Abstract
This cohort study examines medication use in term neonates with hypoxic-ischemic encephalopathy (HIE) and seizures before and after implementation of a Neonatal Neurocritical Care Service (N=108), which included increased seizure monitoring. Nearly all neonates received phenobarbital (96% pre- versus 95% post-Neonatal Neurocritical Care Service) and total loading dose did not vary among groups (33 [95% CI 29–37] versus 30 [26–34] mg/kg). After adjustment for seizure burden, neonates managed during the Neonatal Neurocritical Care Service era, on average, received 30 mg/kg less cumulative phenobarbital (95% CI 15–46 mg/kg) and were on maintenance 5 fewer days (95% CI 3–8 days) than those who were treated prior to implementation of the service. In spite of the enhanced ability to detect seizures due to improved monitoring and increased vigilance by bedside practitioners, implementation of the Neonatal Neurocritical Care Service was associated with decreased use of potentially harmful phenobarbital treatment among neonates with HIE.
Keywords: Hypoxic-Ischemic Encephalopathy, Electroencephalogram, Epilepsy monitoring, Neonatal seizures
Introduction
Neurocritical care is a relatively new subspecialty that combines expertise in neurology and critical care medicine. While there is strong evidence that adults with acute neurological conditions who are treated in a specialized neurocritical unit have reduced morbidity and mortality,1,2 the impact of neurocritical care in the neonatal population is not known. Several reasons for improved outcomes have been proposed, including: (1) higher patient volumes in designated centers, (2) adherence to protocols, and (3) multimodal neurological monitoring.2
Seizure management is an important focus in neonatal neurocritical care for several reasons. First, the neonatal period represents the highest lifetime risk for acute symptomatic seizures, and seizures often herald serious underlying brain injury and adverse outcome.3,4 Second, seizures are very difficult to identify, since bedside clinical detection of seizures is not accurate (trained providers are correct only 50% of the time in identifying which paroxysmal clinical events have an electrographic correlate),5 and seizures among critically ill neonates are frequently subclinical.6 Finally, commonly used medications like phenobarbital have partial efficacy,7 demonstrate neurotoxicity in animal models,8 and hold potential for worsening cognitive and motor function in toddlers.9 Although published guidelines recommend continuous, prolonged electroencephalogram (cEEG) with video monitoring as the gold standard for accurate detection of seizures among critically ill populations of all ages, including neonates,10–12 barriers to implementation of cEEG include need for specialized equipment, and substantial resources and expertise to apply the neonatal montage and interpret findings; thus, not all centers can routinely monitor patients.13
The potential impact of specialized neurocritical care using cEEG on medical management for seizures in neonates is not known. In theory, accurate detection should lead to tailored treatment, however some physicians harbor concern that increased seizure recognition with cEEG could lead to increased treatment with potentially neurotoxic agents such as phenobarbital. Limited evidence suggests prolonged neurophysiology monitoring using amplitude-integrated EEG (aEEG) does not influence seizure medication treatment,14,15 however the impact of cEEG within a Neonatal Neurocritical Care Service has not been studied.
This cohort study examines the association between medication use in term neonates with moderate-severe hypoxic-ischemic encephalopathy (HIE) and seizures that were managed before and after implementation of a Neonatal Neurocritical Care Service, which included a therapeutic hypothermia program, prolonged, continuous video-EEG (cEEG) monitoring, seizure management guidelines, and physician and nursing education. We hypothesized that detection and treatment of seizures without clinical correlate would be offset by decreased treatment of non-seizure clinical events and, as such, we would observe no change in cumulative phenobarbital dose among neonates with HIE and seizures monitored with prolonged EEG after the establishment of a Neonatal Neurocritical Care Service as compared to those managed prior to the new service.
Methods
Subjects
Neonates admitted to the University of California, San Francisco (UCSF) Benioff Children’s Hospital Intensive Care Nursery (ICN) were considered for inclusion in the study. The ICN database, which contains information on all neonates, was compiled prospectively in a systematic manner using a protocol and pre-determined variable definitions in accordance with the California Perinatal Quality Care Collaborative (CPQCC). The ICN database was continuously sampled for a diagnosis of HIE. The charts of those neonates diagnosed with HIE were assessed for study inclusion. UCSF’s Committee on Human Research approved waiver of consent and data collection.
