Abstract
Objective
Metabolic Syndrome (MetS), the clustering of obesity, high blood pressure, and disordered glucose and lipid/lipoprotein metabolism within a single individual, is associated with poorer cognitive function. It has been hypothesized that cognitive impairment in MetS occurs primarily within the context of inflammation. MetS risk factors are also associated with thinning of the cerebral cortex. However, the mechanisms by which MetS and inflammation affect the brain are poorly understood. The present study used statistical mediation to examine the relationship between MetS risk factors, cortical thickness in a priori regions of interest (ROIs) and inflammation. ROIs were chosen from the previous literature.
Research Design and Methods
Forty-three adults between the ages of 40 and 60 years underwent a health screen, neuropsychological testing and structural magnetic resonance imaging. Serum levels of pro-inflammatory markers (interleukin 1, interleukin 2, interleukin 6 and C-Reactive Protein) were measured using enzyme-linked immunosorbent assays.
Results
A higher number of MetS risk factors were associated with thinning in the inferior frontal ROI (β=−0.35, p = 0.019) as well as higher levels of serum interleukin 2 (β=0.31, p=0.04). A higher level of serum interleukin 2 was also associated with reduced thickness in the inferior frontal gyrus (β=−0.41, p=0.013). After accounting for the effects of interleukin 2, the number of MetS risk factors was no longer associated with cortical thickness in the inferior frontal gyrus indicating successful statistical mediation.
Conclusions
The results indicate a potentially important role for inflammation in linking MetS to cortical thinning and cognitive vulnerability.
Introduction
Cardiovascular risk factors such as hypertension and diabetes have been consistently associated with poor cognitive function in midlife and increased risk of dementia in older age (Knopman, 2001; Hofman, 1997). Metabolic Syndrome (MetS), a clustering of cardiovascular risk factors such as diabetes, hypertension, obesity and dyslipidemia within a single individual, appears to confer an even greater risk for cognitive impairment than the sum of its individual components (Yaffe, 2007). Individuals with MetS components such as diabetes and high triglycerides have an increased risk for Alzheimer’s disease (Raffaitin, 2009). Higher numbers of MetS risk factors are also associated with poorer cognition in midlife (Yaffe, 2009). As cognitive function is the most important predictor of functional ability and quality of life (Black, 2002) it is of utmost importance to uncover the mechanisms by which MetS-related cognitive decline occurs and to develop targeted interventions. Those mechanisms, however, are as of yet unclear.
One hypothesis, advanced by Yaffe and colleagues (Yaffe, 2004), is that cognitive impairment in MetS occurs primarily within the context of inflammation. Systemic markers of inflammation have been associated with poorer cognitive function in patients with Alzheimer’s Disease (Holmes, 2009). Pro-inflammatory cytokines such as interleukin 1 (IL-1) cause activation of inducible nitric oxide sythase (iNOS) astrocytes, which could indirectly potentiate NMDA-induced neurotoxicity (Hewett, 1994). Higher serum concentrations of interleukin 6 (IL-6), on the other hand, also have been linked to NMDA-induced neurotoxicity through increases in calcium influx (Qiu, 1998). Induction of interleukin-2 (IL-2), on the other hand, is associated with disruption of blood brain barrier functionality, which could lead to structural damage (Ellison, 1987). Thus, it is reasonable to hypothesize that higher serum levels of pro-inflammatory cytokines may induce changes in the cerebral cortex such as cortical thinning, which in turn may lead to cognitive decline.
In the present study, we employed structural MRI to determine whether alterations in cortical thickness are discernible in cognitively intact middle-aged adults with varying numbers of MetS risk factors and if those changes might be related to peripheral inflammation. Based on published research on MetS and cortical thickness (Dickerson, 2009; Leritz, 2011), we hypothesized that participants with a greater number of MetS risk factors will have cortical thinning in the bilateral regions in the inferior frontal, and middle frontal gyri, as well as supra marginal and cingulate regions of interest as described in published literature (Leritz 2011). We further hypothesized that this association will be statistically mediated by serum levels of pro-inflammatory cytokines, namely IL-1, IL-2, IL-6 and C-Reactive Protein (CRP). A successful mediation would imply that the structural changes in the cerebral cortex seen in MetS occur through inflammatory processes. This could lead to the development of targeted interventions towards peripheral inflammation, to slow down the process of cortical thinning.
