Abstract
Executive function (EF) and cognitive processing speed (CPS) are two cognitive performance domains that decline with advanced age. Reduced EF and CPS are known to correlate with age-related frontal-lobe volume loss. However, it remains unclear whether white matter microstructure in these regions is associated with age-related decline in EF and/or CPS. We utilized quantitative tractography metrics derived from diffusion-tensor MRI to investigate the relationship between the mean fiber bundle lengths (FBLs) projecting to different lobes, and EF/CPS performance in 73 healthy aging adults. We measured aspects of EF and CPS with the Trail Making Test (TMT), Color-Word Interference Test, Letter-Number Sequencing (L-N Seq), and Symbol Coding. Results revealed that parietal and occipital FBLs explained a significant portion of variance in EF. Frontal, temporal and occipital FBLs explained a significant portion of variance in CPS. Shorter occipital FBLs were associated with poorer performance on the EF tests TMT-B and CWIT 3. Shorter frontal, parietal, and occipital FBLs were associated with poorer performance on L-N Seq and Symbol Coding. Shorter frontal and temporal FBLs were associated with lower performance on CPS tests TMT-A and CWIT 1. Shorter FBLs were also associated with increased age. Results suggest an age-related FBL shortening in specific brain regions related to poorer EF and CPS performance among older adults. Overall, results support both the frontal aging hypothesis and processing speed theory, suggesting that each mechanism is contributing to age-related cognitive decline.
Keywords: Fiber Bundle Lengths, DTI, White Matter, Cognitive Processing Speed, Executive Function, Aging
1. Introduction
It is widely established that cognitive changes occur with the aging process (McDowell, Xi, Lindsay, & Tukko, 2004). Executive function (EF) is particularly sensitive to age-related changes (Raz, Gunning-Dixon, Head, Dupuis, & Acker, 1998). Along with EF, tasks that require rapid responses driven by cognitive processing speed (CPS) are likely to be compromised with advanced age (Jacobs et al., 2013; Lee et al., 2012; Lu, Lee, Tishler, Meghpara, Thompson, & Bartzokis, 2013).
Multiple processes mediate age-related cognitive decline; however, particular attention has focused on frontal lobe dysfunction as a major driver of cognitive changes in advanced age. The frontal aging hypothesis (Greenwood, 2000) suggests that cognitive performance on tasks associated with frontal brain regions (e.g., EF) is most vulnerable to aging processes. Normal age-related frontal lobe deterioration leads to neuropsychological impairments that are similar in nature and severity to individuals with frontal lobe lesions (Dempster, 1992). Others have noted, however, that aging effects are not restricted to the frontal lobe, but also involve other brain areas such as the temporal region (West, 1996). As such, the frontal aging hypothesis may be too restrictive, ignoring the complementary role between the prefrontal cortex and other areas such as the inferotemporal cortex (Fuster, Baurer, & Jervey, 1985; Yajeya, Quintana, & Fuster, 1988). Nonetheless, the importance of the frontal lobe to cognitive aging cannot be discarded.
The processing speed theory put forth by Salthouse (Salthouse, 1996) has gained support as an explanation of age-related cognitive difficulties beyond processes encapuslated by the frontal aging hypothesis. This theory suggests that overall cognitive performance is degraded due to slower execution of tasks such as divided thinking, inhibition, and resistance to distractions. Jacobs et al. (2013) identified empirical support for both the frontal aging hypothesis and processing speed theory by emphasizing the important relationship between EF and CPS. A similar study found considerable overlap between execution of tasks associated with EF and those related to CPS when investigating white matter (WM) integrity (Albinet, Boucard, Bouquet, & Audiffren, 2012). Results indicated that age-related variance in measures of EF overlapped with variance in measures of CPS, implying that deficits in EF may be due to a slowing of CPS. Additional studies have underlined the importance of both EF and CPS in meditating the cognitive changes seen with advanced age. (Bugg, Zook, DeLosh, Davalos, & Davis, 2006; Charlton et al., 2008; Schretlen et al., 2000).
Along with cognitive decline, prior studies have identified reduced brain volume in older age (Allen, Bruss, Brown, & Damasio, 2005; Brickman et al., 2006; Cowell et al., 1994). White matter atrophy typically follows a geometric progression with age, and more overt changes (i.e., increased WM hyperintensity burden) are observed during middle adulthood (Jernigan et al., 2001; Raz & Rodrigue, 2006; Sowell et al., 2003). Multiple studies have demonstrated patterns of increased WM volume until the fourth decade, followed by reduced volume over time (Allen et al., 2005; Guttman et al., 1998). Histologic and magnetic resonance imaging (MRI) patterns of myelin integrity indicate that oligodendrocytes in later-myelinated areas (i.e., frontal lobes) are more susceptible to volumetric decline associated with advanced age (Bartzokis, Beckson, Neuechterlein, Edwards, & Mintz, 2001; Bartzokis et al., 2003; Bartzokis et al., 2007; Cowell et al., 1994; Jernigan et al., 2001; Raz & Rodrigue, 2006).
The microstructural basis of age-related WM changes and their relationship to EF and CPS as well as other cognitive abilities is unclear. In MRI diffusion-tensor imaging (DTI), the microscopic movements of water molecules are measured in living human brain tissues. The degree to which diffusion is directionally-dependent in ordered tissues is indicated by the scalar measure of anisotropy. Diffusion in cerebral WM is highly anisotropic due to the fast diffusion of water molecules along neuronal fiber bundles, whereas diffusion is slower across fiber bundles. Directionally-averaged mean diffusivity (MD) is another scalar measure that provides information about total diffusion rate within a voxel independent of tissue orientation. Water diffusion in the tissue exhibits anisotropy (directionality), providing sensitivity to microstructural changes in WM. Measurements of anisotropy and MD in WM are sensitive to age-related changes in WM (Bennett, Madden, Vaidya, Howard, & Howard, 2010; Correia et al., 2008; Madden, Bennett, & Song, 2009; Mosely, 2002). As such, both anisotropy and MD can detect microscopic effects of aging in both normal and diseased states. Higher anisotropy reflects more-intact WM pathways (and vice versa) (Madden et al., 2009). Conversely, with greater integrity, MD values typically decrease in WM (Sullivan & Pfefferbaum, 2006). Further, WM degeneration evidenced on DTI correlates with age-related cognitive decline (Madden et al., 2009; Salat et al., 2005; Voineskos et al., 2012). Charlton et al. (2006) revealed that cognitive performances on tests of working memory, EF, and CPS correlated with changes in anisotropy and MD. It has also been demonstrated that anisotropy relates to cognitive switching in older adults (Madden et al., 2009).