Inclusion Criteria
The study population was neonates with moderate-severe encephalopathy who had clinical and/or electrographic seizure(s), admitted to the UCSF ICN from July 2004 to December 2011, and who met or would have met our institutional criteria for treatment with therapeutic hypothermia. Therapeutic hypothermia was instituted on November 1, 2007. Our criteria for treatment are similar to those of the randomized trials,16,17 as follows: (1) birth at ≥ 36 weeks postmenstrual age; (2) the presence of one or more of the following: an Apgar score <5 at 10 minutes of life, a history of prolonged resuscitation at birth, the presence of severe acidosis defined as a cord pH, or first gas (within 60 minutes of birth) pH of <7.0, or a base deficit of greater than −12 from cord blood, or first gas; and (3) the presence of moderate-severe encephalopathy identified by the attending neonatologist or pediatric neurologist within the first 6 hours after birth.
Exclusion Criteria
Subjects were excluded for the following reasons: (1) ineligible for therapeutic hypothermia due to birth weight <1800 grams; coagulopathy with active bleeding; required extracorporeal membrane oxygenation (ECMO) prior to 6 hours of life; severe congenital anomalies; syndromes or known metabolic disorders; (2) missing Medication Administration Records; (3) enrolled in a blinded study that randomized patients to prophylactic phenobarbital versus placebo after resolution of acute symptomatic seizures.
Selection and Group Assignment
The neonatal monitoring program -- which includes cEEG and concurrent bedside display of a simplified montage aEEG -- was initiated coincident with implementation of the therapeutic hypothermia. Prior to this date, seizure monitoring typically consisted of brief EEG for ≤2 hours when clinical suspicion of seizures arose. In contrast, neonates evaluated after the implementation of our program were continuously monitored with conventional video EEG applied according to the 10–20 system, modified for neonates, from the time of admission at least until rewarming was complete, or until the neonate was 24 hours free of EEG seizures (in keeping with recommendations from the ACNS10). A multidisciplinary Neonatal Neurocritical Care Service was established in July 2008, 9 months after implementation of therapeutic hypothermia and monitoring, which resulted in increased involvement of neurologists in the care of neonates, implementation of standardized seizure treatment guidelines (which recommend phenobarbital as the first-line medication up to total bolus dosing of 40–50mg/kg, followed by fosphenytoin and levetiracetam as second and third line, and early discontinuation of medications after resolution of EEG confirmed seizures), and specialized training for neonatal bedside nurses, among other systemic changes.18,19 Neonates born prior to implementation of the Neonatal Neurocritical Care Service were in the “pre-Neonatal Neurocritical Care Service era” group and neonates born on or after this date were in the “post-Neonatal Neurocritical Care Service era” group. Data from a subset of subjects were previously reported.20
Measurements
Patient demographics were extracted from the UCSF ICN database and systematic chart review. Encephalopathy severity was assigned based on chart documentation of the worst mental status observed during the 7 days following birth, according to CPQCC guidelines. Neonates who were not responsive to arousal maneuvers were designated as having severe encephalopathy, and neonates who were hyperalert and/or lethargic were designated as having moderate encephalopathy. Neonates with unclear encephalopathy severity (n=6) due to poor documentation or use of paralytic medication were categorized as having moderate encephalopathy.
Neonates who were treated with therapeutic hypothermia received whole-body cooling via a cooling unit and blanket (CSZ Blanketrol III, Cincinnati). Rectal temperature remained at 33.5 ± 0.5°Celsius for 72 consecutive hours. Morphine was given throughout cooling to provide adequate sedation and to minimize shivering.