Research Design and Methods
Participants
Forty-three adults between the ages of 40 and 60 years were recruited through flyers and newspaper advertisements. Individuals with a history of coronary artery disease, angina pectoris, myocardial infarctions, heart failure and cardiac surgery were excluded. Additional exclusion criteria included history of neurological disease (e.g Parkinson’s disease, neurodegenerative illness, clinically significant traumatic brain injury), major co-morbid psychiatric illness (schizophrenia, anxiety), substance abuse (i.e diagnosed abuse and/or previous hospitalization for substance abuse), metabolic disorder (thyroid disorder), smoking (within the last 2 years) and MRI contraindications. Participants who passed the initial screen were enrolled in the study after providing written consent.
Procedures
The study procedures were approved by the local institutional review board. Participants completed a medical history questionnaire. They also underwent a cognitive screen, a health visit including a fasting blood draw to obtain serum levels of pro-inflammatory cytokines and brain imaging on separate days, completing the study within 1 month.
Cognitive Screen
Details of cognitive assessments administered have been previously published (Eagan, 2012). Briefly, a measure of full-scale intelligence quotient (FSIQ) and a mini mental state evaluation were obtained. Both tests were administered and scored by a trained research assistant using standard administration and scoring criteria.
Health Screen
Fasting blood concentrations of glucose, triglyceride, HDL-cholesterol, and LDL-cholesterol were determined using the standard enzymatic technique. Arterial blood pressure was measured using a standard oscillometric blood pressure monitor (VP-2000, Colin Medical Instruments, San Antonio, TX) after at least 15 minutes of rest. Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of height in meters. MetS risk factors were classified according to the 2009 criterion issued jointly by the International Diabetes Association, World Health Federation, International Artherosclerosis Society and the International Association for the Study of Obesity (Alberti, 2009): abdominal obesity (BMI >30); elevated triglycerides (≥150mg/dL and/or treatment for elevated triglycerides); reduced high density lipoprotein (HDL) cholesterol (< 40mg/dL for men and <50mg/dL for women and/or pharmacological treatment for cholesterol management); elevated blood pressure (systolic blood pressure ≥ 130mmHg and/or diastolic blood pressure ≥ 85 mmHg and/or antihypertensive drug treatment); elevated fasting glucose (≥100 mg/dL or treatment with a hypoglycemic agent).
A 3 mL fasting blood sample was also collected from the antecubital vein by venipucture. Serum was separated within 2 hours of the collection, and aliquots were stored at −80°C until later analysis. Prior to the analysis, the serum sample was diluted 100-fold. Serum concentration of C Reactive Protein (CRP) was measured using high sensitivity human ELISA kits (Alpha Diagnostics, San Antonio, TX). Serum concentration of interleukin 1 (IL-1), interleukin 2 (IL-2), interleukin 6 (IL-6), were measured using commercially available human enzyme-linked immunosorbent assays (EMD Millipore Corporation).
Neuroimaging data acquisition
Magnetic Resonance Imaging (MRI) data for each participant was acquired in a single session on a 3T GE Signa Excite MRI scanner equipped with a standard head coil. T1 – weighted anatomical scans of the entire brain were collected using a high-resolution spoiled gradient echo sequence (256 × 256 matrix, field of view = 24 × 24 cm2, 1 mm slice thickness, 0 gap).
Neuroimaging data processing
Structural images were processed using Freesurfer Image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu). Image processing involves first motion correction of one volumetric T1–weighted image, computerized removal of non brain tissue using a hybrid watershed/surface deformation procedure, automated Talairach transformation, intensity normalization, tessellation of the gray matter, white matter boundary, automated topology correction and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class. Once the cortical models are complete, a number of deformable procedures can be performed for further data processing and analysis including surface inflation, registration to a spherical atlas which utilized individual cortical folding patterns to match cortical geometry across participants, and creation of a variety of surface based data including maps of curvature and sulcal depth (Fischl, 2000). This method uses both intensity and continuity information from the entire three dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as closest distance from the gray/white boundary to the gray/cerebrospinal fluid boundary at each vertex in the tessellated surface. Freesurfer output was visually examined by a trained graduate student. Minor errors detected in segmentation were manually fixed using the edit tool, without the need for control points, and reprocessed.
