changes in the amplitude of melatonin and core body temperature (CBT) rhythms, suggesting circadian disruption (see Hasler, Smith, et al., 2012 for review). These limited data are generally cohesive with animal studies, which have provided strong evidence that both acute and chronic alcohol use disrupts circadian timing through direct effects on the central clock (Brager, Ruby, Prosser, & Glass, 2010; Ruby, Brager, DePaul, Prosser, & Glass, 2009). While difficult to directly compare to human studies and generally lacking physiological circadian rhythm measures (i.e., melatonin or core body temperature), animal studies provide evidence that alcohol interferes with the clock’s ability to adjust in response to shifting entrainment cues (e.g., light).
In summary, extant evidence indicates that alcohol consumption disrupts sleep, is even more stimulating and sleep-disturbing in adolescents, and concurrently disrupts the ability of the circadian system to adapt to shifting schedules.
Reward as a mechanism
A sizeable and ever expanding literature supports a role for sleep and circadian rhythms in the modulation of reward. Sleep and circadian disturbances are associated with altered reward function, and thereby may influence involvement of alcohol and other drugs. Based on these observations, we have proposed a theoretical model linking circadian misalignment, reward function, and adolescent alcohol involvement (see Fig. 1; Hasler & Clark, 2013). Specifically, we propose that adolescents, as a result of the tendency for delayed circadian phase, are chronically subjected to a misalignment between the sleep/wake schedule and internal circadian timing. The delayed circadian phase-associated sleep loss impacts the neural circuitry of reward processing, manifesting in increased alcohol use and other risk-taking behaviors.
Figure 1.
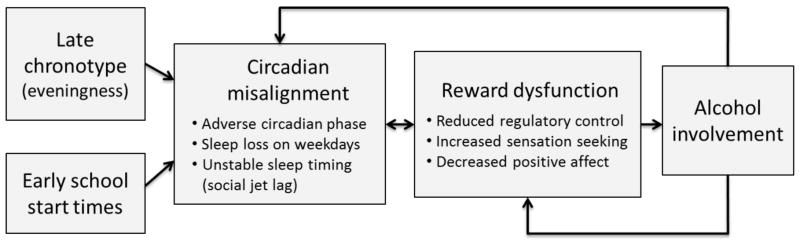
A conceptual model of a pathway from circadian misalignment to adolescent alcohol involvement via altered reward function
Adolescence is characterized by developmentally specific changes in reward-related behavior, including initiation of alcohol involvement and increased risk-taking, that are assumed to reflect ongoing changes in the structure and function of reward-related circuitry (Ernst & Fudge, 2009; Forbes & Dahl, 2005; Spear, 2000). We recognize that a host of important developmentally specific factors, such as peer interactions, are key determinants in whether these typical adolescent changes in reward function result in pathology. Our contention is that circadian misalignment is a particularly important and under-recognized risk factor during this developmental stage. That is, sleep and circadian challenges may tip developmentally typical changes in reward function into pathological patterns of behavior, including excessive alcohol use, which can in turn exacerbate the sleep and circadian disturbance. This vicious cycle, if uninterrupted, may accelerate the development of AUDs that persist well beyond the circadian misalignment induced by early school start times.
The support for our model can be broadly categorized into three lines of evidence.
First, the molecular and neurophysiologic underpinnings of the circadian system are integral to normal reward system function. Circadian genes are expressed widely throughout the brain, notably in components of the mesocorticolimbic reward system such as the ventral tegmental area (VTA) and striatum (e.g., Lynch, Girgenti, Breslin, Newton, & Taylor, 2008; McClung et al., 2005; Uz, Akhisaroglu, Ahmed, & Manev, 2003). Animal models (see Logan et al., 2014 for review) have demonstrated that disruptions to the function of circadian genes are associated with corresponding disruptions to key elements of the reward system. The mechanisms of these disruptions may include alterations to the expression of tyrosine hydroxylase and dopamine firing in the VTA (Coque et al., 2011; McClung et al., 2005), or altered dopamine levels and D1 to D2 receptor ratio in the nucleus accumbens (Hampp & Albrecht, 2008). Animal models have uncovered that orexin, a neuropeptide involved in the homeostatic regulation of sleep, serves to modulate both circadian gene function and mesolimbic pathways (Belle et al., 2014; Kim, Brown, & Lawrence, 2012). Furthermore, circadian gene mutants often demonstrate altered reward behavior, such as increased preference for alcohol or hypersensitization to cocaine (McClung et al., 2005; Spanagel et al., 2005). A handful of human studies have similarly linked circadian gene variants to reward-related behavior and brain function. A Per2 polymorphism was associated with more alcohol use in patients with AUDs (Spanagel et al., 2005), while various other circadian gene polymorphisms appeared to be differentially related to AUDs or alcohol use in social drinkers without an AUD diagnosis (Kovanen et al., 2010). In the sole published study in adolescents (ages 11 to 13 years), Forbes and colleagues (Forbes et al., 2012) found that the genotype for a particular Per2 polymorphism (rs2304672) is differentially related to activation of the medial prefrontal cortex (mPFC) in response to a monetary reward task.