Physician, nursing, transport, and referring hospital notes were reviewed to determine observation of seizure prior to hospital discharge. Clinical events that were suspicious for seizure were determined based on Mizrahi and Kellaway characterizations,21 and electrographic seizure diagnosis was based on EEG reports written by neurophysiologists blinded to the study hypothesis. Seizure monitoring in the pre-Neonatal Neurocritical Care Service era was at the discretion of the treating physician, typically video-EEG for ≤2 hours when clinical suspicion of seizure arose. In contrast, seizure monitoring in the post-Neonatal Neurocritical Care Service era involved continuous monitoring with both aEEG and conventional video-EEG applied according to the international 10–20 system, modified for neonates, from the time of admission until the completion of rewarming or until the neonate was 24 hours seizure-free, whichever was shorter.22 In both eras, initiation of seizure medication treatment was at the discretion of the treating neonatologist and neurologist, typically after a suspected seizure (and never prophylactically) with the goal of treating seizures (not achieving a specific drug level). Details regarding dosages were abstracted from Medication Administration Records. Total cumulative phenobarbital dose was defined as the total medication dose that a subject received from the time of birth until hospital discharge, including boluses and maintenance dosing. Seizure burden was categorized based on the following 3 definitions: neonates with status epilepticus (continuous seizure activity for at least 30 minutes or recurrent seizures for over 50% of 1–3 hours of recording time)23 those with ≥5 seizures (clinical and/or electrographic) and who failed to meet criteria for status epilepticus were said to have “many seizures”; and those with <5 total seizures were said to have “few seizures.”
Study data were collected and managed using REDCap (Research Electronic Data Capture).24
Analysis
Unadjusted comparisons between the groups were conducted using chi-squared and Fisher’s exact tests (for categorical variables with expected counts ≥10 and counts < 10, respectively), and the t-test for numeric variables with approximately normal distributions, and rank-sum tests for numeric variables with non-normal distributions.
The differences in cumulative phenobarbital dose and days on phenobarbital maintenance between groups were determined using multiple linear regression models adjusted for seizure burden category. The percent change in cumulative phenobarbital dose was determined using a linear regression model of the log-transformation of cumulative phenobarbital dose (with 10 added to avoid issues with zero doses), adjusted for seizure burden. The odds ratio of being discharged on a seizure medication was determined using a multiple logistic regression model adjusted for seizure burden. Seizure burden was a priori identified as a potential confounder since evidence suggested therapeutic hypothermia may decrease seizure burden,25,26 and the majority of neonates (80%) were treated with therapeutic hypothermia in the post-Neonatal Neurocritical Care Service era, while 8% were treated with hypothermia in the pre-Neonatal Neurocritical Care Service era.
The fit of the models were checked for linearity, normality, constant variance, influential points, and covariate overlap. Of note, bootstrap confidence intervals based on 1000 repeats were calculated for all regressions given that residuals were slightly skewed right. These estimates were essentially identical to the 95% confidence intervals from the models and assured us that models were robust to violations of normality. Potential influential points were identified using DFBETA calculations. Sensitivity analysis revealed that exclusion of these points minimally changed point estimates and did not affect qualitative conclusions. Thus, no data points were excluded from analysis.
For all analyses, p-values <0.05 were considered significant and all tests were two-sided. Analyses were performed using Stata 12 (StataCorp, College Station, Texas).