Cortical thickness was extracted from the following a priori regions of interest to be analyzed for purposes of this study: bilateral middle frontal gyri, bilatertal inferior frontal gyri, supramarginal and posterior cingulate. These regions of interest were selected based on their published association with MetS risk factors (Leritz, 2011). Using the Analysis of Functional NeuroImages (AFNI) software (Cox, 1996), spherical regions of interest, 5 mm in diameter, were created around the Talairach atlas central coordinates of the regions exhibiting MetS-related changes in cortical thickness in the report by Leritz and colleagues (2011). The coordinates for these regions of interest are presented in table 1.
Table 1. Coordinates of regions of interest.
| Region | Location (left/right/bilateral) |
X | Y | Z | Mean thickness (mm) |
|---|---|---|---|---|---|
| Inferior frontal | Bilateral | +/−44 | 24 | 2 | 2.45 |
| Middle frontal | Bilateral | +/−37 | −29 | 26 | 1.89 |
| Cingulate | Bilateral | +/−6 | 28 | 31 | 3.06 |
| Supramarginal | Bilateral | +/−51 | −48 | 31 | 2.50 |
Statistical analyses
Statistical analyses were conducted in three steps: 1) Cortical thickness in bilateral regions was examined for laterality effects (significant differences in thickness between the left and right hemisphere) using paired t-tests. Since none were found, cortical thickness values within left and right hemisphere regions were averaged together in order to reduce the number of conducted comparisons; 2) Associations between mean cortical thickness within the resultant ROIs and number of MetS risk factors were explored using linear regressions; 3) Associations between cortical thickness in the one ROI with significant MetS effects (inferior frontal gyrus) and levels of pro-inflammatory cytokines were examined using linear regressions after rank-transforming cytokine values in order to fulfill assumptions of normality. 4) Finally, a single mediation analysis was performed to assess if IL-2, the only inflammatory variable related to reduced inferior frontal gyrus thickness, explains the association between number of MetS risk factors and cortical thickness in the inferior frontal ROI.
Mediation was assessed using both the traditional causal steps approach and non-parametric bootstrapping procedures (Preacher, 2004). Multiple regression was used to assess mediation according to traditional causal steps approach. The causal steps approach postulates that four conditions must be met to determine mediation; (i) a significant relationship between the independent variable (number of MetS risk factors) and the dependent variable (thickness), (ii) a significant relationship between the independent variable and the potential mediator (serum levels of IL-2), (iii) a significant relationship between the potential mediator and the dependent variable and (iv) a nonsignificant relationship between the independent variable and the dependent variable after controlling for the potential mediator. An additional assessment on the significance of the mediation model was conducted by using confidence intervals. Confidence intervals were obtained using Preacher and Hayes bootstrapping method for assessing indirect effects (Preacher, 2004). A 95% confidence interval that does not include 0 was used as the criterion for significance.
A Sidak adjusted two-tailed level of 0.02, recommended for intercorrelated outcomes (Sidak, 1967), was used for the initial step of the analyses to account for multiple comparisons due to the high number of ROIs. A less conservative two-tailed α level of 0.05 was used as the criterion for statistical significance in the mediation analysis, as well as the analyses of the serum levels of the cytokines, as these analyses were performed on only a single ROI. All statistical analyses were carried out using IBM SPSS 19.0 software (SPSS Inc.).
Results
Descriptive Statistics
Participants had a mean age of 48.17 years (S.D 9.28), with 14.48 years of education (S.D 3.5). On average, they reported 2.63 (S.D 1.43) MetS risk factors. Twenty one participants identified as Caucasian, 14 as Hispanic/Latin American, 1 as African American and 4 as “Other”. Participants were cognitively intact at the time of assessment, with a mean MMSE score of 28.22 out of 30 (S.D 1.42) and a full-scale intelligence quotient (FSIQ) of 114.11 (S.D 11.82, average range). Further information about the demographic and physiological characteristics of the sample can be found in Table 2.