Second, daily rhythms are present in reward-related experience, behavior, and underlying physiology. In animal models, drug-seeking behavior, locomotor sensitization, and sexual behavior all display daily rhythms. These rhythms parallel patterns in expression of the dopamine transporter, dopamine receptors, and circadian genes throughout the mesolimbic dopaminergic pathway (e.g., Ikeda et al., 2013; Sleipness, Sorg, & Jansen, 2007; Webb et al., 2009). In humans, positive affect, an experiential phenomenon related to activation of the reward system, also shows a daily rhythm. Positive affect is lowest in early morning, rises throughout the day peaking in the afternoon and/or evening, and declines prior to bedtime (e.g., Clark, Watson, & Leeka, 1989; Hasler, Germain, et al., 2012). This same pattern is observed in positive affect-related behavior such as socializing and laughing (Hasler, Mehl, Bootzin, & Vazire, 2008), as well as drinking behavior (Arfken, 1988). Furthermore, carefully controlled laboratory studies have confirmed that this pattern in positive affect does not simply reflect time-varying sociocultural factors, but is partially dictated by endogenous circadian timing (Boivin et al., 1997; Murray et al., 2009), paralleling the core body temperature rhythm. Notably, one of these studies observed a corresponding rhythm in the heart rate response to a reward task (Murray et al., 2009). Finally, a handful of neuroimaging studies support a similar daily rhythm in the reward system. Brain glucose metabolism has been observed to be greater in striatal regions during the evening (Buysse et al., 2004; Germain et al., 2007; Hasler, Germain, et al., 2012). Further, our own pilot fMRI study reported evidence that the striatum responds more strongly to monetary reward in the afternoon relative to the morning (Hasler, Forbes, & Franzen, 2014).
Third, disrupted sleep and circadian rhythms are associated with symptoms, behaviors, and neural processing putatively associated with alterations in reward function. Profound sleep and circadian disturbances are exhibited in the context of AUDs and/or other SUDs, as well as affective disorders. These disorders are all characterized by altered reward processing (Koob & Le Moal, 2001; Nestler & Carlezon, 2006). In healthy adolescents and adults, sleep disturbance and sleep loss are associated with increased risk-taking and (typically) hyper-responsivity to reward. Holm and colleagues (2009) reported that shorter sleep duration and lower sleep quality were associated with reduced striatal response to monetary reward in healthy 11–13-year-olds. The authors speculate that the blunted striatal response could lead to compensatory reward seeking. A study of healthy adolescents (14–16 years old) found that lower sleep quality was associated with reduced dorsolateral PFC activation on a Go/No Go task, suggesting impaired cognitive control. Increased insula activation during a risk-taking task paired with reward was also observed, suggesting increased reward sensitivity (Telzer, Fuligni, Lieberman, & Galván, 2013). Self-reported decision-making, risk-taking, and reward sensitivity showed parallel associations with sleep quality. Adult studies using acute sleep deprivation paradigms have consistently demonstrated increased neural responses to reward or positive stimuli along with attenuated response to loss (Gujar, Yoo, Hu, & Walker, 2011; Mullin et al., 2013; Venkatraman, Chuah, Huettel, & Chee, 2007; Venkatraman, Huettel, Chuah, Payne, & Chee, 2011).
Eveningness, sleep timing, and social jet lag measures are associated with altered reward function. Evening-types exhibit delayed positive affect rhythms (Porto, Duarte, & Menna-Barreto, 2006) and evening-types with an insomnia diagnosis exhibit both delayed and blunted positive affect rhythms (Hasler, Germain, et al., 2012), suggesting an alteration in the timing and degree of reward activation. Eveningness has also been linked to increased risk taking (Killgore, 2007; Wang & Chartrand, 2014), higher novelty seeking (Caci, Robert, & Boyer, 2004; Randler & Saliger, 2010), and lower harm avoidance (Adan, Natale, Caci, & Prat, 2010). Two studies using the BIS/BAS scales (Carver & White, 1994) to examine relationships between chronotype and behavioral activation sensitivity reported seemingly conflicting findings. Randler and colleagues reported higher scores on the BAS Fun-Seeking scale among evening-types in a mostly female sample of university students, but lower BAS Drive (Randler, Baumann, & Horzum, 2014). In contrast, evening-types in an adult sample ranging from healthy to severely depressed individuals showed lower scores for BAS Total and BAS Reward Responsiveness (Hasler et al., 2010). These mixed findings raise the possibility that the chronotype-BAS relationship may differ in the context of depression.
Finally, several neuroimaging studies in adolescents support a circadian-reward link. The aforementioned fMRI study of healthy 11–13-year-olds (Forbes et al., 2012) reported that later sleep timing was associated with reduced mPFC response to monetary reward. A follow-up analysis in an overlapping sample focused on weekday-weekend differences in sleep timing (i.e., social jet lag). Hasler and colleagues (Hasler, Dahl, et al., 2012) reported that larger weekday-weekend differences in midsleep timing were associated with decreases in both mPFC and striatal response to monetary reward, even after accounting for total sleep time. Finally, evening-types in a sample of 20-year-old males exhibited reduced mPFC response and increased striatal response monetary reward (Hasler, Sitnick, Shaw, & Forbes, 2013). Crucially, these patterns of mPFC and striatal reactivity correlated with greater alcohol consumption and more alcohol dependence symptoms, respectively.
Other key points for our model
Several additional points deserve emphasis in considering our model. Watson’s early observations identified daily rhythms in positive affect, but not negative affect, and suggested that this reflected an adaptive influence of the clock to drive individuals out into the world at times with maximal reward opportunities (Watson, 2000). We concur that circadian modulation of reward typically has adaptive value, although this drive to later sleep times can go awry when faced with artificially imposed constraints (e.g., early school start times). Second, we have broadly defined “reward function”. However, we recognize the conceptual and empirical support for dividing reward function into appetitive (wanting) and consummatory (liking) processes (Berridge & Robinson, 2003). While definitive evidence does not yet exist, Watson’s hypothesis and the extant literature are consistent with circadian modulation of the pursuit of rewards (i.e., wanting). Finally, our model emphasizes the effects of sleep and circadian factors on reward function and alcohol involvement, though the reverse pathway is also well documented (see Alcohol effects on sleep and circadian rhythms section). We speculate that these forward and reverse pathways may perpetuate a positive feedback cycle that exacerbates problems in each domain and leads to behavioral, psychological, and physiological dysregulation.
Other mechanisms
In addition to reward function, several other mechanisms may influence the connection between adolescent alcohol use and sleep and circadian dysfunction.