Results
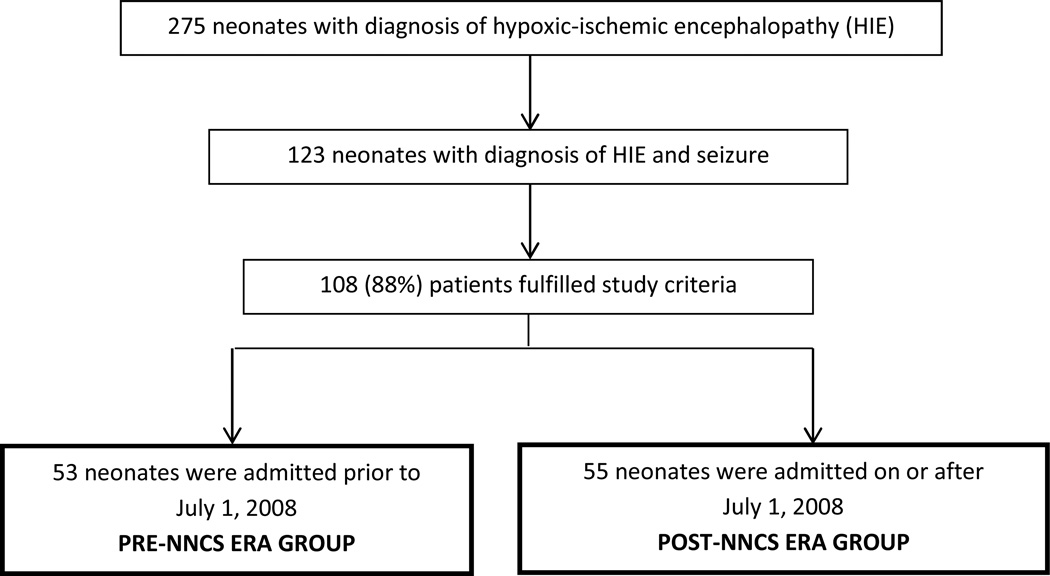
Of 123 neonates diagnosed with HIE and seizure(s), 108 (88%) met study criteria (Figure 1). Fifteen patients were excluded: 5 had an HIE event secondary to post-natal cardiopulmonary arrest, 2 had no documented encephalopathy during the first 6 hours after birth, 3 did not meet criteria for perinatal asphyxia, 2 had severe congenital anomalies, syndromes, or known metabolic disorders, 1 had coagulopathy with active bleeding, 1 had a missing Medication Administration Record, and 1 was enrolled in a study that randomized patients to prophylactic phenobarbital versus placebo after resolution of acute symptomatic seizures.
Figure 1.
Flow diagram depicting patient selection and group assignments. NNCS Neonatal Neurocritical Care Service.
Fifty-three neonates were categorized as pre-Neonatal Neurocritical Care Service era group, and 55 were categorized as post-Neonatal Neurocritical Care Service era group. The majority of neonates born in the pre-Neonatal Neurocritical Care Service were not treated with therapeutic hypothermia (92%), while 3 (6%) received complete cooling therapy and 1 (2%) received partial cooling therapy (terminated due to transition to palliative care). Within the post-Neonatal Neurocritical Care Service era group, 44 (80%) completed the therapeutic hypothermia protocol, 8 (15%) partially completed the therapeutic hypothermia protocol, and 3 (5%) were not treated with therapeutic hypothermia due to late referral or late recognition of HIE. In the 9 cases of partial treatment, therapeutic hypothermia was stopped prior to 72 hours due to severe cardiopulmonary instability or transition to palliative care. The UCSF Neonatal Neurocritical Care Service evaluated all neonates born in the post-Neonatal Neurocritical Care Service era. The 4 neonates born prior to the launch of the service who received hypothermia were monitored by cEEG and evaluated by a Pediatric Neurologist from admission through completion of hypothermia treatment, however the full model of neurocritical care was not yet established, and treatment was not based on consensus guidelines.19
Baseline characteristics of neonates show that 10-minute Apgar scores were lower and encephalopathy severity was higher in the post-Neonatal Neurocritical Care Service compared with pre-implementation era group (Table 1).
Table 1.
Characteristics of 108 neonates with seizures due to hypoxic-ischemic encephalopathy after and before implementation of a Neonatal Neurocritical Care Service.