DTI can also measure the orientation of fiber bundles, which forms the basis for tracking neuronal fiber pathways (Conturo et al., 1999; Mori, Crain, Chacko, & Van Zijl, 1999). Quantitative tractography metrics (QTM) can be utilized to further investigate the microstructural integrity of the aging brain. Region-of-interest (ROI) measurements of DTI scalar metrics can be less sensitive to pathologic changes than measurements involving tractography (Tate et al., 2010). In this method, track lines are computed and geographically rendered to represent neuronal fiber bundles. Basic DTI scalar metrics are combined with information about the curvature and detailed directionality of the WM paths in order to provide a more complete description of WM fiber bundles (Correia et al., 2008). This technique has successfully identified changes in WM tracts (Bolzenius et al., 2013; Correia et al., 2008; Salminen et al., 2013; Tate et al., 2010).
Prior to QTM technology, fiber length (FBL) could only be investigated using animal and autopsy studies (Conturo et al., 1999). Such post-mortem studies have revealed frontal WM fiber length reductions that are associated with older age (Marner, Nyengaard, Tang, & Pakkenberg, 2003; Tang, Nyengaard, Pakkenberg, & Gundersen, 1997). Based on these data, it has been proposed that the lengths of axonal fibers are particularly decreased in shorter, smaller-diameter myelinated fibers (Marner et al., 2003). As a sensitive noninvasive measure, tractography can help determine the location of FBL changes, whether these changes are a function of normal aging, and whether these changes are capable of impacting cognitive performance. We have recently reported significant alterations in FBL among older individuals compared to younger individuals (Baker et al., 2014), but no study has examined these imaging metrics in combination with cognitive performance.
The present study utilized QTM to determine whether lobar FBLs are significantly associated with cognitive performance in the domains of EF and CPS among healthy older adults. Based on previous studies focused on WM volume, cognitive ability, and age, it can be postulated that reduced FBLs in WM tracts are associated with reduced cognitive ability. Further, age-related compromise of fiber length may represent a biomarker of cognitive deficits observed in older adults (Marner et al., 2003; Meier-Ruge, Ulrich, Brühlmann, & Meier, 1992). As such, we hypothesized that shorter FBL by lobe would be significantly associated with poorer performance on cognitive measures of EF and CPS in a healthy-aging population.
2. Methods
2.1 Participants
Baseline MRI and behavioral data were acquired from individuals between the ages of 51 and 85 years who were recruited for a longitudinal study on cognitive aging. Participants were recruited from the local community using print and radio ads; additional participants were recruited from the Research Participant Registry of the Washington University Institute of Clinical and Translational Sciences (ICTS). The Mini Mental Status Examination (MMSE; Folstein, Folstein, & McHugh, 1975) was administered to exclude individuals with possible dementia. One participant was removed from the study prior to data analysis, due to inability to perform one of the cognitive tests. The final sample size was 73 participants (male n = 25, female n = 48). The sample was primarily composed of Caucasian Americans (n = 54) and African Americans (n = 14). Demographics are listed in Table 1. Indivduals with missing data were (max = 2 individuals) due to administration error or computer error removed from the analysis involving that test (see Table 2). Exclusion criteria included any medical condition that could significantly affect cognitive functioning, history of traumatic brain injury resulting in loss of consciousness greater than five minutes, learning disorders, substance abuse issues, and a diagnosis of any major psychiatric disorder (e.g., all Axis I and II disorders with the exception of treated depression). Full exclusion criteria are described in (Paul et al., 2011). The study was approved by the Institutional Review Boards of the respective institutions. All participants provided informed consent and were financially compensated for their participation.
Table 1.
Demographic information and mean fiber bundle lengths.
|
N = 73 participants (25 males, 48 females)
| ||||
|---|---|---|---|---|
| Variable | Mean | SD | Min | Max |
| Demographicsa | ||||
| Age at MRI (years) | 63.56 | 8.59 | 51 | 85 |
| Educationb (years) | 15.38 | 2.53 | 11 | 20 |
| Mean Fiber Bundle Length (mm) | ||||
| Frontal Lobe | 33.21 | 2.85 | 27.7 | 40.21 |
| Insula | 28.07 | 6.44 | 16.19 | 47.26 |
| Temporal Lobe | 27.41 | 2.96 | 21.06 | 35.58 |
| Parietal Lobe | 31.37 | 3.32 | 22.22 | 41.46 |
| Occipital Lobe | 34.46 | 5.11 | 21.42 | 48.74 |
Demographics were calculated after one outlier was removed prior to analysis (see text).
Education was measured as highest year of school completed.
Table 2.
Summary of cognitive scores
| Cognitive task | Mean score | SD | Min | Max |
|---|---|---|---|---|
| TMT-B | 85.33 | 53.1 | 36 | 300 |
| CWIT 3 | 58.07 | 14.7 | 33 | 113 |
| CWIT 4 | 67 | 18.82 | 43 | 130 |
| LN-Seq | 10.63 | 2.01 | 7 | 16 |
| TMT-A | 34.96 | 14.96 | 17 | 116 |
| CWIT 1 | 29.94 | 4.57 | 21 | 46 |
| Symbol Coding | 47.56 | 8.77 | 28 | 68 |
TMT Trail Making Test, CWIT Color-Word Interference Test, LN-Seq Letter Number Sequencing
2.2 MRI Protocol
Imaging acquisition took place using a head-only Magnetom Allegra 3T MRI scanning system (Siemens Medical Solutions, Erlangen, Germany) at Washington University - St. Louis. For quality assurance, identical pulse sequences and movement minimization tactics (e.g., tape across the forehead) were consistent throughout the course of the study. To ensure data reliability, daily quality control tests were performed, and stability was maximized by avoiding modifications to scanning software and equipment. This scanner has high-performance gradients (max strength 40 mT/m in a 100-microsecond rise time on all three axes simultaneously; maximum slew rate 400 T/m/s on each axis), limiting the session time to one hour. Head placement was confirmed by a preliminary scout scan composed of three orthogonal planes.
