Abstract
Background
Loss to follow-up (LTFU) in HIV-positive cohorts is an important surrogate for interrupted clinical care which can potentially influence the assessment of HIV disease status and outcomes. After preliminary evaluation of LTFU rates and patient characteristics, we evaluated the risk of mortality by LTFU status in a high resource setting.
Methods
Rates of LTFU were measured in the Australian HIV Observational Database for a range of patient characteristics. Multivariate repeated measures regression methods were used to identify determinants of LTFU. Mortality by LTFU status was ascertained using linkage to the National Death Index. Survival following combination antiretroviral therapy initiation was investigated using the Kaplan-Meier (KM) method and Cox proportional hazards models.
Results
Of 3,413 patients included in this analysis, 1,632 (47.8%) had at least one episode of LTFU after enrolment. Multivariate predictors of LTFU included viral load (VL)>10,000 copies/ml (Rate ratio (RR) 1.63 (95% confidence interval (CI):1.45–1.84) (ref ≤400)), time under follow-up (per year) (RR 1.03 (95% CI: 1.02–1.04)) and prior LTFU (per episode) (RR 1.15 (95% CI: 1.06–1.24)). KM curves for survival were similar by LTFU status (p=0.484). LTFU was not associated with mortality in Cox proportional hazards models (univariate hazard ratio (HR) 0.93 (95% CI: 0.69–1.26) and multivariate HR 1.04 (95% CI: 0.77–1.43)).
Conclusions
Increased risk of LTFU was identified amongst patients with potentially higher infectiousness. We did not find significant mortality risk associated with LTFU. This is consistent with timely re-engagement with treatment, possibly via high levels of unreported linkage to other health care providers.
Introduction
Loss to follow-up (LTFU) in HIV-positive cohorts is an important surrogate for interrupted clinical care which can potentially influence the assessment of HIV disease status and outcomes. Interrupted clinical follow-up of HIV-positive patients can delay the timely initiation of antiretroviral therapy (ART) in ART-naive patients, as well as disrupt ongoing ART in treatment experienced patients and thereby impair treatment response.
Prior studies have reported an association between episodes of LTFU and poorer outcomes in HIV-positive patients in both low and high resource settings [1–7]. In particular, survival of LTFU patients might be poor compared to patients in care if there is significant disease resurgence during episodes of LTFU. The ascertainment of survival by LTFU status is an important objective of this study as well as of similar studies of HIV-positive populations in high resource settings [5, 8, 9].
Inaccurate assumptions about outcomes in LTFU patients can bias findings derived from in-care populations [10]. Evaluation of risk of LTFU can assist in identification and adjustment of biases introduced by different outcomes compared with patients in follow-up. However, predicted outcomes in LTFU patients might also be confounded by unreported patient linkage to other health care providers. By identifying mortality using national death registries, reliable rates of survival in LTFU patients can be compared to patients in routine care which might also allow some inference to be made about the extent to which patients are truly disengaged from care [11–13].
Patient populations with extended durations of LTFU are also of importance because they may include groups with relatively low treatment adherence who are more likely to have viral rebound and who are, therefore, potentially a source of ongoing HIV transmission. Identification of specific patients who may be at increased risk of LTFU can prompt preventative strategies and can direct the introduction of supports to pre-empt discontinuous clinical attendance and improve treatment adherence [14, 15]. Determination of risk of LTFU is, therefore, important at the patient level to allow early intervention to prevent LTFU.
After preliminary investigation of rates and determinants of LTFU in a high resource setting we compared mortality in patients by LTFU status. For this we used the Australian HIV Observational Database which has detailed long-term attendance data, a large patient population and wide regional coverage. We used linkage to the National Death Index to compare mortality in LTFU with that of patients under routine care.
Methods
The Australian HIV Observational Database (AHOD) is an observational clinical cohort study of patients with HIV infection seen at 29 clinical sites throughout Australia. AHOD utilises methodology which has been described in detail elsewhere [16]. Briefly, data are transferred electronically to The Kirby Institute, University of New South Wales every 6 months. Prospective data collection commenced in 1999, with retrospective data provided where available.
Ethics approval for the AHOD study was granted by the University of New South Wales Human Research Ethics Committee, and all other relevant institutional review boards. Written informed consent was obtained from participating individuals. All study procedures were developed in accordance with the revised 1975 Helsinki Declaration. The Australian Institute of Health and Welfare and all relevant institutional review boards granted specific ethics approval for this particular study which included linkage of consented AHOD patient identifiers to be made to the National Death Index to identify fact of death and date of death.
Study population
This analysis included patients who had been recruited to AHOD as part of general AHOD recruitment prior to 31 March 2013 with at least one recorded clinical visit thereafter. Patients from non-Australian sites were excluded as were patients recruited as part of AHOD sub-studies which often target specific non representative patient groups.
We used all recorded site visits from enrolment in AHOD until the earlier of identified date of death or cohort censoring date (31 March 2013) or site specific censoring date as a surrogate for clinical follow-up. Episodes of LTFU were defined as greater than 365 days between any recorded treatment visit and the earlier of censor date and next recorded visit. A 365 day gap represents more than 2 missed visits based on routine clinical attendance (according to patient, site and era) as well as 2 consecutive AHOD reporting periods without updated data, and has been used by cohort studies from similar resource settings [8, 9, 17, 18]. Episodes of LTFU commenced at 180 days after the last relevant recorded visit. Covariates analysed were: sex; age (“<20”, “20–29”, “30–39”, “40–49”, “50–59”, “60–69”, “70–79”); mode of HIV exposure (“men who have sex with men (MSM)”, “heterosexual”, “intravenous drug use (IDU)”, “other/unknown”); hepatitis C virus (HCV) antibody (“never positive/never tested”, “positive ever”); hepatitis B virus surface antigen (HBsAg) (“never positive/never tested”, “positive ever”); year of first HIV positive test (“<1996”, “≥1996”; this categorisation was based on dichotomisation about the population mean), combination antiretroviral therapy (cART) naïve (“yes”, “no”); calendar year (“≤2004”, “>2004”; this categorisation was based on dichotomisation about the population mean); time under followup, prior episodes of LTFU; CD4 cell count (CD4+) (closest from 180 days prior or up to 30 days after – “0–199”,”200–349”, “350–499”, “≥500” cells/µl); and viral load (VL) (closest 180 days prior or up to 30 days after – “≤400”, “401–1000”, “1001–10,000”, >10000 copies/ml). Categorical cut points for continuous variables, unless otherwise stated, were based on widely used clinical thresholds to facilitate generalizability of results, but with sufficient observations per category level to facilitate meaningful statistical analysis. Time dependent risk factors unless otherwise stated were updated in the analysis at the time of most recent visit.