Table 2. Participant characteristics.
| Mean (SD) | |
|---|---|
| Age (years) | 48.17 (9.28) |
| Education (years) | 14.48 (1.5) |
| Gender | 25 males |
| Serum IL-1, μg/dl | 0.76 (1.53) |
| Serum IL-2, μg/dl | 1.41 (2.19) |
| Serum IL-6, μg/dl | 4.77 (3.59) |
| Serum CRP, μg/dl | 2.65 (2.17) |
| BMI | 29.84 (5.86) |
| HDL cholesterol, mg/dl | 44.25 (15.49) |
| LDL cholesterol, mg/dl | 123.76 (38.07) |
| Triglycerides, mg/dl | 173.96 (95.11) |
| Glucose, mg/dl | 108.77 (34.18) |
| Systolic blood pressure, mmHg | 126.88 (16.76) |
| Diastolic blood pressure mmHg | 76.30 (9.75) |
Cortical thickness and MetS risk factors
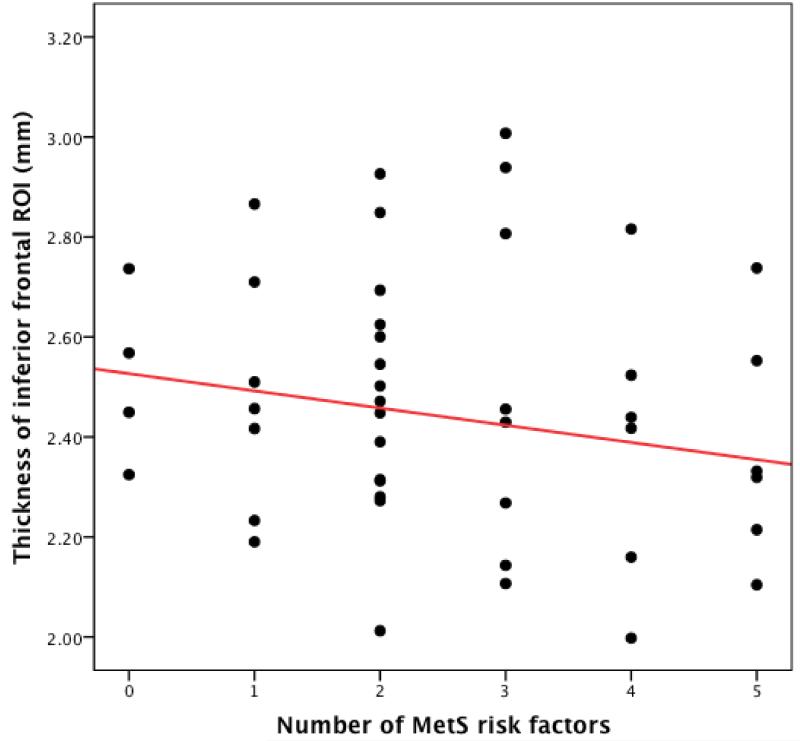
Mean thickness in each of the bilateral ROIs is presented in table 1. Higher number of MetS risk factors was associated with lower thickness in the inferior frontal gyrus (β=−0.35, p = 0.019) (Figure 1). As covariates such as age (t=0.56, p = 0.58), FSIQ (t=0.23, p =0.82) and gender (t=−0.61, p =0.54) were not significantly correlated with thickness in this ROI, these covariates were not included in further analyses.
Figure 1. Scatterplot depicting the relationship between MetS risk factors and thickness in the inferior frontal ROI.