Impulsivity
Questionnaire (Stautz & Cooper, 2013) and behavioral measures (Nigg et al., 2004) of impulsivity have been linked to increased alcohol use in adolescence. One study has provided initial support for the involvement of sleep and circadian disruption in this relationship. A behavioral measure of impulsivity, response inhibition, evaluated during adolescence (12–14 years) mediated the relationship between parent-rated sleep problems in childhood (3–8 years) and alcohol problems in young adulthood (18–20 years) (Wong et al., 2010). Sleep disturbance and sleep loss have been observed to be associated with impulsivity. For example, several sleep deprivation studies conducted with young adults have shown sleep loss to be associated with poorer response inhibition using Go/No Go paradigms (e.g., Anderson & Platten, 2011; Drummond, Paulus, & Tapert, 2006), with occasional non-replications (Renn & Cote, 2013; Rossa, Smith, Allan, & Sullivan, 2014). In adults, self-reported measures of disturbed sleep, such as poor sleep quality or insomnia severity, have been associated with self-reported measures of impulsivity (Kamphuis, Dijk, Spreen, & Lancel, 2014; Schmidt, Gay, & Van der Linden, 2008). In 516 community adolescents (Moore, Slane, Mindell, Burt, & Klump, 2011), greater sleep disturbance by mothers’ and adolescents’ reports was negatively associated with effortful control.
Fewer studies have examined the relationship between circadian functioning and impulsivity, and none to our knowledge in adolescent samples. Across adult studies employing self-reported measures of chronotype and impulsivity, greater eveningness has been consistently associated with heightened impulsivity (i.e., Adan et al., 2010; Caci et al., 2004, 2005). No studies have yet explored the relation between physiological markers of the circadian system and impulsivity.
Negative affect
A consistent, though weak, relationship between negative affect and alcohol use has been observed in adolescence (e.g., Ahonen, Nebot, & Gimenez, 2007; Hallfors, Waller, Bauer, Ford, & Halpern, 2005; Tschann et al., 1994). This association is postulated to arise from a self-medication process in youth, particularly with solitary drinking (Creswell, Chung, Clark, & Martin, in press).
Disturbed sleep and sleep loss have been consistently linked to increased negative affect in adults (Goldstein & Walker, 2014). Recent work has started to extend these findings to sleep-affect relationships in adolescence. A study conducted by Talbot and colleagues (Talbot, McGlinchey, Kaplan, Dahl, & Harvey, 2010) restricted sleep opportunity in early adolescents (ages 10–13), mid-adolescents (ages 13–16), and adults (ages 30–60). Across all groups, they observed that global negative affect did not differ between the rested and sleep-deprived conditions, except for the ‘miserable’ item, which was increased following sleep loss. Rather, positive affect was decreased in the sleep-deprived condition across groups. An experience-sampling study of 286 healthy adolescents (13–16 years) showed that poorer sleep quality (rated on a 7-point scale) predicted more negative affect and less positive affect the next day (van Zundert, van Roekel, Engels, & Scholte, 2013). Overall, this work provides initial, though mixed, evidence for a relationship between sleep and negative affect in adolescence.
Several facets of circadian dysfunction, including eveningness, delayed or advanced sleep phase, and circadian misalignment, have long been implicated in the pathophysiology of adult mood disorders (e.g., Drennan et al., 1991; Emens, Lewy, Kinzie, Arntz, & Rough, 2009; Hasler et al., 2010; Healy & Waterhouse, 1995; Kitamura et al., 2010). However, this association may not be mediated via negative affect – the apparent absence of circadian rhythms in negative affect (in contrast to positive affect) has led some to suggest a more modest or absent role for circadian modulation in the negative affect system (Hasler et al., 2010; Murray et al., 2009). In any case, the link between negative affect and circadian rhythms has not been established in adolescence.
Self-medication
Sleep-alcohol links could arise in part because sleep-disturbed individuals turn to alcohol as a sleep aid. Despite the plausibility of this self-medication mechanism, extant data suggests that a relatively small proportion of drinkers report using alcohol for this purpose. A general population study of 2,181 adults (18–45 years) observed that 13% of respondents used alcohol as a sleep aid (Johnson, Roehrs, Roth, & Breslau, 1998). Similarly, in a sample of 1,039 undergraduate students tracking alcohol use and sleep in daily diaries, 11% reported using alcohol as a sleep aid, with higher rates of use in male students relative to female students (16% vs. 9.8%) (Taylor & Bramoweth, 2010). We have also examined the extent of self-medication in a sample of 285 adolescents involved in a longitudinal study at the Pittsburgh Adolescent Alcohol Research Center (Hasler et al., unpublished data). At baseline, 11.3% of the 12–19-year-old sample reported ‘frequently’ or ‘almost always’ using alcohol when they had trouble sleeping on the Inventory of Drinking Situations (Annis, 1982). Notably, the proportion of teens reporting alcohol use as a sleep aid varied based on extent of alcohol use. Teens who drank 2 or more drinks per day reported using alcohol as a sleep aid 30% of the time, as compared to 10% or less of the teens in the lower drinking categories. Furthermore, frequency of alcohol use as a sleep aid decreased during follow-up; by the 5-year visit, less than 5% of the total sample and 16% of the heavy drinkers reported using alcohol when they had trouble sleeping. We speculate that these reductions may relate to alcohol becomingly less and less effective at promoting sleep over time, which is consistent with the findings of a recent animal study (Sharma et al., 2014).
Psychological dysregulation
An alternative conceptualization considers sleep problems and related difficulties to be phenotypic manifestations of a general propensity toward difficulties with self-regulation (i.e., psychological dysregulation: Clark & Winters, 2002). The psychological dysregulation theory proposes that neurobiological immaturity or deficits in cortical networks subserving cognitive, emotional, and behavioral control are manifested as developmental-specific phenotypes (Clark, Chung, Thatcher, Pajtek, & Long, 2012). In childhood, these manifestations include attention control problems, irritability, impulsive behavior, and possibly sleep difficulties. In adolescence, dysregulation manifests as earlier onset of substance use and more rapid transitions from first use to substance use disorders.