Statistical Methods
Determinants of loss to follow-up
Rates of LTFU episodes were calculated by demographic and clinical patient characteristics. Patients enrolled over 1 year prior to censoring (who were therefore at risk of becoming lost to follow-up) were followed from enrolment until the earlier of death, cohort censoring date (31 March 2013) or site specific censoring date (31 March 2007 for one site subgroup of patients and 31 March 2009 for another site). Factors associated with LTFU were examined using repeated measures Poisson regression, with generalised estimating equations methodology. This allowed for multiple episodes of LTFU per patient and accounted for within and between patient variability. Exchangeable variance structure was assumed, but with robust calculated variances to minimise possible incorrectly assumed variance structure. For GEE models, time was coarsened to annual intervals. Time dependent variables were updated at the most recent visit except for CD4+ and VL where the median of test results over the year was used.
Data linkage to the National Death Index (NDI)
To develop estimates of mortality adjusted for mortality in LTFU patients, the subgroup of AHOD general recruitment with identified approval for data linkage was linked to the NDI using definite linkage for a range of linkage keys for each patient. Linkage was made using first two letters of given name, first two letters of surname, the day, month, and year of birth, and sex after preliminary quality assessment of the performance of a range of linkage keys by the Australian Institute of Health and Welfare (AIHW) linkage team[19].
Where possible we manually assessed linkage matches using comparison of linked date of death against AHOD date variables (including enrolment date, recent visit date and date of death).
We estimated sensitivity of matched NDI mortality status against AHOD mortality status using linkage results for patients with a recorded AHOD death as indicated by the completion of an AHOD Cause of Death (CoDe) form. CoDe forms are completed by a site clinician and have detailed information on clinical status at time of death as well as attached autopsy reports where relevant. These are reviewed by AHOD coordinators and if required further reviewed by HIV specialist clinicians to verify primary and secondary causes of death. Because of the rigour of this process, recorded AHOD death was taken to indicate confirmed death. In addition we assumed 100% specificity of matching after validating all linked deaths against AHOD date variables to verify the absence of false positive matches.
We then developed estimates for the number of extra deaths identified by linkage using the sensitivity from the analysis above to incorporate rates of true positives and of false negatives in LTFU patients arising from the linkage process.
Finally, we resolved estimates by LTFU status and calculated a likely adjustment multiplier as the ratio of estimated extra deaths versus linkage deaths in LTFU to apply to rates of mortality amongst patients LTFU to adjust for missing deaths associated with patients LTFU.
Mortality estimates
We compared crude rates of death for patients who had commenced cART and who had consented to data linkage by time updated LTFU status. Follow-up status was categorised as either “not LTFU”, “returned to follow-up” (for patients with at least one prior episode of LTFU but who are in follow up at any given time) or “LTFU”. Analysis baseline was cART initiation but with left truncation applied such that entry was delayed until enrolment in instances where patients had commenced cART prior to enrolment. Patients were followed until cohort censor date or site specific censor date or death. An administrative censoring time of 17 years after cART commencement was also applied.
Univariate and multivariate Cox proportional hazards models were developed to evaluate the relationship between LTFU and mortality while controlling for potential confounders. Forward stepwise selection of covariates was used with forced inclusion of LTFU status to develop a parsimonious model but which was inclusive of important predictors of survival. Results from model fitting using backward selection methods were compared. Covariates examined were age at first cART; sex; IDU mode of exposure; year HIV positive (<1996/≥1996); year of first cART (<2000, ≥2000); prior AIDS defining illness (ADI) at first cART; HCV (ever); HBsAg (ever); prior mono/dual (Mono/Duo) treatment at first cART; and CD4+ at first cART. Analyses were stratified by treatment centre.
A sensitivity analysis was conducted to investigate mortality by LTFU status limited to patients who prospectively commenced cART. In this analysis analogous Cox proportional hazards models were developed to those described above but where only patients with consent to data linkage who had commenced cART at or after enrolment were included.
Analyses were conducted using Stata version 12.1 (Stata Corp LP, College Station, TX, United States).
Results
Total AHOD recruitment was 3, 894 patients of whom 286 were omitted from analyses because they were from non-Australian sites or were part of targeted AHOD sub studies. Of the remaining 3,608 patients, those who were recruited at least 1 year prior to censor date were included in analysis of determinants of LTFU (3,413 patients (94.6%)) and followed for a total of 23,922 person years (PY) from enrolment. Of these patients 1,632 (47.8%) had at least one episode of LTFU. In total over the duration of the analysis, 2,349 episodes of LTFU were observed and in 1,283 (54.6%) of these episodes patients returned to care.
Patients were predominantly male (3,208 (94.0%)), with mean age of 42.3 years at enrolment and predominant route of HIV transmission was via MSM (2,581 (75.6%)) (Table 1). The median duration between attendances was 62 days (interquartile range (IQR) 17–100) with 42.5% of visits being between 62 and 180 days apart, 5.7% of visits being between 180 and 365 days apart and 2.0% of visits being over 365 days apart. The overall crude rate of LTFU (including episodes resulting in return to care) was 9.82 episodes per 100 PY (/100 PY) (95% confidence interval (CI): 9.43–10.22)
Table 1.