MetS risk factors and inflammation
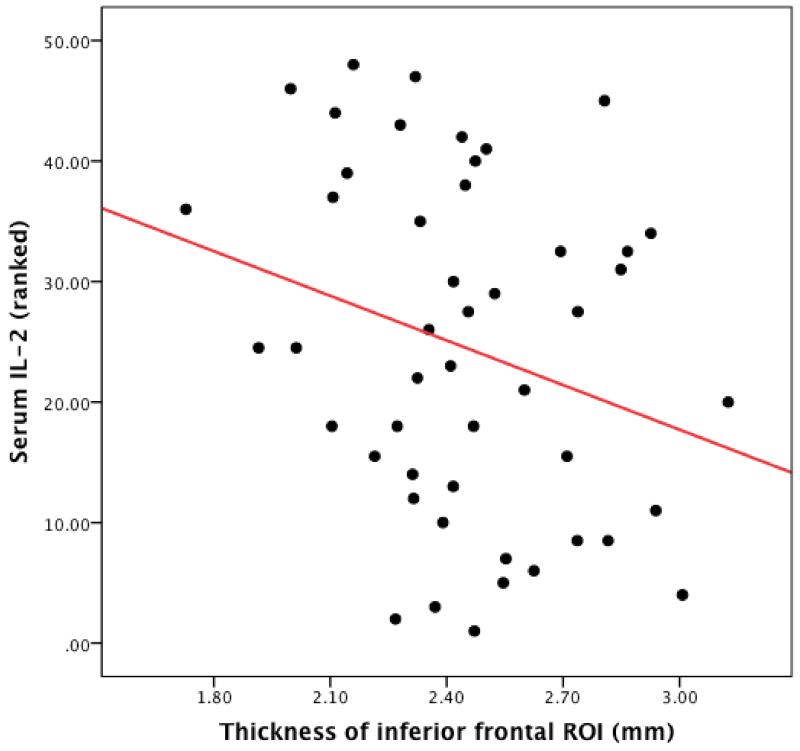
The relationship between MetS risk factors and higher levels of the pro-inflammatory cytokines IL-1, IL-2,IL-6 and CRP were assessed using individual linear regressions. The only inflammatory cytokine that was significantly associated with MetS risk factor number was serum IL-2 (β=0.31, p=0.04). This association is depicted in Figure 2.
Figure 2. Scatterplot depicting the relationship between serum levels of IL-2 and thickness in the inferior frontal ROI.
Mediation Analysis
As the inferior frontal ROI was the only ROI significantly associated with number of MetS risk factors (β=−0.35, p=0.019), this ROI was selected for further analysis. Higher number of MetS risk factors was also associated with higher serum levels of IL-2 (β= 0.31, p =0.04). Higher levels of serum IL-2 were also associated with reduced thickness in the inferior frontal ROI (β=−0.41, p=0.013). After accounting for the effects of IL-2, number of MetS risk factors no longer accounted for any unique variance (β= −0.25 p=0.124). The association between IL-2 and cortical thickness remained significant in the model (β= −0.35 p=0.019) indicating successful statistical mediation. The significance of this mediation effect was further confirmed by the 95% confidence intervals (95% C.I = −0.061 - −0.0012) derived by Preacher and Hayes bootstrapping method.
Conclusions
The main finding of this study was that higher numbers of cardiovascular risk factors (obesity, hypertension, hyperglycemia, dyslipidemia) in middle age were related to significantly thinner cortical mantle in the inferior frontal cortex. More importantly, this relationship was significantly mediated by increases in the peripheral inflammatory marker IL-2, implicating inflammation as a significant contributor to midlife brain vulnerability.
Cortical thickness
Cortical thickness defined on MRI is a relatively new measure of brain integrity estimated through automated reconstruction of the gray/white and pial surfaces in the brain and measuring the distance between the two surfaces. This allows for better accuracy in measuring the cerebral cortex, which is folded and difficult to assess through manual methods (Fischl, 2000). The measurement is of interest because changes in cortical thickness are observed in neurodegenerative conditions such as Alzheimer’s disease (Dickerson, 2009). Cortical thinning has been highlighted as an early marker of Alzheimer’s disease symptomatology and has been observed in asymptomatic amyloid positive older adults who are considered to be at high risk for dementia (Dickerson, 2009). In addition, cortical thinning in frontal regions has been documented in relation to lower global cognitive functioning in patients with Alzheimer’s disease (De Leon, 1997).
Our findings of reductions in cortical thickness related to higher number of MetS risk factors are consistent with the previous literature reporting cortical thinning in the inferior frontal cortex associated with high cholestrol (Leritz, 2011). They are also consistent with previous studies linking MetS to higher numbers of silent infacts (Kim, 2012) and ultrastructural tissue damage in the frontal and temporal lobes on diffusion tensor imaging (Kwon, 2006). As cortical thinning is also related to poorer cognitive function (Segura, 2009), this study could further elucidate the mechanisms underlying the association between MetS risk factors and cognitive function. Broadly, our findings contribute to the growing body of literature redefining MetS as a syndrome with significant implications for the brain. Our results also highlight the association between inflammation and brain integrity. Mounting evidence suggests that inflammation is associated with adverse cognitive outcomes (Yaffe, 2004; Yaffe 2003). Most importantly, our results point towards inflammation as a possible mechanism explaining the cumulative effects of higher numbers of MetS risk factors on brain integrity.