| Post- Neonatal Neurocritical Care Service Era (n=55) |
Pre- Neonatal Neurocritical Care Service Era (n=53) |
p-value | |
|---|---|---|---|
| Male sex | 27(49) | 35(66) | 0.08 |
| Gestational age, weeks | 39.6 ± 1.4 | 39.7 ± 1.5 | 0.8 |
| Cesarean section | 31(56) | 27(51) | 0.6 |
| 10-minute Apgar score | 4(3 to 5) | 6(4 to 7) | 0.001 |
| Base Excess* | −18 ± 7 | −18 ± 7 | 0.6 |
| Encephalopathy Severity | 0.03 | ||
| -Moderate | 32(58) | 41(77) | |
| -Severe | 23(42) | 12(23) | |
| Therapeutic hypothermia | 52 (95%) | 4 (8%) | <0.001 |
| Seizure Burden** | 0.4 | ||
| -Few Seizures (<5) | 30(55) | 36(68) | |
| -Many Seizures (>5) | 19(35) | 13(25) | |
| Status Epilepticus | 6(11) | 4(8) | |
| Length of stay, days | 8(6 to 11) | 13(6 to 19) | 0.01 |
| Death before hospital discharge | 16(29) | 11(21) | 0.3 |
Values are given in terms of n (%), mean ± standard deviation, or median (25th percentile to 75th percentile).
Values based on worst base excess from cord gas or first blood gas.
Seizure based on chart documentation of clinical events that were suspicious for seizure(s) or on electroencephalogram (EEG) confirmed seizures.
In the post-Neonatal Neurocritical Care Service era, fewer neonates were first diagnosed with seizures based on clinical manifestations alone (45% versus 85%; p<0.001), and approximately one-third of neonates were first diagnosed with seizures based on EEG monitoring alone (33% versus 4%; p<0.001). Of note, neonates with only seizures without clinical correlate were almost only observed in the Neonatal Neurocritical Care Service era (27% versus 2%; p<0.001).
In both eras, the majority of neonates with seizures were treated with a single seizure medication (89% post- versus 81% pre-Neonatal Neurocritical Care Service era; p=0.3) and 2 concurrent seizure medications at most (7% versus 17%; p=0.1). Nearly all neonates received phenobarbital (95% versus 96%; p=0.7). Total phenobarbital loading dose did not vary between eras (30 (95% CI 26 to 34) versus 33 (95% CI 29 to 37) mg/kg; p=0.3). Neonates born in the Neonatal Neurocritical Care Service era, on average, received 27 mg/kg less cumulative phenobarbital than those managed prior to the Neonatal Neurocritical Care Service (95% CI 10 to 43 mg/kg; p=0.002; 30 mg/kg less after adjustment for seizure burden 95% CI 15 to 46 mg/kg; p<0.001).
This amounted to a >30% reduction (95% CI 16 to 46%; p=0.001). This effect was not due to cooling alone: subjects treated with therapeutic hypothermia did receive a lower cumulative phenobarbital dose (p=0.01), however the effect of cooling was no longer present after adjustment for Neonatal Neurocritical Care Service era (0.5 [95% CI −32 to 33) mg/kg more cumulative phenobarbital, p=0.97), whereas the effect of the interventions associated with the Neonatal Neurocritical Care Service remained near significant (p=0.05) with no change in estimated effect size. Those born in the Neonatal Neurocritical Care Service era were treated with maintenance phenobarbital for an average of 5 fewer days compared with those in the pre- Neonatal Neurocritical Care Service era (95% CI 3 to 7; p<0.001; no change after adjustment for seizure burden, 95% CI 3 to 8 days; p<0.001). Survivors were also much less likely to be maintained on a seizure medication following hospital discharge in the Neonatal Neurocritical Care Service era as compared with the pre-Neonatal Neurocritical Care Service era (8% versus 55%; OR=0.04, 95% CI 0.01 to 0.2; p<0.001, after adjustment for seizure burden).
Among those neonates who survived until hospital discharge (n=81), the association between Neonatal Neurocritical Care Service management and phenobarbital use was similar (37 [95% CI 17–57] mg/kg less cumulative phenobarbital, p<0.001; and 7 [95% CI 4–10] fewer days on therapy, p<0.001, after adjustment for seizure burden). Among survivors with only clinical events that were suspicious for seizures (i.e., without documented EEG seizures) (n=38), the estimated effect sizes were larger (39 [95% CI 3–74] mg/kg less cumulative phenobarbital, p=0.03; and 8 [95% CI 2–13] fewer days on therapy, p=0.01, after adjustment for seizure burden). In this subgroup, 27% (3/11) in the Neonatal Neurocritical Care Service era versus 74% (20/27) in the pre-Neonatal Neurocritical Care Service era were continued on maintenance phenobarbital in the absence of an EEG-confirmed seizure (p=0.007).