Patient numbers, follow-up time, episodes of loss to follow-up (LTFU) and crude rates of LTFU by characteristic categories for patients enrolled in the Australian HIV Observational database between 1999 and 2013 with over 1 year of follow-up1
| N (%) | Person years (PY) | LTFU | Rate (95% CI) per 100 PY | ||
|---|---|---|---|---|---|
| All | 3,413 (100.00) | 23,922 | 2,349 | 9.82 (9.43–10.22) | |
| Sex | |||||
| Male | 3,208 (94.0) | 22,620 | 2,241 | 9.91 (9.51–10.33) | |
| Female | 205 (6.0) | 1,302 | 108 | 8.30 (6.87–10.02) | |
| Age2 | |||||
| Mean (SD) | 42.3 (10.2) | ||||
| <30 | 321 (9.4) | 656 | 144 | 21.95 (18.64–25.84) | |
| 30–39 | 1,247 (36.5) | 5,240 | 737 | 14.06 (13.08–15.12) | |
| 40–49 | 1,126 (33.0) | 9,089 | 881 | 9.69 (9.07–10.36) | |
| 50–59 | 519 (15.2) | 5,997 | 427 | 7.12 (6.48–7.83) | |
| 60–69 | 164 (4.8) | 2,407 | 128 | 5.32 (4.47–6.32) | |
| ≥70 | 36 (1.1) | 532 | 32 | 6.01 (4.25–8.5) | |
| Exposure | |||||
| MSM | 2,581 (75.6) | 18,566 | 1,783 | 9.60 (9.17–10.06) | |
| IDU | 209 (6.1) | 1,301 | 172 | 13.22 (11.39–15.36) | |
| Heterosexual | 499 (14.6) | 3,236 | 333 | 10.29 (9.24–11.46) | |
| Other | 124 (3.6) | 820 | 61 | 7.44 (5.79–9.56) | |
| HCV (ever) | |||||
| No | 3,026 (88.7) | 21,252 | 2,066 | 9.72 (9.31–10.15) | |
| Yes | 387 (11.3) | 2,670 | 283 | 10.6 (9.43–11.91) | |
| HBsAg (ever) | |||||
| No | 3,269 (95.8) | 22,906 | 2,237 | 9.77 (9.37–10.18) | |
| Yes | 144 (4.2) | 1,015 | 112 | 11.03 (9.17–13.28) | |
| Year HIV Positive | |||||
| <1996 | 1,735 (50.8) | 14,435 | 1,341 | 9.29 (8.81–9.80) | |
| ≥1996 | 1,508 (44.2) | 8,419 | 888 | 10.55 (9.88–11.26) | |
| Missing | 170 (5.0) | 1,067 | 120 | 11.25 (9.40–13.45) | |
| Prior cART2 | |||||
| No | 831 (24.4) | 1,435 | 117 | 8.15 (6.80–9.77) | |
| Yes | 2,582 (75.7) | 22,486 | 2,232 | 9.93 (9.52–10.35) | |
| CD4 cell count (cells/µl)2,3 | |||||
| median (IQR) | 480 (320–665) | ||||
| <200 | 374 (11.0) | 1,669 | 147 | 8.81 (7.49–10.35) | |
| 200–349 | 574 (16.8) | 3,258 | 307 | 9.42 (8.43–10.54) | |
| 350–499 | 807 (23.6) | 4,821 | 398 | 8.26 (7.48–9.11) | |
| ≥500 | 1509 (44.2) | 12,300 | 996 | 8.10 (7.61–8.62) | |
| Missing | 149 (4.4) | 1,873 | 501 | 26.75 (24.51–29.2) | |
| Viral load (copies/ml)2,4 | |||||
| median (IQR) | ≤400 (≤400–8,000) | ||||
| ≤400 | 1910 (56.0) | 16,601 | 1,198 | 7.22 (6.82–7.64) | |
| 401–1,000 | 175 (5.1) | 545 | 50 | 9.18 (6.96–12.11) | |
| 1,001–10,000 | 431 (12.6) | 1,599 | 167 | 10.45 (8.98–12.16) | |
| >10,000 | 759 (22.2) | 3,379 | 392 | 11.60 (10.51–12.81) | |
| Missing | 138 (4.0) | 1,799 | 542 | 30.13 (27.70–32.78) | |
| Calendar Year2 | |||||
| ≤2004 | 2,250 (65.9) | 8,157 | 904 | 11.08 (10.38–11.83) | |
| >2004 | 1,163 (34.1) | 15,765 | 1,445 | 9.17 (8.71–9.65) | |
| Episodes of LTFU5 | |||||
| 0 | 1,781 (52.2) | 20,448 | 1,632 | 7.98 (7.60–8.38) | |
| 1 | 1,126 (33.0) | 2,452 | 506 | 20.63 (18.91–22.51) | |
| ≥2 | 506 (14.8) | 1,021 | 211 | 20.66 (18.05–23.64) | |
Loss to follow-up defined as ≥365 days until next clinic visit or prior to death or cohort/site censoring. Patients excluded if censoring or death occurred less than 1 year after enrolment.
N=number at enrolment, PY & LTFU & Rate based on time updated value
Median CD4 cell count at enrolment=480 cells/µl (interquartile range (IQR) 320–665)
Median viral load at enrolment=≤400copies/ml (IQR ≤400–8,000)
N=total number ever, PY & LTFU & Rate based on time updated value
Predictors of lost to follow-up
Increased crude rates of LTFU were associated with younger patients (age <30 years (21.95 episodes/100 PY (95% CI: 18.64–25.84)), and age 30–39 years (14.06 episodes/100 PY (95% CI: 13.08–15.12)), IDU mode of exposure (13.22 episodes/100 PY (95% CI: 11.39–15.36)), VL>10,000 copies/ml (11.60 episodes/100 PY (95% CI: 10.51–12.81)), prior episodes of LTFU (20.63 episodes/100 PY (95% CI: 18.91–22.51) with 1 prior episode), and earlier calendar year periods of follow-up (≤2004 11.08 episodes/100 PY (95% CI:10.38–11.83)) (Table 1).
In a multivariate model, increased risk of LTFU was associated with heterosexual mode of exposure (HR 1.17 (95% CI: 1.04–1.33) (ref MSM), p=0.012), patients who had initiated cART (1.76 (95% CI: 1.44–2.16), p<0.001), increased VL (copies/ml) (1,001–10,000 1.34 (95% CI: 1.16–1.55), >10,000 1.63 (95% CI: 1.45–1.84) (ref ≤400), p<0.001), time under follow-up (per additional year) (1.03 (95% CI: 1.02–1.04), p<0.001) and prior episodes of LTFU (per additional episode) (1.15 (95% CI: 1.06–1.24), p<0.001). Female sex (0.68 (95% CI: 0.55–0.83), p<0.001) and increased age (per year older) (0.74 (95% CI: 0.71–0.78), p<0.001) were associated with decreased risk of LTFU. Missing current annual CD4+ (cells/µl) (1.67 (95% CI: 1.33–2.08) ref <200), p<0.001) and missing current annual VL (3.35 (95% CI: 2.89–3.88) (ref ≤400), p<0.001) were also associated with increased risk of LTFU in this model (Table 2).