2.3 Structural MRI Acquisition
Structural whole brain scans were collected through use of a T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence; a T2-weighted multislice turbo spin echo (TSE) sequence; and a T2-weighted multislice fluid-attenuated inversion-recovery (FLAIR) TSE sequence. Slice coverage, field of view (FOV), and other acquisition parameters are described in our initial study (Paul et al., 2011) in which the structural MRI acquisition parameters were established.
2.4 Diffusion-Weighted Imaging (DWI) Acquisition
Axial diffusion-weighted images were acquired using a customized in-house single-shot multislice echo-planar pulse sequence. The tensor was encoded using 31 non-collinear diffusion-encoded directions consisting of 4 tetrahedral directions (Conturo, McKinstry, Akbudak, & Robinson, 1996) and 27 interspersed directions (diffusion weighting of b = 996 s/mm2). Five I0 acquisitions with a diffusion weighting of ~0 s/mm2 were also employed to capture a reference T2-weighted image volume with high signal-to-noise ratio (SNR). The following parameters were used: TE = 86.2 ms; TR = 7.82 s; 64 contiguous 2.0-mm slices; and an acquisition matrix of 128 x 128 with a field of view of 256 x 256 mm (isotropic 2.0 x 2.0 x 2.0 mm voxels). Two scan repeats were acquired, resulting in 10 T2-weighted (I0) volumes and 62 diffusion-weighted volumes. In addition, a custom protocol was used to allow for floating-point DWI image reconstruction. Prior studies have utilized this methodology (Bolzenius et al., 2013; Salminen et al., 2013).
2.5 Post-processing, Track Calculation/Extraction, and Length Measurement
Subject movement during scan time was corrected by registering each participant’s DWI and diffusion-encoding vectors to the I0 image using FSL FLIRT (mutual information metric; Jenkinson, Bannister, Brady, & Smith, 2002). Using dti_recon from the Diffusion Toolkit (DTK; Wang, Benner, Sorensen, & Wedeen, 2007), voxel-wise tensors and fractional anisotropy (FA) were computed from the diffusion-encoding vectors, b-values, and DWIs. The diffusion tensor field was calculated using trilinear interpolation, from which curvilinear track lines (“tracks”) were constructed to represent WM fiber bundles. Tracks were computed using FACT (Mori et al., 1999), a continuous algorithm with one seed per voxel. Tracks were terminated if the FA fell below a threshold of 0.15, or if the step angle exceeded a threshold of 35 degrees over 1 mm. In order to isolate the tracks associated with a specific lobe, tracks were extracted if they passed into a lobar spatial selection volume (SSV; Conturo et al., 1999; Lori et al., 2002), and if they were a minimum of 10 mm in length. Because both low SNR and low pathway anisotropy can lead to foreshortening of tracks due to premature track termination (Lori et al., 2002), we used high-SNR acquisition conditions, conservative stopping criteria, and a length criterion to minimize this foreshortening effect. These approaches support the reliability of the length measurements. The stopping and length criteria are also consistent with previously used extraction methods (Correia et al., 2008), and provided optimal results that correspond to well-known WM structures.
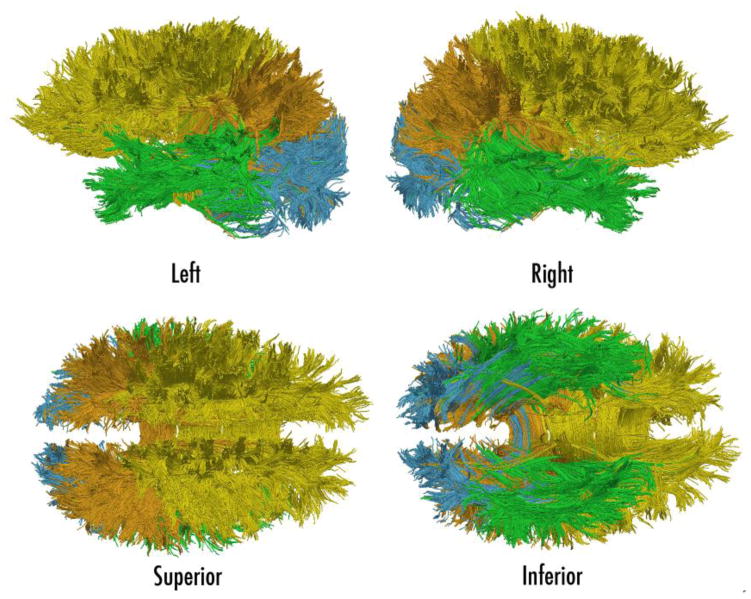
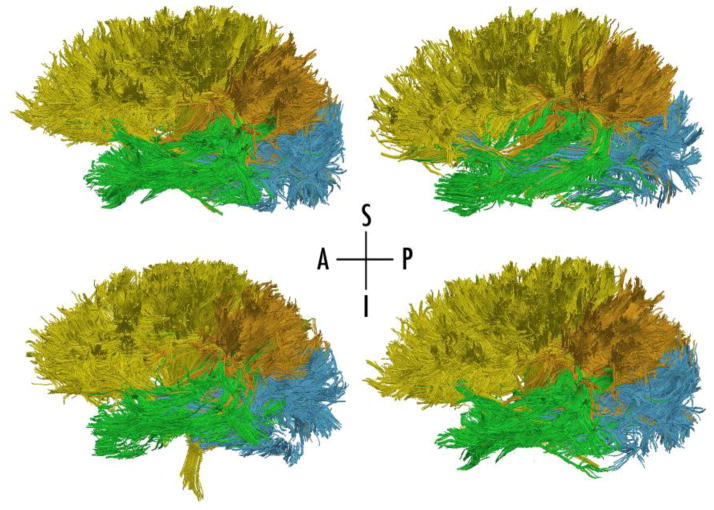
To define the lobar SSVs, the FA image was registered to the ICBM T1 atlas (Mazziotta et al., 2001) utilizing FSL FLIRT (Correia et al., 2008). The lobar labels were mapped to each subject’s DWI space (Jenkinson et al., 2002) with nearest-neighbor interpolation. Brain regions included the frontal, temporal, parietal, and occipital lobes bilaterally, as well as the insular region. This provided a set of ten SSVs. Tracks were assigned to a specific lobe if at least one endpoint of the track was inside either the right or left lobe. A track could have both endpoints solely within one lobe of one hemisphere, or a track could project ipsilaterally to another lobe (in the same hemisphere), or could even project contralaterally (across the midline) to the same or different lobe. For example, a track with both endpoints in the right temporal lobe was assigned to be “temporal”. A track with endpoints in the right and left temporal lobe was also assigned to be “temporal”. A track with endpoints in the right temporal lobe and right parietal lobe was assigned to be both “temporal” and “parietal”. The logic of assigning tracks to lobes is further described in (Baker et al., 2014; Bolzenius et al., 2013; Salminen et al., 2013). For each subject, the mean track length was computed from the arc lengths and was normalized to intracranial volume. Prior to statistical comparison, FBLs were corrected for head size by using normalized algorithms combing the total number of streamtubes, summed length of the streamtubes, and weighted length metrics for linear and fractional anisotropy in each WM track. Further details of the normalization process are explained in previous work from our group (Bolzenius et al., 2013; Baker et al., 2014; Correia et al., 2008; Salminen et al., 2013). Visulizations of mean FBL by lobe are depicted for a single subject (see Figure 1) and for inter subject variability (see Figure 2).