Inflammation
In this study, higher levels of peripheral IL-2 were related to significantly lower cortical thickness in the inferior frontal gyrus. IL-2 is naturally released as part of the body’s immune response (McGreer, 1999) and is produced by T-cells in response to antigenic or mitogenic stimulation. Moreover, administration of IL-2 is associated with poorer working memory and planning ability in cancer patients (Capuron, 2001). As fMRI studies (Fletcher et. al, 1998; Henson et al, 1999) have shown increased activation of the inferior frontal gyri during working memory retrieval tasks, it is possible to suggest that higher levels of IL-2 would cause selective structural damage to the inferior frontal gyrus as a precursor to cognitive decline.
One potential mechanism via which inflammation might have a negative impact on brain integrity is through impairing endothelial function. Inflammation is associated with poorer endothelial function through diminished production of nitric oxide (NO) (Venugopal, 2002), a critical vasodilator. NO is critical for regulating blood flow in the central nervous system. Cerebral endothelial cells secrete NO, which helps to maintain resting vascular tone and regulate local flow adjustments in response to hypercapniac demands (Lavi, 2003; White, 1998; White 2000). Poorer endothelial function has been associated with diminished BOLD response during a cognitively demanding task in middle-aged adults (Gonzales, 2010). IL-2 in particular, could affect endothelial function through increasing blood brain barrier permeability. Unlike other cytokines, IL-2 does not require a saturable transport system to cross the blood brain barrier (Forman, 2008). Thus, IL-2 levels can accumulate more quickly in the brain compared to other cytokines, causing more damage to endothelial cells. Chronically high levels of IL-2 are also associated with capillary leakage syndrome (Waguespack, 1994). This may impair cerebral microcirculation, which would have an effect on endothelial cells located in and around the blood brain barrier.
Alternatively, inflammation may exert its deleterious effects on brain integrity through oxidative stress. Inflammation triggered by a high fat diet is also related with diminished serum levels of brain derived neutrophic factor (BDNF) (Wu, 2004), a protein that is vital for neuronal survival and differentiation. Supplementation with anti oxidants in the same study reversed cerebral oxidation and restored BDNF levels, indicating that oxidative damage subsequent to inflammation may directly impair neuronal viability (Wu, 2004); thus impacting cortical thickness.
Comparison with previous findings
To the best of the author’s knowledge, this study is the first to directly examine inflammation as a potential mediator of the relationship between MetS and cortical thickness. Each arm of the mediation relationship that was found, however, is consistent with other work implicating MetS (Yaffe, 2009; Black 2002; Yaffe, 2004; Holmes, 2009) and inflammation (Yaffe, 2004; Yaffe, 2003) in cognitive decline and/or neurodegeneration.
An unanticipated finding was the lack of association between cortical thickness in the inferior frontal gyrus and levels of CRP. Previous studies have shown that CRP in particular is associated with poorer cognitive function in people with high BMI (Wu, 2004) as well as MetS (Yaffe, 2003) and induces neurochemical changes that are associated with poorer cognition (Sweat, 2008). However, the published correlation with IL-2 induction and cognitive function in domains associated with the inferior frontal gyrus (Capulon, 2001) supports our results. It is possible that the unique effects of IL-2 on the blood brain barrier (Ellison, 1987) are driving the changes in the particular region of the cerebral cortex as seen here. It is also possible that as CRP is associated with increased risk for coronary heart disease (Karakas, 2009; Rost, 2001) that the effects of CRP on the brain are seen at a subcortical level, which is beyond the scope of the current study.
Limitations
The main limitation of this study is the small sample size (n =43) which restricted the number and types of analyses that we could perform. Larger sample sizes would allow us to examine the synergistic effects of inflammatory cytokines via moderated mediation and mixed models. The small sample size also reduced the power of the statistical analyses that were performed. Longitudinal studies would be helpful in examining the effect of the observed cortical thinning on long term cognitive health.