Table 2.
Predictors of loss to follow-up (LTFU) after enrolment for univariate and multivariate Poisson regression analyses for patients enrolled in the Australian HIV Observational database between 1999 and 2013 and with over 1 year of follow-up (N=3,413)1
| Univariate IRR (95% CI) |
p | P | Multivariate IRR (95% CI) |
p | P | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 1 | 1 | ||||
| Female | 0.82 (0.67–1.00) | 0.051 | 0.68 (0.55–0.83) | <0.001 | ||
| Age2 | ||||||
| Per 10 years older | 0.78 (0.75–0.82) | <0.001 | 0.74 (0.71–0.78) | <0.001 | ||
| Exposure | ||||||
| MSM | 1 | <0.001 | 1 | 0.018 | ||
| IDU | 1.38 (1.15–1.64) | <0.001 | 1.16 (0.99–1.36) | 0.059 | ||
| Heterosexual | 1.04 (0.92–1.18) | 0.505 | 1.17 (1.04–1.33) | 0.012 | ||
| Other | 0.76 (0.57–1.01) | 0.063 | 0.87 (0.66–1.16) | 0.350 | ||
| HCV (ever) | ||||||
| No | 1 | |||||
| Yes | 1.10 (0.95–1.26) | 0.199 | ||||
| HBsAg (ever) | ||||||
| No | 1 | |||||
| Yes | 1.16 (0.93–1.45) | 0.200 | ||||
| Year HIV Positive | ||||||
| <1996 | 1 | 0.547 | ||||
| ≥1996 | 1.04 (0.94–1.14) | 0.429 | ||||
| Missing | 1.10 (0.89–1.37) | 0.371 | ||||
| Started cART2 | ||||||
| No | 1 | 1 | ||||
| Yes | 1.46 (1.20–1.77) | <0.001 | 1.76 (1.44–2.16) | <0.001 | ||
| CD4 cell count (cells/µl)3 | ||||||
| <200 | 1 | <0.001 | 1 | <0.001 | ||
| 200–349 | 1.02 (0.86–1.22) | 0.822 | 1.17 (0.98–1.39) | 0.090 | ||
| 350–499 | 0.89 (0.75–1.06) | 0.200 | 1.05 (0.88–1.26) | 0.587 | ||
| ≥500 | 0.91 (0.77–1.08) | 0.283 | 1.08 (0.90–1.29) | 0.400 | ||
| Missing | 3.37 (2.81–4.04) | <0.001 | 1.67 (1.33–2.08) | <0.001 | ||
| Viral load (copies/ml)3 | ||||||
| ≤400 | 1 | <0.001 | 1 | <0.001 | ||
| 401–1,000 | 1.13 (0.88–1.44) | 0.334 | 1.20 (0.94–1.53) | 0.139 | ||
| 1,001–10,000 | 1.27 (1.10–1.47) | 0.001 | 1.34 (1.16–1.55) | <0.001 | ||
| >10,000 | 1.57 (1.41–1.74) | <0.001 | 1.63 (1.45–1.84) | <0.001 | ||
| Missing | 4.77 (4.34–5.25) | <0.001 | 3.35 (2.89–3.88) | <0.001 | ||
| Calendar Year2 | ||||||
| ≤2004 | 1 | |||||
| >2004 | 1.08 (1.01–1.16) | 0.032 | ||||
| Time under follow-up2 | ||||||
| Per additional year | 1.03 (1.02–1.04) | <0.001 | 1.03 (1.02–1.04) | <0.001 | ||
| Episode of LTFU2 | ||||||
| Per additional episode | 1.25 (1.16–1.34) | <0.001 | 1.15 (1.06–1.24) | <0.001 |
Loss to follow-up defined as ≥365 days until next clinic visits or prior to death or cohort/site censoring. GEE models, Poisson family, log link. Patients excluded if censoring or death occurred less than 1 year after enrolment.
Time updated variable based on most recent available measure
Time updated variable based on median annual value
Data linkage
Of 3,608 general recruitment AHOD patients, 3,404 (94.3%) were consented and linked to the NDI. Of these patients 2,529 (74.3%) had a current AHOD mortality status, while 875 (25.7%) did not. The estimated linkage sensitivity was 84.5% based on 246 of 291 confirmed AHOD deaths being matched to NDI deaths.
Of 2,501 linked patients not LTFU, 263 (10.5%) had a death recorded in AHOD. Of the remaining 903 patients who were LTFU, 28 (3.1%) had an AHOD death recorded, and 42 (4.7%) extra deaths were identified by linkage. A likely extra 8 (0.9%) deaths had occurred but were missed by linkage based on the above sensitivity. This suggests that, for AHOD, estimates of mortality in patients LTFU should be revised upwards by a factor of 1.11 (i.e. 78/70).