In a sample of boys ages 10–12 years old recruited through a high-risk family design, Vanyukov and colleagues (2009) utilized an empirical approach to identify characteristics associated with paternal drug use disorders. The analyses identified 45 items that were demonstrated to compose a unidimensional index (i.e., Transmissible Liability Index: TLI: Vanyukov et al., 2003). TLI was shown to predict substance use disorders in adolescence and young adulthood. In a twin study (i.e., ages 9–18-years-old: Vanyukov et al., 2009), TLI showed a high degree of heritability (i.e., 0.79). While the majority of TLI items were disruptive behavior disorder characteristics (26 items), 5 sleep items describing restlessness during sleep were included. Sleep architecture differences have been noted among adolescents at high risk for AUD by family history compared to those at low risk (Colrain & Baker, 2011; Tarokh et al., 2012).
Consistent with the hypothesis that sleep difficulties constitute an early marker for psychological dysregulation, Wong and colleagues (2004) found that sleep problems in childhood (ages 3 to 5 years) predicted more attention problems, more negative affect (i.e., anxiety and depression), and, in early adolescence, higher rates of substance use onset, including cigarettes, alcohol, and marijuana. Childhood sleep difficulties, represented by “overtiredness”, predicted deficits in response to a response inhibition task (Wong et al., 2010). Childhood overtiredness also predicted alcohol-related problems in young adulthood.
Clinical implications
Our model has preventive implications based on the assumption that, in vulnerable adolescents, minimizing circadian misalignment has beneficial effects on risk for AUDs. Simply put, sleep-wake schedules that are better matched with internal timing should lead to fewer consequences from phase misalignment and associated sleep disruption. Potential prevention approaches range from encouraging adolescents to maintain more consistent weekday-weekend schedules (the individual-level intervention), to policy changes to delay school start times (the system-level intervention). School districts that have implemented later start times have reported improvements in academic achievement, reductions in daytime sleepiness, reductions in depression (Carrell, Maghakian, & West, 2011; Owens, Belon, & Moss, 2010), and reductions in motor vehicle accidents (Danner & Phillips, 2008).
Given that school start times consonant with typical adolescent circadian timing are unlikely to achieve widespread implementation in the near future, individual-level intervention options are important to consider. Alcohol use itself can lead to sleep and circadian disturbance (Ebrahim et al., 2013; Hasler, Smith, et al., 2012), raising the possibility of a vicious cycle. With their circadian rhythms further disrupted due to binge drinking, adolescents with AUDs may require both alcohol abstinence and correction of their circadian alignment. Fortunately, sleep and circadian rhythms are amenable to behavioral and non-pharmacological interventions (e.g., bright light: Sack et al., 2007b; Smith, Huang, & Manber, 2005). Melatonin is also effective for correcting delayed sleep and circadian timing in adolescents (Szeinberg, Borodkin, & Dagan, 2006; van Geijlswijk, Korzilius, & Smits, 2010), and can remain an attractive pharmacological option for adolescents with AUDs when sleep aids from the benzodiazepine family are contraindicated (Clark, 2012). Notably, correction of delayed phase by melatonin has been demonstrated to have antidepressant effects in adults (Rahman, Kayumov, & Shapiro, 2010) and improves school attendance in adolescents (Szeinberg et al., 2006); effects on alcohol and drug use remain unexamined. Finally, circadian interventions may be less stigmatizing and more amenable to adolescents than traditional drug and alcohol treatment (Bootzin & Stevens, 2005).
Future directions
Although we believe the extant literature provides compelling support for the above model, definitive support will require targeted research across a number of areas. In particular, more studies are needed that focus on adolescent samples, include physiological circadian measures, and employ experimental designs that can demonstrate causal mechanisms; longitudinal designs that can demonstrate long-term trajectories following adolescent circadian misalignment may also be informative. Other important issues include sex differences in the interactions among circadian, reward, and addiction processes (e.g., Kokras & Dalla, 2014), interactions among alcohol and other drugs (including energy drinks) with relation to sleep, circadian rhythms, reward function, and peer influences in this context. Patterns of sleep and drug use may “spread” through adolescent peer networks (Mednick, Christakis, & Fowler, 2010), which may be explained in part by the pervasiveness of communication through electronic devices. Mobile phone use after bedtime is common among adolescents, and is associated with delayed sleep timing and increased sleep problems and daytime sleepiness (Munezawa et al., 2011; Pieters et al., 2012; Van den Bulck, 2007).
Concluding remarks
Although definitive demonstration of causal relationships linking circadian misalignment, reward dysregulation, and AUD awaits prospective and experimental studies, we believe that the extant literature offers strong support for further attention to this area. “Timing matters” – with respect to within-day circadian processes, and to within-week changes in sleep-wake schedule. More broadly, sleep and circadian rhythms are important for understanding adolescence. The field of adolescent alcohol use would benefit from more consistently considering these potentially important factors in understanding the development and maintenance of adolescent AUDs.
Highlights.
Later sleep/circadian timing during adolescence are at odds with school start times.
Adolescents consequently suffer from circadian misalignment and sleep problems.
Circadian misalignment and sleep problems are linked to increased alcohol use.
Circadian rhythms and sleep modulate reward-related behavior and brain function.
Sleep/circadian effects on reward function may increase risk for adolescent AUDs.