Discussion
Implementation of a Neonatal Neurocritical Care Service alters seizure treatment decisionmaking, and is associated with a clinically relevant reduction in cumulative phenobarbital dose among neonates with seizures due to HIE. In spite of increased sensitivity for seizure detection during the Neonatal Neurocritical Care Service era due to improved monitoring using cEEG and increased vigilance by bedside practitioners, there was a decrease in cumulative phenobarbital dose (with no difference in total bolus doses), shorter duration of maintenance dosing, and fewer neonates discharged home on a seizure medication. In addition, among survivors with clinical events that were suspicious for seizures only (i.e., no EEG confirmed seizures), the reduction in cumulative phenobarbital dose and days on maintenance therapy were more substantial, suggesting reduced harm by more accurate identification of who should and should not receive treatment. Finally, subjects managed by the Neonatal Neurocritical Care Service had a shorter length of stay, although this study was not designed to account for all potential confounders for this outcome.
Our findings are in keeping with previous studies showing that specialized neurocritical care, and a bundled care approach can improve outcomes.1,2,27 However, like other studies that examine the impact of neurocritical care, the implementation of our service involved a number of changes, including cEEG, as well as education for bedside physicians and nurses, and seizure management guidelines; thus, a single causal explanation for reduced medication use cannot be definitively identified.
Two previous studies have reported on prolonged monitoring and seizure medication use in neonates. In their single-center observational study, Shellhaas et al report that aEEG implementation did not alter seizure medication use compared to no aEEG in a population of neonates with seizures.14 Specifically, there were no differences between the groups in terms of the number of seizure medications per patient, nor the proportion of patients who were maintained on phenobarbital after discharge home. Similarly, a small, multicenter RCT reported no difference in the number of seizure medications per patient among neonates with HIE monitored with aEEG compared to those whose caregivers were blinded to aEEG.15 Our data also showed no differences between the eras in terms of the number of seizure medications per patient. In contrast, our study revealed a significantly lower proportion of patients maintained on phenobarbital at the time of discharge from the hospital. The reasons for the difference may be related to added certainty in treatment decision making due to use of cEEG (rather than the aEEG simplified montage); the fact that our population was more homogenous in terms of seizure etiology (a physician may be more likely to stop a medication in a neonate with seizures due to HIE as compared with other etiologies); or due to other changes related to the implementation of the Neonatal Neurocritical Care Service, for example education of nurses and physicians, or implementation of guidelines.
We recognize that our study has limitations related to its retrospective design. First, measurements were based on chart review and, while data extraction was standardized, variation in chart documentation limited our ability to account for all potential confounders. Second, we and others have reported that therapeutic hypothermia may be associated with a reduction in seizures20,25,26; we addressed this potential confounder by adjusting for seizure burden. Since our clinical practice is to treat only detected seizures, any change in seizure frequency that was related to hypothermia should be accounted for by the adjustment. Furthermore, the effect of therapeutic hypothermia was not significant after adjustment for Neonatal Neurocritical Care Service, suggesting no effect of hypothermia alone. Finally, studies examining the effect of therapeutic hypothermia on phenobarbital pharmacokinetics have yielded mixed results, with 2 reporting no clinically relevant effect, and a third reporting higher levels and prolonged half-life of the medication.28–30 As such, we cannot exclude an effect of prolonged phenobarbital metabolism on our results, however, since the effect of therapeutic hypothermia was not significant, we expect that the reduction in phenobarbital is also independent of phenobarbital phamacokinetics.