Mortality
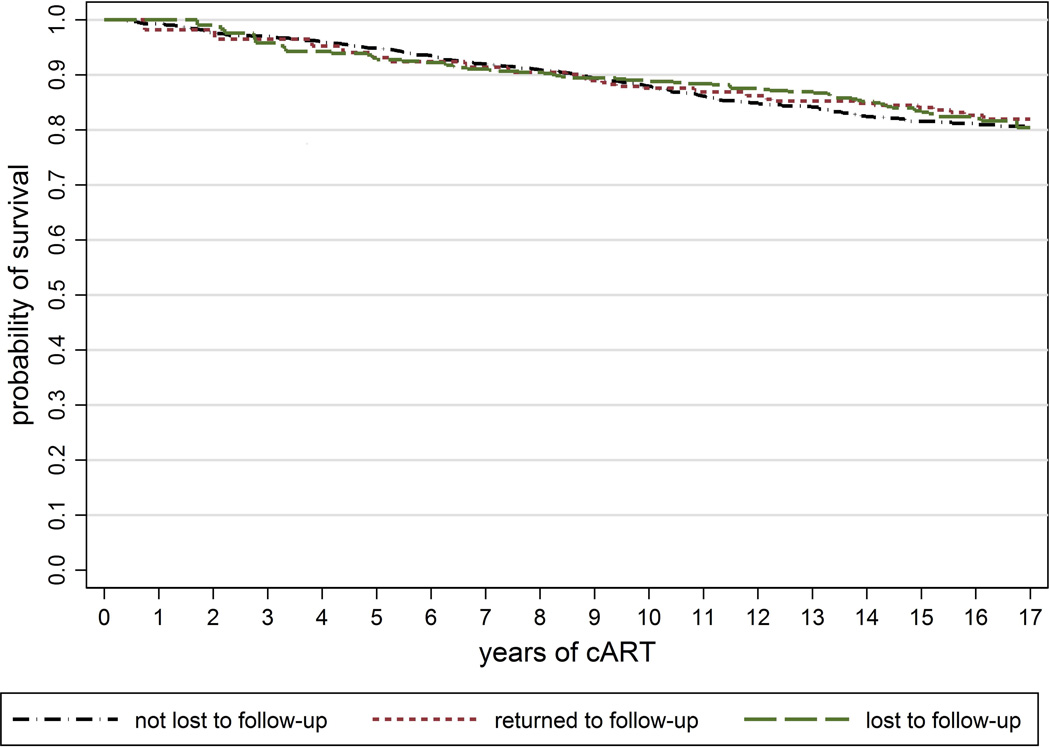
Of 3,404 AHOD patients linked to the NDI, 3,030 (89.0%) had initiated cART prior to censor date and were included in survival analyses. Over the duration of survival analysis (from cART commencement but with patients entering the at-risk population at time of enrolment in instances of later enrolment and being followed until censoring) 323 deaths were observed: 228 (70.6%) in patients with no prior LTFU, 32 (9.9%) in patients returned to follow-up, and 63 (19.5%) in patients LTFU. Overall mortality was 12.87 deaths/1000 PY (95% CI: 11.54–14.35). Crude rates of mortality by time updated LTFU status for enrolled patients were similar by follow-up status: 13.35 deaths/1000 PY (95% CI: 11.73–15.20) for patients with no prior LTFU; 11.41 deaths/1000 PY (95% CI: 8.07–16.14) for patients returned to follow-up; and 12.08 deaths/1000 PY (95%: 9.44–15.47) for patients LTFU. Kaplan Meier curves of the unadjusted relationship between LTFU and survival showed similar survival by LTFU status (Figure 1) (log rank p=0.484).
Figure 1.
LTFU status was not associated with mortality in a univariate cox proportional hazard model (p=0.893) (Table 3). Baseline covariates associated with increased mortality were increased age, IDU mode of exposure, prior ADI, HCV or HBV ever, earlier year of infection, earlier year of first cART and lower CD4+. LTFU status was not associated with survival in a multivariate model which was adjusted for Age, IDU exposure status, HBV, year of first cART and CD4+ (p=0.844). Both forward and backwards model selection techniques selected identical models. In a sensitivity analysis which restricted inclusion to patients who had commenced cART at or after enrolment LTFU was not found to be a significant predictor of mortality. Models were strongly limited by insufficient patient numbers and endpoints (476 patients, 21 deaths) and models were not robust to small variations in inclusion criteria.
Table 3.
Univariate and multivariate hazard ratios (HR) of mortality following cART commencement for patients enrolled in the Australian HIV Observational Database between 1999 and 2013 with consent to data linkage (N=3,030)1
| Univariate models | Multivariate model | ||||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | P | HR (95% CI) | P | P | ||
| LTFU2,3 | No | 1 | 0.893 | 1 | 0.844 | ||
| Returned | 0.95 (0.64–1.41) | 0.803 | 1.12 (0.75–1.69) | 0.570 | |||
| Yes | 0.93 (0.69–1.26) | 0.649 | 1.04 (0.77–1.43) | 0.782 | |||
| Gender | Male | 1 | |||||
| Female | 0.93 (0.57–1.51) | 0.759 | |||||
| Age4 | |||||||
| Per year | 1.03 (1.02–1.04) | <0.001 | 1.04 (1.03–1.05) | <0.001 | |||
| IDU5 | No | 1 | 1 | ||||
| Yes | 1.73 (1.21–2.48) | 0.003 | 1.95 (1.34–2.84) | <0.001 | |||
| Prior ADI4 | No | I | |||||
| Yes | 1.50 (1.14–1.97) | 0.003 | |||||
| HCV (ever) | No | I | |||||
| Yes | 1.45 (1.09–1.94) | 0.012 | |||||
| HBsAg (ever) | No | 1 | 1 | ||||
| Yes | 1.71 (1.14–2.55) | 0.009 | 1.71 (1.14–2.57) | 0.009 | |||
| Year HIV Positive | <1996 | 1 | 1 | ||||
| ≥1996 | 0.57 (0.43–0.76) | <0.001 | 0.67 (0.50–0.91) | 0.009 | |||
| Year of first cART | <2000 | 1 | 1 | ||||
| ≥2000 | 0.64 (0.46–0.90) | 0.009 | 0.66 (0.45–0.96) | 0.028 | |||
| CD4 cell count4 | 0–199 | 1 | <0.001 | 1 | <0.001 | ||
| 200–349 | 0.59 (0.44–0.79) | <0.001 | 0.57 (0.42–0.77) | <0.001 | |||
| ≥350 | 0.50 (0.38–0.67) | <0.001 | 0.49 (0.36–0.66) | <0.001 | |||
| Prior Mono/Dual4 | No | 1 | |||||
| Yes | 1.43 (1.13–1.79) | 0.002 | |||||
Cox proportional hazards ratios. Baseline= cART commencement. Patients left truncated at enrolment. Multivariate model selection using forward stepwise selection with forced inclusion of LTFU status.
LTFU if no recorded AHOD date other than AHOD death or NDI death within 1 year of cohort/site censor date or date of death. Lost to follow-up status time updated from 180 days after date of last AHOD visit date other than death. 1 subset of patients censored at 31 March 2007. 1 site censored at 31 March 2010. Administrative censored at 17 years of cART. Analyses stratified by treatment centre.
Time updated variable
At cART initiation
Mode of exposure via intravenous drug use
Discussion
Characteristics of patients in the Australian HIV Observational Database who were at increased risk of LTFU were consistent with groups experiencing increased viral load and exhibiting higher infectiousness. We found no difference between mortality rates in patients according to follow-up status. This might indicate high levels of timely and often unreported re-engagement in care amongst these patients.