Also, the reported effect represents the results of a statistical mediation procedure. While generally indicative of a cause and effect relationship, this may or may not be the case here. While it is plausible to suggest that elevated IL-2 levels may be responsible for thinning in the inferior frontal ROI, it is also possible that both may be related to genetic influences or developmental factors. Confidence in the reported mechanism can be gained through longitudinal studies where temporal precedence between elevated IL-2 levels and cortical thinning in the inferior frontal ROI can be clearly established.
Acknowledgments
S.S.K wrote the manuscript and conducted all data analyses. D.E.E and M.M.G assisted with data collection, imaging data analyses, and data interpretation. K.G contributed to data collection and manuscript review and discussion. H.T. contributed to project conceptualization and funding, supervision of data collection, data interpretation and manuscript review. A.P.H was the principal investigator for the project; she contributed to project conceptualization and funding, supervision of data collection, data analysis and interpretation, and manuscript review.
This work was made possible by funding provided by the American Heart Association (09BGIA2060722), the National Institute of Neurological Disorders and Stroke (R01 NS075565), the National Institute on Aging (F31AG040890, MMG), and the University of Texas at Austin.
Footnotes
The authors report no conflicts of interest.
References
- 1.Knopman D, Boland LL, Mosly T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR. Cardiovascular risk factors and cognitive decline in middle aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 2.Hofman A, Ott A, Breteler MM. Artherosclerosis, Apoliprotein E and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K. Metabolic Syndrome and Cognitive Disorders: Is the sum greater than it’s parts? Alzheimer’s Disease and Associated Disorders. 2007;21:167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- 4.Raffaitin C, Gin H, Empana J-P, Helmer C, Berr C, Tzourio C, Portet C, Dartigues J-F, Alperovitch A, Barbager-Gateau P. Metabolic Syndrome and Risk for Incident Alzheimer’s Disease or Vascular Dementia: The Three-city study. Diabetes Care. 2009;32:169–174. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Weston AL, Blackwell T, Krueger KA. The Metabolic Syndrome and the development of Cognitive Impairment among Older Women. Clinical Neurology. 2009;72:A99. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black SA, Rush RD. Cognitive and functional decline in adults 75 years and older. Journal of the American Geriatrics Society. 2002;50:1978–1986. doi: 10.1046/j.1532-5415.2002.50609.x. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. Journal of the American Medical Association. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 8.Holmes C, Butchart J. Systemic Inflammation and Alzheimer’s disease. Biochemical Society Transactions. 2009;39:898–901. doi: 10.1042/BST0390898. [DOI] [PubMed] [Google Scholar]
- 9.Hewett SI, Csernansky CA, Choi DW. Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron. 1994;13:487–494. doi: 10.1016/0896-6273(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Z, Sweeney DD, Netzeband JG, Gruol DL. Chronic Interleukin 6 alters NMDA receptor mediated responses and enhances neurotoxicity in developing CNS neurons. Journal of Neuroscience. 1998;18:10445–10456. doi: 10.1523/JNEUROSCI.18-24-10445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellison MD, Povlishock JT, Merchant RE. Blood brain barrier dysfunction in cats following recombinant interleukin-2 infusion. Cancer Research. 1987;47:5765–5770. [PubMed] [Google Scholar]
- 12.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, hyman BT, Morris JC, Fischl B, Buckner RL. The Cortical signature of Alzheimer’s Disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Fischl B, McGlinchey RE, Milberg WP. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54:2659–2671. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eagan DE, Gonzales MM, Tarumi T, Tanaka H, Stautberg S, Haley AP. Elevated C-Reactive Protein relates to increased cerebral myo-inositol levels in middle-aged adults. Cardiovascular Psychiatry and Neurology. 2012 doi: 10.1155/2012/120540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 16.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11044–11099. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox RW. AFNI: Software for analysis and visualization of functional magnetic neuroimages. Computers and biomedical research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 18.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 19.Sidak Z. Rectangular confidence region for the means of multivariate normal distributions. Journal of the American Statistical Association. 1967;62:626–633. [Google Scholar]