Fig. 1.
Illustration of the tractography results for a single subject presented in four views. Whole-brain tractography was segmented by connectivity in each lobe to create frontal (yellow), parietal (orange), occipital (blue), and temporal (green) groups.
Fig. 2.
Illustration of a left lateral view of tractography results in four different subjects to depict inter-subject variability. Whole-brain tractography was segmented by connectivity in each lobe to create frontal (yellow), parietal (orange), occipital (blue), and temporal (green) groups.
2.6 Cognitive Testing
Neuropsychological testing was completed within 30 days of MRI acquisition. The battery was administered to each participant using standardized testing procedures. Measurements of EF and CPS were obtained through the following tasks.
2.6.1 Measures of EF
Trail Making Test Part B
The Trail Making Test (TMT) depends primarily on frontal lobe activation, and contains two conditions, A and B (Charlton et al., 2008; Moscovitch & Winocur, 1992). In the B condition of the Trail Making Test (TMT-B), participants are required to sequentially connect numbers and letters in an alternating pattern. Time to completion served as the dependent variable.
Color-Word Interference Test Conditions 3 & 4
The third portion of Color-Word Interference Test (CWIT 3), a subtest from the Delis-Kaplan Executive Function System (D-KEFS), predominantly focuses on interference by having participants name the specified ink color of incongruently colored words (i.e. the word “red” depicted in green ink). The fourth portion of this test (CWIT 4) increases the difficulty by adding an additional level of inhibition. Participants must follow the same directions as in CWIT 3, but are told to read the word instead of naming the ink color when the stimulus is contained inside a box. Completion time in seconds was used as the dependent variable in both conditions.
Letter-Number Sequencing
Letter-Number Sequencing (L-N Seq) is a subtest obtained from the Wechsler Adult Intelligence Scale (WAIS-III) that measures working memory. Completion of the task requires participants to mentally organize a string of random orally-presented letters and numbers in alphabetical and numerical order. Total number of correct responses served as the dependent variable.
2.6.2 Measures of CPS
Trail Making Test Part A
The TMT-B condition is described above. The TMT-A condition assesses attention, information processing, graphomotor speed, and visuospatial tracking while participants try to correctly connect 25 sequentially numbered circles as fast as possible. Completion time in seconds served as the dependent variable.
Color-Word Interference Test Condition 1
The first portion of the Color-Word Interference Test (CWIT 1) predominantly focuses on mental speed and control by having participants name the patches of ink color in order of presentation, as quickly as possible without making errors. Completion time in seconds served as the dependent variable.
Symbol Coding
This subtest of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) measures processing speed. The task consists of boxes containing a numeral in the top line and a symbol in the bottom line. The participant must then write the symbol corresponding to each numeral in the worksheet provided. The participant must work as quickly as possible without making errors. The number of correct items recorded in 90 seconds served as the dependent variable.
2.7 Statistical Analyses
Multivariate linear models were used to examine the relationship between age and cognitive measures of the EF and CPS with FBL. As described above, the EF domain consisted of TMT-B, CWIT 3, CWIT 4, and L-N Seq scores, while the CPS domain consisted of CWIT 1, TMT-A, and Symbol Coding scores. In the age-FBL multivariate analysis, the mean FBL measures by lobe were entered as dependent variables, and age was entered as a predictor. In the FBL-cognition multivariate analysis, each FBL measure by lobe was entered as a predictor, and raw scores for each cognitive task were entered as dependent variables. Additional follow-up univariate linear models tested to what extent age predicted each mean FBL, and to what extent mean FBL by lobe predicted each cognitive measure. In each age-FBL univariate model, the FBL measure was entered as the response, and age was entered as the predictor. In each FBL-cognition univariate model, the cognitive measure was entered as the response, and each mean FBL was entered as a predictor. To adjust for the effect of age in the FBL-cognition analysis, we also performed multivariate and univariate FBL-cognition models with age included as an additional covariate (see supplemental information for results). Mean FBL and cognitive test measures were logarithmically (base-10) transformed before inclusion in the models since all the measures were positive and demonstrated curved non-linear relationships between the responses and predictors. This transformation, suggested by Tukey’s Bulging Rule, yields better linear representation and better satisfaction of the assumptions of the linear model. All statistical computations were carried out in R (version 2.15.2, http://cran.r-project.org), and all p-values are two-sided.
3. Results
Age explained a significant portion of the FBL variance (Pillai-Bartlett statistic = 0.16, approximate F(5,67) = 2.0, p = 0.039 in the multivariate linear model for all FBL measures. A 1-year increase in age predicted a statistically-significant 0.14% decrease in mean frontal FBL (t(71) = −2.90, p = 0.005) and a statistically-significant 0.18% decrease in mean temporal FBL (t(71) = −2.92, p = 0.005). A 1-year increase in age was also marginally significant with decreases in mean occipital FBL (t(71) = −1.69, p = 0.09) and in mean parietal FBL (t(71) = −1.70, p = 0.09). The relation between age and insular FBL was not statistically significant (p = 0.96). The univariate results are detailed in Table 3.