Acknowledgments
This work was supported by grants from the National Institutes of Health, including K01DA032557 (Hasler), R01AA016482 (Clark), U01AA021690 (Clark), and P50DA05605 (Clark), and a grant from the Commonwealth of Pennsylvania (PA-HEAL SPH00010, Clark).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction. 1994;89:455–462. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiology International. 2012;29:1153–1175. doi: 10.3109/07420528.2012.719971. [DOI] [PubMed] [Google Scholar]
- Adan A, Natale V, Caci H, Prat G. Relationship between circadian typology and functional and dysfunctional impulsivity. Chronobiology International. 2010;27:606–619. doi: 10.3109/07420521003663827. [DOI] [PubMed] [Google Scholar]
- Ahonen EQ, Nebot M, Gimenez E. Negative mood states and related factors in a sample of adolescent secondary-school students in Barcelona (Spain) Gaceta Sanitaria. 2007;21:43–52. doi: 10.1157/13099120. [DOI] [PubMed] [Google Scholar]
- Anderson C, Platten CR. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behavioural Brain Research. 2011;217:463–466. doi: 10.1016/j.bbr.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Annis H. Inventory of Drinking Situations (IDS-100) Toronto: Addiction Research Foundation of Ontario; 1982. [Google Scholar]
- Arfken CL. Temporal pattern of alcohol consumption in the United States. Alcoholism: Clinical and Experimental Research. 1988;12:137–142. doi: 10.1111/j.1530-0277.1988.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Conroy D, Rutt J, Aloia MS, Brower KJ, Armitage R. An open trial of cognitive-behavioral treatment for insomnia comorbid with alcohol dependence. Sleep Medicine. 2007;8:176–180. doi: 10.1016/j.sleep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behaviour Research and Therapy. 2011;49:227–233. doi: 10.1016/j.brat.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle MD, Hughes AT, Bechtold DA, Cunningham P, Pierucci M, Burdakov D, et al. Acute suppressive and long-term phase modulation actions of orexin on the mammalian circadian clock. The Journal of Neuroscience. 2014;34:3607–3621. doi: 10.1523/JNEUROSCI.3388-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Archives of General Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clinical Psychology Review. 2005;25:629–644. doi: 10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcoholism: Clinical and Experimental Research. 2010;34:1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biological Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Britton WB, Bootzin RR, Cousins JC, Hasler BP, Peck T, Shapiro SL. The contribution of mindfulness practice to a multicomponent behavioral sleep intervention following substance abuse treatment in adolescents: a treatment-development study. Substance Abuse. 2010;31:86–97. doi: 10.1080/08897071003641297. [DOI] [PubMed] [Google Scholar]
- Broms U, Kaprio J, Hublin C, Partinen M, Madden PA, Koskenvuo M. Evening types are more often current smokers and nicotine-dependent – a study of Finnish adult twins. Addiction. 2011;106:170–177. doi: 10.1111/j.1360-0443.2010.03112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Research & Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcoholism: Clinical and Experimental Research. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. The American Journal of Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Nofzinger EA, Germain A, Meltzer CC, Wood A, Ombau H, et al. Regional brain glucose metabolism during morning and evening wakefulness in humans: preliminary findings. Sleep. 2004;27:1245–1254. doi: 10.1093/sleep/27.7.1245. [DOI] [PubMed] [Google Scholar]
- Caci H, Adan A, Bohle P, Natale V, Pornpitakpan C, Tilley A. Transcultural properties of the composite scale of morningness: the relevance of the “morning affect” factor. Chronobiology International. 2005;22:523–540. doi: 10.1081/CBI-200062401. [DOI] [PubMed] [Google Scholar]
- Caci H, Bouchez J, Baylé FJ. Inattentive symptoms of ADHD are related to evening orientation. Journal of Attention Disorders. 2009;13:36–41. doi: 10.1177/1087054708320439. [DOI] [PubMed] [Google Scholar]
- Caci H, Robert P, Boyer P. Novelty seekers and impulsive subjects are low in morningness. European Psychiatry. 2004;19:79–84. doi: 10.1016/j.eurpsy.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European Heart Journal. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- Carrell SE, Maghakian T, West JE. A’s from ZZZZ’s? The causal effect of school start time on the academic achievement of adolescents. American Economic Journal: Economic Policy. 2011;3:62–81. [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Annals of the New York Academy of Sciences. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chan JK, Trinder J, Andrewes HE, Colrain IM, Nicholas CL. The acute effects of alcohol on sleep architecture in late adolescence. Alcoholism: Clinical and Experimental Research. 2013;37:1720–1728. doi: 10.1111/acer.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness-morningness” dimension in “depressive” college students. Journal of Affective Disorders. 1999;52:19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Clark DB. Pharmacotherapy for adolescent alcohol use disorder. CNS Drugs. 2012;26:559–569. doi: 10.2165/11634330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Clark DB, Chung T, Thatcher DL, Pajtek S, Long EC. Psychological dysregulation, white matter organization and substance use disorders in adolescence. Addiction. 2012;107:206–214. doi: 10.1111/j.1360-0443.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Lynch KG, Donovan JE, Block GD. Health problems in adolescents with alcohol use disorders: self-report, liver injury, and physical examination findings and correlates. Alcoholism: Clinical and Experimental Research. 2001;25:1350–1359. [PubMed] [Google Scholar]
- Clark DB, Winters KC. Measuring risks and outcomes in substance use disorders prevention research. Journal of Consulting and Clinical Psychology. 2002;70:1207–1223. doi: 10.1037//0022-006x.70.6.1207. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motivation and Emotion. 1989;13:205–234. [Google Scholar]
- Colrain IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychology Review. 2011;21:5–21. doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DA, Hairston IS, Arnedt JT, Hoffmann RF, Armitage R, Brower KJ. Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiology International. 2012;29:35–42. doi: 10.3109/07420528.2011.636852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockΔ19 mouse model of mania. Neuropsychopharmacology. 2011;36:1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell KG, Chung T, Clark DB, Martin CS. Solitary alcohol use in teens is associated with drinking in response to negative affect and predicts alcohol problems in young adulthood. Clinical Psychological Science. doi: 10.1177/2167702613512795. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Medicine. 2007;8:602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiology International. 2010;27:1469–1492. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum RM, Storr CL, Chan YF, Ford DE. Sleep disturbance and risk for alcohol-related problems. The American Journal of Psychiatry. 2004;161:1197–1203. doi: 10.1176/appi.ajp.161.7.1197. [DOI] [PubMed] [Google Scholar]
- Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004;99:1121–1132. doi: 10.1111/j.1360-0443.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- Danner F, Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. Journal of Clinical Sleep Medicine. 2008;4:533–535. [PMC free article] [PubMed] [Google Scholar]
- Davis S, Mirick DK. Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes & Control. 2006;17:539–545. doi: 10.1007/s10552-005-9010-9. [DOI] [PubMed] [Google Scholar]
- Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. Journal of Affective Disorders. 1991;23:93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcoholism: Clinical and Experimental Research. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. Journal of Sleep Research. 2006;15:261–265. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: Effects on normal sleep. Alcoholism: Clinical and Experimental Research. 2013;37:539–549. doi: 10.1111/acer.12006. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, Dishion TJ, Figueredo AJ, Gray P, Griskevicius V, et al. The evolutionary basis of risky adolescent behavior: implications for science, policy, and practice. Developmental Psychology. 2012;48:598–623. doi: 10.1037/a0026220. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Research. 2009;168:259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience & Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakier N, Wild LG. Associations among sleep problems, learning difficulties and substance use in adolescence. Journal of Adolescence. 2011;34:717–726. doi: 10.1016/j.adolescence.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, Almeida JR, Ferrell RE, Nimgaonkar VL, Mansour H, et al. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biological Psychiatry. 2012;71:451–457. doi: 10.1016/j.biopsych.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Balu S, Greusing S, Rothen N, Cajochen C. Consequences of the timing of menarche on female adolescent sleep phase preference. PloS one. 2009;4:e5217. doi: 10.1371/journal.pone.0005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Rose JS, Swift R, Stout RL, Millman RP, Stein MD. Trazodone for sleep disturbance after alcohol detoxification: a double-blind, placebo-controlled trial. Alcoholism: Clinical and Experimental Research. 2008;32:1652–1660. doi: 10.1111/j.1530-0277.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007;22:268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- Germain A, Nofzinger EA, Meltzer CC, Wood A, Kupfer DJ, Moore RY, et al. Diurnal variation in regional brain glucose metabolism in depression. Biological Psychiatry. 2007;62:438–445. doi: 10.1016/j.biopsych.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annual Review of Clinical Psychology. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. The Journal of Neuroscience. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Developmental Neuroscience. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallfors DD, Waller MW, Bauer D, Ford CA, Halpern CT. Which comes first in adolescence--sex and drugs or depression? American Journal of Preventive Medicine. 2005;29:163–170. doi: 10.1016/j.amepre.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hampp G, Albrecht U. The circadian clock and mood-related behavior. Communicative & Integrative Biology. 2008;1:1–3. doi: 10.4161/cib.1.1.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115:1555–1561. doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Allen JJ, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Research. 2010;176:166–173. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Bootzin RR, Cousins JC, Fridel K, Wenk GL. Circadian phase in sleep-disturbed adolescents with a history of substance abuse: a pilot study. Behavioral Sleep Medicine. 2008;6:55–73. doi: 10.1080/15402000701796049. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcoholism: Clinical and Experimental Research. 2013;37:558–565. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, et al. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biological Psychology. 2012;91:334–341. doi: 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Forbes EE, Franzen PL. Time-of-day differences and short-term stability of the neural response to monetary reward: a pilot study. Psychiatry Research. 2014;224:22–27. doi: 10.1016/j.pscychresns.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Germain A, Nofzinger EA, Kupfer DJ, Krafty RT, Rothenberger SD, et al. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. Journal of Sleep Research. 2012;21:515–526. doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Martin CS, Wood DS, Rosario B, Clark DB. A longitudinal study of insomnia and other sleep complaints in adolescents with and without alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2014;38:2225–2233. doi: 10.1111/acer.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Mehl MR, Bootzin RR, Vazire S. Preliminary evidence of diurnal rhythms in everyday behaviors associated with positive affect. Journal of Research in Personality. 2008;42:1537–1546. [Google Scholar]
- Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research. 2013;214:357–364. doi: 10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Medicine Reviews. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nature Reviews Neuroscience. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Healy D, Waterhouse JM. The circadian system and the therapeutics of the affective disorders. Pharmacology & Therapeutics. 1995;65:241–263. doi: 10.1016/0163-7258(94)00077-g. [DOI] [PubMed] [Google Scholar]
- Hofstetter JR, Mayeda AR, Happel CG, Lysaker PH. Sleep and daily activity preferences in schizophrenia: associations with neurocognition and symptoms. The Journal of Nervous and Mental Disease. 2003;191:408–410. doi: 10.1097/01.NMD.0000071591.91247.67. [DOI] [PubMed] [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. The Journal of Adolescent Health. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda E, Matsunaga N, Kakimoto K, Hamamura K, Hayashi A, Koyanagi S, et al. Molecular mechanism regulating 24-hour rhythm of dopamine D3 receptor expression in mouse ventral striatum. Molecular Pharmacology. 2013;83:959–967. doi: 10.1124/mol.112.083535. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21:178–186. doi: 10.1093/sleep/21.2.178. [DOI] [PubMed] [Google Scholar]