Overall, the observed rate of episodes of LTFU (which included multiple episodes per patient) was relatively low (9.82 episodes/100 PY (95% CI: 9.43–10.22)). In the UK Collaborative HIV Cohort (CHIC) study a higher rate of LTFU was observed (16.7 episodes/100 PY (95% CI: 16.4–17.2)) [8]. However that analysis defined follow-up by duration between CD4+ test dates rather than all clinical visits which might decrease observable attendance. Compared to many other cohorts, AHOD has wide national coverage of the epidemic and comprises a large proportion of Australian patients under care (approximately 15–20% [20, 21]), and internal linkage is used to capture duplicated cohort recruitment amongst participating sites. Also, in Australia there are relatively low barriers to continuous engagement with care providers because of accessible subsidised treatment that might reduce financially related attrition, although in the UK for example ART is free. It is also reasonable to expect lower rates of LTFU via emigration in AHOD patients given relatively high Australian resourcing compared to other regional national health services. These characteristics increase the likelihood and identification of re-enrolment of transient patients across AHOD sites.
Demographically, risk of LTFU was associated with males, younger age and mode of exposure (heterosexual and marginally also IDU). These characteristics have been associated with residential transience [22, 23] and are consistent with shorter term engagement with localised healthcare as well as with relatively poor adherence to ART [24–29] and higher transmission risk behaviours [27, 30–32]. This suggests that LTFU events are likely to correlate with increased risk of viral rebound and have serious implications for the HIV epidemic with higher community VL and infectiousness, and consequent ongoing HIV transmission.
We also observed higher risk of LTFU by higher median annual VL which suggests that possibly less adherent patients are more likely to become LTFU. In this study there was no difference in risk associated with level of median annual CD4+, but instead we observed increased risk associated with missing CD4+ tests (and similarly, missing VL tests). This was facilitated by defining LTFU based on durations between any recorded clinical attendances rather than just durations between attendances with recorded CD4+ testing. Our results suggest that at-risk patients are less likely to engage in a structured or consistent approach to treatment, to the extent that this is reflected by routine CD4+/VL monitoring. To contrast, Hill et al observed a moderate decrease in risk associated with decreasing CD4+ [8] in a similarly resourced setting using a definition that incorporated episodic LTFU. However, that analysis was based on follow-up defined by duration between CD4+ test dates rather than all clinical visits which may affect the association with risk of LTFU, as well as preclude comparison of relative rates of attendance without CD4+ testing.
We also found that having commenced cART compared to being cART-naive, duration of follow-up and prior episodes of LTFU were associated with increased risk. Conversely, Hill et al observed decreased risk associated with cART initiation [8, 9]. This might to an extent be attributable to relative differences in the recruitment to both cohorts, with over 40% of followup time in the CHIC study being of cART naïve patients compared to 6% in this study. Our findings describe more experienced patients and show that there is persistent habitual LTFU amongst this population. It is possible that these patients re-present at the same centre following periods of low treatment adherence which can result in viral rebound.
The median duration between all attendances in this study was 62 days (IQR 17–100) which accords with accepted Australian guidelines over the period of study [33], although this figure is likely strongly weighted by patients requiring more intensive care, whereas longer term, stable patients are likely to be seen at more extended intervals (42.5% of visits were between 2 and 6 months apart). Generally, observed attendance patterns in this study reflect that LTFU, as defined, is associated with strong departure from recommended and normal therapy. It is likely that many patients are not adherent to treatment for sizeable proportions of these episodes given that HIV prescriptions in Australia are generally made for much shorter intervals and are invalid after one year duration [34, 35].
In this study we ascertained vital status of all patients by linkage to the NDI to investigate potentially poorer survival in LTFU patients. We internally validated linkage against known AHOD deaths and estimated linkage sensitivity as 84.5%, and given relatively low migration as described above, we propose this as a reliable estimate of true patient mortality. We found that true mortality was likely to be over 17% higher than recorded mortality. Overall mortality was observed to be around 13 deaths per 1000 patient years (incorporating adjustment for possible false negatives from NDI linkage) and we observed similar rates of mortality by LTFU status. In particular, LTFU status was not a significant predictor of survival in multivariate analyses adjusting for age, IDU exposure, HBsAg status, year positive, year of first cART and baseline CD4+. This suggests that additional risk associated with potential disease re-emergence during these episodes is often able to be mitigated. This may be via delayed re-engagement with the same treating centre (as suggested via high rates of episodic LTFU), or via unreported linkage to other health care although this was not investigated by this study.
A limitation of this study is that more detailed attendance data was not incorporated into analyses. In particular recorded failure to attend scheduled visits is likely to correlate strongly with at risk patients. Use of this predictor would obviate the use of duration based definitions of LTFU which may be inappropriate for any given patient specific schedule of attendance. This data is not collected in AHOD although it is recommended that this aspect of clinical attendance be investigated further. The consideration of non-attendance over intervals less than 365 days, which might potentially be informative, was limited by the bi-annual period of AHOD reporting. In practice non-reporting of true attendances for any single reporting period (half year intervals) was seen to be sufficiently common in preparatory analysis that calculated rates of non-attendance might be incorrectly and significantly increased. However, this is likely to be mitigated at durations above a year, which permit sites to respond to quality assurance procedures, and generally rates of calculated LTFU in AHOD are low compared to comparable cohorts using the 365 day definition of LTFU. Also, some socio-demographic risk factors were not able to be included in this analysis. In particular there are strong posited reasons for ethnicity being associated with risk of LTFU, although in the comparable studies of Mocroft and Hill results are conflicting [8, 9]. In AHOD, the population is predominantly Caucasian and any given ethnic minority category may have insufficient numbers to include in adjusted analyses. There are also reasonably high levels of underreporting of ethnicity, often by site policy, which might introduce bias to analyses incorporating this variable. Generally however, our results can be taken to be representative of the broader in treatment population in Australia.
A strength of this analysis was the ability to link AHOD data to the National Death Index, which permitted the ascertainment of mortality in patients LTFU. We have therefore been able to develop rates of mortality which are unbiased by patient attrition and we have also shown that this bias is actually likely to be quite low in this cohort. Many similar studies have listed linkage to death registries as an important but unattained goal.