- 20.De Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, De Santi S, McRae T, Ferris SH, Reisberg B, et al. Neurobiology of Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Lee JW, Kim GH, Roh JH, Kim MJ, Seo SW, Kim ST, Jeon S, Lee JM, Heilman KM, Na DL. Cortical asymmetries in normal, mild cognitive impairment, and Alzheimer’s Disease. Neurobiology of Aging. 2012;33:1959–1966. doi: 10.1016/j.neurobiolaging.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Kwon HM, Kim BJ, Lee SH, Choi SH, Yoon BW. Metabolic syndrome as an independent risk factor of silent brain infarction in healthy people. Stroke. 2006;37:466–470. doi: 10.1161/01.STR.0000199081.17935.81. [DOI] [PubMed] [Google Scholar]
- 23.Segura B, Jurado MA, Freixenet N, Falcon C, Junque C, Arboix A. Microstructural white matter changes in metabolic syndrome: a diffusion tensor imaging study. Neurology. 2009;73:438–444. doi: 10.1212/WNL.0b013e3181b163cd. [DOI] [PubMed] [Google Scholar]
- 24.Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 25.McGeer EG, McGeer PL. Brain inflammation in Alzheimer disease and the therapeutic implications. Curr Pharm Des. 1999:5821–5836. [PubMed] [Google Scholar]
- 26.Capuron L, Ravaud A, Dantzer R. Timing and specificity of cognitive changes induced by Interleukin 2 and Interferon-treatments in cancer patients. Psychosomatic Medicine. 2001;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher PC, et al. The functional roles of prefrontal cortex in episodic memory II. Retrieval. Brain. 1998;121:1249–1256. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- 28.Henson RN, et al. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999;122:1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- 29.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration That C-Reactive Protein Decreases eNOS Expression and Bioactivity in Human Aortic Endothelial Cells. Circulation. 2002;106:1439–1441. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 30.Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: Chemoregulation versus mechanoregulation. Circulation. 2003;107:1901–1905. doi: 10.1161/01.CIR.0000057973.99140.5A. [DOI] [PubMed] [Google Scholar]
- 31.White RP, Deane C, Vallance P, Markus HS. Nitric oxide synthase inhibition in humans reduces cerebral blood flow but not the hyperemic response to hypercapnia. Stroke. 1998;29:467–472. doi: 10.1161/01.str.29.2.467. [DOI] [PubMed] [Google Scholar]
- 32.White RP, Vallance P, Markus HS. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clinical Science. 2000;99:555–560. [PubMed] [Google Scholar]
- 33.Gonzales M, Tarumi T, Tanaka H, Sugawara J, Swann-Sternberg T, Goudarzi Katyoon, et al. Functional imaging of working memory and peripheral endothelial function in middle-aged adults. Brain and Cognition. 2010;73:146–151. doi: 10.1016/j.bandc.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forman DE, Cohen RA, Hoth KF, Haley AP, Poppas A, Moser DJ, Gunstad J, Paul RH, Jefferson AL, Tate DF, Ono M, Wake N, Gerhard-Herman M. Vascular health and cognitive function in older adults with cardiovascular disease. Artery Research. 2008;2:35–43. doi: 10.1016/j.artres.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waguespack PJ, Banks WA, Kastin AJ. Interleukin-2 does not cross the blood-brain barrier by a saturable transport system. Brain Research Bulletin. 1994;34:103–109. doi: 10.1016/0361-9230(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 36.Ellison MD, Krieg RJ, Merchant RE. Cerebral vasomotor responses after recombinant interleukin 2 infusion. Cancer Res. 1990;50:4377–4381. [PubMed] [Google Scholar]
- 37.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 38.Sweat V, Starr V, Bruehl H, Arentoft A, Tirsi A, Javier E, Convit A. C-Reactive Protein is linked to lower cognitive performance in overweight and obese women. Inflammation. 2008;31:198–207. doi: 10.1007/s10753-008-9065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karakas M, Koenig W. CRP in Cardiovascular disease. Herz. 2009;34:607–613. doi: 10.1007/s00059-009-3305-7. [DOI] [PubMed] [Google Scholar]
- 40.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, D’Agostino RB, Franzblau C, Wilson PWF. Plasma concentrations of C-Reactive Protein and Risk of Ischemic Stroke and Transient Ischemic Attack. Stroke. 2001;32:2575–2579. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]