- Kamphuis J, Dijk DJ, Spreen M, Lancel M. The relation between poor sleep, impulsivity and aggression in forensic psychiatric patients. Physiology & Behavior. 2014;123:168–173. doi: 10.1016/j.physbeh.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Kenney SR, LaBrie JW, Hummer JF, Pham AT. Global sleep quality as a moderator of alcohol consumption and consequences in college students. Addictive Behaviors. 2012;37:507–512. doi: 10.1016/j.addbeh.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney SR, Lac A, Labrie JW, Hummer JF, Pham A. Mental health, sleep quality, drinking motives, and alcohol-related consequences: a path-analytic model. Journal of Studies on Alcohol and Drugs. 2013;74:841–851. doi: 10.15288/jsad.2013.74.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD. Effects of sleep deprivation and morningness-eveningness traits on risk-taking. Psychological Reports. 2007;100:613–626. doi: 10.2466/pr0.100.2.613-626. [DOI] [PubMed] [Google Scholar]
- Kim AK, Brown RM, Lawrence AJ. The role of orexins/hypocretins in alcohol use and abuse: an appetitive-reward relationship. Frontiers in Behavioral Neuroscience. 2012;6:78. doi: 10.3389/fnbeh.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, et al. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiology International. 2010;27:1797–1812. doi: 10.3109/07420528.2010.516705. [DOI] [PubMed] [Google Scholar]
- Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. British Journal of Pharmacology. 2014;171:4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lönnqvist J, et al. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol and Alcoholism. 2010;45:303–311. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. American Journal of Epidemiology. 2009;169:1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiology International. 2011;28:771–778. doi: 10.3109/07420528.2011.602445. [DOI] [PubMed] [Google Scholar]
- Logan RW, Williams WP, 3rd, McClung CA. Circadian rhythms and addiction: mechanistic insights and future directions. Behavioral Neuroscience. 2014;128:387–412. doi: 10.1037/a0036268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Girgenti MJ, Breslin FJ, Newton SS, Taylor JR. Gene profiling the response to repeated cocaine self-administration in dorsal striatum: a focus on circadian genes. Brain Research. 2008;1213:166–177. doi: 10.1016/j.brainres.2008.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Fernandes M, Kranzler HR, Covault JM, Welsh DK. Circadian clock period inversely correlates with illness severity in cells from patients with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2013;37:1304–1310. doi: 10.1111/acer.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, Perry GS. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Preventive Medicine. 2011;53:271–273. doi: 10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Christakis NA, Fowler JH. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010;5:e9775. doi: 10.1371/journal.pone.0009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Skene DJ. Nonphotic entrainment in humans? Journal of Biological Rhythms. 2005;20:339–352. doi: 10.1177/0748730405277982. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ. Exposure to shift work as a risk factor for diabetes. Journal of Biological Rhythms. 2013;28:356–359. doi: 10.1177/0748730413506557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M, Slane J, Mindell JA, Burt SA, Klump KL. Sleep problems and temperament in adolescents. Child Care, Health and Development. 2011;37:559–562. doi: 10.1111/j.1365-2214.2010.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin BC, Phillips ML, Siegle GJ, Buysse DJ, Forbes EE, Franzen PL. Sleep deprivation amplifies striatal activation to monetary reward. Psychological Medicine. 2013;43:2215–2225. doi: 10.1017/S0033291712002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munezawa T, Kaneita Y, Osaki Y, Kanda H, Minowa M, Suzuki K, et al. The association between use of mobile phones after lights out and sleep disturbances among Japanese adolescents: a nationwide cross-sectional survey. Sleep. 2011;34:1013–1020. doi: 10.5665/SLEEP.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, et al. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9:705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Natale V, Ballardini D, Schumann R, Mencarelli C, Magelli V. Morningness-eveningness preference and eating disorders. Personality and Individual Differences. 2008;45:549–553. [Google Scholar]
- Negriff S, Dorn LD, Pabst SR, Susman EJ. Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Research. 2011;185:408–413. doi: 10.1016/j.psychres.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, et al. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. Journal of Abnormal Psychology. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- O’Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behavioral Sleep Medicine. 2005;3:113–133. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- Owens JA, Belon K, Moss P. Impact of delaying school start time on adolescent sleep, mood, and behavior. Archives of Pediatrics & Adolescent Medicine. 2010;164:608–614. doi: 10.1001/archpediatrics.2010.96. [DOI] [PubMed] [Google Scholar]
- Pasch KE, Laska MN, Lytle LA, Moe SG. Adolescent sleep, risk behaviors, and depressive symptoms: are they linked? American Journal of Health Behavior. 2010;34:237–248. doi: 10.5993/ajhb.34.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters D, De Valck E, Vandekerckhove M, Pirrera S, Wuyts J, Exadaktylos V, et al. Effects of pre-sleep media use on sleep/wake patterns and daytime functioning among adolescents: the moderating role of parental control. Behavioral Sleep Medicine. 2012;12:427–443. doi: 10.1080/15402002.2012.694381. [DOI] [PubMed] [Google Scholar]
- Pieters S, Van Der Vorst H, Burk WJ, Wiers RW, Engels RC. Puberty-dependent sleep regulation and alcohol use in early adolescents. Alcoholism: Clinical and Experimental Research. 2010;34:1512–1518. doi: 10.1111/j.1530-0277.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- Porto R, Duarte L, Menna-Barreto L. Circadian variation of mood: comparison between different chronotypes. Biological Rhythm Research. 2006;37:425–431. [Google Scholar]
- Rahman SA, Kayumov L, Shapiro CM. Antidepressant action of melatonin in the treatment of Delayed Sleep Phase Syndrome. Sleep Medicine. 2010;11:131–136. doi: 10.1016/j.sleep.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Randler C. Morningness-eveningness comparison in adolescents from different countries around the world. Chronobiology International. 2008;25:1017–1028. doi: 10.1080/07420520802551519. [DOI] [PubMed] [Google Scholar]
- Randler C, Baumann VP, Horzum MB. Morningness-eveningness, Big Five and the BIS/BAS inventory. Personality and Individual Differences. 2014;66:64–67. [Google Scholar]
- Randler C, Saliger L. Relationship between morningness–eveningness and temperament and character dimensions in adolescents. Personality and Individual Differences. 2010;50:148–152. [Google Scholar]