In summary, increased risk of LTFU was identified amongst patients with increased viral load and these patients might therefore have higher infectiousness than other groups. However, we did not find a significant mortality risk associated with LTFU suggesting that there is relatively low detriment to individuals that is associated with LTFU events. This is consistent with timely re-engagement with treatment possibly via high levels of unreported linkage to other health care providers.
Acknowledgements
Australian HIV Observational Database contributors
Asterisks indicate steering committee members in 2014.
New South Wales: D Ellis, General Medical Practice, Coffs Harbour; M Bloch, S Agrawal, T Vincent, Holdsworth House Medical Practice, Darlinghurst; D Allen, JL Little, Holden Street Clinic, Gosford; D Smith, R Hawkins, K Allardice, Lismore Sexual Health & AIDS Services, Lismore; D Baker*, V Ieroklis, East Sydney Doctors, Surry Hills; DJ Templeton*, CC O’Connor, S Phan, RPA Sexual Health Clinic, Camperdown; E Jackson, K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba; M Grotowski, S Taylor, Tamworth Sexual Health Service, Tamworth; D Cooper, A Carr, F Lee, K Hesse, St Vincent’s Hospital, Darlinghurst; R Finlayson, S Gupta, Taylor Square Private Clinic, Darlinghurst; R Varma, J Shakeshaft, Nepean Sexual Health and HIV Clinic, Penrith; K Brown, V McGrath, S Halligan, N Arvela Illawarra Sexual Health Service, Warrawong; L Wray, R Foster, H Lu, Sydney Sexual Health Centre, Sydney; D Couldwell, Parramatta Sexual Health Clinic; DE Smith*, V Furner Albion Street Centre; Clinic 16 – Royal North Shore Hospital, S Fernando; Dubbo Sexual Health Centre, Dubbo; J Watson*, National Association of People living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; B Mulhall*, Department of Public Health and Community Medicine, University of Sydney; M Law*, K Petoumenos*, S Wright*, H McManus*, C Bendall*, M Boyd*, The Kirby Institute, University of NSW. Northern Territory: N Ryder, R Payne, Communicable Disease Centre, Royal Darwin Hospital, Darwin. Queensland: M O’Sullivan, S White, Gold Coast Sexual Health Clinic, Miami; D Russell, S Doyle-Adams, C Cashman, Cairns Sexual Health Service, Cairns; D Sowden, K Taing, K McGill, Clinic 87, Sunshine Coast-Wide Bay Health Service District, Nambour; D Orth, D Youds, Gladstone Road Medical Centre, Highgate Hill; M Kelly, D Rowling, N Latch, Brisbane Sexual Health and HIV Service, Brisbane; B Dickson*, CaraData. South Australia: W Donohue, O’Brien Street General Practice, Adelaide. Victoria: R Moore, S Edwards, R Woolstencroft Northside Clinic, North Fitzroy; NJ Roth*, H Lau, Prahran Market Clinic, South Yarra; T Read, J Silvers*, W Zeng, Melbourne Sexual Health Centre, Melbourne; J Hoy*, K Watson*, M Bryant, S Price, The Alfred Hospital, Melbourne; I Woolley, M Giles*, T Korman, J Williams*, Monash Medical Centre, Clayton. Western Australia: D Nolan, J Robinson, Department of Clinical Immunology, Royal Perth Hospital, Perth. New Zealand: G Mills, C Wharry, Waikato District Hospital Hamilton; N Raymond, K Bargh, Wellington Hospital, Wellington.
Coding of Death Form (CoDe) reviewers:
AHOD reviewers: D Sowden, J Hoy, L Wray, I Woolley, K Morwood, N Roth, K Choong, CC O'Connor, MA Boyd.
Funding Source
The Australian HIV Observational Database is funded as part of the Asia Pacific HIV Observational Database, a program of The Foundation for AIDS Research, amfAR, and is supported in part by a grant from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID) (Grant No. U01-AI069907) and by unconditional grants from Merck Sharp & Dohme; Gilead Sciences; Bristol-Myers Squibb; Boehringer Ingelheim; Janssen-Cilag; ViiV Healthcare. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The views expressed in this publication do not necessarily represent the position of the Australian Government.
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- 1.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalal RP, Macphail C, Mqhayi M, Wing J, Feldman C, Chersich MF, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47(1):101–107. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 3.Brennan AT, Maskew M, Sanne I, Fox MP. The importance of clinic attendance in the first six months on antiretroviral treatment: a retrospective analysis at a large public sector HIV clinic in South Africa. J Int AIDS Soc. 2010;13:49. doi: 10.1186/1758-2652-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egger M, Spycher BD, Sidle J, Weigel R, Geng EH, Fox MP, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8(1):e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards JK, Cole SR, Westreich D, Moore R, Mathews C, Geng E, et al. Loss to clinic and five-year mortality among HIV-infected antiretroviral therapy initiators. PLoS One. 2014;9(7):e102305. doi: 10.1371/journal.pone.0102305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDS. 2013;27(8):442–449. doi: 10.1089/apc.2013.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanoy E, Lewden C, Lievre L, Tattevin P, Boileau J, Aouba A, et al. How does loss to follow-up influence cohort findings on HIV infection? A joint analysis of the French hospital database on HIV, Mortalite 2000 survey and death certificates. HIV Med. 2009;10(4):236–245. doi: 10.1111/j.1468-1293.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 8.Hill T, Bansi L, Sabin C, Phillips A, Dunn D, Anderson J, et al. Data linkage reduces loss to follow-up in an observational HIV cohort study. J Clin Epidemiol. 2010;63(10):1101–1109. doi: 10.1016/j.jclinepi.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Mocroft A, Kirk O, Aldins P, Chies A, Blaxhult A, Chentsova N, et al. Loss to follow-up in an international, multicentre observational study. HIV Med. 2008;9(5):261–269. doi: 10.1111/j.1468-1293.2008.00557.x. [DOI] [PubMed] [Google Scholar]
- 10.Porter K, Johnson AM, Phillips AN, Darbyshire JH. The practical significance of potential biases in estimates of the AIDS incubation period distribution in the UK register of HIV seroconverters. AIDS. 1999;13(14):1943–1951. doi: 10.1097/00002030-199910010-00018. [DOI] [PubMed] [Google Scholar]