In spite of these limitations, this study provides evidence that implementation of a Neonatal Neurocritical Care Service (which included a therapeutic hypothermia program, prolonged, continuous video-EEG (cEEG) monitoring, seizure management guidelines, and physician and nursing education) is associated with decreased phenobarbital use, even in the setting of an increased ability to detect seizures due to cEEG monitoring, and added vigilance by the bedside practitioner. Current practice at most centers is to conduct brief EEG monitoring (up to 1–2 hours) in the setting of clinical events that are suspected seizures, and medical treatment (typically with phenobarbital and with or without EEG confirmation of seizures), for 1–6 months on average, following discharge from hospital.31 The reduction in phenobarbital use associated with implementation of the Neonatal Neurocritical Care Service may benefit patients by reducing exposure to potentially harmful effects of this medication. We hypothesize that cEEG provided treating clinicians the input and reassurance needed for a more tailored approach to seizure treatment decision-making, as opposed to the less targeted approach used prior to implementation of continuous monitoring, and that enriched monitoring data from cEEG may provide treating physicians with a clearer understanding of presence of seizures (and whether treatment is required), seizure control, and response to seizure medications. The additional information likely alters management by treating physicians, who can reduce harm by limiting or discontinuing phenobarbital in neonates who display clinical events but never have EEG confirmed seizures, as well as after resolution of electrographic seizures. Additionally, cEEG monitoring can identify neonates who are experiencing electrographic seizures without overt clinical manifestations. Absent monitoring, seizures in these neonates may not be identified and treated.
Additional studies are needed to investigate the utility of the individual components of a Neonatal Neurocritical Care Service, including prolonged, continuous video-EEG monitoring, education and implementation of guidelines, as well as the impact on long-term neurodevelopmental outcomes.
ACKNOWLEDGMENTS
We would like to thank Jessica Kan for her contributions to data collection, Dr. Donna Ferriero for careful review of the manuscript, and the CHIH Foundation Award in Neuroscience for investing in young researchers. Amy J. Markowitz, J.D. provided editorial support.
Dr. Glass receives research funding from NIH K23NS066137 and the Pediatric Epilepsy Research Foundation, has received travel expenses and/or honoraria for lectures not funded by industry, and has received payment for medical-legal review work. She serves on the editorial board of Pediatric Neurology.
FUNDING
The National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1TR000144, supported SOW. NIH/NINDS K23NS066137, the Pediatric Epilepsy Research Foundation and the Neonatal Brain Research Institute at UCSF support HCG. SLB is supported by K23NS082500. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
AUTHOR CONTRIBUTIONS
Sharon O Wietstock, MSc - Drafted and revised the manuscript for content, participated in study concept and design, planned and performed analysis and interpretation of data, performed statistical analysis, and approved the submitted manuscript.
Sonia L Bonifacio, MD - Revised the manuscript for content, assisted with interpretation of data, and approved the submitted manuscript.
Charles E McCulloch, PhD - Revised the manuscript for content, participated in study concept and design, planned and performed analysis and interpretation of data, assisted with interpretation of data, and approved the submitted manuscript.
Michael Kuzniewicz, MD, MPH - Revised the manuscript for content, participated in study concept and design, assisted with interpretation of data, and approved the submitted manuscript.
Hannah C Glass, MDCM, MAS- Drafted and revised the manuscript for content, participated in study concept and design, assisted with analysis and interpretation of data, performed statistical analysis, and approved the submitted manuscript.
Ms. Orbach reports no disclosures.
Dr. Bonifacio reports no disclosures.
Dr. McCulloch reports no disclosures.
Dr. Kuzniewicz reports no disclosures.
ETHICAL APPROVAL
UCSF Committee on Human Research approved waiver of consent and data collection.