- Renn RP, Cote KA. Performance monitoring following total sleep deprivation: effects of task type and error rate. International Journal of Psychophysiology. 2013;88:64–73. doi: 10.1016/j.ijpsycho.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Roane BM, Taylor DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31:1351–1356. [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Medicine Reviews. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Current Biology. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Rogers HL, Reilly SM. A survey of the health experiences of international business travelers. Part One--Physiological aspects. AAOHN Journal. 2002;50:449–459. [PubMed] [Google Scholar]
- Rossa KR, Smith SS, Allan AC, Sullivan KA. The effects of sleep restriction on executive inhibitory control and affect in young adults. The Journal of Adolescent Health. 2014;55:287–292. doi: 10.1016/j.jadohealth.2013.12.034. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2009;297:R729–R737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007a;30:1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007b;30:1484–1501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxvig IW, Pallesen S, Wilhelmsen-Langeland A, Molde H, Bjorvatn B. Prevalence and correlates of delayed sleep phase in high school students. Sleep Medicine. 2012;13:193–199. doi: 10.1016/j.sleep.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Gay P, Van der Linden M. Facets of impulsivity are differentially linked to insomnia: evidence from an exploratory study. Behavioral Sleep Medicine. 2008;6:178–192. doi: 10.1080/15402000802162570. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM. Rapid tolerance development to the NREM sleep promoting effect of alcohol. Sleep. 2014;37:821–824. doi: 10.5665/sleep.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Research. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clinical Psychology Review. 2005;25:559–592. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Medicine. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stautz K, Cooper A. Impulsivity-related personality traits and adolescent alcohol use: a meta-analytic review. Clinical Psychology Review. 2013;33:574–592. doi: 10.1016/j.cpr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Szeinberg A, Borodkin K, Dagan Y. Melatonin treatment in adolescents with delayed sleep phase syndrome. Clinical Pediatrics. 2006;45:809–818. doi: 10.1177/0009922806294218. [DOI] [PubMed] [Google Scholar]
- Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep deprivation in adolescents and adults: changes in affect. Emotion. 2010;10:831–841. doi: 10.1037/a0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Van Reen E, Acebo C, LeBourgeois M, Seifer R, Fallone G, et al. Adolescence and parental history of alcoholism: insights from the sleep EEG. Alcoholism: Clinical and Experimental Research. 2012;36:1530–1541. doi: 10.1111/j.1530-0277.2012.01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier R, Willoughby T. Are all evening-types doomed? Latent class analyses of perceived morningness-eveningness, sleep and psychosocial functioning among emerging adults. Chronobiology International. 2013;31:232–242. doi: 10.3109/07420528.2013.843541. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Bramoweth AD. Patterns and consequences of inadequate sleep in college students: substance use and motor vehicle accidents. Journal of Adolescent Health. 2010;46:610–612. doi: 10.1016/j.jadohealth.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. The effects of poor quality sleep on brain function and risk taking in adolescence. NeuroImage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschann JM, Adler NE, Irwin CE, Jr, Millstein SG, Turner RA, Kegeles SM. Initiation of substance use in early adolescence: the roles of pubertal timing and emotional distress. Health Psychology. 1994;13:326–333. doi: 10.1037//0278-6133.13.4.326. [DOI] [PubMed] [Google Scholar]
- Urbán R, Magyaródi T, Rigó A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiology International. 2011;28:238–247. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- Van den Bulck J. Adolescent use of mobile phones for calling and for sending text messages after lights out: results from a prospective cohort study with a one-year follow-up. Sleep. 2007;30:1220–1223. doi: 10.1093/sleep/30.9.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010;33:1605–1614. doi: 10.1093/sleep/33.12.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reen E, Rupp TL, Acebo C, Seifer R, Carskadon MA. Biphasic effects of alcohol as a function of circadian phase. Sleep. 2013;36:137–145. doi: 10.5665/sleep.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert RM, van Roekel E, Engels RC, Scholte RH. Reciprocal Associations Between Adolescents’ Night-Time Sleep and Daytime Affect and the Role of Gender and Depressive Symptoms. Journal of Youth and Adolescence. 2013 doi: 10.1007/s10964-013-0009-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Kirisci L, Moss L, Tarter RE, Reynolds MD, Maher BS, et al. Measurement of the risk for substance use disorders: phenotypic and genetic analysis of an index of common liability. Behavior Genetics. 2009;39:233–244. doi: 10.1007/s10519-009-9269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neuroscience and Biobehavioral Reviews. 2003;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Huettel SA, Chuah LY, Payne JW, Chee MW. Sleep deprivation biases the neural mechanisms underlying economic preferences. The Journal of Neuroscience. 2011;31:3712–3718. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinescu BI, Szentagotai A, David D. Sleep disturbance, circadian preference and symptoms of adult attention deficit hyperactivity disorder (ADHD) Journal of Neural Transmission. 2012;119:1195–1204. doi: 10.1007/s00702-012-0862-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Chartrand TL. Morningness-eveningness and risk taking. The Journal of Psychology. 2014:1–18. doi: 10.1080/00223980.2014.885874. ahead-of-print. [DOI] [PubMed] [Google Scholar]
- Watson D. Mood and Temperament. New York: Guilford Press; 2000. [Google Scholar]
- Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. Journal of Biological Rhythms. 2009;24:465–476. doi: 10.1177/0748730409346657. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiology International. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcoholism: Clinical and Experimental Research. 2004;28:578–587. doi: 10.1097/01.alc.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Nigg JT, Zucker RA. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcoholism: Clinical and Experimental Research. 2010;34:1033–1044. doi: 10.1111/j.1530-0277.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B, Rea MS, Plitnick B, Figueiro MG. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Applied Ergonomics. 2013;44:237–240. doi: 10.1016/j.apergo.2012.07.008. [DOI] [PubMed] [Google Scholar]