- 11.Simard EP, Pfeiffer RM, Engels EA. Mortality due to cancer among people with AIDS: a novel approach using registry-linkage data and population attributable risk methods. AIDS. 2012;26(10):1311–1318. doi: 10.1097/QAD.0b013e328353f38e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca MG, Coeli CM, de Fatima de Araujo Lucena F, Veloso VG, Carvalho MS. Accuracy of a probabilistic record linkage strategy applied to identify deaths among cases reported to the Brazilian AIDS surveillance database. Cad Saude Publica. 2010;26(7):1431–1438. doi: 10.1590/s0102-311x2010000700022. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease C, Prevention. Electronic record linkage to identify deaths among persons with AIDS--District of Columbia, 2000–2005. MMWR Morb Mortal Wkly Rep. 2008;57(23):631–634. [PubMed] [Google Scholar]
- 14.Jordan WC. Reaching lost-to-care populations. Clin Infect Dis. 2007;45(Suppl 4):S275–S280. doi: 10.1086/522550. [DOI] [PubMed] [Google Scholar]
- 15.Harding R, Krakauer EL, Sithole Z, De Lima L, Selman L. The 'lost' HIV population: time to refocus our clinical and research efforts. AIDS. 2009;23(1):145–146. doi: 10.1097/QAD.0b013e32831de90b. [DOI] [PubMed] [Google Scholar]
- 16.Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 17.Cullen BL, Codere G, Wallace LA, Baguley S, Clutterbuck DJ. British Association for Sexual Health and HIV Scottish investigation 2010–2011: reasons for non-attendance in individuals lost to follow-up for HIV care for more than 12 months in Scotland. Int J STD AIDS. 2013;24(6):481–484. doi: 10.1177/0956462412472812. [DOI] [PubMed] [Google Scholar]
- 18.Lebouche B, Yazdanpanah Y, Gerard Y, Sissoko D, Ajana F, Alcaraz I, et al. Incidence rate and risk factors for loss to follow-up in a French clinical cohort of HIV-infected patients from January 1985 to January 1998. HIV Med. 2006;7(3):140–145. doi: 10.1111/j.1468-1293.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- 19.EO2013-2-11: Risk in Loss to Follow-up in the Australian HIV Observational Database Linkage Report. The Australian Institute of Health and wellfare; 2013. [Google Scholar]
- 20.Falster K, Gelgor L, Shaik A, Zablotska I, Prestage G, Grierson J, et al. Trends in antiretroviral treatment use and treatment response in three Australian states in the first decade of combination antiretroviral treatment. Sexual health. 2008;5(2):141–154. doi: 10.1071/sh07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Australian HIV Observational Database Annual Report. Institute TK. The University of New South Wales; 2013. editor. [Google Scholar]
- 22.Pollio DE. The relationship between transience and current life situation in the homeless services-using population. Social Work. 1997;42(6):541–551. doi: 10.1093/sw/42.6.541. [DOI] [PubMed] [Google Scholar]
- 23.Tulloch AD, Fearon P, David AS. Timing, prevalence, determinants and outcomes of homelessness among patients admitted to acute psychiatric wards. Soc Psych Psych Epid. 2012;47(7):1181–1191. doi: 10.1007/s00127-011-0414-4. [DOI] [PubMed] [Google Scholar]
- 24.Chesney MA. Factors affecting adherence to antiretroviral therapy. Clin Infect Dis. 2000;30(Suppl 2):S171–S176. doi: 10.1086/313849. [DOI] [PubMed] [Google Scholar]
- 25.Keiser O, Spycher B, Rauch A, Calmy A, Cavassini M, Glass TR, et al. Outcomes of antiretroviral therapy in the Swiss HIV Cohort Study: latent class analysis. AIDS Behav. 2012;16(2):245–255. doi: 10.1007/s10461-011-9971-5. [DOI] [PubMed] [Google Scholar]
- 26.Andrews JR, Wood R, Bekker LG, Middelkoop K, Walensky RP. Projecting the benefits of antiretroviral therapy for HIV prevention: the impact of population mobility and linkage to care. J Infect Dis. 2012;206(4):543–551. doi: 10.1093/infdis/jis401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rachlis B, Brouwer KC, Mills EJ, Hayes M, Kerr T, Hogg RS. Migration and transmission of blood-borne infections among injection drug users: understanding the epidemiologic bridge. Drug Alcohol Depend. 2007;90(2–3):107–119. doi: 10.1016/j.drugalcdep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 28.South SJ, Crowder KD. Residential mobility between cities and suburbs: race, suburbanization, and back-to-the-city moves. Demography. 1997;34(4):525–538. [PubMed] [Google Scholar]
- 29.Wood E, Yip B, Gataric N, Montaner JS, O'Shaughnessy MV, Schechter MT, et al. Determinants of geographic mobility among participants in a population-based HIV/AIDS drug treatment program. Health Place. 2000;6(1):33–40. doi: 10.1016/s1353-8292(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 30.Madden HC, Phillips-Howard PA, Hargreaves SC, Downing J, Bellis MA, Vivancos R, et al. Access to HIV community services by vulnerable populations: evidence from an enhanced HIV/AIDS surveillance system. AIDS Care. 2011;23(5):542–549. doi: 10.1080/09540121.2010.525609. [DOI] [PubMed] [Google Scholar]
- 31.German D, Davey MA, Latkin CA. Residential transience and HIV risk behaviors among injection drug users. AIDS Behav. 2007;11(6 Suppl):21–30. doi: 10.1007/s10461-007-9238-3. [DOI] [PubMed] [Google Scholar]
- 32.Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376(9737):268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. [cited 2014 11/03/2014];2013 Antiretroviral Guidelines with Australian Commentary: Australasian Society for HIV Medicine. 2014 updated 2/12/2013; Available from: http://arv.ashm.org.au/arv-guidelines/initiating-art-in-treatment-naive-patients.
- 34.Medicare_Australia. [cited 2014 12 May, 2014];Highly Specialised Drugs (HSD) program: Commonwealth of Australia. 2014 updated 19 December, 2013; Available from: http://www.medicareaustralia.gov.au/provider/pbs/highly-specialised-drugs/#N100E3.
- 35. [cited 2014 12 May, 2014];Medicare_Australia. Browse by Section 100 Item List: Commonwealth of Australia. 2014 Available from: http://pbs.gov.au/browse/section100-hb?initial=a.