Table 3.
Univariate linear model analysis of how age predicted fiber bundle length.
| Response | Coefficient | SE | t | p value |
|---|---|---|---|---|
| Frontal FBL | −0.0014 | 0.0003 | −2.9049 | 0.0049* |
| Insula FBL | −0.0001 | 0.00013 | −0.0545 | 0.9567 |
| Occipital FBL | −0.0016 | 0.0009 | −1.6941 | 0.0946 |
| Parietal FBL | −0.0011 | 0.0006 | −1.7018 | 0.0932 |
| Temporal FBL | −0.0018 | 0.0006 | −2.9229 | 0.0046* |
FBL fiber bundle length
Statistically significant p < 0.05
In the EF domain, before age adjustment, mean parietal FBL explained a significant portion of the behavioral variance (Pillai-Bartlettt statistic = 0.17, approximate F(4, 64) = 3.40, p = 0.015) in the multivariate linear model. After adjusting for age, mean parietal FBL remained significant (Pillai-Bartlettt statistic = 0.15, approximate F(4,63) = 2.79, p = 0.034). Mean occipital FBL was marginally significant in the multivariate analysis (Pillai-Bartlettt statistic = 0.11, approximate F(4, 64) = 2.0, p = 0.10) before the age adjustment but was no longer statistically significant after adjusting for age (Pillai-Bartlettt statistic = 0.09, approximate F(4,63) = 1.57, p = 0.19). All other FBLs were not significant in this EF analysis (p-values > 0.39) with or without age adjustment. For the CPS domain before age adjustment, mean frontal FBL (Pillai-Bartlettt statistic = 0.13, approximate F(3, 66) = 3.00, p = 0.029) and mean occipital FBL (Pillai-Bartlettt statistic = 0.15, approximate F(3, 66) = 4.00, p = 0.013) explained a significant portion of the behavioral variance, while all other FBLs were not significant (p-values > 0.19). After correcting for age, mean occipital FBL (Pillai-Bartlettt statistic = 0.12, approximate F(3, 65) = 2.83, p = 0.045) remained significant, while all other FBLs were not significant (p-values > 0.21). The multivariate results are detailed in Table 4.
Table 4.
Multivariate linear model analysis of fiber bundle length and cognitive domains.
| Pillai-Bartlett Statistic | Approximate F Statistic | df | p value | |
|---|---|---|---|---|
| Executive Function | ||||
| Frontal FBL | 0.062 | 1.053 | 4, 64 | 0.387 |
| Occipital FBL | 0.112 | 2.022 | 4, 64 | 0.102 |
| Parietal FBL | 0.174 | 3.367 | 4, 64 | 0.015* |
| Temporal FBL | 0.041 | 0.692 | 4, 64 | 0.600 |
| Insular FBL | 0.055 | 0.940 | 4, 64 | 0.447 |
| Cognitive Processing Speed | ||||
| Frontal FBL | 0.127 | 3.186 | 3, 66 | 0.029* |
| Occipital FBL | 0.149 | 3.854 | 3, 66 | 0.013* |
| Parietal FBL | 0.040 | 0.925 | 3, 66 | 0.434 |
| Temporal FBL | 0.044 | 1.002 | 3, 66 | 0.398 |
| Insula FBL | 0.069 | 1.640 | 3, 66 | 0.189 |
FBL fiber bundle length
Statistically significant p < 0.05
In the EF domain, a 1% decrease in mean parietal FBL predicted an average decrease of 0.51% in L-N Seq scores (t(69) = 2.54, p = 0.013) and a 1% decrease in mean occipital FBL predicted a marginally significant 0.62% increase in TMT-B completion time (t(71) = −1.94, p = 0.056). There were also trends in the occipital and frontal FBLs with respect to L-N Seq, and a trend in the occipital FBL with respect to CWIT 3.
In the CPS domain, a 1% decrease in mean frontal FBL predicted a 1.39% increase in completion time for TMT-A (t(70) = −3.11, p = 0.0027). A 1% decrease in mean temporal FBL predicted a 0.86% increase (t(70) = −2.33, p = 0.023) in TMT-A completion time. For CWIT 1, a 1% decrease in mean frontal FBL predicted 0.51% increase in completion time (t(69) = −2.55, p = 0.013). For Symbol Coding, a 1% decrease in mean frontal FBL predicted a 0.59% decrease (t(70) = 2.31, p = 0.024), and a 1% decrease in mean occipital FBL predicted a 0.46% decrease (t(70) = 3.41, p = 0.001). Details of the univariate results, and trends that did not reach statistical significance, are shown in Table 5.
Table 5.
Univariate linear model analysis of fiber bundle length and specific cognitive tests.