REFERENCES
- 1.Kramer AH, Zygun DA. Do neurocritical care units save lives? Measuring the impact of specialized ICUs. Neurocrit Care. 2011;14:329–333. doi: 10.1007/s12028-011-9530-y. [DOI] [PubMed] [Google Scholar]
- 2.Kramer AH, Zygun DA. Neurocritical care: why does it make a difference? Current opinion in critical care. 2014;20:174–181. doi: 10.1097/MCC.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 3.Annegers JF, Hauser WA, Lee JR, et al. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935–1984. Epilepsia. 1995;36:327–333. doi: 10.1111/j.1528-1157.1995.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 4.Tekgul H, Gauvreau K, Soul J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–1280. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- 5.Malone A, Ryan CA, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–2101. doi: 10.1111/j.1528-1167.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 6.Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–261. doi: 10.1111/j.1528-1157.1988.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 7.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341:485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 8.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Annals of the New York Academy of Sciences. 2003;993:103–114. doi: 10.1111/j.1749-6632.2003.tb07517.x. discussion 23-4. [DOI] [PubMed] [Google Scholar]
- 9.Maitre NL, Smolinsky C, Slaughter JC, et al. Adverse neurodevelopmental outcomes after exposure to phenobarbital and levetiracetam for the treatment of neonatal seizures. J Perinatol. 2013;33:841–846. doi: 10.1038/jp.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society's Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011;28:611–617. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 11.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claassen J, Taccone FS, Horn P, et al. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive care medicine. 2013;39:1337–1351. doi: 10.1007/s00134-013-2938-4. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz P, Hanafy KA, Claassen J. Continuous EEG monitoring: is it ready for prime time? Current opinion in critical care. 2009;15:99–109. doi: 10.1097/MCC.0b013e3283294947. [DOI] [PubMed] [Google Scholar]
- 14.Shellhaas RA, Barks AK. Impact of amplitude-integrated electroencephalograms on clinical care for neonates with seizures. Pediatr Neurol. 2012;46:32–35. doi: 10.1016/j.pediatrneurol.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Rooij LG, Toet MC, van Huffelen AC, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125:e358–e366. doi: 10.1542/peds.2009-0136. [DOI] [PubMed] [Google Scholar]
- 16.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 17.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 18.Glass HC, Bonifacio SL, Shimotake T, et al. Neurocritical care for neonates. Curr Treat Options Neurol. 2011;13:574–589. doi: 10.1007/s11940-011-0144-7. [DOI] [PubMed] [Google Scholar]
- 19.Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care for neonates. Neurocrit Care. 2010;12:421–429. doi: 10.1007/s12028-009-9324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orbach SA, Bonifacio SL, Kuzniewicz MW, et al. Lower Incidence of Seizure Among Neonates Treated With Therapeutic Hypothermia. J Child Neurol. 2013 doi: 10.1177/0883073813507978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–1844. doi: 10.1212/wnl.37.12.1837. [DOI] [PubMed] [Google Scholar]
- 22.Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76:556–562. doi: 10.1212/WNL.0b013e31820af91a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisani F, Cerminara C, Fusco C, et al. Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology. 2007;69:2177–2185. doi: 10.1212/01.wnl.0000295674.34193.9e. [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low E, Boylan GB, Mathieson SR, et al. Cooling and seizure burden in term neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F267–F272. doi: 10.1136/archdischild-2011-300716. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasakumar P, Zempel J, Wallendorf M, et al. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr. 2013;163:465–470. doi: 10.1016/j.jpeds.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Weaver SJ, Dy SM, Rosen MA. Team-training in healthcare: a narrative synthesis of the literature. BMJ quality & safety. 2014 doi: 10.1136/bmjqs-2013-001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shellhaas RA, Ng CM, Dillon CH, et al. Population pharmacokinetics of phenobarbital in infants with neonatal encephalopathy treated with therapeutic hypothermia. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14:194–202. doi: 10.1097/PCC.0b013e31825bbbc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filippi L, la Marca G, Cavallaro G, et al. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: a pharmacokinetic study during whole body hypothermia. Epilepsia. 2011;52:794–801. doi: 10.1111/j.1528-1167.2011.02978.x. [DOI] [PubMed] [Google Scholar]
- 30.van den Broek MP, Groenendaal F, Toet MC, et al. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. Clinical pharmacokinetics. 2012;51:671–679. doi: 10.1007/s40262-012-0004-y. [DOI] [PubMed] [Google Scholar]
- 31.Guillet R, Kwon JM. Prophylactic phenobarbital administration after resolution of neonatal seizures: survey of current practice. Pediatrics. 2008;122:731–735. doi: 10.1542/peds.2007-3278. [DOI] [PubMed] [Google Scholar]