| Coefficient | SE | t | p value | |
|---|---|---|---|---|
| Tests ofExecutive Function | ||||
|
| ||||
| TMT-B | ||||
| Frontal FBL | −1.058 | 0.581 | −1.821 | 0.073 |
| Insula FBL | −0.043 | 0.228 | −0.188 | 0.851 |
| Occipital FBL | −0.621 | 0.320 | −1.940 | 0.056 |
| Parietal FBL | 0.539 | 0.482 | 1.118 | 0.267 |
| Temporal FBL | −0.756 | 0.462 | −1.634 | 0.107 |
| CWIT 3 | ||||
| Frontal FBL | −0.500 | 0.328 | −1.525 | 0.132 |
| Insula FBL | −0.030 | 0.128 | −0.233 | 0.816 |
| Occipital FBL | −0.287 | 0.179 | −1.601 | 0.114 |
| Parietal FBL | −0.234 | 0.270 | −0.866 | 0.389 |
| Temporal FBL | −0.304 | 0.259 | −1.171 | 0.245 |
| CWIT 4 | ||||
| Frontal FBL | −0.473 | 0.339 | −1.395 | 0.168 |
| Insula FBL | −0.083 | 0.131 | −0.637 | 0.526 |
| Occipital FBL | −0.277 | 0.185 | −1.496 | 0.139 |
| Parietal FBL | −0.143 | 0.282 | −0.505 | 0.615 |
| Temporal FBL | −0.308 | 0.267 | −1.152 | 0.253 |
| L-N Seq | ||||
| Frontal FBL | 0.468 | 0.254 | 1.847 | 0.069 |
| Insula FBL | 0.106 | 0.101 | 1.045 | 0.300 |
| Occipital FBL | 0.274 | 0.146 | 1.872 | 0.066 |
| Parietal FBL | 0.513 | 0.202 | 2.540 | 0.013* |
| Temporal FBL | 0.283 | 0.205 | 1.385 | 0.171 |
|
| ||||
| Tests ofCognitive Processing Speed | ||||
|
| ||||
| TMT-A | ||||
| Frontal FBL | −1.396 | 0.449 | −3.112 | 0.003* |
| Insula FBL | −0.151 | 0.178 | −0.849 | 0.399 |
| Occipital FBL | −0.204 | 0.256 | −0.798 | 0.427 |
| Parietal FBL | 0.027 | 0.382 | 0.071 | 0.944 |
| Temporal FBL | −0.863 | 0.370 | −2.329 | 0.023* |
| CWIT 1 | ||||
| Frontal FBL | −0.511 | 0.200 | −2.554 | 0.013* |
| Insula FBL | −0.064 | 0.082 | −0.782 | 0.437 |
| Occipital FBL | −0.149 | 0.111 | −1.339 | 0.185 |
| Parietal FBL | −0.109 | 0.168 | −0.649 | 0.519 |
| Temporal FBL | −0.205 | 0.164 | −1.250 | 0.215 |
| Symbol Coding | ||||
| Frontal FBL | 0.592 | 0.256 | 2.315 | 0.024* |
| Insula FBL | −0.163 | 0.103 | −1.590 | 0.116 |
| Occipital FBL | 0.456 | 0.134 | 3.414 | 0.001* |
| Parietal FBL | 0.328 | 0.211 | 1.550 | 0.126 |
| Temporal FBL | 0.150 | 0.212 | 0.706 | 0.482 |
FBL fiber bundle length, TMT Trail Making Test, CWIT Color-Word Interference Test, LN-Seq Letter Number Sequencing
Statistically significant p < 0.05
4. Discussion
To our knowledge this is the first study to investigate the relationship between lobar fiber bundle length (FBL) and cognitive performance in a sample of healthy older individuals. Both EF measures and CPS measures revealed lower performance in association with shortened FBLs across various lobes. Specifically, for EF measures, performance on TMT-B was associated with shorter fiber bundles originating or terminating in the occipital lobe. The EF measure of L-N Seq was associated with shorter FBLs in frontal, occipital, and parietal lobes. Regarding CPS measures, lower performance on TMT-A was associated with shorter FBLs for frontal and temporal lobes whereas CWIT 1 was associated with shorter FBLs in the frontal lobe. Finally, Symbol Coding was associated with shorter FBLs in frontal, occipital, and parietal lobes.
In terms of anatomical region, a reduced length of fiber bundles connecting to/from the frontal lobe was found to be associated with poorer performance in both EF (LN-Seq) and CPS (TMT-A, CWIT 1, and Symbol Coding) cognitive domains. FBLs originating/terminating in parietal lobe were also associated with performance in EF (LN-Seq) and CPS (Symbol Coding) domains. FBLs related to the occipital lobe were associated with both EF (LN-Seq, TMT-B) and CPS (Symbol Coding) scores. Only for the temporal lobe did FBLs show a relation to only one domain (CPS score of TMT-A). Collectively, the findings from the present study suggest that cognitive decline is related to compromised FBL in various brain regions among older adults.
Previous histologic studies have suggested that shortening of myelinated fiber length may be a mechanism of aging (Marner et al., 2003; Tang et al., 1997). However, such autopsy studies are usually limited by lack of neuropsychological test data in the same subjects. Application of the imaging appraoch in the present study alows for assessment of FBL and neuropsycholgoical perofrmance in vivo. The results of the multivariate and univariate analyses of the relation between age and lobar FBL herein are consistent with our prior cross-sectional age analyses of the same participants. We previously showed a correlation between FBL shortening and increased age for multiple lobes (Bolzenius et al., 2013; Salminen et al., 2013), with the strongest statistical significance in frontal and temporal lobes similar to Table 2. We also previously found a relation between shortened FBLs and increased age for specific WM pathways using correlation analysis (Salminen et al., 2013) and univariate analysis (Baker et al., 2014). Taken together, our present and prior results indicate age-related FBL shortening at the lobar and pathway levels. Our present finding of decreased mean lobar FBL as a predictor of poorer cognitive performance indicates a relation between age-related FBL shortening and cognition, and provides a more direct link between FBL and cognitive aging. The role of WM as the primary facilitator of communication between brain regions suggests that minor changes in axonal integrity may interfere with cognitive abilities in EF and CPS domains (Albinet et al., 2012; Fields, 2008; Madden et al., 2009). Therefore, the observed association between reduced frontal FBL and decreased performance on specific tests of EF and CPS is consistent with previous results that suggest that decreased frontal WM integrity predicts poorer cognitive performance in these domains. Further support for our findings is gained from previous autopsy studies that determined that reduced neuronal lengths in the frontal WM are present in brain specimens from older donors (Marner et al., 2003; Tang et al., 1997).
Both the frontal aging hypothesis and the processing speed theory align with the findings presented in this study. The observed relations between FBL and EF performance (TMT-B and LN-Seq) described above are consistent with the frontal aging hypothesis (Greenwood, 2000), which postulates that the cognitive abilities associated with the frontal lobe are most sensitive to aging. Additionally, specific cognitive changes associated with frontal FBL (e.g., L-N Seq and TMT-B) are congruent with previous studies and with the frontal aging hypothesis. In addition, the relation between FBL and reduced performance on CPS tasks (TMT-A, CWIT 1, and Symbol Coding) described above is consistent with the processing speed theory. Although previous studies have sought to prioritize either the frontal aging hypothesis or the cognitive processing theory, recent studies have reported a potential mutual relationship between these two theories (Albinet et al., 2012; Bugg et al., 2006; Schretlen et al., 2000). The results of the present study support the idea of a mutual relationship between EF and CPS functions. For example, both EF and CPS performance are associated with age-related shortening of FBLs. Moreover, both EF and CPS performance are associated with the lengths of fiber bundles connecting to/from the frontal lobe. While frontal FBLs were associated with scores on one EF test (LN-Seq), frontal FBLs were associated with scores one three CPS tests (TMT-A, CWIT 1, and Symbol Coding), even though EF is considered a prototypical function of the frontal lobe. Thus, the present study suggests that the frontal aging hypothesis and the cognitive processing theory should be considered collectively rather than as opposing, mutually-exclusive hypotheses to describe age-related changes in cognitive function.
The specific mechanism by which changes in FBL integrity influence cognitive function is not clear. Certainly global WM deterioration can interfere with the synchrony and speed of communication, resulting in slowed responses (Albinet et al., 2012; Bartzokis, 2004; Fields, 2008). Further, diminished response speed can lead to disruption of tasks that require mental manipulation and control (Bartzokis et al., 2007; Lu et al., 2013). Furthermore, studies have shown that deterioration in myelinated networks occurs with advanced age (Guttman et al., 1998; Nucifora, Verma, Lee, & Melhem, 2007). Aging affects WM microstructure, leading to inefficient communication between distinct brain regions, and a concomitant reduction in performance on tasks of EF and CPS. The fact that a number of key WM tracts (e.g., superior longitudinal fasciculus) span multiple lobes underscores the importance of a whole-brain conceptualization. Tract-specific studies will likely further clarify the mechanisms of age-related cognitive decline.
Our findings are consistent with prior results suggesting that compromised frontal lobe WM integrity is associated with reduced cognitive skills that require higher-order thinking (Dempster, 1992). Further, temporal lobe WM deterioration is also involved with reduced EF in healthy aging (West, 1996), a finding in line with our results herein. It is less clear why mean occipital FBL was significantly associated with age-related patterns of decline in EF and CPS. It is noteworthy that the occipital FBL measures herein involve many fiber pathways that enter/exit the occipital lobe from/to other lobes (e.g., frontal, temporal).
A few limitations of the present study should be noted. First, the generalizability of our results may be limited by the demographic weighting toward females and toward more educated individuals (Table 1). Thus, the results may not completely generalize to a large community-based population. Second, the present study did not examine specific WM tracts. Previously, our group found an overall age-related decline in FBL specific to the anterior thalamic radiation (Baker et al., 2014). This significant WM tract plays a key role in the prefrontal-subcortical circuits with damage to this region revealing executive dysfunction and impaired CPS (Behrens et al., 2003; Duering et al., 2011; Tekin & Cummings, 2002). In addition, WM alterations in association tracts such as the inferior fronto-occipital fasciculus (IFC), superior longitudinal fasciculus (SLF), and uncinate fasciculus (UF) have revealed impaired EF and CPS (Peters et al., 2014; Perry et al., 2009; Sun et al., 2014). It is possible that neuropsychological impairment is associated with shorter FBL in specific networks (Zakzanis et al., 2005; O’Sullivan et al., 2001).
Overall, our study provides the first in-vivo evidence that shortened FBLs in otherwise healthy aging are significantly associated with reduced cognitive performance. Future studies involving additional cognitive domains should be examined using QTM. Longitudinal investigation of healthy adults may also determine whether progressive FBL shortening follows the same timecourse as age-related cognitive decline.
Supplementary Material
Acknowledgments
This study was supported by the following grants: NIH/NINDS grant numbers R01 NS052470 and R01 NS039538, NIH/NIMH grant number R21 MH090494. Recruitment database searches were supported in part by NIH/NCRR grant UL1 TR000448.
Footnotes
Disclosure Statement
Ashley M. Behrman, Christina Usher, Thomas E. Conturo, Stephan Correia, David H. Laidlaw, Elizabeth M. Lane, Jacob Bolzenius, Jodi M. Heaps, Lauren E. Salminen, Laurie M. Baker, Ryan Cabeen, Erbil Akbudak, Xi Luo, Peisi Yan, and Robert H. Paul declare that they have no actual or potential conflicts of interest on this manuscript.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
References
- Albinet CT, Boucard G, Bouquet C, Audiffren M. Processing speed and executive functions in cognitive aging: How to disentangle their mutual relationship? Brain and Cognition. 2012;79(1):1–11. doi: 10.1016/j.bandc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26(9):1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Baker LM, Laidlaw DH, Conturo TE, Hogan J, Zhao Y, Luo X, Correia S, Cabeen R, Lane EM, Heaps JM, Bolzenius J, Salminen LE, Akbudak E, McMichael AR, Usher C, Behrman A, Paul RH. White matter changes with age utilizing quantitative diffusion MRI. Neurology. 2014;83(3):247–252. doi: 10.1212/WNL.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breaksown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Neuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Archives of General Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer’s Disease: A magnetic resonance imaging study. Archives of Neurology. 2003;60(3):393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Geschwind DH, Tingus K, Huang D, Mendez MF, Mintz J. Apolipoprotein E affects both myelin breakdown and cognition: Implications for age-related trajectories of decline into dementia. Biological Psychiatry. 2007;62(12):1380–1387. doi: 10.1016/j.biopsych.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Human Brain Mapping. 2010;31(3):378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzenius JD, Laidlaw DH, Cabeen RP, Conturo TE, McMichael AR, Lane EM, Paul RH. Impact of body mass index on neuronal fiber bundle lengths among healthy older adults. Brain Imaging and Behavior. 2013;7(3):300–306. doi: 10.1007/s11682-013-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60(5):444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Bugg JM, Zook NA, DeLosh EL, Davalos DB, Davis HP. Age differences in fluid intelligence: Contributions of general slowing and frontal decline. Brain and Cognition. 2006;62(1):9–16. doi: 10.1016/j.bandc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66(2):217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiology of Aging. 2008;29(10):1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proceedings of the National Academy of Sciences of the United States of American. 1999;96(18):10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conturo TE, McKinstry RC, Akbudak E, Robinson BH. Encoding of anisotropic diffusion with tetrahedral gradients: a general mathematical diffusion formalism and experimental results. Magnetic Resonance in Medicine. 1996;35:399–412. doi: 10.1002/mrm.1910350319. [DOI] [PubMed] [Google Scholar]
- Correia S, Lee SY, Voorn T, Tate DF, Paul RH, Zhang S, Laidlaw DH. Quantitative tractography metrics of white matter integrity in diffusion-tensor MRI. Neuroimage. 2008;42(2):568–581. doi: 10.1016/j.neuroimage.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. The Journal of Neuroscience. 1994;14(8):4748–4755. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive-development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- Duering M, Zieren N, Hervé D, Jouvent E, Reyes S, Peters N, Pachai C, Chabriat H, Dichgans M. Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL. Brain. 2011;134(Pt 8):2366–2375. doi: 10.1093/brain/awr169. [DOI] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in Neuroscience. 2008;31(7):361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Baurer RH, Jervey JP. Functional interactions between the inferotemporal and prefrontal cortex in a cognitive task. Brain Research. 1985;330(2):299–307. doi: 10.1016/0006-8993(85)90689-4. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. Journal of the International Neuropsychological Society. 2000;6(6):705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Guttman CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50(4):972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Jacobs HI, Leritz EC, Williams VJ, Van Boxel MP, van der Elst W, Jolles J, Salat DH. Association between white matter microstructure, executive functions, and processing speed in older adults: The impact of vascular health. Human Brain Mapping. 2013;34(1):77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22(4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Lee T, Mosing MA, Henry JD, Trollor JN, Lammel A, Ames D, Sachdev PS. Genetic influences on five measures of processing speed and their covariation with general cognitive ability in the elderly: The older Australian twins study. Behavior Genetics. 2012;42(1):96–106. doi: 10.1007/s10519-011-9474-1. [DOI] [PubMed] [Google Scholar]
- Lori NF, Akbudak E, Shimony JS, Cull TS, Synder AZ, Guillory RK, Conturo TE. Diffusion tensor fiber tracking of human brain connectivity: Acquisition methods, reliability analysis and biological results. NMR in Biomedicine. 2002;15(7–8):494–515. doi: 10.1002/nbm.779. [DOI] [PubMed] [Google Scholar]
- Lu PH, Lee GJ, Tishler TA, Meghpara M, Thompson PM, Bartzokis G. Myelin breakdown mediates age-related slowing in cognitive processing speed in healthy older men. Brain and Cognition. 2013;81(1):131–138. doi: 10.1016/j.bandc.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychology Review. 2009;19(4):415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. Journal of Cognitive Neuroscience. 2009;21(2):289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. Journal of Comparative Neurology. 2003;462(2):144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philosophical Transactions of The Royal Society Biological Sciences. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell I, Xi G, Lindsay J, Tukko H. Canadian study of health and aging: Study description and patterns of early cognitive decline. Aging, Neuropsychology, and Cognition. 2004;11:149–168. [Google Scholar]
- Meier-Ruge W, Ulrich J, Brühlmann M, Meier E. Age-related white matter atrophy in the human brain. Annals of the New York Academy of Sciences. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, Van Zijl P. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The neuropsychology of memory and aging. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. New Jersey: Erlbaum; 1992. pp. 315–372. [Google Scholar]
- Mosely M. Diffusion tensor imaging and aging – A review. NMR in Biomedicine. 2002;15(7–8):535–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-Tensor MR imaging and tractography: Exploring brain microstructure and connectivity. Radiology. 2007;245(2):367–384. doi: 10.1148/radiol.2452060445. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Paul R, Lane EM, Tate DF, Heaps J, Romo DM, Akbudak E, Conturo TE. Neuroimaging signatures and cognitive correlates of the montreal cognitive assessment screen in a nonclinical elderly sample. Archives of Clinical Neuropsychology. 2011;26(5):454–460. doi: 10.1093/arclin/acr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, Prendergast DM, Szeszko PR, Malhotra AK. Age-related differences in white matter tract mincrostructure are associated with cognitive performnance from childhood to adulthood. Biological Psychiatry. 2014;75(3):248–256. doi: 10.1016/j.biopsych.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry ME, McDonald CR, Hagler DJ, Jr, Gharapetian L, Kuperman JM, Koyama AK, McEvoy LK. White matter tracts assocated with set-shifting in healthy aging. Neuropsychologia. 2009;47(13):2835–2842. doi: 10.1016/j.neuropsychologia.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Grevea DN, Van der Kouwe AJ, Hevelone ND, Zaleta AK, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Salminen LE, Schofield PR, Lane EM, Heaps JM, Pierce KD, Cabeen R, Paul RH. Neuronal fiber bundle lengths in healthy adult carriers of the ApoE4 allele: A quantitative tractography DTI study. Brain Imaging and Behavior. 2013;7(3):274–281. doi: 10.1007/s11682-013-9225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. Journal of the International Neuropsychological Society. 2000;6(1):52–61. doi: 10.1017/s1355617700611062. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience & Biobehavioral Reviews. 2006;30(6):749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sun X, Liang Y, Wang J, Chen K, Chen Y, Zhou X, Jia J, Zhang Z. Early frontal structural and functional changes in mild white matter lesions relevant to cognitive decline. Journal of Alzheimers Disease. 2014;40(1):123–134. doi: 10.3233/JAD-131709. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: A stereological investigation. Neurobiology of Aging. 1997;18(6):609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Tate DF, Conley J, Paul RH, Coop K, Zhang S, Zhou W, Tashima K. Quantitative diffusion tensor imaging tractography metrics are associated with cognitive performance among HIV-infected patients. Brain Imaging and Behavior. 2010;4(1):60–79. doi: 10.1007/s11682-009-9086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. Journal of Psychosomatic Research. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Senton ME, Kennedy JL, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiology of Aging. 2012;33(1):21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion toolkit: A software package for diffusion imaging data processing and tractography (Abstract #3720). Poster presented at the Joint Annual Meeting of the International Society for Magnetic Resonance Medicine; 2007. May, http://trackvis.org/ [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Yajeya J, Quintana J, Fuster JM. Prefrontal representation of stimulus attributes during delay tasks: II. The role of behavioral significance. Brain Research. 1988;474(2):222–230. doi: 10.1016/0006-8993(88)90437-4. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43(13):1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.